Abstract
The incidence of colorectal polyps is rising. Certain types of polyps are considered to be the precursor lesions for colorectal cancers. To investigate the prevalence and related factors of colorectal polyps in Chinese subjects, we first performed a cross-sectional study. A total of 3066 subjects were documented, and the prevalence of colorectal polyps was 18.1%. Then we evaluated the incidence and risk factors of polyps via a retrospective cohort study in the same population. 561 subjects who received at least twice surveillance colonoscopies with available reports during the study period and had no polyp at the first endoscopy were included in the retrospective cohort study, of whom 19.1% developed colorectal polyps. Regular smoking was independently associated with the presence and development of colorectal polyps. Further analyses indicated that polyps were associated with smoking status, daily cigarette consumption, and drinking habit. Moreover, smoking tends to be more relavent to rectal, small and single polyp. In conclusion, colorectal polyp is a common disease in China. Exploring the epidemiology and risk factors may improve the prevention of colorectal polyps, even colorectal cancer.
Subject terms: Colonoscopy, Gastrointestinal diseases, Gastroenterology, Risk factors
Introduction
The incidence of colorectal polyp is rapidly increasing worldwide. Colorectal cancer (CRC) was found to be the third most common cancer among men and the fourth most common cancer among women1. Adenomatous polyps are thought to be the precursor lesions for the majority of CRCs2, developing mostly through an adenoma-carcinoma sequence3. Recent studies proposed that hyperplastic polyps also contribute to CRCs through serrated or microsatellite instable pathways4,5. Some polyps may cause gastrointestinal symptoms such as hematochezia, stomachache, abdominal distension, affecting health and quality of life. More seriously, other asymptomatic polyps may quietly develop into malignancies, which are more likely to be ignored. The detection and resection of precancerous polyps under colonoscopy is a critical way to reduce the incidence of CRC and its subsequent morbidity and mortality6,7. However, to explore the prevalence and risk factors for colorectal polyps could be a better way to prevent and manage this disease in advance.
Population characteristics, living habits, and health conditions are closely related to the presence and development of colorectal polyps and cancers. In recent studies, some risk factors for adenomatous polyps were reported, including sex, metabolic syndrome, Helicobacter pylori infection, smoking, alcohol drinking, etc.8–10. As for CRC, a population-based colorectal cancer screening program in China suggested that age, gender, BMI, family history, meat intake and smoking were associated with colorectal neoplasms11. Smoking is proposed to be closely associated with colorectal polyps, neoplasia, and CRCs11–13. However, most evidence came from case-control or cross-sectional studies, and there is still no cohort study to evaluate the risk factors for colorectal polyps in China.
Therefore, we first evaluated the prevalence of colorectal polyps and factors associated with the presence of this disease in a cross-sectional study. Then we investigated the incidence and risk factors for the colorectal polyps via a retrospective cohort study. Associations between the risk factors and features of colorectal polyps were further assessed.
Methods
We designed two linked studies to investigate the prevalence and incidence of colorectal polyps, and risk factors for its presence and development in a Chinese population. The first was a cross-sectional study to determine the prevalence of colorectal polyps and factors associated with its presence. Then we conducted a retrospective cohort study of baseline polyp-free subjects from the first study who underwent at least twice surveillance colonoscopies to assess the incidence and risk factors for colorectal polyps.
Study population
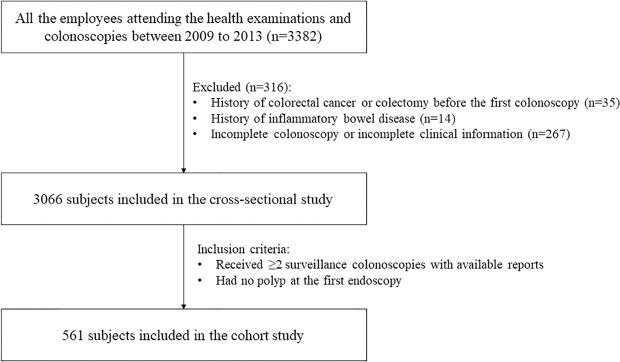
This retrospective study was performed among the employees of Zhenhai Refining & Chemical Company (Ningbo, China). All of the employees attending the health examinations and colonoscopies between January 2009 to December 2013 were screened for enrollment. Exclusion criteria were: (1) history of colorectal cancer or colectomy before the first surveillance colonoscopy; (2) history of inflammatory bowel disease; (3) incomplete colonoscopy or incomplete clinical information. A total of 3066 eligible subjects were enrolled in the cross-sectional study to investigate the prevalence of colorectal polyp and potential factors associated with its presence. Then in the same population, subjects who had at least twice surveillance colonoscopies and had no polyp at the first endoscopy were selected for the retrospective cohort study (Fig. 1).
Figure 1.
Flowchart of subject selection.
