Abstract
DNA damage regulated autophagy modulator 1 (DRAM1) is a stress-inducible regulator of autophagy and cell death. DRAM1 has been implicated in cancer, myocardial infarction, and infectious diseases, but the molecular and cellular functions of this transmembrane protein remain poorly understood. Previously, we have proposed DRAM1 as a host resistance factor for tuberculosis (TB) and a potential target for host-directed anti-infective therapies. In this study, we generated a zebrafish dram1 mutant and investigated its loss-of-function effects during Mycobacterium marinum (Mm) infection, a widely used model in TB research. In agreement with previous knockdown analysis, dram1 mutation increased the susceptibility of zebrafish larvae to Mm infection. RNA sequencing revealed major effects of Dram1 deficiency on metabolic, immune response, and cell death pathways during Mm infection, and only minor effects on proteinase and metabolic pathways were found under uninfected conditions. Furthermore, unchallenged dram1 mutants did not display overt autophagic defects, but autophagic targeting of Mm was reduced in the absence of Dram1. The phagocytic ability of macrophages in dram1 mutants was unaffected, but acidification of Mm-containing vesicles was strongly reduced, indicating that Dram1 is required for phagosome maturation. By in vivo imaging, we observed that Dram1-deficient macrophages fail to restrict Mm during early stages of infection. The resulting increase in bacterial burden could be reverted by knockdown of inflammatory caspase a (caspa) and gasdermin Eb (gsdmeb), demonstrating pyroptosis as the mechanism underlying premature cell death of Mm-infected macrophages in dram1 mutants. Collectively, these data demonstrate that dissemination of mycobacterial infection in zebrafish larvae is promoted in the absence of Dram1 due to reduced maturation of mycobacteria-containing vesicles, failed intracellular containment, and consequent pyroptotic death of infected macrophages. These results provide new evidence that Dram1 plays a central role in host resistance to intracellular infection, acting at the crossroad of autophagy and cell death.
Subject terms: Autophagy, Cell death, Tuberculosis, Infection
Introduction
Autophagy is an intracellular degradation mechanism that functions to maintain homeostasis and intersects with the initiation of cell death programs when homeostasis is perturbed1,2. Autophagy can be induced by various stressors, such as nutrient deprivation and UV damage, but also infection. Detection of microbial invaders by the innate immune system activates the autophagy machinery to capture intracellular pathogens in double-membrane autophagosomes and target them to lysosomal degradation3. Autophagy proteins can also be recruited to single-membrane compartments when pathogens are engulfed by phagocytic cells3. These autophagic defense mechanisms form promising targets for the development of novel host-directed therapies for infectious diseases, many of which are currently complicated by the increasing occurrence of antibiotic resistance4,5. This is especially true for tuberculosis (TB), the most lethal infectious disease worldwide. The causative agents of human TB or TB-like disease in poikilothermic animals, Mycobacterium tuberculosis (Mtb) and Mycobacterium marinum (Mm), are widely studied to increase understanding of the role of autophagy in host defense3,6–10.
DNA damage regulated autophagy modulator 1 (DRAM1) is a stress-inducible regulator of autophagy and cell death. DRAM1 and other members of the DRAM family have been linked to cancer, myocardial infarction, HIV infection, and TB, but their molecular and cellular functions remain poorly understood6,11–16. Among the five DRAM family members, human DRAM1 was first identified as a p53-induced protein that localizes predominantly to lysosomes and promotes autophagic flux as well as UV-damage-induced apoptosis11. In response to mycobacterial infection, DRAM1 transcription is induced by nuclear factor kappa B (NFκB), a central hub in the signaling network regulating the immune system6. DRAM1 colocalizes with Mtb in infected human macrophages and is required for host resistance of zebrafish larvae against Mm infection6. Mtb and Mm share the RD1 virulence locus, required for ESX1-dependent escape from phagosomes into the cytosol17. Selective autophagy, dependent on ubiquitin receptors, such as p62, may counteract this pathogenic mechanism by delaying the escape process or sequestering cytosolic bacteria3,8. In addition, selective autophagy has been shown to deliver anti-microbial ubiquitinated peptides to bacteria-containing compartments18. We have recently shown that selective autophagy receptors are required to control Mm infection in zebrafish and that the host-protective function of Dram1 requires p62 in this infection model6,19.
Cytosolic escape of mycobacteria may accelerate the initiation of host cell death programs8. In TB, the death of an infected macrophage triggers its phagocytosis by other macrophages that subsequently also undergo cell death, resulting in a cascade of cell death events and the formation of inflammatory infection foci, called granulomas20. The fate of individual infected macrophages is therefore a major determinant of whether granulomas can contain the infection or facilitate dissemination of the infection. It has been previously shown that mycobacteria-infected macrophages can undergo several types of regulated cell death, like apoptosis, necroptosis, and pyroptosis, resulting in different infection outcomes21. Apoptosis of infected cells is generally regarded as a host-protective defense mechanism against mycobacterial infection, and virulent Mtb therefore actively inhibit apoptosis20,22,23. In contrast, necroptosis and pyroptosis are lytic forms of cell death that create an inflammatory environment that may facilitate extracellular growth and disease progression24,25.
While our previous work demonstrated a role for Dram1 in autophagic defense against mycobacterial infection, its potential implication in the regulation of cell death during TB pathogenesis has not been explored. In this study, we generated a dram1 mutant zebrafish line to address this question. Analysis of the mutant fish showed that Dram1 is required for maturation of Mm-containing vesicles and for macrophages to restrict Mm infection. Without functional Dram1, Mm-infected macrophages prematurely die via a mechanism dependent on inflammatory Caspase a (Caspa) and Gasdermin eb (Gsmdeb) activities, indicative of pyroptosis. Collectively, our data support that Dram1 protects against mycobacterial infection by modulating autophagic targeting and maturation of Mm-containing vesicles. In the absence of Dram1, infected macrophages rapidly become overburdened by the bacteria and initiate pyroptotic cell death, resulting in increased dissemination of the infection.
Results
dram1 null mutants display increased susceptibility to mycobacterial infection
To study the host resistance function of Dram1, we generated a zebrafish mutant line using CRISPR/Cas9 technology (Fig. S1a). The selected dram1∆19n/∆19n allele (designated dram1ibl53) contains a 21 nucleotides deletion combined with a two nucleotides insertion in the first coding exon (Figs. 1a and S1b), which results in undetectable levels of Dram1 protein, supporting that this represents a null allele (Fig. 1b). The mutant was outcrossed to transgenic lines with an autophagy reporter Tg(CMV:GFP-map1lc3b)26 or a macrophage marker Tg(mpeg1:mCherryF)27, hereafter referred to as GFP-Lc3 and mpeg1:mCherry. The offspring from incrossed heterozygous fish (dram1+/∆19n) strictly followed Mendelian inheritance (Fig. S1d, e), and homozygous mutants were fertile. Body size measurements indicated no apparent difference in development between dram1∆19n/∆19n and dram1+/+ larvae (Fig. S1c). Furthermore, the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay did not reveal an effect of dram1 mutation on the basal level of cell death in zebrafish larvae (Fig. 1c, d). In the absence of detectable developmental aberrations, we proceeded to investigate the response of dram1 mutants to Mm infection. Consistent with previous knockdown results6, dram1∆19n/∆19n larvae showed significantly increased susceptibility to infection (Fig. 1e, f). Furthermore, Dram1-deficient larvae displayed accumulation of bacteria inside intersegmental blood vessels, indicative of extracellular bacterial growth (Fig. 1e). We detected no differences in bacterial burden between dram1+/+ and unrelated wild types, indicating that the genetic background did not affect its susceptibility to infection (Fig. 1f). Next, we demonstrated that injection of dram1 mRNA could rescue the infection susceptibility phenotype of dram1∆19n/∆19n, while injection of a control mRNA containing the Δ19n deletion could not (Fig. 1g). Collectively, our analysis of dram1∆19n/∆19n zebrafish larvae confirms that Dram1 is necessary for host defense during Mm infection.
