Abstract
Cluster thinning and girdling are common and simple practices applied to improve berry quality in table grape cultivation. However, there is limited information about the accumulation and biosynthesis of the entire aromatic profile under cluster thinning and girdling, notably in table grapes. This research investigated the influences of cluster thinning and girdling (alone or in combination) on aroma profiles, particularly the changes in biosynthesis and accumulation of Muscat-flavored related compounds from véraison to harvest in ‘Jumeigui’ grape. Cluster thinning and girdling (alone or in combination) significantly increased the concentrations of total soluble solids (TSS) and key aromatic compounds at harvest, with higher concentrations of both under cluster thinning than girdling. Berry weight and titratable acidity (TA) were unaffected by cluster thinning, girdling, or in combination at harvest. Linalool, the most abundant and active odorant related to Muscat flavor, accumulated in 28.6% and 20.2% higher concentrations from cluster thinning than control and girdling at maturity, respectively. Furthermore, higher DXS3 transcript abundance in cluster thinning groups might contribute to the increased accumulation of terpenes and linalool in ‘Jumeigui’ grape. The results will contribute to further understand the mechanism of source/sink ratio modulation on aroma accumulation and better apply cluster thinning and girdling for grape production.
Subject terms: Plant physiology, Secondary metabolism
Introduction
Aroma is one of the important indicators contributing to table grape and wine quality1,2. Although several hundred aroma compounds have been identified, the most important families of compounds responsible for the aroma of grapes are terpenes, C13 norisoprenoids, methoxypyrazines, C6 alcohols and aldehydes, esters, and thiols1,3. Aroma compounds in grapes exist as free and bound glycosides. In table grapes, free forms might be the only vital ingredients to determine the flavor despite odourless bound glycosylated forms could be hydrolyzed to odour-active free forms during fermentation4,5.
Monoterpene alcohols, notably linalool, with low perception thresholds, contribute to floral characters and are particularly prevalent in Muscat flavored varieties. Monoterpenes in grapevine berries have been demonstrated to biosynthesize via the plastidial 2-C-methyl-D-erythritol-4-phosphate (MEP) pathway6. Due to the correlation between gene expression and monoterpenes accumulation, 1-deoxy-D-xylulose 5-phosphate (DXP) synthase (DXS), DXP reductoisomerase (DXR), 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) and geranyl pyrophosphate synthase (GPPS) are putative upstream rate-limiting enzymes7–9. Moreover, many of terpene synthases (TPSs) involved in late steps of monoterpenols biosynthesis have been functionally characterized10,11. Among them, the linalool/nerolidol synthase gene VvCSLinNer was reported to play an important role in linalool accumulation8,12.
The effects of agronomic practices such as leaf removal, irrigation, and exogenous hormones on primary and secondary metabolism in grapes have been well studied during the past decades3. Regulation of the source/sink ratio via cluster thinning is a common practice to enhance the accumulation of secondary metabolites, especially flavonols and anthocyanins13–18. However, there is little research concerning the impact of cluster thinning on aromatic compounds in berries. Cluster thinning enhanced sensory attributes in Grenache wines, but reduced them in Tempranillo wines19. The effect of cluster thinning on free and glycosylated volatile terpenes showed a significant increase in ‘Sauvignon Blanc’ berries, and the contents of terpenes were the highest in thinned treatment conducted one week before véraison20. Furthermore, girdling is another viticulture practice to improve grape quality through regulating the source/sink ratio21–23. Unfortunately, there is a lack of research about the influence of girdling on aroma profiles in grapes. Recently, peduncle girdling of post-veraison ‘Riesling’ bunches was reported to significantly increase monoterpenoid accumulation and reduce esters and higher alcohols24.
In China and other Asian countries, table grapes are mostly hybrid cultivars between Vitis vinifera and Vitis labrusca, owing to their high sugar levels and disease resistance25. Among them, ‘Jumeigui’, a progeny obtained from ‘Muscat Hamburg’ (4×) × ‘Kyoho’ (4×), is one of the most consumer-preferred table grape cultivars with rich Muscat flavor and planted widely in China. To obtain quality grapes with good appearance and desirable flavor, cluster thinning and girdling are common and efficient practices used in table grape cultivation. However, there is little information about the changes in aromatic profile accumulation and biosynthesis under cluster thinning and girdling, notably in table grapes. This work was aimed to explore and demonstrate the influences of cluster thinning and girdling (alone or in combination) on aroma profiles, as well as the changes in biosynthesis and accumulation of Muscat-flavored related compounds throughout the entire ripening period in ‘Jumeigui’ grape. The results will contribute to further understand the mechanism of source/sink ratio modulation on aroma accumulation and better apply cluster thinning and girdling for grape production.
Results
Yield, berry growth, and quality
Due to removal of half clusters, the yield per vine of thinned groups, CT (cluster thinning) and CTG (cluster thinning + girdling), were reduced by 48% and 49% against control group (CK), respectively (Table 1). There was no significant difference between G (only girdled group) and CK. In addition, the LA per vine was similar among the four treatments at harvest (Table 1). Likewise, the LA/Yield ratio was similar between G and CK. However, the LA/Yield ratio increased 0.9- and 1.0-fold in CT and CTG, respectively, compared to CK.
Table 1.
Vine parameters under cluster thinning and girdling at harvest.
| Treatment | Cluster/vine | Yield/vine (kg) | Leaf area/vine (m2) | Leaf area/yield (m2/kg) |
|---|---|---|---|---|
| CK | 72a | 37.52 ± 2.27a | 18.84 ± 0.84a | 0.50 ± 0.05a |
| G | 72a | 38.14 ± 1.79a | 18.98 ± 0.65a | 0.50 ± 0.04a |
| CT | 36b | 19.33 ± 1.09b | 18.55 ± 1.31a | 0.96 ± 0.12b |
| CTG | 36b | 19.00 ± 0.73b | 19.06 ± 0.86a | 1.00 ± 0.03b |
CK, the control treatment (unthinned and ungirdled); G, trunk girdled one week before véraison; CT, 50% clusters thinned one week before véraison; CTG, 50% clusters thinned and trunk girdled one week before véraison. Different letters within a column indicate statistically different values (p < 0.05) according to Duncan’s test.
Berry weight was unaffected by cluster thinning or girdling during the ripening period (Table 2). Both cluster thinning and girdling increased TSS compared to CK, especially from 71 DAA to harvest. At full ripe, TSS in CTG, CT, and G were 19.7%, 12.7%, and 7.5% higher than CK, respectively. TSS of the thinned groups (CT) was higher than that of the girdled groups (G). TA in the thinned and girdled berries were similar with CK at the onset of véraison (57 DAA), except for the CTG with sharp dropping (Table 2). Then the TA of the thinned or girdled groups declined sharply at 64 DAA and there was no significant difference among G, CT and CTG from 64 DAA to harvest. The drop of TA in CK berries delayed and had no significant difference with other treatments until harvest.
Table 2.
