Abstract
In this study, Ca2+ mediated NO signalling was studied in response to metalloid (As) stress in Brassica seedlings. Arsenic toxicity strongly suppressed the growth (fresh weight, root and shoot length), photosynthetic pigments, Chl a fluorescence indices (Kinetic traits: Fv, Fm, Fv/Fo, Fm/Fo, ФPo or Fv/Fm, Ψo, ФEo, PIABS, Area and N and redox status (AsA/DHA and GSH/GSSG ratios) of the cell; whereas energy flux traits: ABS/RC, TRo/RC, ETo/RC and DIo/RC along with Fo, Fo/Fv, Fo/Fm, ФDo and Sm) were enhanced. Further, addition of EGTA (Ca2+ scavenger) and LaCl3 (plasma membrane Ca2+ channel blocker) to As + Ca; while c‒PTIO (NO scavenger) and l‒NAME (NO synthase inhibitor) to As + SNP treated seedlings, siezed recovery on above parameters caused due to Ca2+ and NO supplementation, respectively to As stressed seedlings thereby indicating their signalling behaviour. Further, to investigate the link between Ca2+ and NO, when c‒PTIO and l‒NAME individually as well as in combination were supplemented to As + Ca treated seedlings; a sharp inhibition in above mentioned traits was observed even in presence of Ca2+, thereby signifying that NO plays crucial role in Ca2+ mediated signalling. In addition, As accumulation, ROS and their indices, antioxidant system, NO accumulation and thiol compounds were also studied that showed varied results.
Subject terms: Plant sciences, Plant physiology
Introduction
Arsenic (As) exposure has become a major threat for world agriculture that causes adverse effect on crop productivity by inhibiting cell functioning. The metalloid (As) mainly arises from geothermal weathering of rocks and human activities1. The toxicity of As depends on As species. Among the two inorganic species, arsenite (AsIII), which prevails in anaerobic environment, enters in plant system through aquaporin channels with greater affinity for thiol groups; while arsenate (AsV) prevalent in aerobic condition/ soil, being analogue of inorganic phosphate (iP), enters via inorganic phosphate transporters, competes with phosphate and replaces phosphate from ATP thereby affecting the energy metabolism of cell2,3. Arsenic accumulation causes growth suppression which involves many biochemical and physiological changes in plants including oxidative stress, injuries to membrane and thereby affecting redox state of the cell4–6, which is detrimental for plant survival under As toxicity. Additionally, As toxicity negatively regulates chlorophyll (Chl) biosynthesis, PS II photochemistry and ribulose 1,5‒bisphosphate carboxylase/ oxygenase (RuBisCO) activity, thereby inhibiting photosynthetic efficiency6–9. Previous studies have explicated the prominence of upholding a favourable antioxidants level and redox status of cell to encounter the damage caused due to metalloid exposure4–6.
Calcium (Ca2+) is a pervasive and pivotal secondary messenger in signal transduction network10 under both stressed and non‒stressed situations. In several studies, it has been shown that Ca2+ is involved in regulation of plant responses such as photosynthetic electron transport rate, enzyme activities of Calvin cycle and activities of key antioxidant enzymes5,11,12, stabilizing the structural integrity of membranes by making bond with phospholipid bilayer13 under various environmental stresses including As. Nitric Oxide (NO), on the other hand is a bioactive gaseous free radical and also as inter and intracellular signalling molecule, and regulates numerous biochemical, physiological and molecular processes in plants under variable conditions4,14,15. Peto et al.16 reported that NO application encounters excessive ROS production by metal/ metalloids in two ways: either acting as a free radical reacting with ROS to neutralize them or as a signalling molecule initiating gene expression in molecular cascade. In proteins, NO induced post‒translational modification is carried out by nitrosylation of their cysteine residue. Wu et al.17 reported that NO application improves photosynthetic rate by (i) channelizing excess energy by increasing carotenoid or other antenna molecules and (ii) increasing quantum yield of PS II, under salinity stress in Solanum melongena seedlings.
From the available literature, it is clear that both Ca2+ and NO plays multiple roles in regulating key physiological processes in stressed as well as non‒stressed situations; however, their cumulative effect in orchestrating plant responses to different environmental cues have not been well established. Therefore, future studies are needed to understand the intensive interaction and interrelation of Ca2+ and NO in various physiological, histochemical and metabolic approaches suffering from arsenic toxicity in plants. Towards this objective, the key components i.e. growth and growth regulating parameters: Chl a fluorescence, redox status of cell, enzymatic and non‒enzymatic antioxidants and levels of thiol compounds were assessed in the present investigation.
Results and discussion
Ca2+ and NO recover As‒induced damage in phenotypic appearance
To examine the Ca2+ mediated NO signalling in alleviating As‒induced toxicity, Brassica L. seedlings were treated with different donors, scavengers and inhibitors of Ca2+ and NO. As expected, metalloid (As) stress caused deteriorating effect on growth and declined the fresh weight, root and shoot length by 29, 33 and 28%, respectively of test seedlings (Fig. 1), which is manifested by increased reactive oxygen species (ROS) production that caused lipid peroxidation, protein oxidation and loss to membrane integrity leading to electrolyte leakage (Figs. 2a,b). However, under As stress, both CaCl2 and SNP treatment counteracted As‒induced negative impact on FW, RL and SL of test seedlings (Figs. 1a‒c), which agree with the greater accumulation of NO than As‒stressed seedlings alone (Fig. 3). The Ca2+ and NO induced positive response on growth have also been reported by Singh et al.5 and Siddiqui et al.15 in mustard and tomato seedlings, respectively. Further, a significant inhibition of growth after EGTA and LaCl3 treatment indicated that both of them arrests Ca2+‒induced positive impact on growth agreeing the fact of involvement of Ca2+ as signalling molecule. Knight et al.11 have also reported that lanthanum (La) and EGTA inhibit the salt‒and mannitol‒induced (Ca2+)cyt elevations in Arabidopsis. In a previous study, Xu et al.18 have reported that ABA protects tall fescue plant from oxidative injuries by promoting NO release (via activating NOS) thereby triggering the activities of antioxidant enzymes. Therefore, also in order to study the possible link between Ca2+ and NO signalling, As + Ca treated Brassica seedlings were treated with NO scavenger: c‒PTIO and synthase inhibitor: l‒NAME. Interestingly, the growth of As + Ca treated seedlings was abolished in presence of c‒PTIO and l‒NAME (Figs. 1a‒c), suggesting that NO is required for the maximal and sustained signalling of Ca2+, which corresponds to reduced NO accumulation in presence of c‒PTIO and l‒NAME (Fig. 3). Lanteri et al.19 also reported that NO is required for the maximal activity of Ca2+‒dependent protein kinase (CDPK) for adventitious root formation in Cucumis sativus. Results of As accumulation suggests that both CaCl2 and SNP counteracted the As accumulation in test seedlings; however in presence of c‒PTIO and/or l‒NAME even Ca2+ was unable in restricting As accumulation, therefore higher As content was observed in c‒PTIO + l‒NAME treated seedlings than As‒stressed seedlings alone (Fig. 1d).
Figure 1.