Fig. 1. Dram1 deficiency leads to increased susceptibility to Mm infection.
a Schematic representation of the zebrafish dram1/Dram1(ENSDARG00000045561/ENSDARP0 0000066996.3) genetic and protein domain architecture and CRISPR/Cas9 target site. Dram1 (240 amino acids) contains six transmembrane domains (indicated with boxes and labeled T1–T6 with amino acid numbers indicated above). The gene is depicted with coding exons as boxes and introns as solid black lines (introns not drawn to scale). The position of the CRISPR/Cas9 target site and the predicted truncated protein is indicated. b Confirmation of dram1 mutation by Western blotting analysis. Protein samples were extracted from 4 dpf dram1∆19n/∆19n and dram1+/+ larvae (>10 larvae/sample). The blots were probed with antibodies against Dram1 and Actin as a loading control. c Representative confocal micrographs of sections from the tail region showing TUNEL staining performed on dram1∆19n/∆19n and dram1+/+ larvae at 3 dpf. Scale bar, 10 μm. d Quantification of TUNEL-positive cells in the indicated region of the tail of dram1∆19n/∆19n and dram1+/+ larvae at 3 dpf (≥7 larvae/group). Data are represented by scatter and boxplots as detailed in the “Methods” section. e Representative stereo images of infected dram1∆19n/∆19n and dram1+/+ larvae at 3 dpi. The arrowhead indicates the accumulation of bacteria in intersegmental veins. f and g Quantification of bacterial burdens at 3 dpi for dram1 mutants, wild type siblings, and unrelated wild types f or for dram1 and dram1∆19n mRNA injected individuals g. The data are accumulated from two independent experiments (>42 larvae/group for f and >62 larvae/group for g) and represented by scatter and boxplots as detailed in the methods section.
Dram1 deficiency affects transcriptional regulation of metabolic, immune response, and cell death pathways during mycobacterial infection
To further explore the dram1∆19n/∆19n phenotype, we performed RNA sequencing. We aimed to identify changes in the gene expression network caused by the absence of functional Dram1, rather than by the increased level of infection in mutants. Therefore, dram1∆19n/∆19n larvae were infected with two different dosages of bacteria. A dosage of 300 CFU, resulting in a higher bacterial burden compared with dram1+/+, and a lower dosage of 150 CFU resulting in a similar bacterial burden as in dram1+/+ infected with 300 CFU (Figs. 2a and S2a). The analysis time point, 4 days post-infection (dpi), correlates with mycobacterial granuloma formation and strong transcriptional activation of the immune response28. Principal component analysis showed clear differences between Mm-infected larvae and PBS-injected controls and between the dram1∆19n/∆19n and dram1+/+ groups (Fig. S2b). Differential gene expression analysis comparing PBS-injected dram1∆19n/∆19n and dram1+/+ groups showed that Dram1 deficiency influences the gene regulation network to a relatively small extent under non-infected conditions (Fig. S2c). Gene ontology and gene set-enrichment analysis (GSEA) revealed that these relatively minor transcriptome differences in non-infected dram1 mutants were related to changes in metabolic and proteolytic pathways (Table S1). During Mm infection, we found that infected wild type larvae displayed a pronounced level of differential gene expression (both upregulated and downregulated genes) compared to their PBS-injected wild type controls, consistent with previous results28. In constrast, alterations in the gene regulation network were less pronounced when comparing infected dram1∆19n/∆19n mutants to their PBS-injected mutant controls, irrespective of whether they where infected with 150 or 300 CFU (Fig. S2c).
Fig. 2. Dram1 deficiency affects gene expression of pathways involved in metabolism, innate immunity, and lytic cell death during infection.
a Bacterial burdens of larvae injected with different CFU doses of Mm that have been analyzed by RNAseq. The data are accumulated from three independent sample sets at 4 dpi (>42 larvae/group) and represented by scatter and boxplots as detailed in the methods section. b Venn diagram of the significantly enriched KEGG pathways in the transcriptome of larvae infected with Mm. The enrichment comparisons were performed on dram1∆19n/∆19n 150 CFU versus dram1∆19n/∆19n PBS, dram1∆19n/∆19n 300 CFU versus dram1∆19n/∆19n, and dram1+/+ 300 CFU versus dram1+/+ PBS. c Visualization of the lytic cellular death signaling pathway transcriptome shows different responses in the transcriptome of infected dram1∆19n/∆19n and dram1+/+. The pathway was adapted from the KEGG pathway necroptosis. In the visualization, the gene expression in the comparison dram1∆19n/∆19n 150 CFU versus dram1∆19n/∆19n PBS, dram1∆19n/∆19n 300 CFU versus dram1∆19n/∆19n, and dram1+/+ 300 CFU versus dram1+/+ PBS are depicted by color gradient (yellow, upregulated, blue downregulated). The expression of all genes of the pathway present in the RNA sequencing was plotted independently of their significance. While the effector genes of the apoptosis pathway did not show high expression changes, the genes from lytic cell death forms, including pyroptosis, showed high expression modulation.
While differences between dram1∆19n/∆19n mutants and controls were observed at both infection doses, the transcriptome response of mutants infected with 300 CFU showed more overlap with infected controls than mutants infected with 150 CFU, despite their higher bacterial burden. For example, while expression of genes related to host defense pathways, such as Nod-like receptor (NLR) signaling, phagosome-related processes, cytokine signaling, and apoptosis, were commonly affected in all Mm-infected larvae, other immune-related pathways, like Toll-like receptor (TLR) and RIG-I-like receptors signaling, were not affected in dram1∆19n/∆19n larvae infected with 150 CFU Mm (Fig. 2b). This difference may reflect a milder initial immune response towards infection with only half the inoculum compared to the other two groups. Despite this, ~60% of the infection-responsive genes in dram1+/+ (1170 out of 1971) were not differentially expressed in dram1∆19n/∆19n mutants (Fig. S2d). Alteration of metabolic pathways related to energy and carbon metabolism (e.g. glycolysis, TCA cycle), a characteristic of mycobacterial infections29, was markedly absent in dram1∆19n/∆19n larvae (Fig. 2b). In contrast, infection of dram1∆19n/∆19n with 300 CFU influenced the expression of genes related to other metabolic processes, such as cholesterol and amino acid biosynthesis (Fig. 2b). For further analysis, we visualized the differential expression of genes that are part of relevant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways for the three Mm infected groups compared to their respective controls. In the KEGG pathway of TLR signaling, the visualization revealed differential expression of several TLRs and downstream genes between the wild type and dram1 mutant infected groups (Fig. S3). In cell death-related KEGG pathways, Dram1 deficiency affected the regulation of programmed cell death during infection, resulting in enhanced expression of genes involved in lytic forms of cell death (Fig. 2c).