Berry fresh weight (FW, g), total soluble solid (TSS, °Brix) and titratable acidity (TA, g/L) of ‘Jumeigui’ grape under cluster thinning and girdling during berry ripening.
| Physicochemical parameter | Treatment | Berry development days after anthesis (DAA) | ||||
|---|---|---|---|---|---|---|
| 57 | 64 | 71 | 78 | 85 | ||
| FW | CK | 6.3 ± 0.4a | 6.8 ± 0.5a | 7.1 ± 0.2a | 7.7 ± 0.3a | 7.7 ± 0.2a |
| G | 6.4 ± 0.5a | 6.9 ± 0.1a | 7.0 ± 0.2a | 7.8 ± 0.2a | 7.7 ± 0.1a | |
| CT | 6.5 ± 0.3a | 7.0 ± 0.4a | 7.1 ± 0.3a | 7.9 ± 0.3a | 7.7 ± 0.2a | |
| CTG | 6.7 ± 0.3a | 7.0 ± 0.4a | 7.1 ± 0.1a | 8.1 ± 0.2a | 7.9 ± 0.1a | |
| TSS | CK | 8.4 ± 0.5a | 11.7 ± 0.2a | 13.5 ± 0.1a | 15.9 ± 0.1a | 17.3 ± 0.1a |
| G | 8.9 ± 0.1ab | 11.9 ± 0.1a | 14.4 ± 0.2b | 17.4 ± 0.1b | 18.6 ± 0.2b | |
| CT | 9.3 ± 0.5b | 12.3 ± 0.5a | 15.9 ± 0.1c | 18.6 ± 0.2c | 19.5 ± 0.2c | |
| CTG | 10.2 ± 0.3c | 13.6 ± 0.3b | 16.3 ± 0.1d | 19.9 ± 0.1d | 20.7 ± 0.1d | |
| TA | CK | 17.1 ± 0.5b | 11.2 ± 0.3b | 9.2 ± 1.5b | 6.7 ± 0.2b | 5.8 ± 0.5a |
| G | 17.5 ± 0.2b | 9.6 ± 0.3a | 7.5 ± 0.2a | 5.8 ± 0.4ab | 5.7 ± 0.3a | |
| CT | 17.0 ± 0.3b | 8.8 ± 0.5a | 7.6 ± 0.4a | 5.4 ± 0.8a | 5.6 ± 0.4a | |
| CTG | 15.5 ± 0.9a | 8.8 ± 1.2a | 6.8 ± 0.1a | 5.4 ± 0.4a | 5.8 ± 0.8a | |
CK, the control treatment (unthinned and ungirdled); G, trunk girdled one week before véraison; CT, 50% clusters thinned one week before véraison; CTG, 50% clusters thinned and trunk girdled one week before véraison. Different letters within a column indicate statistically different values (p < 0.05) according to Duncan’s test.
Aromatic compounds
Esters and C6 compounds were the predominant components of ‘Jumeigui’ grape at harvest, which accounted for 87.3–90.4% of the total components in the four treatments (Supplementary Fig. S1). However, the main component in berries at the onset of véraison were C6 compounds, which decreased gradually accompanied with increasing esters and terpenes from véraison to harvest. Likewise, the proportion of aldehydes declined constantly from the onset of véraison. In addition, the percentage of alcohols was negligible throughout the ripening period.
Regarding C6 compounds at maturity, the aroma-active compounds (OVAs > 1) were hexanal, (Z)-3-hexenal, (E)-2-hexenal and (E)-2-hexenol. Among them, hexanal was the most abundant and active compounds, which was accumulated at higher levels in G, CT and CTG against CK (Supplementary Table S1). Similarly, cluster thinning and girdling had positive effects on the contents of (E)-2-hexenal, (Z)-3-hexenal and (E)-2-hexenol in contrast with control. Despite the low concentration, (Z)-3-hexenal made positive contributions to the flavor of grape berries due to its low threshold (Supplementary Table S1). On the contrary, hexanol, detected at high levels, had nearly no contribution to the aroma owing to its high threshold. Furthermore, cluster thinning and girdling had significant impacts on the concentrations of total C6 compounds, which in CTG, CT and G were 48.9%, 36.4% and 13.3% higher than those in CK, respectively (Table 3).
Table 3.
Concentrations (μg/kg) of volatile compounds under cluster thinning and girdling in the pulp juice of ‘Jumeigui’ grape at harvest.
| Compounds | Treatment | |||
|---|---|---|---|---|
| CK | G | CT | CTG | |
| C6 compounds | ||||
| Hexanal | 1656 ± 48a | 1860 ± 48b | 2049 ± 30c | 2084 ± 39c |
| (Z)-3-Hexenal | 7.9 ± 2.0a | 8.6 ± 2.3ab | 10.9 ± 2.5ab | 13.3 ± 3.1b |
| (E)-2-Hexenal | 682 ± 23a | 815 ± 31b | 1095 ± 41c | 1198 ± 50d |
| Hexanol | 441 ± 30a | 405 ± 45a | 643 ± 61b | 828 ± 40c |
| (Z)-3-Hexenol | 11.9 ± 0.2a | 16.2 ± 0.8b | 15.0 ± 0.7b | 14.8 ± 0.9b |
| (E)-2-Hexenol | 134 ± 12a | 217 ± 54b | 189 ± 16ab | 229 ± 53b |
| Subtotal | 2933 ± 59a | 3322 ± 91b | 4001 ± 68c | 4367 ± 92d |
| Alcohols | ||||
| 1-Octen-3-ol | 3.28 ± 0.16a | 3.14 ± 0.12a | 4.84 ± 0.29b | 7.29 ± 0.32c |
| Heptanol | 3.58 ± 0.26b | 3.04 ± 0.11a | 2.87 ± 0.19a | 4.08 ± 0.31c |
| 2-Ethyl hexanol* | 1.63 ± 0.05a | 1.37 ± 0.18a | 1.27 ± 0.36a | 1.60 ± 0.10a |
| Octanol | 0.83 ± 0.11b | 0.57 ± 0.07a | 1.10 ± 0.11c | 1.52 ± 0.18d |
| Phenylethyl alcohol | 0.83 ± 0.24a | 1.20 ± 0.13b | 1.70 ± 0.02c | 1.53 ± 0.11c |
| Subtotal | 10.2 ± 0.4a | 9.3 ± 0.5a | 11.8 ± 0.2b | 16.0 ± 0.8c |
| Esters | ||||
| Ethyl acetate | 2275 ± 197a | 3343 ± 88b | 5159 ± 113d | 4885 ± 134c |
| Ethyl propionate* | 3.47 ± 0.23a | 4.85 ± 0.21b | 5.14 ± 0.64b | 8.64 ± 0.98c |
| Ethyl isobutyrate | nd | 15.1 ± 8.7a | 27.2 ± 6.8a | 55. 9 ± 7.0b |
| Ethyl butyrate | 79.2 ± 10.5a | 256 ± 6b | 318 ± 21c | 376. ± 32d |
| Ethyl 2-methylbutanoate* | 2.26 ± 0.42a | 8.95 ± 0.44b | 12.2 ± 4.9b | 13.1 ± 4.6b |
| Ethyl pentanoate | 31.4 ± 3.3a | 110 ± 3c | 76.1 ± 10.1b | 33.7 ± 4.5a |