Effect of CaCl2(Ca2+) and SNP (NO) on the phenotypic appearance: (A) fresh weight, (B) root length, (C) shoot length and (D) As content of As‒challenged Brassica seedlings subjected to different modulators (EGTA: ethylene glycol‒bis(2‒aminoethylether)‒N,N,N′,N′‒tetraacetic acid, a Ca scavenger, LaCl3: lanthanum chloride, a plasma membrane Ca channel blocker, c‒PTIO: 2‒4‒carboxyphenyl‒4,4,5,5‒tetramethylimidazoline‒1‒oxyl‒3‒oxide, a NO scavenger and L‒NAME: Nω‒nitro‒L‒arginine methyl ester hydrochloride, a nitric oxide synthase inhibitor). Data signifies the mean ± standard error of five replicates. Bars followed by different letters are significantly different at p < 0.05 level according to Tukey test. Where ‘nd’ is ‘no detection’ of As.
Figure 2.
(a) Effect of CaCl2 and SNP on the contents: (A) super oxide radical (SOR), (B) hydrogen peroxide (H2O2), (C) malondialdehyde (MDA) equivalents and (D) electrolyte leakage of the leaves of As‒challenged Brassica seedlings subjected to different modulators. Data signifies the mean ± standard error of five replicates. Bars followed by different letters are significantly different at p < 0.05 level according to Tukey test. (b) Histochemical detection of (A) SOR, (B) H2O2 and (C) lipid peroxidation (MDA equivalents) and (D) loss of membrane integrity (electrolyte leakage) showing the effect of CaCl2 and SNP on the leaves of As‒challenged Brassica seedlings subjected to different modulators. [(I) Control, (II) As, (III) As + Ca, (IV) As + Ca + EGTA, (V) As + Ca + EGTA + LaCl3, (VI) As + Ca + c‒PTIO, (VII) As + Ca + l‒NAME, (VIII) As + Ca + c‒PTIO + l‒NAME, (IX) As + SNP, (X) As + SNP + c‒PTIO and (XI) As + SNP + l‒NAME].
Figure 3.
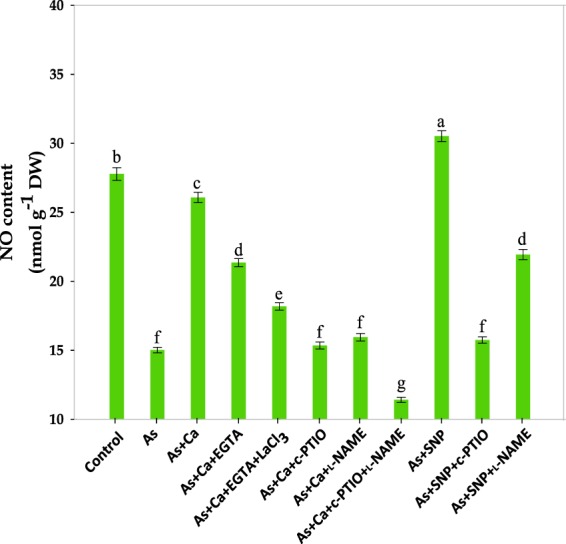
Effect of different modulators of Ca2+ and NO on NO content of the leaves of As‒challenged Brassica seedlings. Data signifies the mean ± standard error of five replicates. Bars followed by different letters are significantly different at p < 0.05 level according to Tukey test.
Ca2+ and NO rescue As‒induced losses of Chls and Car and recovers photosynthetic rate and PS II photochemistry
To further elucidate the role of NO in Ca2+‒induced signalling on photosynthetic performance, photosynthetic pigments: Chls (a and b) and Car were examined. Arsenic induced reduction in the levels of Chl a, b and Car was in accordance with the reduced plant growth (Fig. 1), signifying that As impaired photosynthetic ability of plants (Table 1) by disrupting chloroplast structure and pigments’ biosynthesis6. Calcium and NO, on the other hand, rescued the As‒induced loss in photosynthetic pigments content that partly attributed to lowering of ROS, which might have prevented photo‒oxidative damage of photosynthetic apparatus14,15. However, As‒induced damage was severe when As + Ca treated seedlings were supplemented either with EGTA + LaCl3 or c‒PTIO + l‒NAME, which corresponds to the decreased values of photosynthetic rate (Table 1).
Table 1.
Effect of CaCl2 and SNP on the levels of photosynthetic pigments content: chlorophyll (Chl) a, Chl b and carotenoids (Car) and photosynthetic performance of As‒challenged Brassica seedlings subjected to different modulators.
| Treatments | Pigments content (µg mg−1 FW) | Photosynthesis (µmol O2 evolved (g FW)−1 h−1) | ||
|---|---|---|---|---|
| Chl a | Chl b | Car | ||
| Control | 1.285 ± 0.020a | 0.428 ± 0.006a | 0.313 ± 0.005a | 32.17 ± 0.53a |
| As | 1.111 ± 0.016cd | 0.312 ± 0.004d | 0.282 ± 0.004cd | 18.72 ± 0.24g |
| As + Ca | 1.187 ± 0.018b | 0.361 ± 0.006bc | 0.293 ± 0.005ab | 30.25 ± 0.43b |
| As + Ca + EGTA | 1.106 ± 0.014cd | 0.311 ± 0.004d | 0.274 ± 0.004cd | 16.15 ± 0.22h |
| As + Ca + EGTA + LaCl3 | 1.031 ± 0.016d | 0.301 ± 0.004d | 0.262 ± 0.003d | 12.04 ± 0.19i |
| As + Ca + c‒PTIO | 1.147 ± 0.018bc | 0.343 ± 0.005c | 0.284 ± 0.004bc | 22.64 ± 0.34ef |
| As + Ca + l‒NAME | 1.164 ± 0.020b | 0.350 + ± 0.006bc | 0.284 + ± 0.004bc | 24.05 ± 0.40de |
| As + Ca + c-PTIO + l‒NAME | 1.070 ± 0.016cd | 0.293 ± 0.004d | 0.264 ± 0.003cd | 15.89 ± 0.26h |
| As + SNP | 1.179 ± 0.017b | 0.363 ± 0.006bc | 0.290 ± 0.005b | 27.42 ± 0.39c |
| As + SNP + c‒PTIO | 1.133 ± 0.015bc | 0.374 ± 0.006b | 0.276 ± 0.003 cd | 21.01 ± 0.26f |
| As + SNP + l‒NAME | 1.169 ± 0.017b | 0.359 ± 0.004bc | 0.291 ± 0.005b | 25.81 ± 0.41cd |
Data signifies the mean ± standard error of five replicates. Values within same column followed by different subscripts are significantly different at p < 0.05 level according to Tukey test.