Data collection
All of the data were collected by reviewing the medical records of the medical center. The procedures of health examination were the same as those used at baseline during the study period. Height and weight were measured on standardised machines. Subjects were stratified to three groups according to BMI: underweight (BMI < 18.5), normoweight (18.5 ≤ BMI < 25) and overweight (BMI ≥ 25). Blood samples were analysed in the same laboratories. In blood tests, following data were collected: white blood cell (WBC) count, red blood cell (RBC) count, platelet, hemoglobin, plasma viscosity, erythrocyte sedimentation rate (ESR), albumin, total cholesterol (TC), triglycerides (TG), creatinine and uric acid.
Smoking and drinking habits, medical histories such as colorectal cancer, colectomy, and inflammatory bowel disease were inquired by a physician. Regular smoking (or current smoking) was defined as smoking at least one cigarette per day. Former smoking status was defined as a cessation of smoking for at least one year. Regular drinking was defined as drinking at least one alcoholic beverage per week.
For each colonoscopy report, we extracted the following data: date, quality of bowel preparation, presence of polyps, diameter of the biggest polyp, position and number of the polyps. Duration of follow-up was defined as the interval time between baseline and the examination when colorectal polyps were first identified, or the last endoscopy before the end of our study period.
Statistical analysis
The statistical analyses were conducted using SPSS 24.0 (SPSS, Chicago, IL, USA). Continuous variables were presented as mean with standard vision or medians with interquartile range, and were compared by Student’s t-test or Mann-Whitney U test. Categorical variables were expressed as frequencies with percentages and were analysed by chi-squared(χ2) test. Logistics regression was used to evaluate the factors associated with the presence of colorectal polyps in the cross-sectional study. Cox proportional hazards regression analyses were applied to assess the risk factors for the development of colorectal polyps in the retrospective cohort study. Those considerable covariates (p < 0.10 or have important clinical significance) were included in multivariate analysis. Interactions among the covariates in multivariate analyses were tested by collinearity diagnosis; no significant collinearity was found. A P value < 0.05 (two-tailed) was considered to be statistically significant.
Ethics
This study was performed according to the Declaration of Helsinki. The study protocol was approved by the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference Number: 2019648). Due to the retrospective nature of the study, informed consent was waived.
Results
Characteristics of subjects in the cross-sectional study
A total of 3066 subjects were documented in the cross-sectional study (Fig. 1), of whom 554 (18.1%) had colorectal polyps. The subjects with colorectal polyps tend to be older, male, regular smoker and drinker, and they had higher WBC and RBC counts, hemoglobin, plasma viscosity, creatinine, uric acid, and lower platelet (Table 1). There was no difference between the parameters of lipid metabolism TC and TG.
Table 1.
Characteristics of subjects in the cross-sectional study.
| Variables | With colorectal polyp (n = 554) | Without colorectal polyp (n = 2512) | P |
|---|---|---|---|
| Age (years) | 60 (53–67) | 55 (47–62) | <0.001 |
| Gender (male/female, n) | 395/159 | 1461/1051 | <0.001 |
| Body mass index (kg/m2) | |||
| 18.5~25 | 361 (65.3) | 1711 (68.3) | 1 (reference) |
| <18.5 | 16 (2.9) | 78 (3.1) | 0.92 |
| ≥25 | 176 (31.8) | 715 (28.6) | 0.131 |
| Total cholesterol (mmol/L) | 5.07 (4.53–5.70) | 5.00 (4.44–5.66) | 0.083 |
| Triglycerides (mmol/L) | 1.20 (0.87–1.74) | 1.18 (0.84–1.70) | 0.26 |
| Regular smoker (%) | 158 (28.5) | 514 (20.5) | <0.001 |
| Regular drinker (%) | 125 (22.6) | 397 (15.9) | <0.001 |
| White blood cell count (×109/L) | 5.85 (4.98–6.80) | 5.60 (4.80–6.60) | 0.005 |
| Red blood cell count (×1012/L) | 4.59 (0.45) | 4.55 (0.45) | 0.037 |
| Platelet (×109/L) | 200 (169–230) | 205 (175–242) | 0.005 |
| Hemoglobin (g/L) | 140 (130–148) | 137 (127–147) | 0.001 |
| Plasma viscosity (mpa.s) | 1.38 (1.32–1.41) | 1.37 (1.31–1.40) | 0.001 |
| Erythrocyte sedimentation rate (mm/h) | 8 (4–14) | 9 (4–14) | 0.299 |
| Albumin (g/L) | 44.80 (43.30–46.50) | 45.00 (43.50–46.60) | 0.123 |
| Creatinine (umol/L) | 63 (54–72) | 62 (52–71) | 0.024 |
| Uric Acid (umol/L) | 321 (267–385) | 312 (258–371) | 0.006 |
Factors associated with the presence of colorectal polyps
Logistic regression analysis was applied to evaluate the factors associated with the presence of colorectal polyps (Table 2). In the univariate model, 12 variables were potentially correlated with the presence of colorectal polyps, which were entered into the multivariable regression analysis. The results indicated that older age, male, regular smoking, higher TC and WBC count were significantly associated with the presence of colorectal polyps.
Table 2.