The autophagic response to Mm infection is altered in dram1 mutants
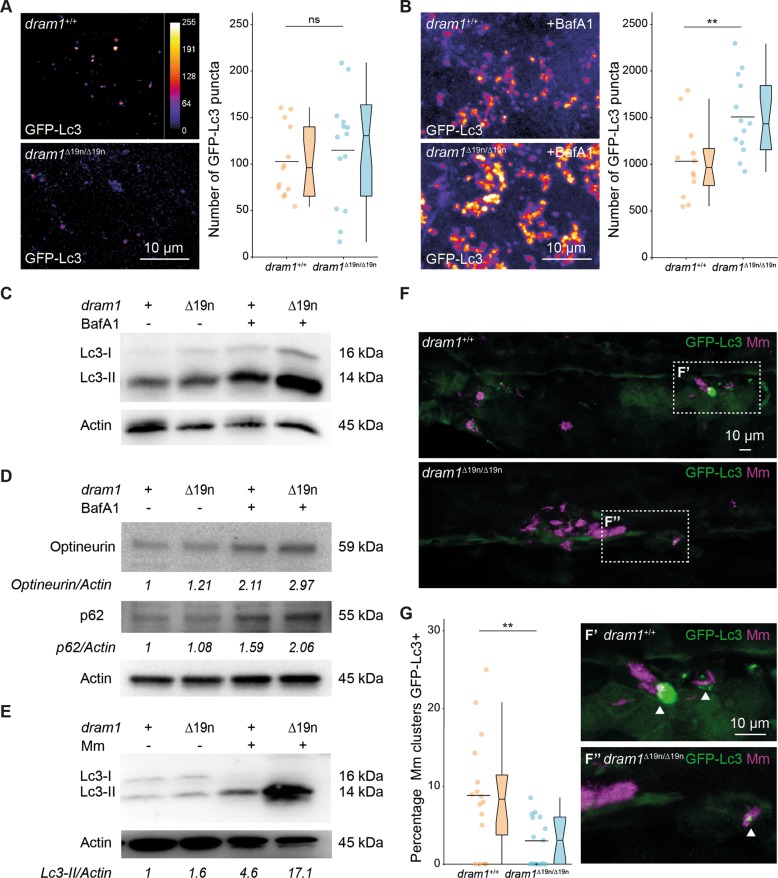
Altered metabolic pathway regulation in dram1 mutants might be a compensatory response to defects in autophagy. Investigating GFP-Lc3 puncta as a marker for autophagosome formation revealed no difference between dram1∆19n/∆19n and dram1+/+ larvae under unchallenged conditions (Fig. 3a). However, dram1∆19n/∆19n larvae responded differently by accumulating higher levels of GFP-Lc3 and Lc3 incorporated in autophagosomes (Lc3-II) compared with dram1+/+ when we applied a cellular stress factor, Bafilomycin A1 (BafA1), which inhibits vacuolar H+ATPase (V-ATPase) to prevent autophagolysosomal maturation (Fig. 3b, c). In agreement, protein levels of ubiquitin-binding receptors, p62 and Optineurin, which are substrates of autophagy30, were unaffected in unchallenged dram1∆19n/∆19n larvae but were elevated compared to the levels in dram1+/+ following BafA1 treatment (Fig. 3d). Similar to BafA1 treatment, Mm infection-induced Lc3-II to higher levels in dram1∆19n/∆19n than in dram1+/+ (Fig. 3e). However, colocalization analysis between GFP-Lc3 and Mm showed that dram1∆19n/∆19n larvae displayed significantly less GFP-Lc3-positive Mm clusters compared to dram1+/+ (Fig. 3f, g), indicating that autophagic targeting of Mm is reduced in the absence of Dram1, despite an overall increase in Lc3-II accumulation. Taken together, dram1 mutants display no overt autophagic defects in unchallenged conditions, but are affected in their autophagic response to cellular stress, including intracellular infection by Mm.
Fig. 3. Dram1 is required for GFP-Lc3 targeting to Mm clusters.
a, b Representative confocal micrographs and quantification of GFP-Lc3 puncta in dram1∆19n/∆19n and dram1+/+ larvae in an unstimulated situation (basal autophagy, a) and following BafA1 treatment b. Each larva was imaged at a pre-defined region of the tail fin (≥11 larvae/group). Results are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. Scale bars, 10 μm. The intensity calibration bar for the Lookup table (LUT) is displayed in panel a. c–e Western blot analysis of autophagy. Protein samples were obtained from 4 dpf dram1∆19n/∆19n and dram1+/+ larvae (>10 larvae/sample). Lc3 c and e, or p62 and Optineurin d protein levels were detected in absence or presence of BafA1, c and d, or in the presence or absence of Mm e. Actin was used as a loading control. Western Blots were repeated three, c and d, or two e times with protein extracts derived from independent experiments. The Lc3II/Actin or p62/Actin and Optineurin/Actin ratio, normalized to the control sample, is indicated below the blots. f–g Representative confocal micrographs and quantification of GFP-Lc3 co-localization with Mm clusters in infected dram1∆19n/∆19n and dram1+/+ larvae. The top images f show the entire region of imaging, while the bottom images f′ and f″ show details of GFP-Lc3 colocalization of Mm clusters in dram1∆19n/∆19n and dram1+/+ larvae. The arrowheads indicate GFP-Lc3-positive Mm clusters. The data is accumulated from two independent experiments (≥15 larvae/group) and represented by scatter and boxplots as detailed in the “Methods” section. Scale bars, 10 μm.
Dram1 deficiency does not affect phagocytosis of Mm
We wanted to exclude that reduced GFP-Lc3 association with Mm might be a consequence of a defect in phagocytosis by macrophages, the primary niche for intracellular Mm growth31. First, we verified that Dram1 deficiency did not alter the total number of macrophages, labeled by mpeg1:mCherryF (Fig. 4a). Similarly, there was no effect on the other main innate immune cell population of zebrafish larvae, the neutrophils (Fig. 4b). Next, we assessed phagocytic activity at 1 h after intravenous delivery of Mm. The results showed that Mm was phagocytosed by macrophages in dram1∆19n/∆19n and dram1+/+ at a similar rate, as indicated by the percentage of intracellular Mm (Fig. 4c). We then determined at which time point during the infection a difference in bacterial burden between dram1∆19n/∆19n and dram1+/+ was first detectable. We found that Dram1 deficiency significantly increased Mm infection burden at 2 dpi but not yet at 1 dpi (Fig. 5a). In conclusion, both dram1∆19n/∆19n and dram1+/+ can phagocytose the injected dose of Mm within the first hour after infection, and the immunocompromised state of Dram1-deficient larvae first becomes apparent 2 days later.
Fig. 4. Dram1 deficiency does not affect the capability of macrophages to phagocytose Mm.
a Representative stereo micrographs of macrophages in the whole tail region and quantification of the number of macrophages in this region. 3 dpf dram1∆19n/∆19n and dram1+/+/ mpeg1:mCherryF larvae were obtained from incrossed dram1+/∆19n animals and the number of macrophages for each larva were counted before determining the genotype. Genotyping was performed by PCR and Sanger sequencing (≥28 larvae/group). Data are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. b Representative stereo images of the whole tail of 3 dpf dram1∆19n/∆19n and dram1+/+ larvae following an immunohistochemical peroxidase activity detection protocol. The number of neutrophils in this region was quantified per individual larva (≥18 larvae/group). Data are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. c Representative confocal micrographs of macrophages located in the blood circulation over the yolk sac of infected dram1∆19n/∆19n and dram1+/+ embryos in mpeg1:mCherryF background at 1 h post-infection (hpi). Scale bars, 10 μm. Quantification of phagocytosis of Mm by macrophages at 1 hpi. dram1∆19n/∆19n and dram1+/+ embryos in mpeg1:mCherryF background were infected with Mm at 30 hpf and fixed at 1 hpi. The percentage of macrophages having phagocytosed Mm clusters was determined per individual larva (≥16 larvae/ group). Results are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001.