Further, to reveal the Ca2+ mediated NO role in structural and functional properties of PS II, the OJIP transient curves as well as biophysical traits deduced from OJIP were studied. A sharp drop in O‒J, J‒I and I‒P transient curves, which denotes the sequential reduction of electron acceptor pool of PS II, indicates that PS II was the major target of As (Fig. 4a). The decline in O‒J‒I‒P transient curve could be attributed to damage at the donor side of PS II restricting the flow of electron between OEC and PS II20–24. Moreover, the drop in OJIP transient curves was intense upon c‒PTIO + l‒NAME supplementation to As + Ca treated seedlings indicating that in absence of NO, the effect of As became more severe (even in presence of Ca2+), which could be due to: (i) inhibition in electron transport rate on the donor side of PS II as reflected by decreased values for area over the fluorescence curve (Area), which consequently decreased the maximum quantum yield for primary photochemistry (ФPo) thereby leading to accumulation of P680+ (Fig. 4b)17,25 and (ii) decline in the size and number of active photosynthetic RCs (Fv/Fo), disrupting electron transfer beyond QA− thus, higher initial fluorescence (Fo) was measured in presence of NO scavenger and NOS inhibitor. Accordingly, a certain suppression in the values for Ψo (that designates trapped exciton moves an electron into the ETC beyond QA−) and the quantum yield of electron transport (ФEo) was detected (Fig. 4b), which consequently declined the pool size of QA− (acceptor side of the PS II17,26,27); suggesting lethargic flow of electrons from PS II to PS I and restriction of QA− reoxidation (QA−‒QA), which could be associated with poor diffusion of PQ across the thylakoid membranes28. Indeed, the higher Fo/Fm designates that QA reduction rate was much higher than its reoxidation rate by QB and PS I activity under As + Ca + c‒PTIO + l‒NAME treatment. Increasing values for the quantum yield of energy dissipation (ФDo) and dissipated energy flux (DIo/RC) (Fig. 4b) under As + Ca + c‒PTIO + l‒NAME and As + Ca + EGTA + LaCl3 treatment, advocate that excess excitation energy was converted to thermal dissipation in order to maintain the energy balance between absorption and consumption, and thus minimize the potential of photo‒oxidative damage8. The decrease in Fm/Fo parameter reflects the damaging effect of As on the structural integrity of the PS II RCs29. Further, the increased Sm (refers the pool of electron transporters between PS II and the acceptor side of PS I) value under As + Ca + c‒PTIO + l‒NAME and As + Ca + EGTA + LaCl3 implies that heterogeneity of PQ increased the electron donation capacity and QA reduction on acceptor side of PS II, suggesting that As along with c‒PTIO + l‒NAME and EGTA + LaCl3 decreased the total electron accepting capacity30.
Figure 4.
Effect of CaCl2 and SNP on the (a) chlorophyll a fluorescence OJIP transient curves and (b) spider plots for OJIP parameters inferred from chlorophyll a fluorescence OJIP transient in the leaves of As‒challenged Brassica seedlings subjected to different modulators. Data signifies the mean ± standard error of five replicates. Bars followed by different letters are significantly different at p < 0.05 level according to Tukey test. (c). The leaf model representing phenomenological energy fluxes per excited cross section (CS) in the leaves of As‒challenged Brassica seedlings subjected to different modulators. [(I) Control, (II) As, (III) As + Ca, (IV) As + Ca + EGTA, (V) As + Ca + EGTA + LaCl3, (VI) As + Ca + c‒PTIO, (VII) As + Ca + L‒NAME, (VIII) As + Ca + c‒PTIO + L‒NAME, (IX) As + SNP, (X) As + SNP + c‒PTIO and (XI) As + SNP + L‒NAME]. The relative value for the measured parameters is the mean of quintuplicates (n = 5). The width of arrow corresponds to the intensity of flux parameters; ABS/CS: absorption flux per CS, TR0/CS: trapped energy flux per CS, ET0/CS, electron transport flux per CS and DI0/CS: dissipated energy flux per CS. Circles embedded in circle (RC/CS) are percentage of active/inactive RCs, where white circles are representing reduced QA RCs (active) and red circles non‒reducing QA RCs (inactive). RCs: reaction centres as described by Sitko et al.62.
The progressive drop in overall performance of PS II (PIABS) upon NO scavenger and NOS inhibitors’ treatment could have resulted from the inactivity of RCs (Fv/Fo), and these RCs then changes into ‘energy sinks/heat sink’, that absorb light but were unable to store the excitation energy and dissipate total energy as heat/fluorescence as deduced by ФPo (Fv/Fm), which consequently changes the average antenna size linked to each active RC (ABS/RC14,31). Therefore, ABS/RC was found to increase under above situation because the reduced number of active RCs favour for increasing the necessary numbers of RC turnovers for complete reduction of the PQ pool (N) (Fig. 4b). The TRo/RC, which refers only to active RCs (QA‒QA−), was increased suggesting that either (i) all the QA might have been reduced (QA−) but were not able to oxidize back (QA) or (ii) the reoxidation of QA− (QA−‒QA) was inhibited under Ca2+ and NO scavenger/synthase inhibitor treated As stressed seedlings, so that QA was unable to transfer electrons efficiently to QB31. The DIo/RC, which reflects the ratio of the dissipation of untrapped excitation energy from all the RCs with respect to the number of active RCs, was increased (Fig. 4b) due to higher energy dissipation from the active RCs under As toxicity14. Furthermore, increased Fo/Fv refers to damaging effects on OEC, which could be due to the decline in uptake of mineral nutrients like Mn, an important component of OEC6, as it suggested by Samborska et al.24 that mineral nutrient deficiency tends to affect the fluorescence parameters variably.
The leaf model for phenomenological energy fluxes showed that As toxicity caused an increase in absorption flux per CS (ABS/CS), trapped energy flux per CS (TR0/CS), electron transport flux per CS (ET0/CS) and dissipated energy flux per CS (DI0/CS) along with the number of inactive/closed RCs (RC/CS) (Fig. 4c).
Calcium and NO, on the other hand alleviated the negative impact of As by restoring the structural attributes of PS II as favoured by increased ФPo, Fm/Fo and Fv/Fo and decreased Fo/Fv values. The positive role of Ca2+ on ФPo may due to the mineral ion homeostasis as discussed by Ahmad et al.13 in tomato seedlings. Upon CaCl2 and SNP application, improvement in Fv parameter was strongly supported by a reduction in Fo, which favoured imitation of the PS II acceptor side30. Furthermore, an improvement in the electron transport rate of the photosynthetic ETC was noticed as deduced from the high Fv/Fo values (Fig. 4b). The restoration in Fm value upon CaCl2 and SNP application suggested that either they might have increased Mn ion and extrinsic proteins of OEC, which affected the electron donation from water to PS II32 or might have caused the conformational changes in D1 protein, thereby altering the properties of PS II electron acceptors33, which augmented PS II activity6. The NO might have improved the electron transport rate from OEC to D1 protein and gene expression belongs to core reaction center (Psb) of PSII complex such as psbA, psbB and psbC as argued by Chen et al.14 in heat‒stressed tall fescue leaves. Upon CaCl2 and SNP addition, a sharp drop in ABS/RC, TRo/RC, ETo/RC and DIo/RC (Fig. 4b) specify that PS II apparatus was able to tackle the balance of energy fluxes for absorption, trapping and transport of electrons through active PS II RCs under As toxicity14,34 thereby improving overall performance of PS II, as agreed with higher PIABS values, which can also be supported by improved Area value under similar conditions (Fig. 4b).
