Abstract
Breast milk is the most important nutrient source for newborn mammals. Studies have reported that milk contains microRNAs (miRNAs), which are potential regulatory components. Currently, existing functional and nutritional two competing hypotheses in milk field though little date have been provided for nutritional hypothesis. In this study, we used the qRT-PCR method to evaluated whether milk miRNAs can be absorbed by newborn piglets by feeding them porcine or bovine milk. The result showed that miRNA levels (miR-2284×, 2291, 7134, 1343, 500, 223) were significantly different between bovine and porcine milk. Four miRNAs (miR-2284×, 2291, 7134, 1343) were significantly different in piglet serum after feeding porcine or bovine milk. After separated milk exosomes by ultracentrifugation, the results showed the selected milk miRNAs (miR-2284×, 2291, 7134, 1343) were present in both exosomes and supernatants, and the miRNAs showed the coincidental expression in IPEC-J2 cells. All our founding suggested that the milk miRNAs can be absorbed both in vivo and in vitro, which will building the foundation for understanding whether these sort of miRNAs exert physiological functions after being absorbed and provided additional evidence for the nutritional hypotheses.
Subject terms: RNA, Transcriptomics
Introduction
Breast milk is the first and most important source of nutrition for newborn mammals1. By differential centrifugation, milk can be divided into milk fat, whey, casein, cells, and debris and further separated by ultra-centrifugation into extracellular vesicles (EVs) and supernatant2.
Exosomes, which are EVs of 30–100 nm in diameter and of endocytic origin, are released by numerous cells and are present in several body fluids, including saliva3,4, plasma5, urine6, amniotic fluid7, malignant ascites8, bronchoalveolar lavage fluid9, and synovial fluids10. Studies have reported that exosomes contain lipids, proteins, mRNA, and microRNA (miRNA)11–14 and that they serve as novel vehicles in cell-to-cell communication15,16. Just like other body fluids, milk contains EVs2,17. Hata et al. detected the presence of mRNA and miRNA in bovine milk-derived vesicles18.
MiRNAs represent a class of endogenous non-coding RNAs of approximately 22 nucleotides in length that are widely distributed in eukaryotes. The biological function of miRNAs is to destabilize mRNAs or halt mRNA translation19,20. Studies have reported that 12 body fluids contain miRNAs, and milk has the highest concentration of total RNA that is rich in miRNAs21. Milk components that contain miRNAs include milk fat globules22, whey23,24, and exosomes11,15. Interestingly, Izumi et al. suggested that miRNAs were also present in the supernatant of ultra-centrifuged bovine raw milk25. Furthermore, Zhou et al. confirmed the presence of 452 pre-miRNAs in human milk exosomes, which lead to 602 mature miRNAs26. Chen et al. reported the presence of 245 miRNAs in bovine milk27, and Kosaka et al. detected 281 of 723 known human miRNAs in human milk by microarray technology28. Porcine milk exosomes contain more than 180 pre-miRNAs29, and 491 miRNAs have been detected in porcine exosomes by Solexa sequencing30. Title et al. concluded that up to 635 miRNAs were expressed in a single milk clot sample, with an average of 506 miRNAs per sample31.
MiRNAs, which target approximately 60% of genes in mammals32,33, are involved in immune function28,34, development35–37, differentiation38–40, proliferation41–43 and metabolism44,45. MiRNAs play important roles in the regulation of immune cell development, innate immune responses, and acquired immune responses46,47. Our previous findings revealed that porcine milk exosomes promote IPEC-J2 proliferation48. Even though milk exosomes increase the stability of miRNAs, it is not known whether miRNAs can be absorbed through the digestive tract. Wolf et al. reported that miRNAs in bovine milk are transferred among animal species by dietary means because bovine milk exosomes can be absorbed by human and rat intestinal cells49. Kosaka et al. suggested that breast milk miRNAs could be transferred from mother to infant through dietary intake28. However, some published gave the opposing viewpoint, they performance the experiments on two transgenic models, miRNA knock-out and over-expressing mice, which those models may be inappropriate to study the physiological transfer the miRNAs to the newborns of their aberrant miRNA expression, thus, the results showed there were no evidence of miRNA absorption31,50. More importantly, there is no information on miRNA absorption in pigs, which are similar to humans in body size. To evaluate whether milk-derived miRNA is absorbed in newborn piglets, we used bovine and porcine milk, which have different miRNA expression profiles, for in vivo and in vitro experiments. This study will provide evidence on miRNA absorption in newborn mammals.