Fig. 5. Macrophages fail to restrict Mm infection in Dram1-deficient larvae.
a Mm bacterial burden for dram1∆19n/∆19n and dram1+/+ at 1 and 2 dpi. Data are accumulated from two independent experiments (>38 larvae/group) and represented by scatter and boxplots as detailed in the “Methods” section. b Representative confocal images of LysoTracker staining performed on infected dram1∆19n/∆19n and dram1+/+ embryos at 1 dpi. The arrowheads indicate Lysotracker-positive (LysoTracker+) Mm clusters. Scale bars, 10 μm. c The percentage of LysoTracker+ Mm clusters was determined in infected embryos (≥15 embryos/group) at 1 dpi. The percentage of Mm clusters positive for LysoTracker staining (LysoTracker+) was determined per individual infected larva. Data are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. d and f Representative confocal images of infected dram1∆19n/∆19n and dram1+/+ embryos/larvae in mpeg1:mCherryF background at 1 dpi d and 2 dpi f. The entire CHT region of fixed embryos or larvae was imaged. The arrowheads indicate intracellular Mm clusters and an asterisk (*) indicates remnants from dead macrophages. Scale bars, 10 μm. e and g Percentage of Mm clusters restricted inside macrophages at 1 dpi e and 2 dpi g (≥10 embryos/group). The percentage of intracellular Mm clusters was determined per individual embryo. Data are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001.
Dram1 is required for macrophages to restrict Mm infection
Since Dram1 protein localizes to lysosomes11, we asked whether Dram1-deficiency affected the maturation of Mm-containing vesicles. We used LysoTracker to determine the extent of colocalization between Mm and acidic vesicles at an early stage of infection (1 dpi), before differences in bacterial burden were detectable. Approximately 60% of Mm clusters were LysoTracker-positive in dram1+/+, while this was reduced to 20% in dram1∆19n/∆19n (Fig. 5b, c). Next, we asked whether reduced maturation of Mm-containing vesicles limits the ability of macrophages in dram1∆19n/∆19n hosts to combat the infection. We found that at 1 dpi the majority of Mm clusters were restricted inside macrophages for both dram1∆19n/∆19n and dram1+/+ hosts (Fig. 5d, e). However, at 2 dpi, the majority of Mm (65%) resided inside macrophages in dram1+/+, while we observed an increased escape of Mm from macrophages in dram1∆19n/∆19n larvae, with only 31% remaining intracellular (Fig. 5f, g). Furthermore, we frequently observed remnants of dead macrophages in the proximity of bacterial clusters in dram1∆19n/∆19n (Fig. 5f), which most likely were contained within the macrophage prior to its death. Together, these data demonstrate that Dram1 is necessary for macrophages to contain the infection and prevent extracellular bacterial growth.
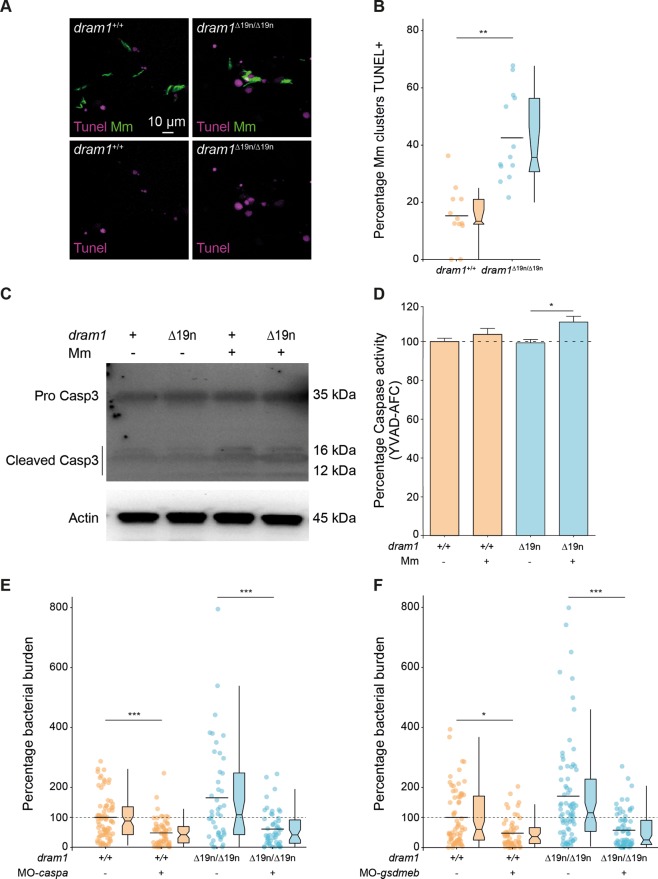
Dram1 deficiency results in increased pyroptotic cell death of Mm-infected macrophages
Transcriptome analysis had revealed that Dram1 deficiency affects lytic cell death pathways, including necroptosis and pyroptosis, while effects on the apoptosis pathway were relatively minor. To delineate the mechanism responsible for increased cell death of Mm-infected macrophages in dram1 mutants, we performed TUNEL staining, which detects damaged DNA present both in apoptotic and pyroptotic cells32,33. We observed TUNEL-positive cells around Mm clusters both in dram1∆19n/∆19n and dram1+/+, but the frequency was around 2.1 times higher in the mutants (Fig. 6a, b). We observed no difference in activation (cleavage) of Caspase 3, an important executioner of apoptosis34,35, between dram1∆19n/∆19n and dram1+/+ in the absence or presence of Mm infection (Fig. 6c). Therefore, we asked if the increased cell death of Mm-infected macrophages dram1∆19n/∆19n was due to pyroptosis. Pyroptotic cell death is associated with the activity of inflammatory caspases, like Caspase 1 and Caspase 4/5/11, and is characterized by the formation of Gasdermin pores in the cell membrane36–38. We have recently found that Caspase a (Caspa) is the Caspase-family member that induces pyroptosis of Mm-infected macrophages in zebrafish via Gasdermin Eb (Gsdmeb)39,40. Thus, we analyzed Caspa activity at 2dpf, the time point where we observed increased cell death in dram1∆19n/∆19n. We detected a minor but significant increase of whole larvae Caspa levels in dram1∆19n/∆19n infected with Mm compared with the non-infected mutant, but such infection-dependent increase was not observed in dram1+/+ (Fig. 6d). Next, we asked if the increased bacterial burden in Dram1-deficient larvae is dependent on Caspa activity. Knockdown of caspa reduced bacterial burden in dram1+/+. Furthermore, caspa knockdown also reduced the enhanced bacterial burden of dram1∆19/∆19, bringing the infection burden in mutants and wild types to a comparable low level (Fig. 6e). Similarly, gsdmeb knockdown reduced bacteria burden in dram1+/+ and rescued the hypersusceptibility phenotype of dram1∆19/∆19 (Fig. 6f). Collectively, these data suggest that dissemination of mycobacterial infection in zebrafish embryos is promoted in the absence of Dram1 due to reduced bacterial containment and consequent pyroptosis of infected macrophages.