Ca2+ and NO improve antioxidant defense system to counteract As‒induced oxidative stress and injuries
In our study, the excess accumulation of As in leaf tissues of Brassica seedlings exhibited severe oxidative stress as evident by enhanced ROS: O2˙− and H2O2 levels (Fig. 2). The As‒induced ROS production caused oxidative injuries by peroxidizing lipid membranes together with loss of membrane integrity which were correlated with significant increase in electrolyte leakage and MDA equivalents levels (Figs. 2a‒b). Further, CaCl2 and SNP addition counteracted the As‒induced loss in cell structure and function by decreasing ROS and the indices of damage as evident by increased NO and decreased As content (Figs. 1d and 3). The increased NO might have formed a less toxic peroxynitrite (ONOO−)16,35 or induced various ROS‒scavenging enzyme activities like superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) (Figs. 5a‒c) as was discussed by Siddiqui et al.15 and Lu et al.36. Interestingly, c‒PTIO and l‒NAME application arrested the effect of Ca2+ and NO in As‒stressed seedlings, which was further confirmed by in‒vivo staining for SOR, H2O2, lipid peroxidation and injury of plasma membrane integrity in leaf tissues (Figs. 2a,b). To overcome the deleterious effect of As, the activities of enzymatic antioxidants: SOD and CAT were found to increase, which were further enhanced upon CaCl2 and SNP addition and more importantly, still an increment in the enzyme activities was noticed after c‒PTIO, l‒NAME, EGTA and LaCl3 treatment (Figs. 5a,b). The increased SOD and CAT activities, which established the frontline enzymatic network that dismutate O2˙− into H2O2 consecutively into H2O, speeded the reduction in ROS accumulation but it was not sufficient to overcome the massive As‒induced c‒PTIO, l‒NAME, EGTA and LaCl3 mediated ROS accumulation; therefore higher ROS accumulation were still noticed (Figs. 2a,b) under these conditions.
Figure 5.
Effect of CaCl2 and SNP on the activities of enzymatic antioxidants: (A) superoxide dismutase (SOD), (B) catalase (CAT), (C) ascorbate peroxidase (APX), (D) dehydroascorbate reductase (DHAR) and (E) glutathione reductase (GR) of the leaves of As‒challenged Brassica seedlings subjected to different modulators. Data signifies the mean ± standard error of five replicates. Bars followed by different letters are significantly different at p < 0.05 level according to Tukey test.
Ca2+ and NO recover As‒induced losses of ascorbate and glutathione contents and maintain redox status
Ascorbate and glutathione are the important redox buffering agents, therefore were analyzed in the present study to know the redox status of the cell. The current study showed that, As seriously impaired the ROS detoxification process by reducing the contents of AsA and GSH along with their redox states: AsA/DHA and GSH/GSSG ratios (Table 2). Upon addition of NO chelator (c‒PTIO) and inhibitor (l‒NAME), further reduction in the contents of AsA and GSH and ratios of AsA/DHA and GSH/GSSG was reported even in presence of Ca2+ (Table 2). Moreover, the activity of APX, which reduces H2O2 to DHA on the expense of AsA, was increased under similar conditions (Fig. 5c) indicating that although APX activity was efficient for H2O2 detoxification; however, this was not enough to counteract H2O2 induced damage, which is obvious from the results of MDA content and electrolyte leakage (Fig. 2). In contrast to this, DHAR activity which recycled DHA into AsA in presence of GSH, was found to decrease suggesting the insufficient regeneration of AsA from DHA; therefore much lower AsA content was noticed under As + Ca + EGTA + LaCl3 and As + Ca + c‒PTIO + L‒NAME treatment and obviously, low AsA/DHA ratio was found (Table 2). The DHAR enzyme is susceptible to high H2O2 concentration37; therefore upon addition of NO chelator/synthesis inhibitor, a remarkable inhibition in DHAR activity was noticed that might have altered the rate of AsA‒GSH cycle, which is apparent from decreased AsA/DHA ratio (Table 2). Calcium and NO, on the other hand restored and up‒regulated DHAR activity to maintain the higher level of AsA; therefore higher AsA/DHA ratio was noticed (Table 2), as was also argued by Ahmad et al.13. Further, during the conversion of DHA into AsA, two molecules of GSH by donating electron converted into GSSG, which in‒turn re‒reduced into GSH by glutathione reductase (GR) enzyme38. In the present investigation, a significant reduction in the content of GSH and subsequent increment in GSSG was found upon As treatment, and effect was more intense under NO scavenger and synthesis inhibitor, thereby causing a severe reduction in GSH/GSSG ratio (Table 2). The decrease in GSH content might either be due to decrease in GSH recycling rate or increase in its degradation rate, as excessive GSH needed during DHA to AsA conversion38. Interestingly, GR activity was increased upon c‒PTIO and l‒NAME treatment (Fig. 5e), which suggests that it was not sufficient to manage the huge GSH consuming effect of As, such as GSH conjugation for GST and PCs synthesis (As‒PCs complex); therefore low GSH/GSSG ratio was obtained (Table 2). Contrastingly, Ca2+ and NO improved the GSH pool by speeding up the rate of GSH recycling from GSSG, which is evident by increased GR activity (Fig. 5e), thus greater GSH/GSSG ratio was obtained to encounter As toxicity, as also suggested by Ahmad et al.13 and Praveen and Gupta4.
Table 2.
Effect of CaCl2 and SNP on the contents of non‒enzymatic antioxidants: ascorbate (AsA: reduced and DHA: dehydroascorbate), glutathione (GSH: reduced and GSSG: oxidized) and their redox status (AsA/DHA and GSH/GSSG) and content of thiol compounds (cysteine: Cys, non‒protein thiols: NPTs and phytochelatins: PCs) of As‒challenged Brassica seedlings subjected to different modulators.