| Ethyl hexanoate | 29.7 ± 5.0a | 62.9 ± 5.7bc | 68.1 ± 7.0c | 56.5 ± 4.4b |
| Hexyl acetate | 1.18 ± 0.11a | 1.96 ± 0.84ab | 2.32 ± 0.35ab | 3.31 ± 1.09b |
| 2-Hexenoic acid ethyl ester* | 3.24 ± 0.29a | 5.55 ± 0.51b | 11.0 ± 1.4c | 14.6 ± 0.5d |
| Ethyl octanoate* | 2.93 ± 2.10a | 4.60 ± 0.16ab | 6.49 ± 1.77b | 6.02 ± 1.56ab |
| Ethyl 3-hydroxybutyrate* | 1.84 ± 0.95a | 4.49 ± 0.29b | 6.11 ± 0.66c | 9.23 ± 0.95d |
| Subtotal | 2430 ± 212a | 3817 ± 88b | 5691 ± 126c | 5462 ± 172c |
| Aldehydes | ||||
| Pentanal | 4.96 ± 0.94ab | 4.46 ± 0.61a | 6.17 ± 0.46c | 6.02 ± 0.83bc |
| Heptanal* | 12.7 ± 3.6a | 13.0 ± 2.3a | 13.6 ± 2.8a | 13.5 ± 1.7a |
| Octanal | 6.82 ± 0.52a | 6.76 ± 1.01a | 8.90 ± 0.86b | 8.13 ± 0.81ab |
| Nonanal | 59.7 ± 15.1a | 48.4 ± 3.2a | 64.7 ± 8.1a | 53.5 ± 7.1a |
| Benzaldehyde | 17.3 ± 3.1a | 16.3 ± 2.6a | 17.8 ± 4.4a | 17.0 ± 6.5a |
| Phenylacetaldehyde* | 4.68 ± 0.21b | 2.77 ± 0.62a | 3.24 ± 0.25a | 3.47 ± 0.17a |
| Subtotal | 106 ± 17a | 91.7 ± 10.0a | 114 ± 16a | 102 ± 10a |
| Terpenes | ||||
| Subtotal | 659 ± 11a | 722 ± 24b | 906 ± 12c | 999 ± 13d |
| Total | 6138 ± 298a | 7962 ± 149b | 10725 ± 192c | 10945 ± 231d |
CK, the control treatment (unthinned and ungirdled); G, trunk girdled one week before véraison; CT, 50% clusters thinned one week before véraison; CTG, 50% clusters thinned and trunk girdled one week before véraison. Different letters within rows indicate statistically different values (p < 0.05) according to Duncan’s test. *Indicated the semi-quantitative determinations with the internal standards.
The total ester content in CTG, CT and G increased 1.2, 1.3 and 0.6-fold, respectively, compared to CK (Table 3). Four compounds with low threshold were the main contributors to flavor. Among them, ethyl butyrate was the most abundant significantly accumulated in G, CT and CTG berries compared with CK. Likewise, the concentrations of other three compounds, ethyl 2-methylbutanoate, ethyl pentanoate and ethyl hexanoate, were positively increased in cluster thinning and girdling groups. Interestingly, ethyl isobutyrate, was not detected in berries of CK, but contributed to aroma of berries in other three treatments, especially in CTG. Ethyl acetate was the most abundant ester, ranging from 87.6–93.6% of the total esters (Table 3). However, it had almost no contribution to aroma due to its high threshold (Supplementary Table S1).
The concentrations of each alcohol were at low intensity and only one compound, 1-octen-3-ol, was aroma-active but had little contribution to flavor. Cluster thinning significantly enhanced the concentration of 1-octen-3-ol, while girdling did not in contrast with CK (Table 3). Although both of proportion and content of aldehydes compounds also present at low level, there were three aroma-active compounds, especially nonanal, which made significant contributions to aroma owing to the low thresholds (Supplementary Table S1). However, cluster thinning and girdling had no significant impact on the concentrations of these compounds (Table 3).
Due to the pleasant Muscat-flavor of ‘Jumeigui’ grape, the impacts of cluster thinning and girdling (alone or in combination) on aromatic compounds were focused on terpenes throughout the ripening period (Table 4). Linalool was the most abundant and active odorant among the detected terpenes compounds. In contrast to other terpenes compounds, linalool dramatically accumulated from the onset of véraison and then accounted for 60.1–66.3% of total terpenes in the four treatments at harvest. Girdling did not significantly increase the concentration of linalool at the beginning of véraison until 78 DAA, whereas cluster thinning did throughout the ripening period (Table 4). At harvest, the berries of CTG, CT and G accumulated 37.4%, 28.6% and 7.1% linalool more than that of CK, respectively. In addition, the contents of linalool in thinned groups, especially in CTG, were significantly higher than that in girdled groups from véraison to harvest. The other aroma-active compounds, including D-limonene, cis-linalool oxide, and geraniol, were also significantly accumulated in thinned berries throughout the ripening period. Although girdling could promote the concentrations of these compounds against CK, there were no significant differences between G and CK at most developmental stages (Table 4). Interestingly, β-myrcene had no contributions to aroma in G and CK berries, whereas did positive contributions in CT and CTG. Furthermore, the berries of G, CT and CTG accumulated 9.5%, 37.5% and 51.6% terpenes more than that of CK at harvest, respectively (Table 3).
Table 4.
Concentrations (μg/kg) of terpenes compounds under cluster thinning and girdling in the pulp juice of ‘Jumeigui’ grape during berry development.