Fig. 6. Dram1 deficiency results in increased pyroptotic cell death.
a Representative confocal images of TUNEL staining in dram1∆19n/∆19n and dram1+/+ larvae at 2 dpi. The entire CHT region of fixed 2 dpi dram1∆19n/∆19n and dram1+/+ larvae was imaged. Scale bars, 10 μm. b Quantification of the percentage of Mm clusters positive for TUNEL staining (TUNEL+) in dram1∆19n/∆19n and dram1+/+ larvae. The percentage of Mm clusters TUNEL+ in the CHT region was determined per individual infected larva (≥12 larvae/group). Data are accumulated from two independent experiments and represented by scatter and boxplots as detailed in the “Methods” section. ns non-significant,*p < 0.05,**p < 0.01,***p < 0.001. c Detection of pro-Caspase 3 and cleaved Caspase 3 protein in dram1∆19n/∆19n and dram1+/+ embryos. Protein samples were extracted from 4 dpf infected and uninfected dram1∆19n/∆19n and dram1+/+ larvae (>10 larvae/sample). The Western blots were probed with antibodies against Caspase 3 and Actin as a loading control. Data is representative of two independent experiments. d Detection of Caspase activity (YVAD-AFC) in dram1∆19n/∆19n and dram1+/+embryos. Protein samples were obtained from 2 dpf control and infected dram1∆19n/∆19n and dram1+/+ embryos in GFP-Lc3 background (35 embryos/sample). The data is accumulated from two independent experiments (n = 4 samples/condition). e Mm bacterial burden at 2 dpi following knockdown of caspa in dram1∆19n/∆19n and dram1+/+ embryos. The data is accumulated from two independent experiments (>44 larvae/group) and represented by scatter and box plots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001. f Mm bacterial burden at 2 dpi following knockdown of gsmdeb in dram1∆19n/∆19n and dram1+/+ embryos. The data is accumulated from two independent experiments (>56 larvae/group) and represented by scatter and box plots as detailed in the “Methods” section. ns non-significant, *p < 0.05,**p < 0.01,***p < 0.001 to mutated dram1.
Discussion
The lysosomal protein DRAM1/Dram1 regulates autophagy and cell survival/death decisions under multiple stress conditions, including diseases like cancer and infection. Its mechanism of action remains largely unknown. Here, we have demonstrated that dram1 mutation in zebrafish impairs resistance to mycobacterial infection. We show that Dram1 deficiency reduces autophagic targeting of Mm and acidification of Mm-containing vesicles, ultimately resulting in pyroptotic cell death of infected macrophages and increased extracellular growth of mycobacteria during early stages of the infection.
The dram1 mutant generated for this study was characterized by transcriptome analysis. Under unchallenged conditions, we found that deficiency of Dram1 affects the network of gene regulation to a small degree, with detectable differences in proteinase and metabolic pathways. Considering that lysosomes act as central regulatory units in signal transduction, this transcriptional response might be a compensatory mechanism for defects in lysosomal function due to the deficiency in Dram141. The differential expression of metabolic pathway genes was markedly enhanced in response to Mm infection. Recent studies have shown that the metabolic status of macrophages is critical for their innate host defense function42. Therefore, the metabolic dysregulation in dram1 mutants could contribute to their hypersusceptibility to Mm infection. Altered regulation of essential innate immunity signaling pathways (including TLR signaling) in dram1 mutants could also contribute to this phenotype. Finally, the upregulated expression of genes in lytic cell death pathways may contribute to pathological inflammation and is consistent with the increased bacterial burden in dram1 mutants.
The function of DRAM1 as a modulator of autophagy has been studied well in vitro11. We therefore tested whether zebrafish dram1 mutants display defects in autophagic processes. Autophagy is a host response to diverse stress factors, including starvation. Zebrafish larvae until 5 dpf can rely on their yolk proteins for nutrients43, and we therefore assumed that their autophagic processes are not activated above a level normal for their developmental stage, unless autophagy is triggered by a stressor such as infection. In agreement, we did not detect any differences when comparing the basal levels of autophagy activity in uninfected dram1 mutant larvae of 4 dpf to those of their wild type siblings. This finding is consistent with an in vitro study of the function of mouse DRAM144, which showed that basal autophagy was not altered in the absence of DRAM1 in primary mouse embryonic fibroblasts (MEFs). DRAM1 deficiency did not change autophagy induction in response to starvation or while blocking mTOR45. However, the lack of DRAM1 affected the activation of autophagy in human cells (HeLa and A549) following the induction of cellular stress by treatment with the mitochondria inhibitor 3-nitropropionic acid (3-NP)46. Besides infection, DNA-damage, and interference with energy metabolism6,11,14, it remains to be further investigated which stress factors can activate DRAM1/Dram1 in vitro and in vivo.
Blocking autophagy flux in dram1 mutant larvae with BafA1 revealed an increase of GFP-Lc3 puncta and Lc3-II protein levels. The dram1 mutants also accumulated higher Lc3-II protein levels than controls under conditions of Mm infection. The prolonged stress conditions imposed to zebrafish larvae during BafA1 treatment or infection may induce a compensatory response in dram1 mutants to produce more autophagosomes. Importantly, despite the increased Lc3-II levels in infected dram1 mutants, imaging in GFP-Lc3 transgenic fish revealed that mycobacteria are targeted by autophagic vesicles nearly three-fold less frequently in dram1 mutants compared to wild type zebrafish larvae. This reduced autophagic targeting of Mm was not due to a different phagocytic ability of zebrafish macrophages. We did, however, find that Dram1 deficiency reduced acidification of Mm-containing vesicles, which was associated with the premature death of infected macrophages. The resulting higher mycobacterial burden of infected zebrafish is in line with increased expansion of extracellularly growing Mm47. While we found that Mm-infected macrophages die more rapidly in the absence of Dram1, human DRAM1 has been shown to trigger lysosomal membrane permeabilization and cell death in HIV-infected CD4+ T cells, thereby lowering viral replication (14). Together, these studies indicate that DRAM1/Dram1 expression levels can have a major impact on cell death processes during infection, with different outcomes depending on the cell type and infectious agent.
DRAM1 was previously shown to mediate apoptosis by blocking the degradation of the pro-apoptotic protein Bax12, but an effect of Dram1 deficiency on apoptosis was not observed in our infection study. Strikingly, we found that Dram1 deficiency leads to increased inflammatory caspase activity and gasdermin-dependent pyroptotic cell death during infection. Previous studies revealed that pyroptosis can be induced by diverse pathogens and forms a critical mechanism to restrict microbial infection48,49. In line with this, there is also evidence that mycobacteria inhibit pyroptosis of infected macrophages via diverse mechanisms50. However, recent studies found that lytic cell death (pyroptosis and necroptosis) helps mycobacteria to evade host immunity and disseminate the infection25,51. Indeed, in the present study we found that pyroptotic cell death promotes the expansion of mycobacteria in Dram1-deficient zebrafish hosts. Moreover, genetic inhibition of Caspase a and Gasdermin Eb could rescue the exacerbated bacterial growth in dram1 mutants, confirming that pyroptosis rather than necroptosis is responsible for the hypersusceptibility phenotype. Taken together, the death of infected macrophages is intricately related to TB pathogenesis and can result either in increased dissemination or restriction of the infection in the zebrafish host. The contradicting evidence discussed above concerning the beneficial or detrimental effects of the different modes of cell death suggests that the balance between different cell death modalities plays a crucial role in determining the outcome of the infection and a careful characterization of the specific type of cell death being studied is critical for a better understanding of TB pathogenesis.
In conclusion, restriction of mycobacteria in infected macrophages during the early stages of infection requires functional Dram1. In this work, we have shown that Dram1 is involved in several processes important to defense against intracellular pathogens, potentially providing an intersection between modulation of autophagy, lysosomal function, and regulated cell death. Future studies are required to precisely elucidate the role of the lysosomal protein Dram1/DRAM1 in this network. Using an in vivo model for the early stages of TB disease we have demonstrated the importance of Dram1 for the elimination of intracellular mycobacteria and the cell fate of infected macrophages.