Factors associated with the presence of colorectal polyps, logistic regression analysis.
| Variables | Mean (s.d.) or median (IQR) or number(%) | Univariable model | Multivariate modela | ||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | 56 (47–63) | 1.037 (1.028–1.046) | <0.001 | 1.039 (1.029–1.049) | <0.001 |
| Gender (male/female, n) | 1856/1210 | 1.787 (1.463–2.184) | <0.001 | 1.545 (1.119–2.134) | 0.008 |
| Body mass index (kg/m2) | |||||
| 18.5~25 | 2072 (67.8) | 1 (reference) | |||
| <18.5 | 94 (3.1) | 0.972 (0.561–1.685) | 0.92 | ||
| ≥25 | 891 (29.1) | 1.167 (0.955–1.425) | 0.131 | ||
| Total cholesterol (mmol/L) | 5.02 (4.45–5.66) | 1.089 (0.988–1.202) | 0.087 | 1.140 (1.025–1.266) | 0.015 |
| Triglycerides (mmol/L) | 1.18 (0.84–1.71) | 1.069 (0.976–1.171) | 0.153 | ||
| Regular smoker (%) | 672 (21.9) | 1.546 (1.255–1.904) | <0.001 | 1.351 (1.050–1.738) | 0.019 |
| Regular drinker (%) | 522 (17.1) | 1.547 (1.234–1.940) | <0.001 | 1.292 (0.999–1.672) | 0.051 |
| White blood cell count (×109/L) | 5.7 (4.80–6.60) | 1.092 (1.027–1.160) | 0.005 | 1.076 (1.003–1.154) | 0.041 |
| Red blood cell count (×1012) | 4.55 (0.45) | 1.240 (1.013–1.517) | 0.037 | 1.038 (0.760–1.417) | 0.816 |
| Platelet (×109/L) | 204 (174–239) | 0.997 (0.996–0.999) | 0.006 | 0.998 (0.996–1.001) | 0.145 |
| Hemoglobin (g/L) | 138 (127–147) | 1.012 (1.005–1.019) | 0.001 | 0.998 (0.986–1.010) | 0.777 |
| Plasma viscosity (mpa.s) | 1.37 (1.31–1.41) | 5.432 (1.796–16.426) | 0.003 | 1.040 (0.273–3.960) | 0.954 |
| Erythrocyte sedimentation rate (mm/h) | 8 (4–14) | 0.996 (0.984–1.008) | 0.996 | ||
| Albumin (g/L) | 44.90 (43.50–46.60) | 0.970 (0.933–1.009) | 0.131 | ||
| Creatinine (umol/L) | 62 (53–71) | 1.010 (1.004–1.017) | 0.002 | 1.003 (0.998–1.009) | 0.275 |
| Uric Acid (umol/L) | 314 (260–373) | 1.001 (1.000–1.003) | 0.011 | 0.999 (0.997–1.000) | 0.084 |
aAdjusted for age, gender, total cholesterol, regular smoker, regular drinker, white blood cell count, red blood cell count, platelet, hemoglobin, plasma viscosity, creatinine, uric acid.
Baseline characteristics of subjects in the cohort study
A total of 561 subjects (338 males and 223 females) who had no colorectal polyps at baseline and underwent at least twice surveillance colonoscopies were included in the cohort study. The average follow-up time was 3.42 years. Baseline characteristics of 561 subjects were presented in Table 3. In this cohort, subjects who developed colorectal polyps were more likely to be male, regular smokers, and had higher BMI, WBC and RBC counts, hemoglobin, plasma viscosity, creatinine, and uric acid at baseline. Besides, the subjects with colorectal polyps had lower TC, albumin, and ESR. Age, TG, regular drinking and platelet were not significantly different between the two groups.
Table 3.
Characteristics of the subjects at baseline in the cohort study.