| Compounds | DAA | Treatment | |||
|---|---|---|---|---|---|
| CK | G | CT | CTG | ||
| β-Myrcene | 57 | 11.8 ± 3.5a | 12.0 ± 3.1a | 13.9 ± 3.3ab | 18.3 ± 0.9b |
| 64 | 19.4 ± 3.5a | 15.3 ± 1.3a | 16.9 ± 0.9a | 17.5 ± 3.2a | |
| 71 | 13.1 ± 1.5a | 18.0 ± 1.7a | 17.7 ± 0.8a | 25.5 ± 5.0b | |
| 78 | 24.1 ± 1.1a | 25.1 ± 0.9a | 38.4 ± 1.8b | 34.5 ± 3.7b | |
| 85 | 22.6 ± 3.6a | 24.8 ± 1.2a | 51.6 ± 6.0b | 50.2 ± 8.4b | |
| D-Limonene | 57 | 54.3 ± 0.8a | 56.5 ± 12.1a | 54.3 ± 11.1a | 68.6 ± 3.5a |
| 64 | 74.3 ± 4.4a | 84.9 ± 4.1b | 90.8 ± 1.1bc | 95.4 ± 5.9c | |
| 71 | 67.2 ± 4.8a | 83.9 ± 10.0b | 90.5 ± 8.0b | 97.1 ± 7.7b | |
| 78 | 73.6 ± 11.0a | 82.6 ± 10.5a | 86.4 ± 4.1a | 88.4 ± 5.5a | |
| 85 | 71.1 ± 4.3a | 79.5 ± 6.3a | 91.9 ± 4.6b | 96.6 ± 7.0b | |
| β-cis-Ocimene* | 57 | 6.25 ± 0.53a | 6.03 ± 0.38a | 7.56 ± 0.68a | 7.11 ± 1.48a |
| 64 | 12.0 ± 1.9ab | 10.2 ± 1.2a | 13.7 ± 1.8b | 15.0 ± 1.5b | |
| 71 | 11.5 ± 3.3a | 12.3 ± 2.6a | 15.9 ± 4.0a | 14.9 ± 0.8a | |
| 78 | 11.6 ± 2.7a | 12.7 ± 2.8ab | 17.2 ± 2.8bc | 17.5 ± 0.9c | |
| 85 | 12.2 ± 1.2a | 13.6 ± 0.4ab | 15.5 ± 1.2b | 16.2 ± 2.6b | |
| p-Cymene | 57 | nd | nd | nd | nd |
| 64 | nd | nd | 1.72 ± 0.06a | 2.52 ± 0.41b | |
| 71 | 2.27 ± 0.10a | 3.28 ± 0.32b | 3.17 ± 0.46b | 3.10 ± 0.25b | |
| 78 | 1.52 ± 0.28a | 2.71 ± 0.22b | 3.16 ± 0.11b | 2.75 ± 0.74b | |
| 85 | 1.87 ± 0.39a | 2.69 ± 0.56ab | 3.32 ± 0.40b | 3.44 ± 0.38b | |
| cis-Linalool oxide* | 57 | nd | nd | nd | nd |
| 64 | nd | nd | nd | nd | |
| 71 | nd | nd | nd | nd | |
| 78 | nd | nd | 2.00 ± 0.22a | 2.28 ± 0.01b | |
| 85 | 1.77 ± 0.07b | 1.47 ± 0.05a | 2.75 ± 0.17c | 2.96 ± 0.09d | |
| Linalool | 57 | 1.77 ± 0.09a | 2.40 ± 0.05b | 5.08 ± 0.16c | 5.96 ± 0.17d |
| 64 | 16.4 ± 2.5b | 13.9 ± 0.2a | 51.9 ± 0.6c | 58.8 ± 0.6d | |
| 71 | 74.7 ± 2.3a | 72.7 ± 6.7a | 155 ± 6b | 170 ± 1c | |
| 78 | 198 ± 3a | 220 ± 5b | 301 ± 6c | 397 ± 4d | |
| 85 | 437 ± 6a | 468 ± 5b | 562 ± 8c | 600 ± 8d | |
| α-Terpineol | 57 | 1.79 ± 0.34a | 2.05 ± 0.29a | 2.15 ± 0.24a | 2.11 ± 0.25a |
| 64 | 2.65 ± 0.55a | 2.43 ± 0.09a | 2.67 ± 0.05a | 2.58 ± 0.13a | |
| 71 | 5.98 ± 0.37a | 5.59 ± 0.48a | 12.3 ± 0.1b | 14.3 ± 0.4c | |
| 78 | 7.49 ± 0.06a | 8.43 ± 0.23a | 17.7 ± 2.2b | 16.3 ± 3.4b | |
| 85 | 16.4 ± 0.5a | 18.2 ± 3.4ab | 19.5 ± 0.9ab | 20.5 ± 0.5b | |
| Nerol | 57 | nd | nd | nd | nd |
| 64 | nd | nd | nd | nd | |
| 71 | nd | nd | 12. 8 ± 0.3a | 14.8 ± 0.7b | |
| 78 | 9.19 ± 0.24a | 10.0 ± 0.2a | 22.3 ± 4.0b | 22.6 ± 3.0b | |
| 85 | 15.6 ± 0.4a | 17.4 ± 0.6a | 22.3 ± 1.0b | 22.2 ± 1.7b | |
| Geraniol | 57 | nd | nd | nd | nd |
| 64 | nd | nd | 30.2 ± 2.1a | 31.1 ± 1.7a | |
| 71 | 54.8 ± 8.2a | 50.2 ± 7.3a | 81.5 ± 1.1b | 97.7 ± 4.1c | |
| 78 | 62.8 ± 12.0a | 68.7 ± 4.4a | 107 ± 11b | 118 ± 21b | |
| 85 | 80.9 ± 5.9a | 96.6 ± 11.5a | 138 ± 2b | 187 ± 17c | |
CK, the control treatment (unthinned and ungirdled); G, trunk girdled one week before véraison; CT, 50% clusters thinned one week before véraison; CTG, 50% clusters thinned and trunk girdled one week before véraison. Different letters within rows indicate statistically different values (p < 0.05) according to Duncan’s test. nd: not detected. * Indicated the semi-quantitative determinations with the internal standards.
For evaluating the changes in global aroma profiles under cluster thinning and girdling (alone or in combination), the OAVs of the aroma compounds with similar descriptors were grouped into aromatic series according to a previous report2. The aroma of ‘Jumeigui’ grapes was mainly characterized by herbaceous, fruity, floral, and sweet (Supplementary Fig. S2). The berries of G, CT and CTG enhanced 18.5%, 31.0% and 27.9% herbaceous series more than that of CK at harvest, respectively. The values of fruity series in G, CT, and CTG were 1.0-, 1.5-, and 1.6-fold higher than those in CK, respectively. In addition, cluster thinning increased 30.7% floral series and 28.6% sweet series compared to CK, respectively. However, the values of floral series and sweet series in berries were similar between G and CK.
Transcript abundance of monoterpene biosynthesis-related genes
Among 6 genes involved in monoterpene biosynthesis, the expression pattern of VvDXS1 and VvCSLinNer was similar with sharp drop of transcript levels from 64 DAA (Fig. 1). Till harvest, the expression of these two genes remained at low levels and was not correlated to total terpenes and linalool concentration. Contrarily, the transcript abundance of VvDXS3 was significantly correlated with terpenes and linalool accumulation in all the four treatments (Supplementary Table S2). The expression of VvDXS3 was significantly upregulated in berries of CT and CTG compared with CK, especially from 71 DAA. There was no significant difference between CK and G throughout the ripening period, except the beginning of véraison (57 DAA). Similarly, cluster thinning positively enhanced the transcript abundance of VvGPPS against CK from 64 to 78 DAA. Meanwhile, girdling only significantly upregulated its expression level at 71 DAA in contrast to CK. Interestingly, the transcript levels in CK berries were significantly higher than that in other treatments at harvest. Unfortunately, the transcript abundance of VvDXR and VvHDR was negligible in berries of all treatments throughout the ripening period.
Figure 1.