Materials and methods
Zebrafish culture and lines
Zebrafish lines in this study (Table S2) were handled in compliance with local animal welfare regulations, as overseen by the Animal Welfare Body of Leiden University (License number: 10612) and maintained according to standard protocols (zfin.org). Generation of the dram1 mutant was approved by the Animal Experimentation Committee of Leiden University (UDEC) under protocol 14198. All experiments were done on embryos or larvae up to 5 days post-fertilization, which have not yet reached the free-feeding stage. Embryos/larvae were kept in egg water (60 µg/ml Instant Ocean sea salts) containing 0.003% 1-phenyl-2-thiourea (PTU, Sigma-Aldrich) at 28.5 °C and treated with 0.02% ethyl 3-aminobenzoate methanesulfonate (Tricaine, Sigma-Aldrich) for anesthesia before bacterial injections, imaging and fixation.
CRISPR/Cas9-mediated mutagenesis of zebrafish dram1
Short guide RNAs (sgRNAs) targeting the first coding exon of zebrafish dram1 (ENSDARG00000045561) were designed using the chop-chop website52 and generated by PCR complementation and amplification of full-length ssDNA oligonucleotides (Sigma-Aldrich, Table S3) as described19. For in vitro transcription of sgRNAs, 0.2 µg template DNA was used to generate sgRNAs using the MEGA short script® T7 kit (AM1354, ThermoFisher) and purified by RNeasy Mini Elute Clean up kit (74204, Qiagen). The Cas9 mRNA was transcribed using mMESSAGE mMACHINE® SP6 Transcription Kit (AM1340, ThermoFisher) from a Cas9 plasmid (39312, Addgene) and purified with RNeasy Mini Elute Clean up kit (74204, Qiagen). A mixture of sgRNA and Cas9 mRNA was injected into one-cell stage AB/TL embryos (sgRNA 150 pg/embryo and Cas9 mRNA 300 pg/embryo). The effect of CRISPR injection was confirmed by PCR and Sanger sequencing. Genotyping was performed by PCR-amplification of the genomic region of interest using the following primers: Forward: 5′-AGTGAACGTCCGTGTCTTTCTT-3′, Reverse: 5′-ACATCTTGTCGATACAAAGCGA-3′; followed by Sanger sequencing to identify mutations (Base Clear, Netherlands)19.
RNA sequencing
Total RNA was extracted from 5 dpf infected and uninfected snap-frozen larvae (20 larvae/sample) from three independent crosses using Qiazol reagent (79306, QIAGEN) according to the manufacturer’s instructions and extracted with RNeasy Mini kit (74104, QIAGEN). RNAs were quantified using a 2100 Bioanalyzer (Agilent, US). At least 10 million reads per sample were sequenced using Illumina Single read 50 nt runs in a Hiseq2500. Sequencing, mapping the reads against the D. rerio GRCz10.80 reference genome and read counting were performed by ZF-screens (Leiden, Netherlands). Analysis of the count libraries was performed in RStudio 1.1.38353 running R 3.4.354 using in-house scripts (available at github.com/gabrifc). An initial quality check of the samples was performed using the tools provided in the edgeR package v3.20.755. Differential gene expression was assessed via pairwise comparisons using DESeq2 v1.18.156. Genes with a FDR-adjusted p-value (adjpval) <0.05 were considered statistically significant. Venn Diagrams were created using the R package VennDiagram v1.6.1857. Gene lists were ranked using the published function “-log10(adjpval)*log2(fold-change)”, compared to the C2 “Curated Gene Sets” collection from the Molecular Signatures Database (MSigDB) using GSEA v3.058. Gene ontology enrichment was analyzed with goseq v1.3.059. Updated gene length and Gene Ontology data from the Zv10 assembly was retrieved from Ensembl with the packages ensembldb v2.2.160 and biomaRt v2.34.261, respectively. When necessary, mapping between different database gene identifiers was also performed using biomaRt v2.34.2. KEGG pathway analysis was performed with the kegga function provided in limma v3.34.562. Gene regulation data of significant pathways was visualized with pathview v1.18.063. Raw data are deposited into Gene Expression Omnibus under accession number GSE129035.
Western blot analysis
Western blot analysis was performed as previously described19. Antibodies used were as follows: polyclonal rabbit anti-DRAM1 (N-terminal) (1:1000, ARP47432-P050, Aviva systems biology), polyclonal rabbit anti-Optineurin (C-terminal) (1:200, lot#100000; Cayman Chemical), polyclonal rabbit anti-p62 (C-terminal) (1:1000, PM045, lot#019, MBL), polyclonal rabbit anti-Lc3 (1:1000, NB100-2331, lot#AB-3, Novus Biologicals), monoclonal Caspase 3 antibody (1:1000, #9662, Lot#12, Cell Signaling), anti-mono-and polyubiquitinated conjugates mouse monoclonal antibody (1:200; BML-PW8810-0100, lot#01031445, Enzo life Sciences), polyclonal actin antibody (1:1000, 4968S, lot#3, Cell Signaling), anti-rabbit IgG, HRP-Linked Antibody (1:1000, 7074 S, Lot#0026, Cell Signaling), anti-mouse IgG, HRP-linked antibody (1:3000, 7076S, Lot#029, Cell Signaling).
Infection conditions and bacterial burden quantification
Mm strain M or Mm strain 20 fluorescently labeled with mWasabi or mCherry, respectively64,65, were microinjected into the blood island of embryos at 28 hpf as previously described66. The injection dose was 200 CFU for all experiments, except for the phagocytosis assay (500 CFU), and the RNA sequencing of infected wild types (300 CFU) and dram1 mutants (150 and 300 CFU). Embryos were manually dechorionated by tweezers before the injection. Infected embryos were imaged using a Leica MZ16FA stereo fluorescence microscope equipped with a DFC420C color camera, and the number of bacterial pixels per infected fish were obtained from the individual embryo stereo fluorescence images using previously described software67.
Confocal laser scanning microscopy and image quantification
Fixed or live embryos were mounted with 1.5% low melting agarose (140727, SERVA) and imaged using a Leica TCS SPE confocal microscope. For quantification of numbers of GFP-Lc3-positive vesicles, the fixed 4 dpf larvae were imaged by confocal microscopy with a ×63 water immersion objective (NA 1.2) in the pre-defined tail fin region to detect the number of GFP-LC3-positive vesicles (Fig. 3a, b). The numbers of GFP-Lc3 vesicles were measured by Fiji/ImageJ software (Fig. 3a, b)68. For quantification of the autophagic response targeted to Mm clusters (Fig. 3f, g), the fixed 2 dpi larvae were imaged by confocal microscopy with a ×40 water immersion objective (NA 0.8) over the whole caudal hematopoietic tissue (CHT) region. The same approach was used to quantify Mm acidification in the CHT region (Fig. 5b, c). To investigate the intramacrophage or extracellular localization of bacteria, fixed larvae were again imaged over the CHT as described above, after which the total number of Mm clusters and the number of clusters inside macrophages were counted. To assay cell death, images from fixed 2 dpi larvae were acquired as above, and the number of cells positive for TUNEL staining in the CHT region was counted manually.
mRNA preparation and injection
dram1 or dram1∆19N (negative control) RNA was isolated from wild type or dram1∆19n/∆19n embryos using QIAzol lysis reagent (79306, QIAGEN) and purified with the RNeasy MinElute Cleanup kit (74204, QIAGEN). cDNA synthesis was performed using the iScript cDNA synthesis kit (1708891, BIO-RAD). Full-length dram1 cDNA and dram1∆19N cDNA was obtained by PCR amplification using Phusion High-Fidelity DNA Polymerase (M0530S, New England Biolabs). The following primers were used: Forward: 5′-CTGCGGCGAGATGTTTTGGTT-3′; Reverse: 5′-CAAAAACAGTGGGACATACAGTGAA-3′. dram1 or dram1∆19N PCR products were ligated into a ZERO BLUNT TOPO vector (450245, ThermoFisher) and the insert was confirmed by Sanger sequencing (Base Clear, Netherlands). dram1 and dram1∆19N mRNA was generated using the SP6 mMessage mMachine kit (AM1340, Thermo Fisher) and Poly(A) Tailing Kit (AM1350, ThermoFisher); purified using the RNeasy Mini Elute Cleanup kit (74204, QIAGEN) and 50 pg mRNA was microinjected into one-cell stage embryos.