Results
MiRNA comparisons between bovine and porcine milk
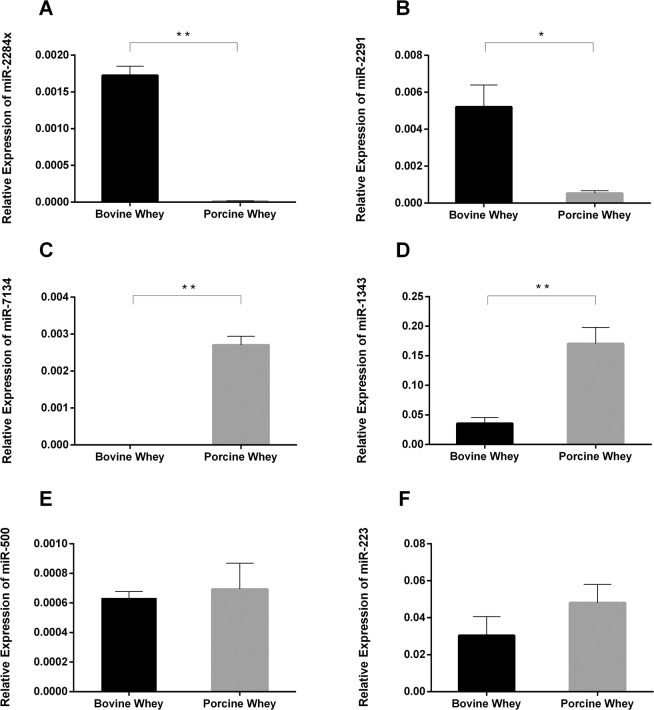
Using bioinformatical comparisons with reported bovine18,27,51 and porcine29,30 milk miRNAs, we selected six miRNAs for further validation: miR-1343, miR-223, miR-2284×, miR-2291, miR-500, and miR-7134. The results of qRT-PCR revealed that miR-2284× and miR-2291 were significantly higher in bovine whey than in porcine whey (Fig. 1A–B). In contrast, miR-7134 and miR-1343 were significantly higher in porcine whey than in bovine whey (Fig. 1C–D). There were no differences in miR-500 and miR-223 levels between bovine and porcine whey samples (Fig. 1E–F).
Figure 1.
Expression of miRNAs in bovine and porcine whey. Levels of miR-2284×(A), miR-2291 (B), miR-7134 (C), miR-1343 (D), miR-500 (E), and miR-223 (F) in bovine and porcine whey. The data were analyzed by t-test with n = 7 biological replicates. The graph was generated using GraphPad Prism 6. *p < 0.05, **p < 0.01.
MiRNAs in piglet serum after feeding porcine or bovine milk
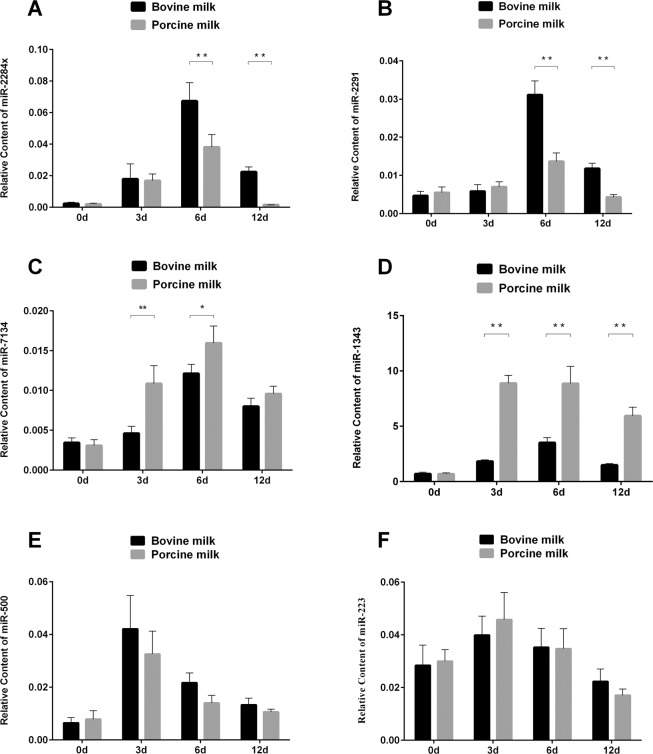
To assess whether milk-derived miRNAs can be absorbed by neonates, we measured the levels of miR-2284×, miR-2291, miR-7134, miR-1343, miR-500, and miR-223 in piglet serum after feeding bovine or porcine milk on four time points (day 0, 3, 6, 12). The results showed that miR-2284×and miR-2291 level were remarkably higher in the bovine milk-feeding group than in the porcine milk-feeding group on day 6 and day 12 and no difference expression on day 0 and 3 (Fig. 2A-B). In contrast, miR-7134 was significantly higher in the porcine milk-feeding group than in the bovine milk-feeding group on days 3 and 6 (Fig. 2C), and miR-1343 was significantly higher in the porcine milk-feeding group at all experimental time points except on day 0 (Fig. 2D). There were no significant differences in the levels of miR-500 and miR-223 between the two groups (Fig. 2E–F). Interestingly, these results were coincidental with the corresponding miRNA levels in bovine and porcine milk whey (Fig. 1). These results indicated that milk-derived miRNAs can be absorbed by newborn piglets and exhibited different content profiles among days, that maybe relevant to diverse physiological requirement after birth.
Figure 2.
Levels of miRNAs in piglet serum after feeding bovine or porcine milk. Relative levels of serum miRNA, miR-2284×(A), miR-2291 (B), miR-7134 (C), miR-1343 (D), miR-500 (E), miR-223 (F) on days 0, 3, 6, and 12 post-birth. The data were analyzed by ANOVA with n = 9 biological replicates. The graph was generated using GraphPad Prism 6. *p < 0.05, **p < 0.01.
MiRNA target prediction
Table 1 shows the predicted target genes for selected miRNAs. MiR-1343 attenuates porcine adipose triglyceride lipase (ATGL) and TGF-β receptors52. Owing to miR-2284×, miR-2291, miR-7134, and miR-1343 had dramatically differences between two kinds of whey and feeding experiments, we selected these four miRNAs for in vitro absorption experiments.
Table 1.