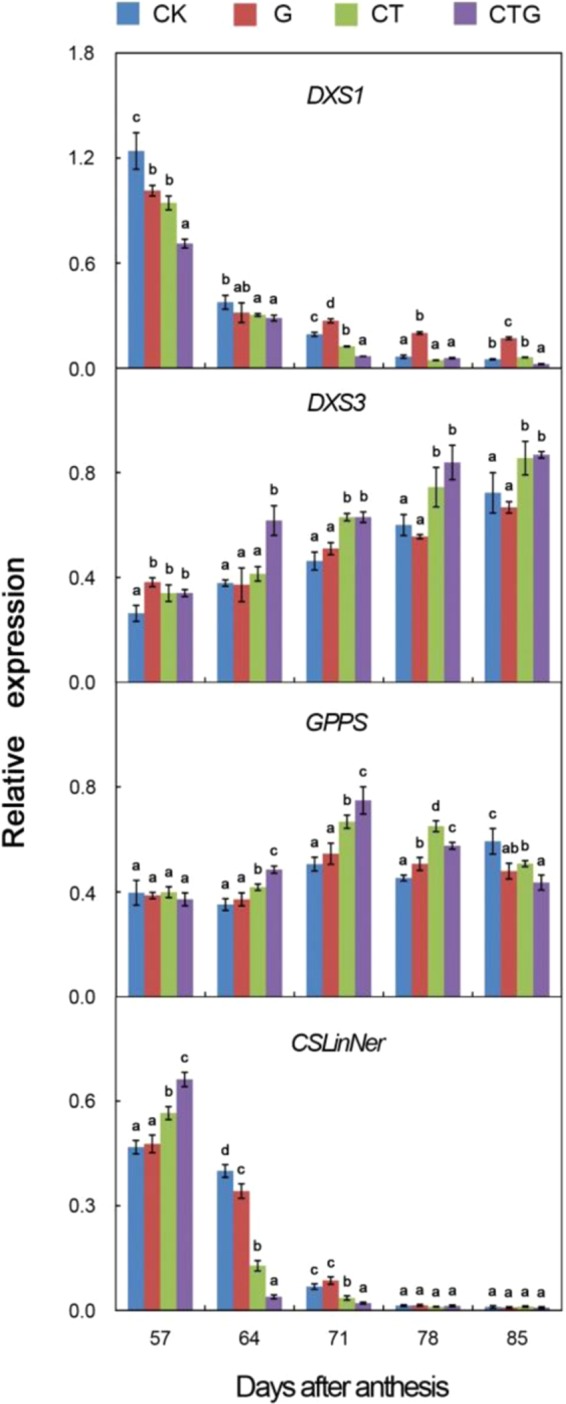
Expression levels of genes involved in monoterpene biosynthesis. CK, the control treatment (unthinned and ungirdled); G, trunk girdled one week before véraison; CT, 50% clusters thinned one week before véraison; CTG, 50% clusters thinned and trunk girdled one week before véraison. Data are mean ± SE of three biological replicates. Different letters on bar chart denotes statistical significance values (p < 0.05) according to Duncan’s test.
Discussion
Cluster thinning is a common viticulture technique of modulating the source-sink balance to improve berry quality and elevate the level of secondary metabolites3. Girdling is another viticulture technique involved in the source/sink ratio regulation, which directs the flow of phloem-transported metabolites to the fruit during the ripening stages. In this study, and in previous studies, girdling at the beginning of véraison advances fruit ripening and increase soluble solids with no yield reduction22,26,27. Likewise, cluster thinning significantly increased TSS of ‘Jumeigui’ berries consistent with previous studies16,28–30. Interestingly, in this study, TSS in thinned groups was significantly greater than that in girdled groups. An LA/Yield above 0.8 m2/kg was determined to be critical for full maturity of grape berries and the impacts of source/sink regulation on sugar accumulation31. In present study, cluster thinning boosted the LA/Yield ratio to 0.96 m2/kg, while girdling had an LA/Yield ratio of 0.50 m2/kg, similar to the control. Therefore, an LA/Yield ratio below 0.8 m2/kg in girdled groups might lower TSS compared to cluster thinning.
In this study, berry weight was the same across treatments throughout the ripening stages. Previous reports suggested cluster thinning and girdling had either no effect on berry weight22,26,30 or berry weight slightly increased13,21,23. The most of berry weight increasing achieved before applying treatment might contribute to similar berry weight across treatments in present study. Likewise, there were no significant differences in titratable acidity among the four treatments at harvest in the present study, similar to prior studies22,30,32. However, others have reported a slight increase23,29 or a significant decrease26 of TA in response to thinning and girdling. Some reports suggest that titratable acidity is less responsive to low LA/Yield ratios than sugars30,33,34. Therefore, both berry weight and titratable acidity might be insensitive to regulation of the source-sink balance with cluster thinning and girdling.
Despite a number of studies about the impact of cluster thinning on phenolic compounds in grapes, there is little research concerning effects on aroma composition, especially in table grapes. Cluster thinning was shown to be favourable for improvement of varietal aromas, including terpenes, ethyl esters, C13-norisoprenoids, and alcohols20,35–37. In this research on ‘Jumeigui’ grapes, cluster thinning significantly enhanced the concentrations of three primary components (C6 compounds, esters and terpenes) at harvest, consistent with prior results20,35–37. Grouping the OAVs of the aroma compounds with similar descriptors into aromatic series was reported to simplify the evaluation of global aroma profiles2. According to the OVAs and odorant series of 37 detected volatile compounds, the herbaceous, fruity, floral, and sweet series made up the aroma of ‘Jumeigui’ grape berries in present study. The values of those four odorant series were significantly increased under cluster thinning. Consequently, cluster thinning had a positive impact on flavor enhancement in ‘Jumeigui’ grape. However, recent research revealed that cluster thinning applied at the onset of véraison did not show a positive effect on grape sensory due to the limited effects on most aromatic compounds38. The high LA/Yield ratio in both of cluster thinning and control treatments was suggested to lead to similar berry maturation between the two treatments, which might contribute to limited differences in the concentrations of aroma and flavonol composition17,38. On the contrary, the LA/Yield ratio under cluster thinning was significantly greater than that under control in this study, probably leading to significantly increasing aroma compounds in thinned berries. In present research, girdling positively increased the values of herbaceous and fruity series, but had little impact on floral and sweet series. Interestingly, the values of those odorant series in thinned groups were greatly higher than the girdled groups. Unfortunately, to our knowledge, there is almost no information about the effect of girdling on aromatic compounds and the difference between cluster thinning and girdling. Therefore, the LA/Yield above 0.8 m2/kg was inferred to be crucial for comparing the impact of source-sink modulations on aroma accumulation as well as sugar. Previous reports have demonstrated that the concentrations of varietal compounds, especially monoterpenes, increased with positive correlation to soluble solids35,39. Consequently, the more aroma accumulation in thinned berries against girdled berries might be the consequence of higher TSS under cluster thinning with the LA/Yield above 0.8 m2/kg in this study.