| Treatments | Contents (nmol g—1 FW) | Redox ratios | Content of thiol compounds (nmol g—1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AsA | DHA | GSH | GSSG | AsA/DHA | GSH/GSSG | Cys | NPTs | PCs | |
| Control | 1455 ± 25a | 106.5 ± 1.8h | 585.5 ± 9.8a | 46.74 ± 0.78i | 13.66 ± 0.22a | 12.52 ± 0.21a | 62.15 ± 1.04g | 761.1 ± 12.7i | 128.8 ± 2.2g |
| As | 1142 ± 15fg | 189.1 ± 2.5cd | 487.7 ± 6.5cd | 82.01 ± 1.09d | 6.04 ± 0.08fg | 5.94 ± 0.07g | 107.67 ± 1.43b | 867.2 ± 11.5h | 297.5 ± 3.9f |
| As + Ca | 1408 ± 20ab | 127.1 ± 1.8g | 566.9 ± 8.2a | 54.89 ± 0.79h | 11.08 ± 0.15b | 10.32 ± 0.14b | 71.38 ± 1.03f | 982.8 ± 14.2fg | 361.1 ± 5.2e |
| As + Ca + EGTA | 1104 ± 15fg | 198.2 ± 2.7bc | 471.0 ± 6.5de | 89.71 ± 1.24c | 5.57 ± 0.07gh | 5.25 ± 0.07h | 113.78 ± 1.57b | 1121.0 ± 15.5bc | 560.4 ± 7.8b |
| As + Ca + EGTA + LaCl3 | 1049 ± 17g | 241.1 ± 3.9a | 442.5 ± 7.1e | 105.88 ± 1.71a | 4.34 ± 0.07i | 4.17 ± 0.06i | 131.89 ± 2.13a | 1191.0 ± 19.3a | 642.6 ± 10.4a |
| As + Ca + c‒PTIO | 1219 ± 18de | 171.8 ± 2.6ef | 512.6 ± 7.7bc | 68.91 ± 1.03ef | 7.09 ± 0.10e | 7.44 ± 0.10e | 95.73 ± 1.43c | 1062.2 ± 15.9de | 480.6 ± 7.2d |
| As + Ca + l‒NAME | 1270 ± 21cd | 158.7 ± 2.7f | 519.4 ± 8.7bc | 63.67 ± 1.06fg | 8.00 ± 0.13d | 8.15 ± 0.13d | 86.89 ± 1.45d | 1037.1 ± 17.4ef | 454.0 ± 7.6d |
| As + Ca + c‒PTIO + l‒NAME | 1063 ± 18g | 208.9 ± 3.5b | 455.4 ± 7.6de | 97.16 ± 1.62b | 5.09 ± 0.08h | 4.68 ± 1.07hi | 126.83 ± 2.12a | 1143.0 ± 19.1ab | 590.5 ± 9.9b |
| As + SNP | 1371 ± 20ab | 139.2 ± 2.0g | 549.0 ± 7.9ab | 61.56 ± 0.88g | 9.84 ± 0.14c | 8.92 ± 0.12c | 78.53 ± 1.13ef | 994.4 ± 14.4fg | 383.8 ± 5.5a |
| As + SNP + c‒PTIO | 1169 ± 15ef | 184.4 ± 2.3de | 493.9 ± 6.3cd | 74.48 ± 0.94e | 6.34 ± 0.08f | 6.63 ± 0.08f | 98.90 ± 1.25c | 1087.1 ± 13.8cd | 518.6 ± 6.6c |
| As + SNP + l‒NAME | 1358 ± 22bc | 134.1 ± 2.2g | 564.4 ± 9.1a | 58.07 ± 0.93gh | 10.12 ± 0.16c | 9.72 ± 0.15b | 81.23 ± 1.31de | 944.8 ± 15.3gh | 322.3 ± 5.2f |
Data signifies the mean ± standard error of five replicates. Values within same column followed by different subscripts are significantly different at p < 0.05 level according to Tukey test.
Ca2+ and NO rescue As‒induced damage by up‒regulating synthesis of thiol compounds
The thiol compounds: Cys, NPTs and PCs act as first barrier against As toxicity thereby reducing the injurious effect to plants2,4. In the present investigation, the content of Cys, NPTs and PCs were found to increase under As stress and further enhancement was noticed when EGTA + LaCl3 or c‒PTIO + L‒NAME were supplemented to As + Ca stressed seedlings (Table 2), which might be due to their demand for Fe–S cluster of photosynthetic apparatus39, protein synthesis, stabilizing tertiary structures of protein, synthesis of GSH, hydroxymethyl‒PCs and other low molecular weight compounds3,36. Moreover, high PCs content demands more GSH to counteract the stressed situation and also higher As accumulation stimulates NPTs for scavenging, by using available GSH pool; thus lower GSH content was obtained upon EGTA + LaCl3 or c‒PTIO + L‒NAME supplementation to As + Ca treated seedlings (Table 2). Additionally, CaCl2 and SNP treatment to As‒stressed test seedlings also showed an improvement in the contents of thiol compounds thereby justifying their role in reducing As toxicity by promoting peptides and proteins to chelate metalloid, which is corroborated with earlier findings of Lu et al.36 and Praveen and Gupta4 in Amaranthus hypochondriacus and Oryza sativa seedlings, respectively.
Table S2 shows the correlation between treatments and tested parameters in the B. juncea L. seedlings. All the treatments affected all the tested parameters significantly. The results clearly showed that arsenic negatively affected the growth and other growth regulating parameters, while Ca and SNP showed positive correlation with growth. Further, addition of EGTA and LaCl3 to As + Ca; while c‒PTIO and l‒NAME to As + SNP treated seedlings significantly declined the growth (as depicted by negative correlation). Further when c‒PTIO and l‒NAME individually as well as in combination were supplemented to As + Ca treated seedlings, more negative values for pearson correlation were observed thereby signifying that NO plays crucial role in Ca2+ mediated signalling.
Materials and methods
Experimental plant, growth conditions and treatments
Healthy seeds of Brassica juncea were surface sterilized with 5% (v/v) sodium hypochlorite (NaOCl) for 5 min, rinsed with distilled water (DW) and left overnight in dark for 48 h by wrapping them in a wet muslin cloth. The germinated seeds were sown in plastic cups having acid sterilized sand and kept in darkness for two days at 25 ± 1 °C. Seedlings were then transferred and allowed to grow in plant growth chamber (CDR model GRW‒300 DGe, Athens, Greece) having photosynthetically active radiation (PAR) of 150 μmol photons m−2 s−1 with 16:8 h day‒night regime and 65–70% relative humidity at 22 ± 1 °C. Seedlings were irrigated with 50% Hoagland and Arnon40 solution on alternate days. After 25 days, seedlings were uprooted and acclimatized in 50% Hoagland solution for 24 h. After that, three healthy and uniform sized seedlings were transferred in each plastic cup having 50 ml of Hoagland solution with or without different combinations of donor, scavenger and inhibitors (doses of As, Ca and NO were selected on the basis of screening experiments; the experimental plants showing phenotypic variation as per the treatments have been shown in Supplementary Fig. S1). All the chemicals were prepared in 50% Hoagland solution. In hydroponic system, following combinations were made: (i) control (nutrient solution alone), (ii) As 50 µM, (iii) As + Ca, (iv) As + Ca + EGTA, (v) As + Ca + EGTA + LaCl3, (vi) As + Ca + c‒PTIO, (vii) As + Ca + l‒NAME, (viii) As + Ca + l‒NAME + c‒PTIO, (ix) As + SNP, (x) As + SNP + c‒PTIO and (xi) As + SNP + l‒NAME. Sodium arsenate (Na2HAsO4.7H2O: a source of As; 50 µM), calcium chloride (CaCl2: a Ca2+ donor; 12 mM), sodium nitroprusside (SNP: a NO donor; 100 µM), ethylene glycol‒bis(2‒aminoethylether)‒N,N,N′,N′‒tetraacetic acid (EGTA: a Ca2+ scavenger; 0.10 mM), 2‒4‒carboxyphenyl‒4,4,5,5‒tetramethylimidazoline‒1‒oxyl‒3‒oxide (c‒PTIO: a NO scavenger; 0.10 mM), lanthanum chloride (LaCl3: a plasma membrane Ca2+ channel blocker; 0.10 mM) and Nω‒nitro‒L‒arginine methyl ester hydrochloride (l‒NAME: a NO synthase inhibitor; 0.10 mM) were used as metal stress, donor, scavenger and inhibitors. Each treatment was performed in five sets and were transferred in growth chamber under similar condition as mentioned above. The seedlings were aerated regularly with air bubbler to avoid hypoxia condition and were harvested after four days of the treatments to examine the Ca2+ and NO‒induced mechanisms in modulating As‒induced responses.