Target genes of selected miRNAs.
| Gene name | Target mRNA | NCBI Reference Sequence | Score | Energy (kCal/Mol) |
|---|---|---|---|---|
| miR-2291 | SIX homeobox 4 (SIX4) | NM_001244614 | 153 | −22.89 |
| Proline rich and Gla domain 4 (PRRG4) | NM_001244836 | 158 | −21.09 | |
| StAR related lipid transfer domain containing 4 (STARD4) | NM_001143726 | 157 | −21.6 | |
| Mannosyl (alpha-1,3-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase (MGAT1) | NM_001078668 | 153 | −22.45 | |
| Phosphatidylinositol-4-phosphate 5-kinase type 1 alpha (PIP5K1A) | NM_001244451 | 160 | −22.26 | |
| Capping actin protein of muscle Z-line beta subunit (CAPZB) | NM_001113444 | 167 | −21.83 | |
| Matrix metallopeptidase 14 (membrane-inserted) (MMP14) | NM_214239 | 159 | −23.71 | |
| Synaptojanin 2 binding protein (SYNJ2BP) | NM_001244991 | 162 | −24.44 | |
| Beta-secretase 1 (BACE1) | NM_001289854 | 164 | −20.33 | |
| Peroxiredoxin 6 (PRDX6) | NM_214408 | 154 | −21.74 | |
| Surfactant protein A1 (SFTPA1) | NM_214265 | 150 | −20.49 | |
| Thymidylate synthetase (TYMS) | NM_001243579 | 158 | −20.55 | |
| CCCTC-binding factor (zinc finger protein) (CTCF) | NM_001244660 | 156 | −23.04 | |
| miR-7134 | Intercellular adhesion molecule 3 (ICAM3) | NM_001145379 | 167 | −22.74 |
| Cell death inducing p53 target 1 (CDIP1) | NM_001244099 | 159 | −20.63 | |
| Solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 (SLC7A1) | NM_001012613 | 153 | −20.01 | |
| Cornichon homolog (Drosophila) (CNIH) | NM_001243525 | 154 | −20.62 | |
| miR-2284× | Versican (VCAN) | NM_001206429 | 154 | −22.94 |
| Pygopus family PHD finger 2 (PYGO2) | NM_001185175 | 153 | −20.34 | |
| SLIT and NTRK like family member 1 (SLITRK1) | NM_001308829 | 165 | −24.86 |
MiRNAs in milk-derived exosomes and exosome-free whey
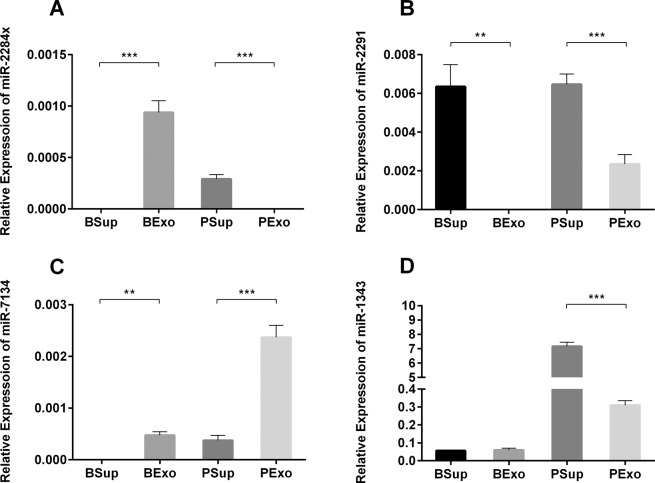
To measure the levels of exosomal and non-exosomal miRNAs in bovine and porcine milk, we collected milk-derived exosomes and supernatants for western blot (Supplementary Figure) and qRT-PCR detection. The results revealed that miR-2284× was significantly higher in bovine milk exosomes than in bovine milk supernatant. Opposite results were obtained with porcine milk (Fig. 3A). MiR-2291 was higher in supernatants than in exosomes of both bovine and porcine milk samples (Fig. 3B). In contrast, miR-7134 was significantly higher in exosomes than in supernatants of both bovine and porcine whey (Fig. 3C). MiR-1343 was significantly higher in supernatants than in exosomes of porcine whey, however, there were no differences in bovine milk (Fig. 3D). These results revealed that milk-derived miRNAs are present in different forms and that the distribution of miRNAs in milk may differ among species.
Figure 3.
Levels of miRNAs in exosomes and supernatants of bovine and porcine milk. Relative levels of miR-2284×(A), miR-2291 (B), miR-7134 (C), and miR-1343 (D). Abbreviations: BSup, bovine milk supernatants; BExo: bovine milk exosomes; PSup: porcine milk supernatants; PExo: porcine milk exosomes. The data were analyzed by t-test with n = 8 biological replicates. The graph was generated using GraphPad Prism 6. **p < 0.01, ***p < 0.001.
Milk-derived exosomes and exosome-free whey affected the concentration of corresponding miRNAs in IPEC-J2 cells
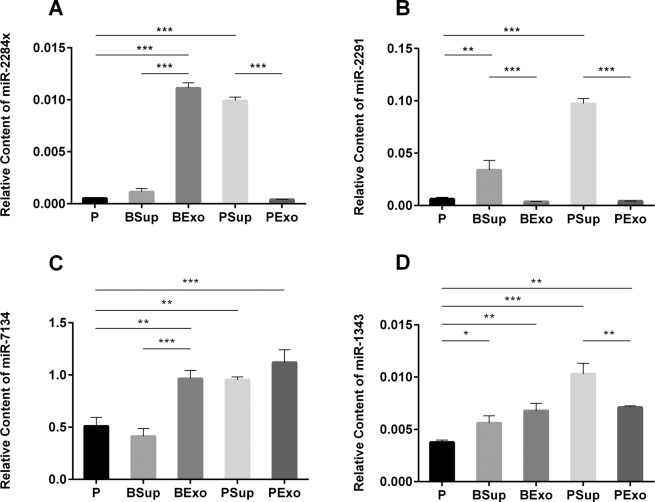
To evaluate whether exosomal and non-exosomal miRNAs are absorbed by IPEC-J2 cells, we measured the relative levels of miR-2284×, miR-2291, miR-7134, and miR-1343 in cells following incubation with bovine and porcine milk exosomes and supernatants. The results revealed that incubation with bovine/porcine milk exosomes and supernatants increased the levels of miRNAs in IPEC-J2 cells (Fig. 4A–D), and U6 among groups had consistent level (data not shown). Higher miRNA levels in the samples resulted in higher miRNA levels in IPEC-J2 cells. Therefore, both exosomal and non-exosomal miRNAs can be absorbed by IPEC-J2 cells.