In China, the ‘Jumeigui’ grape is widely planted and consumer-preferred due to its rich Muscat flavor, which are primarily attributed to extensive terpenes. Consistent with a previous report2, monoterpenes were detected in ‘Jumeigui’ grape with linalool as the most active odorant in pulp juice in this study. Moreover, cluster thinning significantly enhanced the contents of most terpenes, especially from 71 DAA, while girdling slightly increased in contrast with control. Like other compounds in this work, the concentration of terpene compounds under girdling was lower than that under cluster thinning. Previous reports have demonstrated that monoterpenes accumulation in grapevine berries were correlated with gene expression involved in MEP pathway, such as DXS, DXR, HDR, and GPPS8,9,40,41. However, in this study, only the expression level of VvDXS3 showed significant correlation with terpenes and linalool accumulation in all the four treatments from véraison to harvest. VvDXS3 has been suggested as a biomarker for monoterpene accumulation due to the high correlation coefficient between the monoterpene content and gene transcript abundance40. Consequently, the significant increase in VvDXS3 transcript abundance under cluster thinning, especially from 71 DAA to harvest, might contribute to increased terpene and linalool accumulation against control, while no significant difference of gene expression between girdling and control at most ripening period might lead to the slight enhancement of terpenes and linalool levels under girdling. Interestingly, VvDXS1 co-localized with a major QTL for monoterpene content42, only expressed at relatively high level at the onset of véraison and its transcript abundance was irrelevant to the increase of terpenes and linalool according to Pearson’s correlation coefficient analysis. No significant increase in VvDXS1 transcript abundance related with monoterpene accumulation throughout the ripening period was also reported in ‘Gewürztraminer’ grapes8. Inconsistent with previous reports, VvDXR and VvHDR expression were not detected throughout the ripening stages in this study. Similarly, the transcript abundance of VvCSLinNer gene, which was reported as the key gene involved in linalool accumulation8,12, was very low throughout the ripening period except the onset of véraison, but was uncorrelated with linalool accumulation. The similar low expression levels of VvCSLinNer gene were found in ripening berries of other cultivars11,43,44. Therefore, these inconsistent reports on the same gene might due to the different cultivars and climatic conditions.
Methods
Plant materials and field trails
The experiments were performed during 2017 in an experimental vineyard at Shanghai Academy of Agricultural Sciences, Shanghai, China (30°51′N, 121°13′E) with table grape cultivar ‘Jumeigui’. The own-rooted grapevines were planted in the spring of 2008 with a north–south orientation and removed one by one at 4 m × 2.8 m spacing five years later. The vines were grown in a rain shelter with a Y-shape training system and managed according to the standard viticulture and disease control practices in Shanghai. The temperature (°C) and relative humidity (%) within the rain-shelter cultivation greenhouse were monitored hourly with data-loggers (HOBO U23-001, Onset Computer Inc., Bourne, MA, USA; Supplementary Fig. S3).
Thirty-six uniform vines were randomly selected and grouped into four treatments: control (CK), girdling (G), cluster thinning (CT), and cluster thinning + girdling (CTG).
The experimental design was a randomized block design with three vines as an experimental unit and replicated three times. Each vine retained 36 shoots and two clusters per shoot after bloom. In addition, each shoot and cluster was adjusted to 20 leaves and 60 berries at the pea-size stage, respectively. At 50 DAA (one week before véraison), 50% clusters of each vine in CT and CTG were removed with 36 clusters per vine left. Meanwhile, the single trunk of each vine in G and CTG was girdled by removing a 5 mm wide section of the phloem using a girdling knife from approximately 50 cm above ground level.
Berries were randomly sampled weekly from the onset of véraison which was one week after treatment (57 DAA, berries softening with ~8 °Brix) to harvest (85 DAA). For each biological replicate, 40 berries were randomly collected at each sampling date and half berries were randomly chosen for physicochemical analysis. The remaining berries were immediately frozen in liquid nitrogen and stored at −80 °C for aroma compound analysis and RNA extraction.
Measurement of physicochemical parameters
Twenty berries of each biological replicate were sampled to determine berry fresh weight (FW; g), total soluble solids (TSS; °Brix) and titratable acidity (TA; g/L tartaric acid). TSS was estimated with a digital refractometer (PAL-1, Atago, Tokyo, Japan) and TA was measured through conventional acid-base titration.
Measurement of vine performance
Fifteen clusters were randomly sampled from each replicate at harvest and weighed to estimate yield data. Five shoots per replicate were randomly selected after harvest and leaf area (LA) was measured with an LA meter (Yaxin-1241, Yaxinliyi Science and Technology, Beijing, China) to estimate average leaf area per shoot.
Aroma compounds extraction and SPME-GC-MS analysis
Extraction and analysis of aromatic compounds was performed in Shanghai Jiao Tong University according to previously reported methods2. Briefly, deseeded pulps of ten berries were homogenized after thawing at 4 °C, centrifuged, and then filtered to achieve a clear juice. The extraction of aroma compounds in the juice was performed through headspace solid phase microextraction (HS-SPME) using a MPS-2 autosampler (Gerstel, Mühlheim, Germany). The 6 mL juice were mixed with 1.5 g NaCl in a 20-mL glass vial, and then 4 µL 2-octanol internal standard solution (53.84 mg/L) was added. The volatiles were extracted for 30 min using a SPME fiber coated with 50/30 μm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS; Supelco, Bellefonte, PA, USA) after 10 min of equilibration at 50 °C. The fiber was then immediately inserted into the gas chromatograph (GC) injection port for desorption at 260 °C for 3 min in splitless mode. Separation of the desorbed volatiles was carried out using an Agilent 7890 GC coupled with a 5975 mass-selective detector (Agilent Technologies, Santa Clara, CA, USA) equipped with a HP-Innowax column (30 m × 0.25 mm i.d., 0.25 µm film thickness; J & W Scientific, Folsom, CA, USA). The temperature program was as follows: 40 °C for 5 min, increased to 240 °C at 5 °C min−1, and then to 260 °C at 20 °C min−1 and held for 5 min. The flow rate of helium as carrier gas was 1 mL min−1. The mass spectrometry (MS) transfer line and ionization source temperature were 260 °C and 230 °C, respectively. Electron impact mass spectrometric data from m/z 20 to 400 were collected at 70 eV ionization voltages.
Compound identifications were performed via comparing mass spectral data with the National Institute of Standards and Technology (NIST) 2011 library and the retention times of the authenticated standards. For compounds that standards were unavailable in the library, tentative identifications were made via comparing their linear retention indices (LRI) and MS spectra with those reported in the literature. Compounds were quantified according to calibration curves, while the compounds without calibration curves were semi-quantified using the internal standard (Supplementary Table S3).