| Variables | With colorectal polyps (n = 107) | Without colorectal polyp (n = 454) | P |
|---|---|---|---|
| Age (years) | 59 (56–65) | 60 (55–67) | 0.898 |
| Gender (male/female, n) | 83/24 | 255/199 | <0.001 |
| Body mass index (kg/m2) | |||
| 18.5~25 | 61 (57.0) | 302 (66.7) | 1 (reference) |
| <18.5 | 2 (1.9) | 12 (2.6) | 0.804 |
| ≥25 | 44 (41.1) | 139 (30.6) | 0.043 |
| Total cholesterol (mmol/L) | 4.97 (0.94) | 5.16 (0.91) | 0.044 |
| Triglycerides (mmol/L) | 4.84 (4.40–5.68) | 5.15 (4.55–5.70) | 0.489 |
| Regular smoker (%) | 32 (29.9) | 52 (11.5) | <0.001 |
| Regular drinker (%) | 30 (28.0) | 93 (20.5) | 0.089 |
| White blood cell count (×109/L) | 5.9 (4.8–6.7) | 5.3 (4.5–6.4) | 0.019 |
| Red blood cell (×1012) | 4.59 (0.41) | 4.44 (0.43) | 0.002 |
| Platelet (×10^9/L) | 201.7 (47.8) | 205.8 (46.0) | 0.411 |
| Hemoglobin (g/L) | 142.0 (134–151) | 137.0 (126.0–147.0) | <0.001 |
| Plasma viscosity (mpa.s) | 1.38 (1.33–1.42) | 1.36 (1.31–1.41) | 0.029 |
| Erythrocyte sedimentation rate (mm/h) | 7.0 (4.0–12.0) | 9.0 (5.0–14.0) | 0.001 |
| Albumin (g/L) | 44.17 (2.160) | 44.68 (1.981) | 0.019 |
| Creatinine (umol/L) | 68.1 (14.5) | 62.5 (13.6) | <0.001 |
| Uric Acid (umol/L) | 348.0 (89.2) | 324.8 (83.2) | 0.011 |
Risk factors for the development of colorectal polyps
During the 1919 person-years of follow-up, 107 (19.1%) subjects developed colorectal polyps. Cox proportional hazards regression analysis was applied to explore the factors associated with the development of colorectal polyps (Table 4). In the univariate models, 5 variables were correlated with the development of colorectal polyps: gender, regular smoking, red blood cell, hemoglobin, and creatinine. While in the multivariate models, regular cigarette smoking (adjusted hazard ratio [AHR] 1.842; 95% CI 1.139–2.980; P = 0.013) and lower albumin (AHR 0.901; 95% CI 0.819–0.991; P = 0.032) were the independent risk factors for the development of colorectal polyps.
Table 4.
Risk factors for the development of colorectal polyps, Cox regression analysis.
| Variables | Mean (s.d.) or median (IQR) or number(%) | Univariable model | Multivariate modela | ||
|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | 60.0 (55.0–66.5) | 0.993 (0.970–1.016) | 0.543 | 0.994 (0.969–1.020) | 0.658 |
| Gender (male/female, n) | 338/223 | 1.669 (1.057–2.636) | 0.028 | 0.869 (0.440–1.717) | 0.686 |
| Body mass index, n (%) | |||||
| 18.5~25 | 363 (64.8) | 1 (reference) | 1 | ||
| <18.5 | 14 (2.5) | 0.723 (0.177–2.957) | 0.652 | 1.001 (0.239–4.186) | 0.999 |
| ≥25 | 183 (32.6) | 1.414 (0.959–2.083) | 0.08 | 1.255 (0.841–1.872) | 0.266 |
| Total cholesterol (mmol/L) | 5.13 (0.92) | 0.869 (0.702–1.076) | 0.197 | ||
| Triglycerides (mmol/L) | 1.25 (0.89–1.81) | 0.898 (0.733–1.101) | 0.301 | ||
| Regular smoker (%) | 83 (14.8) | 2.150 (1.420–3.254) | <0.001 | 1.842 (1.139–2.980) | 0.013 |
| Regular drinker (%) | 123 (21.9) | 1.191 (0.780–1.816) | 0.418 | 0.812 (0.508–1.299) | 0.385 |
| White blood cell count (×109/L) | 5.4 (4.6–6.5) | 1.081 (0.965–1.211) | 0.178 | ||
| Red blood cell (×1012) | 4.47 (0.43) | 1.681 (1.062–2.661) | 0.027 | 1.174 (0.516–2.669) | 0.702 |
| Platelet (×109/L) | 205.0 (46.4) | 0.998 (0.994–1.002) | 0.282 | ||
| Hemoglobin (g/L) | 138.0 (128.0–147.0) | 1.022 (1.007–1.038) | 0.005 | 1.018 (0.988–1.048) | 0.244 |
| Plasma viscosity (mpa.s) | 1.37 (1.31–1.41) | 3.185 (0.342–29.692) | 0.309 | ||
| Erythrocyte sedimentation rate (mm/h) | 9.0 (5.0–14.0) | 0.976 (0.945–1.007) | 0.127 | ||
| Albumin (g/L) | 44.59 (2.024) | 0.921 (0.839–1.010) | 0.081 | 0.901 (0.819–0.991) | 0.032 |
| Creatinine (umol/L) | 63.6 (13.9) | 1.015 (1.001–1.028) | 0.03 | 1.009 (0.992–1.027) | 0.277 |
| Uric Acid (umol/L) | 329.2 (84.8) | 1.001 (0.999–1.003) | 0.246 | ||
aAdjusted for age, gender, body mass index, regular smoker, regular drinker, red blood cell count, hemoglobin, plasma viscosity, albumin, creatinine.
Further analysis between smoking and colorectal polyps
We further explored the association between cigarette smoking ways and colorectal polyp features in different regression models (Table 5). After adjusted for major confounding factors, the risk for colorectal polyps in current smokers was significantly higher than that in never-smokers (AHR 1.786; 95%CI 1.087–2.936; P = 0.022). People who smoked more than 20 cigarettes per day were more likely to develop colorectal polyps (AHR 1.878; 95%CI 1.018–3.463; P = 0.044) than those who smoked less (AHR 1.811; 95%CI 1.003–3.270; P = 0.049). Since smoking and drinking are always mentioned together, we found that subjects with both smoking and drinking habits had a significantly higher risk for colorectal polyps (AHR 2.073; 95%CI 1.196–3.593; P = 0.009).