Growth analysis
After four days of the treatments, growth of Brassica seedlings was analyzed by measuring fresh weight (FW), root length (RL) and shoot length (SL). The FW of the seedling was recorded by single pan digital balance (Model CA 223, Contech, India), while RL and SL were recorded by meter scale.
Estimation of As content
For the estimation of As content, 100 mg dried plant samples from each treatment were digested in tri‒acid mixture (HClO4:H2SO4:HNO3::1:1:5 ratio, v/v) at 80 °C according to the method of Allen et al.41. Arsenic content in digested sample was estimated by atomic absorption spectrometer (AAS, iCE 3000 series, model–3500, Thermo Scientific, UK).
Estimation of photosynthetic pigment contents
After extracting 20 mg fresh leaves in chilled acetone (80%), absorbance of the supernatants was recorded at 470, 646 and 663 nm to determine the Chls and carotenoid contents according to the formulas suggested by Lichtenthaler42.
Assay of photosynthesis and PS II photochemistry (polyphasic fast chlorophyll a fluorescence induction and JIP‒kinetics)
Photosynthesis
The rate of photosynthetic oxygen yield in leaves of test seedlings was measured using Clark type oxygen electrode (Digital Oxygen System, Model‒10, Rank Brothers, UK) in terms of oxygen evolution in presence of light as suggested by Kurra‒Hotta et al.43.
Polyphasic fast chlorophyll a fluorescence induction and JIP‒kinetics
To check the performance of photosynthesis, Chl a fluorescence measurements were carried out in 30 min dark‒adapted leaves using leaf fluorometer (FluorPen FP 100, Photon System Instrument, Czech Republic). The analysis of OJIP transient took into consideration by measuring the fluorescence values at 50 μs (FO, step O), 2 ms (F2ms, step J), 30 ms (F30ms, step I) and maximal level (FM, step P). The shape of OJIP rise shows the complexity of reduction kinetic of PS II (acceptor side). The O‒J favours photochemical reduction of QA (primary electron acceptor), J‒I agrees with the QB quenching mechanism or complete closure of PS II reaction centre (RC) and I‒P favours reduction of pool and size of final electron acceptor of PS I22.
The following biophysical parameters deduced from OJIP transient curves were calculated: (1). Technical/absolute parameters: (i) initial fluorescence (Fo), (ii) maximum fluorescence (Fm), (iii) variable fluorescence (Fv; fraction of total number of closed RCs), (iv) number of QA redox turnover until Fm is reached (N) and (v) area above the fluorescence induction curve between Fo and Fm, reflecting the size of plastoquinone (PQ) pool (Area); (2). Quantum yields and efficiencies/probabilities of PS II: (i) quantum yield of primary photochemistry (ФPo or Phi_Po), (ii) yield of electron transport per trapped exciton (the probability that a trapped exciton moves an electron into the electron transport chain beyond QA−; Ψo or Psi_o), (iii) quantum yield of electron transport (ФEo or Phi_Eo) and (iv) the probability that an absorbed photon is dissipated (ФDo); (3). Specific energy fluxes or activities per RC (per QA reducing PS II RC): (i) total absorption by PS II antenna Chls divided by the number of active (in sense of QA reduction) RC (ABS/RC; refers average antenna size), (ii) trapped energy flux per active RC [refers only to active (QA‒QA−) RCs; TRo/RC], (iii) electron transport flux from QA− to plastoquinone (PQ) per active RC (ETo/RC), (iv) total dissipation to the amount of active RCs (DIo/RC) and (v) energy necessary for the closure of all the RCs (Sm); (4). Structural and functional heterogeneity of PS II: (i) antenna heterogeneity is associated with QA reduction in relation to electron flow to PQ pool or (ii) reducing side (functioning of reduction side i.e. QB− reducing and non—reducing centres): (a) efficiency of water splitting complex (Fo/Fv), (b) the rate of oxidation/reduction of PQ (Fo/Fm), (c) size and number of active photosynthetic reaction centres (Fv/Fo) and the ratio of fluorescence yields for open and closed states (Fm/Fo); (5). Overall performance index (PIABS; the potential for energy conservation from photons absorbed by PS II to the reduction of intersystem electron acceptors) following the method of Strasser et al.20 and kalaji et al.27.
Estimation of reactive oxygen species (ROS: SOR and H2O2) and indices (MDA equivalents content and electrolyte leakage) of damage
Biochemical analysis
The estimation of ROS: superoxide radical (SOR: O2˙−) and hydrogen peroxide (H2O2) contents were adopted from Elstner and Heupel44 and Velikova et al.45 and the amount was calculated with the help of standard curve of NaNO2 and H2O2, respectively. The estimation of indices of damage: lipid peroxidation (measured in terms of MDA equivalents content) and loss of membrane integrity (measured in terms of electrolyte leakage) in leaf tissues were adopted from Heath and Packer46 and Gong et al.47, respectively.
Histochemical detection
To perform the histochemical detection for SOR, H2O2, lipid peroxidation and loss of membrane integrity, nitro blue tetrazolium (NBT; 0.1%), 3,3′‒diaminobenzidine (DAB; 1%), Schiff’s reagent and Evan’s blue tests were carried out as suggested by Frahry and Schopfer48, Thordal‒Christensen et al.49, Pompella et al.50 and Yamamoto et al.51, respectively. After staining, leaves were bleached with boiling ethanol and photographed.
Estimation of activities of enzymatic antioxidants
The extraction and estimation of superoxide dismutase (SOD; EC 1.15.1.1) activity in presence of riboflavin and methionine was adopted from Giannopolitis and Ries52, which is mainly based on photoreduction of NBT. Catalase (CAT; EC 1.11.3.6) activity was assayed in presence of H2O2 as suggested by Aebi53 using an extinction coefficient (ϵ) 39.4 mM−1 cm−1. Ascorbate peroxidase (APX; EC 1.11.1.11) activity was assayed in presence of ascorbate and H2O2 as suggested by Nakano and Asada54 using (ϵ) of 2.8 mM−1 cm−1. Estimation of dehydroascorbate reductase (DHAR; EC 2.5.1.18) activity is based on the reduction of dehydroascorbate (DHA) into reduced ascorbate (AsA) as suggested by Nakano and Asada54 and the activity was determined using (ϵ) of 7.0 mM−1 cm−1. The assay of glutathione reductase (GR; EC 1.6.4.2) activity is mainly based on oxidation of NADPH in presence of oxidized glutathione (GSSG) using (ϵ) of 6.2 mM−1 cm−1 as suggested by Schaedle and Bassham55.
One unit (U) of activity of SOD is the amount of SOD required to inhibit 50% NBT, CAT (U) is equivalent to 1 nmol H2O2 dissociated min−1, APX (U) is 1 nmol ascorbate oxidized min−1, DHAR (U) is 1 nmol DHA reduced min−1 and GR (U) is defined as 1 nmol NADPH oxidized min−1.