Gene expression analyses
Total RNA was isolated from pulp using universal plant total RNA extraction kit (BioTeke, Beijing, China). RNA quality and concentration were assessed using agarose gel electrophoresis and a NanoDrop 2000 spectrophotometry (Thermo Scientific, Wilmington, DE, USA). cDNA was synthesized using Takara PrimeScript RT reagent Kit with gDNA Eraser (Takara, Dalian, China). The primer sequences used in present study are listed in Supplementary Table S48. Quantitative real-time PCR (qRT-PCR) was conducted using a LightCycler 480 System (Roche, Mannheim, Germany) with SYBR Premix Ex Taq II (Takara, Dalian, China) as previously described16. Gene transcripts were quantified upon normalization to the internal reference genes VvGAPDH and VvActin145,46 using geNorm software according to a previous report47.
Statistical analysis
Data in this experiment were presented as the average of three replicates. One-way ANOVA (p < 0.05) and Duncan’s multiple range tests were conducted with SPSS 18.0 (SPSS Inc., IL, USA).
Supplementary information
Acknowledgements
This research was supported by the Modern Agricultural Industry Technology System (Grape) (CARS-30-9). We thank Dr. Benjamin L. Gutierrez from Plant Genetic Resources Unit, United States Department of Agriculture-Agricultural Research Service for editing this manuscript.
Author contributions
X.X. conceived, designed and performed the experiments, analyzed and interpreted the data, drafted and revised the manuscript. Q.Z. helped perform the real-time RT-PCR experiments, participated in the interpretation of data and the drafting of the manuscript. Y.H. participated in extraction and analysis of aromatic compounds. Y.T. participated in conducting the field trails and sampling the material. A.J. conceived the study, participated in its design and revised the manuscript. All authors have read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaojun Xi, Email: xxj220401@126.com.
Aili Jiang, Email: putaojal@163.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-63826-7.
References
- 1.González-barreiro C, Rial-otero R, Cancho-grande B, Simal-gándara J. Wine aroma compounds in grapes: a critical review. Crit. Rev. Food Sci. Nutr. 2015;11:202–218. doi: 10.1080/10408398.2011.650336. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, et al. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016;6:31116. doi: 10.1038/srep31116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alem H, Rigou P, Schneider R, Ojeda H, Torregrosa L. Impact of agronomic practices on grape aroma composition: a review. J. Sci. Food Agric. 2019;99:975–985. doi: 10.1002/jsfa.9327. [DOI] [PubMed] [Google Scholar]
- 4.Ghaste M, et al. Chemical composition of volatile aroma metabolites and their glycosylated precursors that can uniquely differentiate individual grape cultivars. Food Chem. 2015;188:309–319. doi: 10.1016/j.foodchem.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Zhu X, Ullah N, Tao Y. Aroma glycosides in grapes and wine. J. Food Sci. 2017;82:248–259. doi: 10.1111/1750-3841.13598. [DOI] [PubMed] [Google Scholar]
- 6.Luan F, Wüst M. Differential incorporation of 1-deoxy-d-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry. 2002;60:451–459. doi: 10.1016/S0031-9422(02)00147-4. [DOI] [PubMed] [Google Scholar]
- 7.Battilana J, et al. Functional effect of grapevine 1-deoxy-D-xylulose 5-phosphate synthase substitution K284N on Muscat flavour formation. J. Exp. Bot. 2011;62:5497–5508. doi: 10.1093/jxb/err231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DM, Chiang A, Lund ST, Bohlmann J. Biosynthesis of wine aroma: transcript profiles of hydroxymethylbutenyl diphosphate reductase, geranyl diphosphate synthase, and linalool/nerolidol synthase parallel monoterpenol glycoside accumulation in Gewürztraminer grapes. Planta. 2012;236:919–929. doi: 10.1007/s00425-012-1704-0. [DOI] [PubMed] [Google Scholar]
- 9.Costa LD, et al. Induction of terpene biosynthesis in berries of microvine transformed with VvDXS1 alleles. Front. Plant. Sci. 2018;8:2244. doi: 10.3389/fpls.2017.02244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin DM, et al. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant. Biol. 2010;10:226. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matarese F, Scalabrelli G, D’Onofrio C. Analysis of the expression of terpene synthase genes in relation to aroma content in two aromatic vitis vinifera varieties. Funct. Plant. Biol. 2013;40:552–565. doi: 10.1071/FP12326. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki K, et al. Effect of light exposure on linalool biosynthesis and accumulation in grape berries. Biosci. Biotech. Biochem. 2016;80:2376–2382. doi: 10.1080/09168451.2016.1217148. [DOI] [PubMed] [Google Scholar]
- 13.Guidoni S, Allara P, Schubert A. Effect of cluster thinning on berry skin anthocyanin composition of Vitis vinifera cv. Nebbiolo. Am. J. Enol. Viticult. 2002;53:224–226. [Google Scholar]
- 14.Pastore C, et al. Increasing the source/sink ratio in Vitis vinifera (cv Sangiovese) induces extensive transcriptome reprogramming and modifies berry ripening. BMC Genomics. 2011;12:631. doi: 10.1186/1471-2164-12-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberto SR, et al. Berry-cluster thinning to prevent bunch compactness of ‘BRS Vitoria’, a new black seedless grape. Sci. Horticulturae. 2015;197:297–303. doi: 10.1016/j.scienta.2015.09.049. [DOI] [Google Scholar]
- 16.Xi X, Zha Q, Jiang A, Tian Y. Impact of cluster thinning on transcriptional regulation of anthocyanin biosynthesis-related genes in ‘Summer Black’ grapes. Plant. Physiol. Biochem. 2016;104:180–187. doi: 10.1016/j.plaphy.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. Effects of cluster thinning on vine photosynthesis, berry ripeness and flavonoid composition of Cabernet Sauvignon. Food Chem. 2018;248:101–110. doi: 10.1016/j.foodchem.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Colombo RC, et al. Analysis of the phenolic composition and yield of ‘BRS Vitoria’seedless table grape under different bunch densities using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2020;130:108955. doi: 10.1016/j.foodres.2019.108955. [DOI] [PubMed] [Google Scholar]
- 19.Diago MP, Vilanova M, Blanco JA, Tardaguila J. Effects of mechanical thinning on fruit and wine composition and sensory attributes of Grenache and Tempranillo varieties (Vitis vinifera L.) Aust. J. Grape Wine Res. 2010;16:314–326. doi: 10.1111/j.1755-0238.2010.00094.x. [DOI] [Google Scholar]
- 20.Kok D. Influences of pre- and post-véraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. J. Food Agric. Env. 2011;9:22–26. [Google Scholar]