Figure 4.
Levels of miRNAs in IPEC-J2 cells following incubation with bovine/porcine milk exosomes and supernatants. MiR-2284× levels increased following treatment with BExo and PSup (A), and miR-2291 levels were significantly higher in both BSup and PSup (B). All treatments significantly increased miR-7134 levels except for BSup (C) and significantly increased miR-1343 levels (D). Abbreviations: P: PBS; BSup: bovine milk supernatants; BExo: bovine milk exosomes; PSup: porcine milk supernatants; PExo: porcine milk exosomes. The data were analyzed by ANOVA with n = 6 biological replicates. The graph was generated using GraphPad Prism 6. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Breast milk not only the primary source of nutrition for newborn mammals but also can as a potential immunoprotector and developmental regulators for infant and mother53, epigenetic regulators54,55, metabolism regulators56, disease biomarkers57 and so on. Studies have reported that mammalian milk, such as human26,28, bovine18,27,51, porcine29,30, murine23,31, and tammar wallaby58, contains miRNAs. However, whether milk miRNAs exert any physiological regulation in newborns has not been elucidated. Milk from different species may have different miRNA profiles25,26,29,30. Our study findings revealed that the levels of four miRNAs were different between porcine and bovine milk.
MiRNA is degraded by RNase. Exosomes, one of major forms of membrane-bound vesicles, are present in several body fluids21. A large proportion of miRNAs are encapsulated in exosomes, and exosomal miRNAs have been detected in different types of mammalian milk through sequencing or microarray technology25–27,29,30. As nanoparticles, exosomes confer protection to miRNAs under the harsh extracellular environment of the digestive tract18,28,51,59. However, a considerable fraction of milk-derived miRNAs is located in the supernatants. Izumi et al. reported that miRNAs in bovine milk were present in both ultra-centrifuged supernatants and exosomes25. In this study, we tested the levels of four miRNAs in exosomes and supernatants. In bovine milk, miR-2284x and miR-7134 were present in exosomes, while miR-2291 was present in supernatants. In porcine milk, miR-2284×, miR-2291, and miR-1343 were mostly present in supernatants, while miR-7134 was present in exosomes. Interestingly, other studies found that non-exosomal miRNAs co-fractionated with protein complexes were resistant against degradation. Even though a minority of specific miRNAs is associated predominantly with microvesicles, the majority of miRNAs are bound to Argonaute2 protein in plasma60,61. In addition, nucleophosmin 162 and high-density lipoprotein63 are two miRNA-binding proteins that play roles in miRNA protection, export, and transport. As the majority composition of breast milk are similar to blood, it is reasonable to speculate that milk-derived miRNAs may be bound to proteins.
However, there were limited points existed in our research about the distribution of miRNAs in milk, some previously publications used different milk isolation methods to revealed the different milk part miRNAs expression patters. For instance, Benmoussa et al. reported the characterization of milk EV contain the bulk of milk miRNAs (include bta-miR-125b, bta-miR-148a, etc.), sediment at 12,000 g and 35,000 g, and found their distribution pattern was different from that of exosome-enriched proteins, but similar to that of several proteins commonly found in milk fat globule membranes (MFGM), including xanthine dehydrogenase (XDH)64. Gerstl et al. applied next generation sequencing and q-PCR identified the miRNA expression profile in the skim and fat fraction of human, goat, and bovine milk as well as infant formulas and found that most of known advantageous miRNAs in exosomes and fat layer were very similarity65. Munch et al. were used the next-gen deep sequencing revealed the miRNAs profile in the lipid fraction of human breast milk and found that known and novel miRNAs were enriched in breast milk fat globules, and expression of several novel miRNA species were regulated by maternal diet66. From above researches we can know that different parts of milk would be contain similar miRNAs species and which would be change the expression the miRNA expressions in the infants after feeding mammals or incubated with other cells, and in our research we only considered the miRNAs in supernatant and exosome part of bovine and porcine milk (Fig. 3 and Fig. 4) for their forms, distribution and absorb ability, which would be need for further experimental research to identify the exactly distribution of those miRNAs in milk part and their transfer approach or functions.