Table 5.
Associations between different smoking ways and the development of colorectal polyps.
| n (%) | Model 1a | Model 2b | Model 3c | ||||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Smoking status | |||||||
| Never | 446 (79.5) | 1 | 1 | 1 | |||
| Former | 32 (5.7) | 1.070 (0.465–2.465) | 0.873 | 0.949 (0.404–2.229) | 0.904 | 0.817 (0.344–1.940) | 0.647 |
| Current | 83 (14.8) | 2.161 (1.420–3.289) | <0.001 | 1.914 (1.187–3.086) | 0.008 | 1.786 (1.087–2.936) | 0.022 |
| Cigarettes per day | |||||||
| 0 | 478 (85.2) | 1 | 1 | 1 | |||
| 1–20 | 44 (7.8) | 2.175 (1.267–3.733) | 0.005 | 1.950 (1.099–3.459) | 0.022 | 1.811 (1.003–3.270) | 0.049 |
| >20 | 39 (7.0) | 2.125 (1.238–3.648) | 0.006 | 1.904 (1.059–3.426) | 0.032 | 1.878 (1.018–3.463) | 0.044 |
| Smoke and drink | |||||||
| no smoking and drinking | 478 (85.2) | 1 | 1 | 1 | |||
| Both smoke and drink | 41 (7.3) | 2.525 (1.540–4.138) | <0.001 | 2.263 (1.325–3.865) | 0.003 | 2.073 (1.196–3.593) | 0.009 |
aUnadjusted.
bAdjusted for age and gender.
cAdjusted for age, gender, body mass index, regular smoker, regular drinker, red blood cell count, hemoglobin, plasma viscosity, albumin, creatinine.
The relation between smoking and colorectal polyps was different in polyp position, size and number (Table 6). Regular smoking induced a higher risk for rectal polyps (AHR 2.975; 95%CI 1.315–6.726; P = 0.009) rather than colonic polyps (AHR 1.544; 95%CI 0.877–2.721; P = 0.133). Besides, smoking tends to be associated with small (<1 cm) polyps (AHR 1.903; 95%CI 1.154–3.136; P = 0.012) and single polyp (AHR 2.112; 95% CI 1.182–3.773; P = 0.012).
Table 6.
Associations between cigarette smoking and colorectal polyp features.
| Variable | n (%) | Model 1a | Model 2b | Model 3c | |||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Polyp position | |||||||
| colon | 81 (75.7) | 1.836 (1.107–3.045) | 0.019 | 1.587 (0.913–2.758) | 0.102 | 1.544 (0.877–2.721) | 0.133 |
| rectum | 32 (29.9) | 3.985 (1.966–8.075) | <0.001 | 3.543 (1.579–7.948) | 0.002 | 2.975 (1.315–6.726) | 0.009 |
| Maximum polyp size | |||||||
| <1 cm | 101 (94.4) | 2.186 (1.425–3.352) | <0.001 | 1.970 (1.218–3.185) | 0.006 | 1.903 (1.154–3.136) | 0.012 |
| ≥1 cm | 6 (5.6) | 3.174 (0.581–17.349) | 0.183 | 2.458 (0.400–15.114) | 0.332 | 2.348 (0.365–15.127) | 0.369 |
| Number of polyp(s) | |||||||
| 1 | 72 (67.3) | 2.485 (1.504–4.106) | <0.001 | 2.200 (1.253–3.863) | 0.006 | 2.112 (1.182–3.773) | 0.012 |
| ≥2 | 35 (32.7) | 2.297 (1.103–4.788) | 0.026 | 2.093 (0.928–4.719) | 0.075 | 1.875 (0.820–4.287) | 0.136 |
aUnadjusted.
bAdjusted for age and gender.
cAdjusted for age, gender, body mass index, regular smoker, regular drinker, red blood cell count, hemoglobin, plasma viscosity, albumin, creatinine.
Discussion
We performed two linked studies to assess the prevalence and risk factors for the presence and development of colorectal polyps. In the cross-sectional study, the prevalence of colorectal polyps was 18.1% in our study population. Age, gender, TC, regular smoking and WBC count were independently associated with the presence of colorectal polyps. The retrospective cohort study revealed that the cumulative incidence of subjects developed colorectal polyps was 19.1% during their follow-up colonoscopy surveillance. Regular cigarette smoking and albumin were independent risk factors for the development of colorectal polyps. Thus the two studies have the consistent result that smoking plays an essential role in the presence and development of colorectal polyps. Further analyses showed those who were current smokers, had more daily smoking consumption, and combined with regular drinking had a higher risk of developing colorectal polyps. Moreover, regular smoking was more relevant to rectal, small (<1 cm) and single polyp.