Estimation of non‒enzymatic antioxidants: ascorbate, glutathione and their redox status
The estimation of contents of ascorbate: reduced (AsA) and oxidised (dehydroascorbate: DHA) was carried out in acidic solution, which is mainly based on Fe3+ reduction into Fe2+ following the method of Gossett et al.56. The estimation of glutathione: reduced (GSH) and oxidized (GSSG) contents were based on the sequential oxidation of GSH by 5,5′‒dithiobis‒2‒nitrobenzoic acid (DTNB) into trinitrobenzoic acid (TNB) as suggested by Brehe and Burch57.
Estimation of thiol compounds: cysteine, non‒protein thiols and phytochelatins
Estimation of cysteine (Cys) content was done in presence of glacial acetic acid (GAA), acid ninhydrin and toluene following the method of Gaitonde58. The content of non‒protein thiols (NPTs) was measured according to Ellman59 in presence of Ellman’s reagent. The amount of total phytochelatins (PCs) was calculated using the formula: total PCs = NPTs‒total GSH60.
Estimation of nitric oxide (NO) content
The NO content was determined using the method described by Zhou et al.61. The 500 mg fresh leaf tissues were homogenized in 50 mM acetic acid buffer (pH 3.6) containing zinc diacetate (4%) and centrifuged for 15 min at 4 °C. The absorbance of reaction mixture containing charcoal and 1 ml of Greiss reagent was monitoring at 540 nm and NO content in the mixture was calculated with the help of standard curve of NaNO2.
Statistical analysis
The experiments were performed in quintuplicates and the results displayed in figures and tables are the means ± standard error of the average values obtained from quintuplicates (n = 5) of individual experiment to check the reproducibility of result. The results were statistically analyzed by one‒way analysis of variance (ANOVA) using software ‘SPSS 16.0. Tukey alpha test was performed for the mean separation for significant differences among the treatments at p < 0.05 significance level. Pearson correlation coefficient (r) test was also applied to test the significance of treatments.
Conclusion
From the present study, it can be concluded that As inhibits growth of Brassica seedlings; Ca2+ and NO on the other hand recovered the growth and growth related parameters. However, with the addition of Ca2+ chelator (EGTA), plasma membrane Ca2+ channel blocker (LaCl3) as well as NO chelator (c‒PTIO) and synthetase inhibitor (L‒NAME), the improvement in growth caused by Ca2+ and NO was further arrested, which suggests that both are involve in signalling network. When NO chelator and synthase inhibitor were added to As‒stressed CaCl2 supplemented seedlings, a steep decline in growth promoting processes: photosynthetic activity, Chl a fluorescence, redox status (AsA/DHA and GSH/GSSG) of the cell was noticed even in presence of Ca2+, thereby signifying that physiological and biochemical attributes of Brassica seedlings are mostly regulated by intensive Ca2+ mediated NO signalling.
Supplementary information
Acknowledgements
Rachana Singh and Parul Parihar are obliged to the Department of Botany, University of Allahabad, Prayagraj and University Grants Commission (UGC), New Delhi for providing necessary lab facilities and Chemical grant, respectively to succeed the present work.
Author contributions
S.P. designed the experiment. R.S. and P.P. performed the experiment. S.P., R.S. and P.P. wrote and finalized the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rachana Singh and Parul Parihar.
Supplementary information
is available for this paper at 10.1038/s41598-020-62831-0.
References
- 1.Singh R, Singh S, Parihar P, Singh VP, Prasad SM. Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol. Environ. Saf. 2015;112:247–270. doi: 10.1016/j.ecoenv.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Raab A, Schat H, Meharg AA, Feldmann J. Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic–phytochelatin complexes during exposure to high arsenic concentrations. New Phytol. 2005;168:551–558. doi: 10.1111/j.1469-8137.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 3.Farooq MA, et al. Arsenic toxicity in plants: cellular and molecular mechanism of its transport and metabolism. Environ. Exp. Bot. 2016;132:42–52. doi: 10.1016/j.envexpbot.2016.08.004. [DOI] [Google Scholar]
- 4.Praveen A, Gupta M. Nitric oxide confronts arsenic stimulated oxidative stress and root architecture through distinct gene expression of auxin transporters, nutrient related genes and modulates biochemical responses in Oryza sativa L. Environ. Pollut. 2018;240:950–962. doi: 10.1016/j.envpol.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Parihar P, Prasad SM. Simultaneous exposure of sulphur and calcium hinder As toxicity: up–regulation of growth, mineral nutrients uptake and antioxidants system. Ecotoxicol. Environ. Saf. 2018;161:318–331. doi: 10.1016/j.ecoenv.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 6.Singh R, Parihar P, Prasad SM. Sulfur and calcium simultaneously regulate photosynthetic performance and nitrogen metabolism status in As–challenged Brassica juncea L. seedlings. Front. Plant Sci. 2018;9:772. doi: 10.3389/fpls.2018.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoeva N, Bineva T. Oxidative changes and photosynthesis in oat plants grown in As–contaminated soil. Bulg. J. Plant Physiol. 2003;29:87–95. [Google Scholar]
- 8.Wang S, Zhang D, Pan X. Effects of arsenic on growth and photosystem II (PS II) activity of Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2012;84:104–111. doi: 10.1016/j.ecoenv.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Farooq MA, et al. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016;7:468. doi: 10.3389/fpls.2016.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kudla J, Batistič O, Hashimoto K. Calcium signals: the lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313X.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- 12.Hochmal AK, Schulze S, Trompelt K, Hippler M. Calcium–dependent regulation of photosynthesis. Biochim. Biophys. Acta. 2015;1847:993–1003. doi: 10.1016/j.bbabio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad P, et al. Exogenous application of calcium to 24–epibrassinosteroid pretreated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018;8:13515. doi: 10.1038/s41598-018-31917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, K., Chen, L., Fan, J. & Fu, J. Alleviation of heat damage to photosystem II by nitric oxide in tall fescue. Photosynth. Res.116, 21–31 (2013). [DOI] [PubMed]
- 15.Siddiqui MH, et al. Exogenous application of nitric oxide and spermidine reduces the negative effects of salt stress on tomato. Hortic. Environ. Biotechnol. 2017;58:537–547. doi: 10.1007/s13580-017-0353-4. [DOI] [Google Scholar]
- 16.Peto A. Involvement of nitric oxide and auxin in signal transduction of copper–induced morphological responses in Arabidopsis seedlings. Ann. Bot. 2011;108:449–457. doi: 10.1093/aob/mcr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu, X. X. et al. Nitric oxide alleviates adverse salt–induced effects by improving the photosynthetic performance and increasing the antioxidant capacity of eggplant (Solanum melongena L.). J. Hortic. Sci. Biotech.88, 352–360 (2013).
- 18.Xu YF, et al. Nitric oxide mediates abscisic acid induced light–tolerance in leaves of tall fescue under high–light stress. Sci. Hortic. 2013;162:1–10. doi: 10.1016/j.scienta.2013.07.042. [DOI] [Google Scholar]
- 19.Lanteri ML, Pagnussat GC, Lamattina L. Calcium and calcium–dependent protein kinases are involved in nitric oxide–and auxin–induced adventitious root formation in cucumber. J. Exp. Bot. 2006;57:1341–1351. doi: 10.1093/jxb/erj109. [DOI] [PubMed] [Google Scholar]
- 20.Strasser, R. J., Srivastava, A. & Tsimilli–Michael, M. Fluorescence transient as a tool to characterize and screen photosynthetic samples in book, Probing photosynthesis: mechanisms, regulation and adaptation, Taylor and Francis, London, 445–483 (ed. M. Yunus, Pathre, U., Mohanty, P., Pathre, U. & Mohanty, P. (2000).