Additionally, the separation method of milk-derived extracellular vesicles is important for RNA enriched and would lead to different biological functions of the EVs. Gerstl et al., obtained skim milk (6,500 g, 30 min, 4 °C to 12,000 g, 1 h, 4 °C) or fat layer (6,500 g, 30 min, 4 °C) by different centrifuged speed, collecting milk exosome by ExoQuick kit shown the miR-148a is highly conserved in human, bovine and goat milk65. Rubio et al. identified miRNAs, piRNAs, tRNAs, snRNAs, and snoRNAs in milk/plasma centrifugation at 16,000 g for 15 minutes at 4 °C55. Herwijnen et al., collected sucrose gradient (1.12–1.18 g/ml) fractions from human and porcine milk showed abundant of let-7 family members and miR-148a67, a series of centrifugations and filtrations combination of ExoQuick regent for human milk exosome isolation was proofed that miRNA-148a is a highly expressed miRNA and down-regulated PTEN (phosphatase and tensin homolog) in normal fetal colon epithelial but not in colon tumor cells, and milk-derived exosomes deleted of miRNA-148a, which inhibited proliferation and DNMT1(DNA methyltransferase 1) expression in cells68. But a recently reported that unfractionated cow milk and derived EV subsets with differential ultracentrifugation 12,000 g (P12K), 35,000 g (P35K), 70,000 g (P70K), and 100,000 g (P100K) exhibited P100K EV were enriched in reference miRNA sequences, and P12K and P35K EV in related isomiR. Milk EV miR-223 was transferred in cells and down-regulated the reporter gene69. All those evidence in hinted the separation methods of milk EV will not only affect the non-miRNA’ concentration and form enriched but also their bioactivity. In our research, the ultracentrifugation was used for exosome and exosome-free separation showed the miRNAs (miR-2284×, miR-2291, miR-7134, miR-1343) absorbability coincide within pig serum and cells suggested those separation conditions facility the specific miRNAs gained in milk and stabilize for their absorption or function’s regulation, but need for further identification.
To investigate whether milk-derived miRNAs are absorbed, we designed an in vivo experiment using piglets and an in vitro experiment using IPEC-J2 cells. The in vivo and in vitro results revealed that milk miRNAs were absorbed by cells in the digestive tract. In addition, miRNAs in exosomes and supernatants were absorbed by IPEC-J2 cells, and the levels of miRNAs in cells were in agreement with the levels of miRNAs in milk (Fig. 3). There is conflicting information on the absorption of milk-derived miRNAs. Baier et al. reported that milk-borne miRNAs can be absorbed by humans59, and Chen et al. reported that miRNAs in milk exosomes can be absorbed by IPEC-J2 cells48. Furthermore, Sun et al. demonstrated that colostrum’s MVs may transfer immune-related miRNAs into cells and exert immunomodulatory effects34. Izumi et al. revealed that bovine milk exosomes containing RNA may enter human macrophages25. Gerstl et al. identified that the high expression miR-148a-3p in milk exosome and fat layer with Exo‐Red labeled can be take into CRL 1831 cells (human normal intestine cell line), K562 (leukemia cells) and Lim 1215 (colon cancer cells) and showed up-regulated in the entered cells65. MiRNAs transferred from maternal milk to neonates via the digestive tract are essential to the development of the immune system26,29. However, Title et al. reported that there is no evidence on milk miRNA absorption in miR-375-knockout and miR-200c/141-knockout mouse models and that miRNAs may be degraded by the digestive system31, because those two miRNAs related to control of the exosome endocytosis or exocytosis would influence their uptake, the KO mice were inappropriate models to study milk exosome uptake70 and they can’t inferred the milk miRNAs only provide nutrition for offspring. Laubier et al. demonstrated that milk-rich miR-30b could not be detected in transgenic pups compared to wild-type pups50. Above two studies propounded that milk-derived miRNA cannot be uptaken by pups. Coincidentally, the authors employed gene-changed mice models, which made the context complicated and the whole process biologically artifactual. Otherwise, Manca. et al. gained the unique distribution profiles and accumulated in intestinal mucosa, spleen, liver, heart or brain after administered mice with transfected fluorophore-labeled microRNAs into bovine milk exosomes, which provided the experimental evidence for the uptake of miRNAs by newborn71. In this study, we utilized wild-type models and provided indirect evidence on the absorption of milk-derived miRNAs.
Based on our in vivo and in vitro results, breast milk miRNAs can be absorbed through the neonatal digestive tract. As key post-transcriptional gene regulators, miRNAs play important roles in several physiological and pathological processes72. Which would be relate to the epigenetic regulatory effect in infants, such as during in the different lactation times different genes will participate the milk fat synthesis and secretion73, and the miRNAs could control the homeostatic regulation of cholesterol and triacylglycerol metabolism74–76. For instance, the miR-148a showed an higher expression than other lactation-related miRNAs during the lactation mammary glands of the Chinese swamp buffalo77, and the miRNA-148a and miRNA-17–5p shown to synergistically increase milk triacylglycerol synthesis via regulation of PPARGC1A and PPARA in goat MECs (mammary epithelial cells)78. All those evidence implicated the regulated function of genes or miRNAs would be suit to the infants’ requirements, and we speculated the results of different miRNAs expressions showed time variation in serum after feeding different species milk in our research would be coincide with the pattern.
Conclusions
In this study, we found that the different miRNAs (miR-2284×, miR-2291, miR-7134 and miR-1343) expression between bovine whey and porcine whey have diverse content profiles in newborn piglets’ serum from two milk-feeding groups. Furthermore, different distribution of miRNAs in porcine and bovine milk format (exosome and exosome-free supernatants) showed the uniform expression pattern in IPEC-J2 cells. These findings contribute to the debate concerning whether milk-source miRNAs can be absorbed by infants, and to building the foundation for understanding whether these sort of miRNAs exert physiological functions after being absorbed.
Materials and Methods
Milk samples
Porcine milk samples were collected from healthy lactating Large White pigs one day following parturition. The pigs were bred at the breeding farm of the Livestock Research Institute (Guangzhou, China). Bovine milk samples were collected from healthy one- to five-day old lactating Holstein cows after parturition. The cows were bred at the breeding farm of Feng Xing Milk Company (Guangzhou, China). All milk samples were stored at −80 °C immediately after collection.