In recent years, the prevalence of colorectal polyps is increasing, which may be influenced by factors like changes in diet and lifestyle habits. Moreover, another important reason is that the improved equipment or colonoscopy techniques cause higher detection rates over time14. The incidence of colorectal polyp and CRC varies in different geographical locations and races. The highest incidence is found in western countries, while the lowest is in Africa and South-Central Asia15. A large study from the United States reported that 44.9% polypectomies were performed on 17275 patients who underwent average-risk screening colonoscopy between 2005–200616. However, endoscopy is much more accessible in western countries. Besides, interval CRC risk was found higher in blacks than in whites17. Recently, there are more Asian studies investigating prevalence and risk factors for colorectal polyps and CRC. A Korean study reported that the prevalence of colorectal adenomas was 34.5% in men and 20.0% in women among subjects at average risk, and the prevalence increased annually over the study period14. A Thai study found that in the population aged 50–65 years old, 18.2% of subjects had adenomatous polyps, of which 7% were high-risk adenoma18. In our study, the prevalence of colorectal polyp was 18.1% at baseline, while almost one-fifth subjects developed colorectal polyps in the subsequent cohort study. Therefore, it is crucial to investigate the risk factors of this disease.
Smoking is a well-known modifiable risk factor for colorectal polyps and CRC19–22. We found that regular cigarette smoking is an independent risk factor for the presence and development of colorectal polyps in Chinese population. Previous studies have revealed dose-response relations among the daily number of cigarettes smoked, the duration of smoking, the pack-years of smoking, and the risk for colorectal polyps23,24. The association was robust in all kinds of polyps (sessile serrated polyps, conventional adenomas, and hyperplastic polyps). Reduced risk of all stages of colorectal carcinogenesis (hyperplastic polyps, non-advanced adenomas, and advanced CRN) was found in people with a healthy lifestyle, including nonsmoking25. Previous studies revealed some potential mechanisms for the association between smoking, colorectal polyps and CRN, such as the reduced methylation of relevant genes26, genetic variants in carcinogen-metabolising enzymes12, the polymorphisms in DNA repair genes EXO1 and ATM27, the mutations in mismatch repair enzymes28, and XPC polymorphisms29, etc. In a word, tobacco contains many carcinogens that are thought to create no less than irreversible genetic damage to the colorectal mucosa, initiating the formation of colorectal polyps19.
It’s worth mentioning that drinking and smoking often together affect the prevalence, occurrence, and development of many diseases. In 2007, the International Agency for Research on Cancer (IARC) indicated that there was sufficient evidence to support the inclusion of CRC in the list of alcohol-related malignancies30,31. Recent meta-analysis showed that alcohol also linked with an increased risk of colorectal adenomas32 and serrated polyps33. However, the association appears to be more influential in European studies compared to those conducted in Asia or the US32. Our study failed to show the effect of alcohol alone on the development of colorectal polyps. Possible reasons could be genetic and lifestyle differences. Nevertheless, we found that when drinking combined with a regular smoking, the risk for colorectal polyp was doubled. This phenomenon may be due to the interactive effects of alcohol and tobacco/nicotine on cross-cue reactivity to alcohol and tobacco craving, subjective feelings of stimulation and sedation, and alcohol and smoking self-administration34. In addition, smoking and alcohol could cause changes in the gastrointestinal microbiome35. However, specific mechanisms need to be further explored.
The current study also demonstrated that regular tobacco consumption tends to cause rectal, small and single polyps. Consistent with our result, a stronger association between smoking and distal rather than proximal polyps was also found in another study36. This phenomenon might be explained that distal colorectal carcinogenesis is highly associated with environmental carcinogens such as various chemicals while proximal carcinogenesis is more relevant to genetic background37,38. A case-control study from Germany suggested that frequent cigarette smoking was an independent risk factor for the occurrence of large (≥20 mm) colorectal polyps, but our results showed a more substantial relation to small (<10 mm) polyps, possibly due to the small number of the subjects with large polyps in our study, as well as the racial and genetic disparities39. Nevertheless, the size and number of polyps are changing with the prolonging of time. Within the time frame of our study, smoking tends to be associated with rectal, small and single polyps.
As is known to all, diseases would result in nutrient consumption. Meanwhile, malnutrition is also a significant problem that could cause a number of clinical consequences in turn, such as deteriorated quality of life, decreased response to treatment, and shorter survival rate40. Serum albumin is a recognised biomarker for assessing the nutritional condition and were considered to be an important factor to predict recovery and survival of CRC41. In our study, decreased albumin was found to be associated with the development of colorectal polyps. In a recent study, Sun F et al. found elevated FAR (FAR = 100*Fibrinogen/Albumin) in newly diagnostic CRC patients compared with the healthy or benign controls, while albumin was low in the cases42. However, the specific association between albumin and the development of colorectal polyps remains to be further studied.