TUNEL assay
Cell death was examined by Terminal deoxynucleotidyl TUNEL staining with the In Situ Cell Death Detection Kit, TMR red (1256792910, SIGMA-ALDRICH) in 2dpi fixed embryos. The assay was performed as follows: embryos were fixed in 4% PFA in PBS solution O/N at 4 °C, de-hydrated and re-hydrated in serial dilutions of methanol (MeOH) (25%, 50%, 75%, 100%, 75%, 50%, 25%) and washed in PBS containing 0.1% Triton-X100. Then, embryos were permeabilized in 10 μg/ml Proteinase K for 40 min at 37 °C followed by a quick rinse in PBS containing 0.1% Triton X100. TUNEL staining was performed according to kit instructions O/N at 37 °C in the dark. Samples were washed and stored in PBST until imaging by confocal microscopy as described above.
LysoTracker staining and Myeloperoxidase (Mpx) activity assay
Infected embryos were immersed in egg water with 10 μM LysoTracker Red DND-99 (L7528, ThermoFisher) for 1 h. Embryos were washed three times with egg water before imaging. Mpx activity assay was performed with the Leukocyte detection Kit (390A, SIGMA-ALDRICH) for detection of neutrophils as previously described69.
Drug treatment
Embryos were bath treated with Bafilomycin A1 (BafA1) (B1793-10UG, SIGMA-ALDRICH) diluted into egg water at the working concentration of 100 nM for 12 h.
Caspase activity assay
Inflammatory caspase activity was assayed with the fluorometric substrate Z-YVAD 7-Amido-4-trifluoromethylcoumarin (Z-YVAD-AFC, Caspase-1 Substrate IV, Colorimetric, sc-311283, Santa Cruz) as described previously70. 35 embryos/group were sonicated and incubated in hypotonic cell lysis buffer (25 mM 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid, 5 mM ethylene glycol-bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 5 mM dithiothreitol, pH 7.5) on ice for 15 min. For each reaction, 10 μg protein was incubated for 90 min at 28 °C with 50 μM YVAD-AFC in 50 μl of reaction buffer (0.2% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 0.2 M 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid, 20% sucrose, 29 mM dithiothreitol, pH 7.5). After the incubation, fluorescence was measured in a Tecan M1000 microplate reader at an excitation wavelength of 400 and emission wavelength of 505 nm.
Morpholino injection conditions
Previously validated caspa and gsdmeb morpholinos (MO)71 were purchased from Gene Tools (Gene Tools, USA). MO oligonucleotide sequence: caspa 5′-GCCATGTTTAGCTCAGGGCGCTGAC-3′, gsdmeb MO 5′-TCATGCTCATGCTAGTCAGGGAGG-3′71. MOs were diluted in Milli-Q water with 0.05% phenol red and 1 nL of 0.6 mM (caspa MO) or 0.7 mM (gsdmeb) MO was microinjected into the yolk of one-cell stage embryos as previously described (39).
Statistical analyses and data representation
Power calculations where performed to ensure that sufficient animals were included in the experiments to be able to detect a effect size of at least 20%. For infection experiments, improperly injected animals were excluded from the experiment. No animals were excluded from the analysis after the initial selection. Wild type and mutant animals were randomly distributed over the different experimental conditions and blinding was applied during data acquisition and analysis when possible. Statistical analyses were performed using GraphPad Prism software (Version 5.01; GraphPad). All experimental data (mean ± SEM) was analyzed using unpaired, two-tailed Mann–Whitney U tests for comparisons between two groups and Kruskal–Wallis one-way analysis of variance with Dunn’s multiple comparison methods as a posthoc test for comparisons between more than two groups. (ns, no significant difference; *p < 0.05; **p < 0.01; ***p < 0.001). For segregation of F1 or F3 heterozygous offspring, data were analyzed with a Chi-square test (ns, no significant difference). Continuous measurements are represented as bar graphs. In non-continuous measurements, the data sets from each group are shown in a scatter plot (left) and a boxplot (right). In the scatter plots each data point represents an individual embryo/larva, with the mean indicated by a horizontal line. Boxplots include 50% of the data points, with a vertical line indicating the 95% confidence interval and a horizontal line indicating the median.
Supplementary information
Acknowledgements
We thank Daniel Klionsky for sharing the CMV:EGFP-map1lc3b transgenic zebrafish line and Georges Lutfalla for the mpeg1:mCherryF line. We are grateful to all members of the fish facility team for zebrafish caretaking. We would like to thank Gerda Lamers and Joost Willemse for advice on confocal imaging and image analysis. R.Z. was supported by a grant from the China Scholarship Council (CSC). M.V. and G.F.-C. were funded by European Marie Curie fellowships (H2020-MSCA-IF-2014-655424 and H2020-COFUND-2015-FP-707404), V.T. was a Marie Curie fellow in the Initial Training Network FishForPharma (PITN-GA-2011-289209), and M.v.d.V. was supported by the Netherlands Technology Foundation TTW (project 13259).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by B. Zhivotovsky
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rui Zhang, Monica Varela
These authors jointly supervised this work: Michiel van der Vaart, Annemarie H. Meijer
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-2477-1).