Experimental feedings and serum collection
Three Large White pigs, which were in first parturition and deliveries on the same day, were used in this study. Six newborn piglets from each litter were randomly selected and assigned to one of two groups, a porcine milk-feeding group and a bovine milk-feeding group, with nine piglets per group. The porcine milk-feeding group received milk from the sow, while the bovine milk-feeding group received bovine milk artificially. Blood samples (5 mL) were collected from the anterior vein of the piglets on day 0, 3, 6, and 12 after birth. The serum was separated by centrifugation and stored at −80 °C.
Whey preparation
Porcine and bovine milk samples were centrifuged twice at 1,200 × g for 10 min at 4 °C to remove milk fat and mammary gland-derived cells. Defatted milk samples were centrifuged at 20,350 × g for 60 min at 4 °C to remove residual fat, casein, and other debris (modified from Izumi et al.51). The clear supernatant (whey) was collected for further use.
Preparation of exosome and exosome-free supernatants
The collected whey was further ultra-centrifuged at 110,000 × g for 2 h at 4 °C in an SW41T rotor (Beckman Coulter Instruments, Fullerton, CA) to precipitate the exosomes30. After ultra-centrifuged we collected the pellet as the milk exosome in under layer and exosome-free supernatants in upper layer of the centrifuge tube. The pellet was washed with PBS and ultra-centrifuged to purify the exosomes while the exosome-free supernatants were carefully stored at –80 °C directly. Finally, the purified exosomes were re-suspended in 30 mL PBS and stored at −80 °C for used.
Cell culture
Porcine small intestinal epithelial (IPEC-J2) cells were cultured at 37 °C and 5% CO2 in Dulbecco’s modified eagle medium/Ham’s F-12 in a 1:1 ratio (Invitrogen, Life Technologies, Carlsbad, CA, USA) supplemented with 5% fetal calf serum (FCS; Invitrogen) and 5 ng/mL epidermal growth factor (EGF; Peprotech, Rocky Hill, NJ, USA). IPEC-J2 cells were seeded at 0.5 × 105 cells/mL in a 10 mL volume in plastic tissue culture flasks (75 cm2 Corning, Corning, NY, USA). After reaching confluency (four days)79, the cells were seeded into 6-well tissue culture plates (9.6 cm2/well) at 2.0–2.3 × 105 cells/well in a 2 mL volume. The cells were allowed to adhere for 24 h, and the media were replaced every other day. When the cells were 90% confluent, we added 0.5 mL exosomes or exosome-free supernatants to each well, the equal volume PBS added as control. We determined that a 25% media substitution was optimum. Exosomes suspended in PBS and supernatants were passed through 0.45-μm and 0.22-μm membrane filters prior to incubation. IPEC-J2 cells were harvested after 8 h. we give all the in vitro experiment for three repeats test.
RNA extraction and qRT-PCR
Total RNA was isolated from samples using Trizol reagent (Invitrogen, Carlsbad, CA). Trizol reagent (1 mL) was added to 300 µL sample (whey/serum) and to each well (cells). Samples were spiked with 50 fmol synthetic cel-miR-39 as an internal control for extraction efficiency (modified from Kroh et al.80), and U6 was used as an internal control for cell assay. Total RNA was first digested with DNase I (Promega, Madison, WI, USA), and 100 ng of total whey/serum RNA or 2 μg of total cell RNA was reverse transcribed into poly (A) tail-added cDNA using the Mir-X miRNA First Strand Synthesis kit (Takara Bio Company, Dalian, China). The resulting cDNA was diluted 10-fold with nuclease-free H2O. The PCR reaction mixture (20 µL) contained 2 μL template cDNA, 10 μL of 2× Taq Plus Master Mix (Vazyme Biotech Co., Nanjing, China)/GoTaq qPCR Master Mix (Promega, Madison, WI), and 0.5 μL 1 mM of each primer. The PCR products were examined on a 3% agarose gel to confirm that a single PCR product was generated. The real-time PCR thermal profile consisted of 95 °C for 2 min, 40 cycles at 95 °C for 15 s, the annealing temperature for 15 s, and 72 °C for 30 s, followed by the melting curve stage. The miRNA forward primer was designed using Primer 5.0 (Table 2).
Table 2.
Primers for qRT-PCR.
| Gene name | Sequence (5 ′ to 3 ′) |
|---|---|
| miR-2284× | TGAAAAGTTCGTTCGGGTTTT |
| miR-2291 | GCTGATAGTGAGCGACTGGGGCAG |
| miR-7134 | ATGCGGAACCTGCGGATACGG |
| miR-1343 | CTCCTGGGGCCCGCACTCTC |
| miR-500 | ATGCACCTGGGCAAGGATTCT |
| miR-223 | TGTCAGTTTGTCAAATACCCCA |
MiRNA target prediction
To predict miRNA target sites, we analyzed miRNA targets using miRanda v3.3a microRNA target scanning algorithm81 with the default parameters and cutoffs (score ≥ 150 and energy ≤−20.0). Sequences of 3’UTRs of porcine were obtained from NCBI (https://www.ncbi.nlm.nih.gov/).
Statistical analysis
Data were expressed as mean ± standard error of the mean (SEM). Significant differences were assessed by t-test for two-group comparisons and by one-way analysis of variance (ANOVA), least significant difference (LSD) or Duncan test or Tukey analysis post hoc test for multiple comparisons using SPSS 19.0. Statistical significance was set at p < 0.05.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors and all the animal procedures were conducted under the protocol (SCAU-AEC-2016-0714, 14 July 2016) approved by Institutional Animal Care and Use Committee (IACUC) of South China Agricultural University.