Age and gender might be unmodifiable factors for polyps. The prevalence of colorectal polyps and CRC generally increases with age18. Our results also showed increasing age associated with the presence of colorectal polyps. Most studies reported a higher prevalence in men than in women, which even can’t be explained by the influence of cardiovascular and lifestyle risk factors43. A cross-sectional analysis of the data from a large colonoscopy-based screening program showed that in each age group, the numbers needed to undergo colorectal-cancer screening for detecting advanced neoplasia was significantly lower in men than in women44. However, we didn’t find relevance between sex and colorectal polyps after adjusted for confound factors, but the trend existed in the first study.
Some factors were found not significantly associated with colorectal polyps in multivariable analysis, such as BMI, lipid metabolic parameters, WBC and RBC. Previous studies indicated a weak association between BMI45,46, serum lipids47 and colorectal polyps, but not all48. Mechanisms related to insulin resistance and inflammation have been postulated to be involved in colorectal carcinogenesis. In our study, TC and WBC were related to the presence of polyps, while others did not independently influence the presence and development of colorectal polyps, but modest relevance could not be excluded.
The present study had some limitations that should be acknowledged. First, as is a retrospective study, we failed to get sufficient polyp histopathology reports of every subject to further evaluate the risk factors for different pathological types of colorectal polyps. Second, data on smoking and drinking were self-reported, which may cause recall bias. Last, we did not explore the mechanism of how smoking and decreased albumin increases the risk of polyps. Future studies are expected to clarify the molecular mechanisms and signal pathways of these factors in the development of colorectal polyps.
In summary, our study indicated that colorectal polyps are prevalent in China, and nearly one-fifth subjects developed polyps during the study period. Smoking was significantly associated with the presence and development of polyps, especially related to the rectal, small and single polyp. The incidence of colorectal polyps was also influenced by smoking status, daily tobacco consumption, and whether smoking was combined with a drinking habit. As potential precursor lesions of CRC, we should pay more attention to the risk factors for colorectal polyps, to better prevent and manage this series of disease.
Author contributions
S.Z., Y.C. and L.Y conceived and designed the study. X.L. and M.M. collected the clinical information. P.J. and C.L. analyzed the data and drafted the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre la Bray F, et al. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman DA, et al. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 4.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138(6):2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 5.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J. Natl Cancer Inst. 2001;93(17):1307–1313. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 6.Zauber AG, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heitman SJ, et al. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2009;7:1272–1278. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Nakai K, et al. Sex differences in associations among metabolic syndrome, obesity, related biomarkers, and colorectal adenomatous polyp risk in a Japanese population. J. Clin. Biochem. Nutr. 2018;63(2):154–163. doi: 10.3164/jcbn.18-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CX, et al. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol. 2019;19(1):14. doi: 10.1186/s12876-018-0918-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colussi D, et al. Lifestyle factors and risk for colorectal polyps and cancer at index colonoscopy in a FIT-positive screening population. United European. Gastroenterol. J. 2018;6(6):935–942. doi: 10.1177/2050640618764711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68(8):1450–1457. doi: 10.1136/gutjnl-2018-317124. [DOI] [PubMed] [Google Scholar]
- 12.Fu Z, et al. Interaction of cigarette smoking and carcinogen-metabolising polymorphisms in the risk of colorectal polyps. Carcinogenesis. 2013;34(4):779–786. doi: 10.1093/carcin/bgs410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung YS, et al. The impact of passive smoking on the risk of colorectal neoplasia in never, former, and current smokers. J. Gastroenterol. Hepatol. 2018;33(5):1023–1030. doi: 10.1111/jgh.14023. [DOI] [PubMed] [Google Scholar]
- 14.Yang MH, et al. The prevalence of colorectal adenomas in asymptomatic Korean men and women. Cancer Epidemiol. Biomarkers Prev. 2014;23(3):499–507. doi: 10.1158/1055-9965.EPI-13-0682. [DOI] [PubMed] [Google Scholar]
- 15.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med. Clin. North. Am. 2005;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Diamond SJ, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest. Endosc. 2011;74(1):135–140. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedewa SA, et al. Racial and Ethnic Disparities in Interval Colorectal Cancer Incidence: A Population-Based Cohort Study. Ann. Intern. Med. 2017;166(12):857–866. doi: 10.7326/M16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siripongpreeda B, et al. High prevalence of advanced colorectal neoplasia in the Thai population: a prospective screening colonoscopy of 1,404 cases. BMC Gastroenterol. 2016;16:101. doi: 10.1186/s12876-016-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Øines M, et al. Epidemiology and risk factors of colorectal polyps. Best. Pract. Res. Clin. Gastroenterol. 2017;31(4):419–424. doi: 10.1016/j.bpg.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Botteri E, et al. Cigarette smoking and adenomatous polyps: a meta-analysis. Gastroenterology. 2008;134(2):388–395. doi: 10.1053/j.gastro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017;152(1):92–104. doi: 10.1053/j.gastro.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E. An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 2001;10(7):725–731. [PubMed] [Google Scholar]