References
- 1.Ohsumi Y. Yoshinori Ohsumi: autophagy from beginning to end. Interview by Caitlin Sedwick. J. Cell Biol. 2012;197:164–165. doi: 10.1083/jcb.1972pi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013;13:722. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis RS, Hafner R. Advancing host-directed therapy for tuberculosis. Nat. Rev. Immunol. 2015;15:255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 5.Paik, S., Kim, J. K., Chung, C. & Jo, E. K. Autophagy: a new strategy for host-directed therapy of tuberculosis. Virulence 1–12, 10.1080/21505594.2018.1536598 (2018). [DOI] [PMC free article] [PubMed]
- 6.van der Vaart M, et al. The DNA damage-regulated autophagy modulator DRAM1 links mycobacterial recognition via TLR-MYD88 to autophagic defense [corrected] Cell Host Microbe. 2014;15:753–767. doi: 10.1016/j.chom.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez MG, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krakauer T. Inflammasomes, autophagy, and cell death: the trinity of innate host defense against intracellular bacteria. Mediators Inflamm. 2019;2019:2471215. doi: 10.1155/2019/2471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López-Jiménez AT, et al. The ESCRT and autophagy machineries cooperate to repair ESX-1-dependent damage at the Mycobacterium-containing vacuole but have opposite impact on containing the infection. PLoS Pathog. 2019;14:e1007501. doi: 10.1371/journal.ppat.1007501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crighton D, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Guan JJ, et al. DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX. Cell Death Dis. 2015;6:e1624. doi: 10.1038/cddis.2014.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert M, Mueller C, Fey MF, Tschan MP. Inhibition of damage-regulated autophagy modulator-1 (DRAM-1) impairs neutrophil differentiation of NB4 APL cells. Leuk. Res. 2012;36:1552–1556. doi: 10.1016/j.leukres.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Laforge M, et al. DRAM triggers lysosomal membrane permeabilization and cell death in CD4(+) T cells infected with HIV. PLoS Pathog. 2013;9:e1003328. doi: 10.1371/journal.ppat.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mrschtik M, Ryan KM. Another DRAM involved in autophagy and cell death. Autophagy. 2016;12:603–605. doi: 10.1080/15548627.2015.1137412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu X, et al. Increased expression of DRAM1 confers myocardial protection against ischemia via restoring autophagy flux. J. Mol. Cell. Cardiol. 2018;124:70–82. doi: 10.1016/j.yjmcc.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Houben D, et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 2012;14:1287–1298. doi: 10.1111/j.1462-5822.2012.01799.x. [DOI] [PubMed] [Google Scholar]
- 18.Ponpuak M, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, et al. The selective autophagy receptors Optineurin and p62 are both required for zebrafish host resistance to mycobacterial infection. PLoS Pathog. 2019;15:e1007329. doi: 10.1371/journal.ppat.1007329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012;12:352–366. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan, L., Ahlbrand, S. & Briken, V. Interaction of Mycobacterium tuberculosis with host cell death pathways. Cold Spring Harb. Perspect. Med.4, 10.1101/cshperspect.a022459 (2014). [DOI] [PMC free article] [PubMed]
- 22.Gan H, et al. Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat. Immunol. 2008;9:1189–1197. doi: 10.1038/ni.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behar SM, et al. Apoptosis is an innate defense function of macrophages against Mycobacterium tuberculosis. Mucosal Immunol. 2011;4:279–287. doi: 10.1038/mi.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stutz MD, Clark MP, Doerflinger M, Pellegrini M. Mycobacterium tuberculosis: rewiring host cell signaling to promote infection. J. Leukoc. Biol. 2018;103:259–268. doi: 10.1002/JLB.4MR0717-277R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He C, Bartholomew CR, Zhou W, Klionsky DJ. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy. 2009;5:520–526. doi: 10.4161/auto.5.4.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernut A, et al. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl Acad. Sci. USA. 2014;111:E943–E952. doi: 10.1073/pnas.1321390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benard EL, Rougeot J, Racz PI, Spaink HP, Meijer AH. Transcriptomic approaches in the Zebrafish model for tuberculosis-insights into host- and pathogen-specific determinants of the innate immune response. Adv. Genet. 2016;95:217–251. doi: 10.1016/bs.adgen.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 29.de Carvalho LP, et al. Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 2010;17:1122–1131. doi: 10.1016/j.chembiol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Gatica D, Lahiri V, Klionsky DJ. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambier CJ, O’Leary SM, O’Sullivan MP, Keane J, Ramakrishnan L. Phenolic glycolipid facilitates mycobacterial escape from microbicidal tissue-resident macrophages. Immunity. 2017;47:552–565 e554. doi: 10.1016/j.immuni.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyrylkova K, Kyryachenko S, Leid M, Kioussi C. Detection of apoptosis by TUNEL assay. Methods Mol. Biol. 2012;887:41–47. doi: 10.1007/978-1-61779-860-3_5. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porter, A. G. & Janicke, R. U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ.6, 99–104 (1999). [DOI] [PubMed]
- 36.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 38.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 39.Varela, M., van der Vaart, M., Groenewoud, A. & Meijer, A. H. Extracellular mycobacterial DNA triggers Caspase-11-dependent pyroptosis of infected macrophages and drives disease progression. Preprint at 10.1101/514125 (2019).
- 40.Forn-Cuni, G., Meijer, A. H. & Varela, M. Zebrafish in inflammasome research. Cells8, 10.3390/cells8080901 (2019). [DOI] [PMC free article] [PubMed]
- 41.Perera RM, Zoncu R. The lysosome as a regulatory hub. Annu. Rev. Cell Dev. Biol. 2016;32:223–253. doi: 10.1146/annurev-cellbio-111315-125125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinlivan VH, Farber SA. Lipid uptake, metabolism, and transport in the larval zebrafish. Front Endocrinol. 2017;8:319. doi: 10.3389/fendo.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagata M, et al. Dram1 regulates DNA damage-induced alternative autophagy. Cell Stress. 2018;2:55–65. doi: 10.15698/cst2018.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galavotti S, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712. doi: 10.1038/onc.2012.111. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XD, Qi L, Wu JC, Qin ZH. DRAM1 regulates autophagy flux through lysosomes. PLoS ONE. 2013;8:e63245. doi: 10.1371/journal.pone.0063245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cookson B, Brennan M. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 49.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Danelishvili L, Everman JL, McNamara MJ, Bermudez LE. Inhibition of the plasma-membrane-associated serine protease cathepsin G by Mycobacterium tuberculosis Rv3364c suppresses caspase-1 and pyroptosis in macrophages. Front. Microbiol. 2011;2:281. doi: 10.3389/fmicb.2011.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahamed, D. et al. Intracellular growth of Mycobacterium tuberculosis after macrophage cell death leads to serial killing of host cells. Elife6, 10.7554/eLife.22028 (2017). [DOI] [PMC free article] [PubMed]
- 52.Labun K, Montague TG, Gagnon JA, Thyme SB, Valen E. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 2016;44:W272–W276. doi: 10.1093/nar/gkw398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RStudio: Integrated Development for R. (RStudio, Inc., Boston, MA, 2015).
- 54.R: A language and environment for statistical computing (The R Foundation for Statistical Computing, Vienna, 2011).
- 55.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. R package version 1.6.20 (2018).
- 58.Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S. & Mesirov, J. P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed]
- 59.Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol.11, R14 (2010). [DOI] [PMC free article] [PubMed]
- 60.Rainer, J., Gatto, L. & Weichenberger, C. X. ensembldb: an R package to create and use Ensembl-based annotation resources. Bioinform Oxf Engl35, 3151–3153 (2019). [DOI] [PMC free article] [PubMed]
- 61.Durinck S, Spellman PT, Birney E, Huber W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009;4:1184–1191. doi: 10.1038/nprot.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo W, Brouwer C. Pathview: an R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics. 2013;29:1830–1831. doi: 10.1093/bioinformatics/btt285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takaki K, Davis JM, Winglee K, Ramakrishnan L. Evaluation of the pathogenesis and treatment of Mycobacterium marinum infection in zebrafish. Nat. Protoc. 2013;8:1114–1124. doi: 10.1038/nprot.2013.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Sar AM, et al. Mycobacterium marinum strains can be divided into two distinct types based on genetic diversity and virulence. Infect. Immun. 2004;72:6306–6312. doi: 10.1128/IAI.72.11.6306-6312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benard EL, et al. Infection of zebrafish embryos with intracellular bacterial pathogens. J. Vis. Exp. 2012 doi: 10.3791/3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stoop EJM, et al. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Models Mech. 2011;4:526–536. doi: 10.1242/dmm.006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui C, et al. Infectious disease modeling and innate immune function in zebrafish embryos. Methods Cell Biol. 2011;105:273–308. doi: 10.1016/B978-0-12-381320-6.00012-6. [DOI] [PubMed] [Google Scholar]
- 70.Tyrkalska SD, et al. Neutrophils mediate Salmonella Typhimurium clearance through the GBP4 inflammasome-dependent production of prostaglandins. Nat. Commun. 2016;7:12077. doi: 10.1038/ncomms12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masumoto J, et al. Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J. Biol. Chem. 2003;278:4268–4276. doi: 10.1074/jbc.M203944200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.