Methods statement
All the experimental procedures were conducted under the protocol (SCAU-AEC-2015–0127, 27 January 2015) approved by the Experimental Operations Management Association (EOMA) of South China Agricultural University.
Supplementary information
Acknowledgements
We thank the breeding farm of the Livestock Research Institute (Guangzhou, China) and the breeding farm of Feng Xing Milk Company (Guangzhou, China) for providing milk samples. This study was funded by National Natural Science Foundation of China (grant No. 31802156, 31872435, 31802032), Natural Science Foundation of Guangdong Province (No. 2019A1515011734), National Key Research and Development Program (grant No. 2016YFD0500503, 2016YFD0501205).
Author contributions
D.L., T.C. carried out the miRNA qRT-PCR and data analysis, and participated in drafted the manuscript. M.X., M.L., J.H. performed the raw data analysis. B.Z., R.S.,Y.Z., D.Y. participated in the sample collected. J.S. performed the biological information analysis. Q.X., Q.J. and Y.Z. conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Delin Lin and Ting Chen.
Supplementary information
is available for this paper at 10.1038/s41598-020-63485-8.
References
- 1.Haug A, Hostmark AT, Harstad OM. Bovine milk in human nutrition–a review. Lipids in health and disease. 2007;6:25. doi: 10.1186/1476-511X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Admyre C, et al. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. The Journal of Immunology. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa Y, Azuma M, Akimoto Y, Kawakami H, Yanoshita R. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biological and Pharmaceutical Bulletin. 2008;31:1059–1062. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- 4.Begne M, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) Journal of proteome research. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia JM, et al. Extracellular plasma RNA from colon cancer patients is confined in a vesicle-like structure and is mRNA-enriched. RNA. 2008;14:1424–1432. doi: 10.1261/rna.755908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller S, et al. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney international. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- 8.Runz S, et al. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecologic oncology. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 9.Prado N, et al. Exosomes from Bronchoalveolar Fluid of Tolerized Mice Prevent Allergic Reaction. The Journal of Immunology. 2008;181:1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 10.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 11.Ohshima K, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PloS one. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez ML, Khosroheidari M, Kanchi Ravi R, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney international. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 13.Xiao D, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PloS one. 2012;7:e46874. doi: 10.1371/journal.pone.0046874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic acids research. 2012;40:D1241–1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang X, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer letters. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatani H, et al. Weaning-induced expression of a milk-fat globule protein, MFG-E8, in mouse mammary glands, as demonstrated by the analyses of its mRNA, protein and phosphatidylserine-binding activity. The Biochemical journal. 2006;395:21–30. doi: 10.1042/BJ20051459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hata T, et al. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochemical and biophysical research communications. 2010;396:528–533. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 19.Jing Q, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 20.Djuranovic S, Nahvi A, Green R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–240. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber JA, et al. The microRNA spectrum in 12 body fluids. Clinical chemistry. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munch EM, et al. Transcriptome profiling of microRNA by Next-Gen deep sequencing reveals known and novel miRNA species in the lipid fraction of human breast milk. PloS one. 2013;8:e50564. doi: 10.1371/journal.pone.0050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izumi H, et al. Time-dependent expression profiles of microRNAs and mRNAs in rat milk whey. PloS one. 2014;9:e88843. doi: 10.1371/journal.pone.0088843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin XL, Wei ZH, Liu L, Liu HY, Liu JX. Comparative studies of two methods for miRNA isolation from milk whey. Journal of Zhejiang University. Science. B. 2015;16:533–540. doi: 10.1631/jzus.B1400355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izumi H, et al. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. Journal of dairy science. 2015;98:2920–2933. doi: 10.3168/jds.2014-9076. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. International journal of biological sciences. 2012;8:118. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell research. 2010;20:1128–1137. doi: 10.1038/cr.2010.80. [DOI] [PubMed] [Google Scholar]
- 28.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7. doi: 10.1186/1758-907X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu Y, et al. Lactation-related microRNA expression profiles of porcine breast milk exosomes. PloS one. 2012;7:e43691. doi: 10.1371/journal.pone.0043691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T, et al. Exploration of microRNAs in porcine milk exosomes. BMC genomics. 2014;15:100. doi: 10.1186/1471-2164-15-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Title AC, Denzler R, Stoffel M. Uptake and function studies of maternal milk-derived microRNAs. J Biol Chem. 2015 doi: 10.1074/jbc.M115.676734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 33.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome research. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Q, et al. Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein & cell. 2013;4:197–210. doi: 10.1007/s13238-013-2119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JF, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature genetics. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Current opinion in cell biology. 2009;21:461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 38.Song L, Tuan RS. MicroRNAs and cell differentiation in mammalian development. Birth defects research. Part C. Embryo today: reviews. 2006;78:140–149. doi: 10.1002/bdrc.20070. [DOI] [PubMed] [Google Scholar]