- 23.Shrubsole MJ, et al. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am. J. Epidemiol. 2008;167:1050–1058. doi: 10.1093/aje/kwm400. [DOI] [PubMed] [Google Scholar]
- 24.Shin A, et al. Associations of cigarette smoking and alcohol consumption with advanced or multiple colorectal adenoma risks: a colonoscopy-based case-control study in Korea. Am. J. Epidemiol. 2011;174(5):552–562. doi: 10.1093/aje/kwr098. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JC, et al. Strong associations of a healthy lifestyle with all stages of colorectal carcinogenesis: Results from a large cohort of participants of screening colonoscopy. Am. J. Gastroenterol. 2018;113(12):1828–1835. doi: 10.1038/s41395-018-0393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paun BC, et al. Relation between normal rectal methylation, smoking status, and the presence or absence of colorectal adenomas. Cancer. 2010;116(19):4495–4501. doi: 10.1002/cncr.25348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, et al. DNA repair gene polymorphisms and tobacco smoking in the risk for colorectal adenomas. Carcinogenesis. 2011;32(6):882–887. doi: 10.1093/carcin/bgr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu JH, et al. Mismatch repair polymorphisms and colorectal polyps: hMLH1-93G>A variant modifies risk associated with smoking. Am. J. Gastroenterol. 2006;101(6):1313–1319. doi: 10.1111/j.1572-0241.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 29.Huang WY, et al. Nucleotide excision repair gene polymorphisms and risk of advanced colorectal adenoma: XPC polymorphisms modify smoking-related risk. Cancer Epidemiol. Biomarkers Prev. 2006;15(2):306–311. doi: 10.1158/1055-9965.EPI-05-0751. [DOI] [PubMed] [Google Scholar]
- 30.Pelucchi C, et al. Alcohol consumption and cancer risk. Nutr. Canc. 2011;63:983–990. doi: 10.1080/01635581.2011.596642. [DOI] [PubMed] [Google Scholar]
- 31.IARC Monograph On the evaluation of carcinogenic risks to humans: international agency for research on cancer. Alcohol consumption and ethyl carbamate. 96 (2010). [PMC free article] [PubMed]
- 32.Zhu JZ, et al. Systematic review with meta-analysis: alcohol consumption and the risk of colorectal adenoma. Aliment. Pharmacol. Ther. 2014;40:325–337. doi: 10.1111/apt.12841. [DOI] [PubMed] [Google Scholar]
- 33.Bailie L, Loughrey MB, Coleman HG. Lifestyle risk factors for serrated colorectal polyps: a systematic review and meta-analysis. Gastroenterology. 2017;152(1):92–104. doi: 10.1053/j.gastro.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Verplaetse TL, McKee SA. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am. J. Drug. Alcohol. Abuse. 2017;43(2):186–196. doi: 10.1080/00952990.2016.1189927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Capurso G, Lahner E. The interaction between smoking, alcohol and the gut microbiome. Best. Pract. Res. Clin. Gastroenterol. 2017;31(5):579–588. doi: 10.1016/j.bpg.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Fliss-Isakov N, et al. Smoking Habits are Strongly Associated With Colorectal Polyps in a Population-based Case-control Study. J. Clin. Gastroenterol. 2018;52(9):805–811. doi: 10.1097/MCG.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 37.Missiaglia E, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann. Oncol. 2014;25(10):1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 38.Oh SW, et al. The comparison of the risk factors and clinical manifestations of proximal and distal colorectal cancer. Dis. Colon. Rectum. 2008;51(1):56–61. doi: 10.1007/s10350-007-9083-5. [DOI] [PubMed] [Google Scholar]
- 39.Fedewa SA, et al. Racial and Ethnic Disparities in Interval Colorectal Cancer Incidence: A Population-Based Cohort Study. Ann. Intern. Med. 2017;166(12):857–866. doi: 10.7326/M16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr. J. 2010;9:69. doi: 10.1186/1475-2891-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu WH, et al. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS‐NSQIP. Nutr. J. 2015;14:91. doi: 10.1186/s12937-015-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun F, et al. Circulating fibrinogen to pre-albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J. Clin. Lab. Anal. 2019;33(1):e22635. doi: 10.1002/jcla.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldmann E, et al. Risk factors cannot explain the higher prevalence rates of precancerous colorectal lesions in men. Br. J. Cancer. 2016;115(11):1421–1429. doi: 10.1038/bjc.2016.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Regula J, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N. Engl. J. Med. 2006;355(18):1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 45.Kitahara CM, et al. Prospective investigation of body mass index, colorectal adenoma, and colorectal cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J. Clin. Oncol. 2013;31(19):2450e9. doi: 10.1200/JCO.2012.48.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He X, et al. Association Between Risk Factors for Colorectal Cancer and Risk of Serrated Polyps and Conventional Adenomas. Gastroenterology. 2018;155(2):355–373. doi: 10.1053/j.gastro.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang W, et al. Association between Obesity, Serum Lipids, and Colorectal Polyps in Old Chinese People. Gastroenterol. Res. Pract. 2013;2013:931084. doi: 10.1155/2013/931084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lieberman DA, et al. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959–2967. doi: 10.1001/jama.290.22.2959. [DOI] [PubMed] [Google Scholar]