- 21.Kalaji HM, et al. Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth. Res. 2014;122:121–158. doi: 10.1007/s11120-014-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalaji HM, et al. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016;38:102. doi: 10.1007/s11738-016-2113-y. [DOI] [Google Scholar]
- 23.Kalaji HM, et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017;132:13–66. doi: 10.1007/s11120-016-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samborska IA, et al. Can just one-second measurement of chlorophyll a fluorescence be used to predict sulphur deficiency in radish (Raphanus sativus L. sativus) plants? Curr. Plant Biol. 2019;19:100096. doi: 10.1016/j.cpb.2018.12.002. [DOI] [Google Scholar]
- 25.Govindjee Sixty–three years since Kautsky: chlorophyll a fluorescence. Aust. J. Plant Physiol. 1995;34:1073–1079. [Google Scholar]
- 26.Kalaji HM, et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018;136:329–343. doi: 10.1007/s11120-017-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalaji HM, et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica. 2018;56:953–961. doi: 10.1007/s11099-018-0766-z. [DOI] [Google Scholar]
- 28.Magyar M, et al. Rate–limiting steps in the dark to light transition of photosystem II–revealed by chlorophyll–a fluorescence induction. Sci. Rep. 2018;8:2755. doi: 10.1038/s41598-018-21195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouzounidou G, Ilias I, Kabataidis M, Chatzimichail A. Comparative study of nutrient deficiencies on growth and photochemistry of tobacco. J. Plant Nutr. 2003;26:1605–1611. doi: 10.1081/PLN-120022371. [DOI] [Google Scholar]
- 30.Ghassemi–Golezani K, Lotfi R. The impact of salicylic acid and silicon on chlorophyll a fluorescence in mung bean under salt stress. Russ. J. Plant Physiol. 2015;62:611–616. doi: 10.1134/S1021443715040081. [DOI] [Google Scholar]
- 31.Paunov M, Koleva L, Vassilev A, Vangronsveld J, Goltsev V. Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018;19:787. doi: 10.3390/ijms19030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enami I, et al. Is the primary cause of thermal inactivation of oxygen evolution in spinach PS II membranes release of the extrinsic 33 kDa protein or of Mn. Biochim. Biophys. Acta. 1994;1186:52–58. doi: 10.1016/0005-2728(94)90134-1. [DOI] [Google Scholar]
- 33.Andréasson LE, Vass I, Styring S. Ca2+ depletion modifies the electron transfer on the both donor and acceptor sides in photosystem II from spinach. Biochim. Biophys. Acta. 1995;1230:155–164. doi: 10.1016/0005-2728(95)00047-M. [DOI] [Google Scholar]
- 34.Misra AN, Srivastava A, Strasser RJ. Utilization of fast chlorophyll a fluorescence technique in assessing the salt/ion sensitivity of mung bean and Brassica seedlings. J. Plant Physiol. 2001;158:1173–1181. doi: 10.1078/S0176-1617(04)70144-3. [DOI] [Google Scholar]
- 35.Zhao G, et al. Nitric oxide is required for melatonin–enhanced tolerance against salinity stress in rapeseed (Brassica napus L.) seedlings. Int. J. Mol. Sci. 2018;19:1912. doi: 10.3390/ijms19071912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, et al. Influences of calcium silicate on chemical forms and subcellular distribution of cadmium in Amaranthus hypochondriacus L. Sci. Rep. 2017;7:40583. doi: 10.1038/srep40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hossain MA, Asada K. Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol. 1984;25:1285–1295. [Google Scholar]
- 38.Foyer CH, Noctor G. Redox homeostasis and antioxidant signalling: a metabolic interface between stress perception and physiological responses. Plant Cell. 2005;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochaix JD. Assembly of the photosynthetic apparatus. Plant Physiol. 2011;155:1493–1500. doi: 10.1104/pp.110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoagland DR, Arnon DI. The water culture method for growing plants without soil. Cal. Agric. Exp. Station Cir. 1950;347:1–39. [Google Scholar]
- 41.Allen, S. E., Grimshaw, H. M. & Rowland, A. P. Chemical analysis in book, Methods in plant ecology, Blackwell Scientific Publication, Oxford, London, 285–344 (ed. Moore, P.D. & Chapman, S.B. (1986).
- 42.Lichtenthaler HK. Chlorophylls and carotenoids pigments of photosynthetic membranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- 43.Kurra–Hotta M, Satoh K, Katoh S. Relationship between photosynthesis and Chl content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987;28:1321–1329. [Google Scholar]
- 44.Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammonium chloride: a simple assay for superoxide dismutase. Ann. Biochem. 1976;70:616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- 45.Velikova, V., Yordanov, I. & Edreva, A. Oxidative stress and some antioxidant system in acid rain–treated bean plants. Plant Sci.151, 59–66 (2000).
- 46.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 47.Gong M, Li YJ, Chen SZ. Abscisic acid–induced thermo–tolerance in maize seedlings is mediated by calcium and associated with antioxidant system. J. Plant Physiol. 1998;153:488–496. doi: 10.1016/S0176-1617(98)80179-X. [DOI] [Google Scholar]
- 48.Frahry G, Schopfer P. NADH–stimulated, cyanide–resistant superoxide production in maize coleoptiles analysed with a tetrazolium based assay. Planta. 2001;212:175–183. doi: 10.1007/s004250000376. [DOI] [PubMed] [Google Scholar]
- 49.Thordal–Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 50.Pompella A, Maellaro E, Casini AF, Comporti M. Histochemical detection of lipid peroxidation in the liver of bromobenzene–poisoned mice. Am. J. Pathol. 1981;129:295–301. [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminium, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–220. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannopolitis CN, Ries SK. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 54.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 55.Schaedle M, Bassham JA. Chloroplasts glutathione reductase. Plant Physiol. 1977;59:1011–1012. doi: 10.1104/pp.59.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gossett DR, Millhollon EP, Cran LM. Antioxidant response to NaCl stress in salt–sensitive cultivars of cotton. Crop Sci. 1994;34:706–714. doi: 10.2135/cropsci1994.0011183X003400030020x. [DOI] [Google Scholar]
- 57.Brehe JE, Burch HB. Enzymatic assay for glutathione. Anal. Biochem. 1976;74:189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- 58.Gaitonde MK. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem. J. 1967;104:627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 60.Hartley–Whitaker J, et al. Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol. 2001;126:299–306. doi: 10.1104/pp.126.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou B, Guo Z, Xing J, Huang B. Nitric oxide is involved in abscisic acid–induced antioxidant activities in Stylosanthes guianensis. J. Exp. Bot. 2005;56:3223–3228. doi: 10.1093/jxb/eri319. [DOI] [PubMed] [Google Scholar]
- 62.Sitko, K. et al. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol.175, 290–302 (2017). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