- 39.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2422–2427. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li G, et al. MicroRNA identity and abundance in developing swine adipose tissue as determined by Solexa sequencing. Journal of cellular biochemistry. 2011;112:1318–1328. doi: 10.1002/jcb.23045. [DOI] [PubMed] [Google Scholar]
- 41.Sluijter JP, et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:859–868. doi: 10.1161/ATVBAHA.109.197434. [DOI] [PubMed] [Google Scholar]
- 42.Liu N, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JF, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. The Journal of cell biology. 2010;190:867–879. doi: 10.1083/jcb.200911036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Molecular genetics and metabolism. 2007;91:209–217. doi: 10.1016/j.ymgme.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krutzfeldt J, Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell metabolism. 2006;4:9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 47.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nature reviews. Immunology. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 48.Chen T, et al. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Scientific reports. 2016;6:33862. doi: 10.1038/srep33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf, T., Baier, S. R. & Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. The Journal of nutrition, 10.3945/jn.115.218586 (2015). [DOI] [PMC free article] [PubMed]
- 50.Laubier J, Castille J, Le Guillou S, Le Provost F. No effect of an elevated miR-30b level in mouse milk on its level in pup tissues. RNA biology. 2015;12:26–29. doi: 10.1080/15476286.2015.1017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Izumi H, et al. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. Journal of dairy science. 2012;95:4831–4841. doi: 10.3168/jds.2012-5489. [DOI] [PubMed] [Google Scholar]
- 52.Stolzenburg LR, Wachtel S, Dang H, Harris A. miR-1343 attenuates pathways of fibrosis by targeting the TGF-beta receptors. The Biochemical journal. 2016;473:245–256. doi: 10.1042/BJ20150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. Int. J. Environ. Res. Public Health. 2015;12:13981–14020. doi: 10.3390/ijerph121113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melnik BC, Schmitz G. MicroRNAs: Milk’s epigenetic regulators. Best Practice & Research Clinical Endocrinology & Metabolism. 2017;31:427–442. doi: 10.1016/j.beem.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Rubio, M. et al. Circulating miRNAs, isomiRs and small RNA clusters in human plasma and breast milk. Plos One 13, 10.1371/journal.pone.0193527 (2018). [DOI] [PMC free article] [PubMed]
- 56.Zempleni, J., Sukreet, S., Zhou, F., Wu, D. & Mutai, E. in Annual Review of Animal Biosciences, Vol 7 Vol. 7 Annual Review of Animal Biosciences (eds H. A. Lewin & R. M. Roberts) 245-+ (2019). [DOI] [PubMed]
- 57.Benmoussa A, Provost P. Milk MicroRNAs in Health and Disease. Comprehensive Reviews in Food Science and Food Safety. 2019;18:703–722. doi: 10.1111/1541-4337.12424. [DOI] [PubMed] [Google Scholar]
- 58.Modepalli V, et al. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii) BMC genomics. 2014;15:1012. doi: 10.1186/1471-2164-15-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. The Journal of nutrition. 2014;144:1495–1500. doi: 10.3945/jn.114.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arroyo JD, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic acids research. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic acids research. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature cell biology. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benmoussa, A. et al. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow’s milk. Journal of Extracellular Vesicles 6, 10.1080/20013078.2017.1401897 (2017). [DOI] [PMC free article] [PubMed]
- 65.Gerstl, R. et al. Characterization and biological function of milk-derived miRNAs. Molecular Nutrition & Food Research 61, 10.1002/mnfr.201700009 (2017). [DOI] [PubMed]
- 66.Munch, E.M. et al. Transcriptome Profiling of microRNA by Next-Gen Deep Sequencing Reveals Known and Novel miRNA Species in the Lipid Fraction of Human Breast Milk. Plos One 8, 10.1371/journal.pone.0050564 (2013). [DOI] [PMC free article] [PubMed]
- 67.Herwijnen, M.J.C. et al. Abundantly Present miRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Frontiers in Nutrition 5, 10.3389/fnut.2018.00081 (2018). [DOI] [PMC free article] [PubMed]
- 68.Reif, S., Shiff, Y.E. & Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. Journal of Translational Medicine 17, 10.1186/s12967-019-2072-3 (2019). [DOI] [PMC free article] [PubMed]
- 69.Benmoussa A, et al. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. Journal of Dairy Science. 2020;103:16–29. doi: 10.3168/jds.2019-16880. [DOI] [PubMed] [Google Scholar]
- 70.Melnik, B.C. et al. Milk miRNAs: simple nutrients or systemic functional regulators? Nutrition & Metabolism13, 10.1186/s12986-016-0101-2 (2016). [DOI] [PMC free article] [PubMed]
- 71.Manca, S. et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Scientific Reports8, 10.1038/s41598-018-29780-1 (2018). [DOI] [PMC free article] [PubMed]
- 72.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bionaz, M. & Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. Bmc Genomics9, 10.1186/1471-2164-9-366 (2008). [DOI] [PMC free article] [PubMed]
- 74.Hernando C. Emerging Role of MicroRNAs in the Regulation of Lipid Metabolism. Hepatology. 2013;57:432–434. doi: 10.1002/hep.25960. [DOI] [PubMed] [Google Scholar]
- 75.Goedeke L, et al. MicroRNA-148a regulates LDL receptor and ABCA1 expression to control circulating lipoprotein levels. Nature Medicine. 2015;21:1280–1289. doi: 10.1038/nm.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagschal A, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nature Medicine. 2015;21:1290–1297. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai X, et al. Identification and analysis of the expression of microRNA from lactating and nonlactating mammary glands of the Chinese swamp buffalo. Journal of Dairy Science. 2017;100:1971–1986. doi: 10.3168/jds.2016-11461. [DOI] [PubMed] [Google Scholar]
- 78.Chen Z, et al. miR-148a and miR-17-5p synergistically regulate milk TAG synthesis via PPARGC1A and PPARA in goat mammary epithelial cells. Rna Biology. 2017;14:326–338. doi: 10.1080/15476286.2016.1276149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diesing AK, et al. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicology letters. 2011;200:8–18. doi: 10.1016/j.toxlet.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Enright AJ, et al. MicroRNA targets in Drosophila. Genome biology. 2003;5:R1. doi: 10.1186/gb-2003-5-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.