- 21.Brar HS, Singh Z, Swinny E, Cameron I. Girdling and grapevine leafroll associated viruses affect berry weight, colour development and accumulation of anthocyanins in ‘Crimson Seedless’ grapes during maturation and ripening. Plant. Sci. 2008;175:885–897. doi: 10.1016/j.plantsci.2008.09.005. [DOI] [Google Scholar]
- 22.Koshita Y, Yamane T, Yakushiji H, Azuma A, Mitani N. Regulation of skin color in ‘Aki Queen’ grapes: Interactive effects of temperature, girdling, and leaf shading treatments on coloration and total soluble solids. Sci. Horticulturae. 2011;129:98–101. doi: 10.1016/j.scienta.2011.03.014. [DOI] [Google Scholar]
- 23.Basile T, Alba V, Gentilesco G, Savino M, Tarricone L. Anthocyanins pattern variation in relation to thinning and girdling in commercial Sugrathirteen® table grape. Sci. Horticulturae. 2018;227:202–206. doi: 10.1016/j.scienta.2017.09.045. [DOI] [Google Scholar]
- 24.Böttcher C, et al. Post-veraison restriction of phloem import into Riesling (Vitis vinifera L.) berries induces transient and stable changes to fermentation-derived and varietal wine volatiles. Aust. J. Grape Wine Res. 2019;25:286–292. doi: 10.1111/ajgw.12392. [DOI] [Google Scholar]
- 25.Yang C, Wang Y, Wu B, Fang J, Li S. Volatile compounds evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011;128:823–830. doi: 10.1016/j.foodchem.2010.11.029. [DOI] [Google Scholar]
- 26.Carreño J, Faraj S, Martinez A. Effects of girdling and covering mesh on ripening, colour and fruit characteristics of ‘Italia’ grapes. J. Hort. Sci. Biotech. 1998;73:103–106. doi: 10.1080/14620316.1998.11510951. [DOI] [Google Scholar]
- 27.Mikolaou N, Zioziou E, Stavrakas D, Patakas A. Effects of ethephon, methanol, ethanol and girdling treatments on berry maturity and colour development in Cardinal table grapes. Aust. J. Grape Wine Res. 2003;9:12–14. doi: 10.1111/j.1755-0238.2003.tb00227.x. [DOI] [Google Scholar]
- 28.Reynolds AG, Price SF, Wardle DA, Watson BT. Fruit environment and crop level effects on Pinot noir. I. Vine performance and fruit composition in British Columbia. Am. J. Enol. Viticult. 1994;45:452–459. [Google Scholar]
- 29.Gamero E, et al. Effect of bunch thinning and water stress on chemical and sensory characteristics of Tempranillo wines. Aust. J. Grape Wine Res. 2014;20:394–400. doi: 10.1111/ajgw.12088. [DOI] [Google Scholar]
- 30.Xi X, Zha Q, Jiang A, Tian Y. Stimulatory effect of bunch thinning on sugar accumulation and anthocyanin biosynthesis in Shenhua grape berry (Vitis vinifera × V. labrusca) Aust. J. Grape Wine Res. 2018;24:158–165. doi: 10.1111/ajgw.12323. [DOI] [Google Scholar]
- 31.Kliewer WM, Dokoozlian NK. Leaf area/crop weight ratios of grapevines: influence on fruit composition and wine quality. Am. J. Enol. Viticult. 2005;56:170–181. [Google Scholar]
- 32.Preszler T, Schmit TM, Heuvel JEV. Cluster thinning reduces the economic sustainability of Riesling production. Am. J. Enol. Viticult. 2013;64:333–341. doi: 10.5344/ajev.2013.12123. [DOI] [Google Scholar]
- 33.Bobeica N, et al. Differential responses of sugar, organic acids and anthocyanins to source-sink modulation in Cabernet Sauvignon and Sangiovese grapevines. Front. Plant. Sci. 2015;6:382. doi: 10.3389/fpls.2015.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker AK, Hofmann RW, van Leeuwen C, Mclachlan ARG, Trought MC. T.Manipulating the leaf area to fruit mass ratio alters the synchrony of soluble solids accumulation and titratable acidity of grape berries. Aust. J. Grape Wine Res. 2015;21:266–276. doi: 10.1111/ajgw.12132. [DOI] [Google Scholar]
- 35.Reynolds AG, et al. Magnitude of viticultural and enological effects. II. Relative impacts of cluster thinning and yeast strain on composition and sensory attributes of Chardonnay Musqué. Am. J. Enol. Viticult. 2007;58:25–41. [Google Scholar]
- 36.Condurso C, et al. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.) Eur. Food Res. Technol. 2016;242:1719–1726. doi: 10.1007/s00217-016-2671-7. [DOI] [Google Scholar]
- 37.Rutan TE, Herbst-Johnstone M, Kilmartin PA. Effect of Cluster Thinning Vitis vinifera cv. Pinot Noir on Wine Volatile and Phenolic Composition. J. Agric. Food Chem. 2018;66:10053–10066. doi: 10.1021/acs.jafc.8b04062. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, et al. Changes in global aroma profiles of cabernet sauvignon in response to cluster thinning. Food Res. Int. 2019;122:56–65. doi: 10.1016/j.foodres.2019.03.061. [DOI] [PubMed] [Google Scholar]
- 39.Boss PK, Böttcher C, Davies C. Various influences of harvest date and fruit sugar content on different wine flavor and aroma compounds. Am. J. Enol. Viticult. 2014;65:341–353. doi: 10.5344/ajev.2014.13137. [DOI] [Google Scholar]
- 40.Sun L, et al. Monoterpene accumulation and its biosynthesis: gene transcript profiles of two grape cultivars during berry development. Acta Horticulturae. 2015;1082:37–42. doi: 10.17660/ActaHortic.2015.1082.2. [DOI] [Google Scholar]
- 41.Schwab W, Wüst M. Understanding the constitutive and induced biosynthesis of mono-and sesquiterpenes in grapes (Vitis vinifera): a key to unlocking the biochemical secrets of unique grape aroma profiles. J. Agric. Food Chem. 2015;63:10591–10603. doi: 10.1021/acs.jafc.5b04398. [DOI] [PubMed] [Google Scholar]
- 42.Battilana J, et al. The 1-deoxy-d-xylulose 5-phosphate synthase gene co-localizes with a major QTL affecting monoterpene content in grapevine. Theor. Appl. Genet. 2009;118:653–669. doi: 10.1007/s00122-008-0927-8. [DOI] [PubMed] [Google Scholar]
- 43.Wen Y, et al. Using the combined analysis of transcripts and metabolites to propose key genes for differential terpene accumulation across two regions. BMC Plant. Biol. 2015;15:240. doi: 10.1186/s12870-015-0631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang E, et al. Effects of sunlight exclusion on the profiles of monoterpene biosynthesis and accumulation in grape exocarp and mesocarp. Food Chem. 2017;237:379–389. doi: 10.1016/j.foodchem.2017.05.127. [DOI] [PubMed] [Google Scholar]
- 45.Guillaumie S, et al. Genetic analysis of the biosynthesis of 2-methoxy-3-isobutylpyrazine, a major grape-derived aroma compound impacting wine quality. Plant. Physiol. 2013;162:604–615. doi: 10.1104/pp.113.218313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martins V, Bassil E, Hanana M, Blumwald E, Gerós H. Copper homeostasis in grapevine: functional characterization of the Vitis vinifera copper transporter 1. Planta. 2014;240:91–101. doi: 10.1007/s00425-014-2067-5. [DOI] [PubMed] [Google Scholar]
- 47.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


