Abstract
The dehydrogenative alkenylation of C-H bonds with alkenes represents an atom- and step-economical approach for olefin synthesis and molecular editing. Site-selective alkenylation of alkanes and aldehydes with the C-H substrate as the limiting reagent holds significant synthetic value. We herein report a photocatalytic method for the direct alkenylation of alkanes and aldehydes with aryl alkenes in the absence of any external oxidant. A diverse range of commodity feedstocks and pharmaceutical compounds are smoothly alkenylated in useful yields with the C-H partner as the limiting reagent. The late-stage alkenylation of complex molecules occurs with high levels of site selectivity for sterically accessible and electron-rich C-H bonds. This strategy relies on the synergistic combination of direct hydrogen atom transfer photocatalysis with cobaloxime-mediated hydrogen-evolution cross-coupling, which promises to inspire additional perspectives for selective C-H functionalizations in a green manner.
Subject terms: Photocatalysis, Green chemistry, Synthetic chemistry methodology
Dehydrogenative alkenylation of C-H bonds is an atom-economical approach to prepare more complex olefins. Here, the authors use a combination of decatungstate and a cobaloxime catalyst for the photocatalytic dehydrogenative alkenylation of alkanes and aliphatic aldehydes with aryl alkenes.
Introduction
Olefins play an essential role in organic synthesis owing to their extremely rich chemistry1. The direct alkenylation of simple C–H bonds with alkenes is one of the most appealing approaches for the construction and derivatization of complex molecules2,3. While transition-metal-catalyzed alkenylations of arenes and heteroarenes have been extensively studied4–6, analogous transformations of alkanes and aldehydes remain rare7–20. Expanding direct alkenylation to Csp3–H and Csp2(O)–H bonds is challenging due to the slow metal-mediated C–H cleavage21, the instability of alkyl/acyl metallic intermediates22,23, and the selectivity issues arising from the ubiquity of Csp3–H bonds in organic molecules24.
Transition-metal-catalyzed dehydrogenative alkenylation of alkanes and aldehydes through an auxiliary-assisted metal insertion was recently disclosed (Fig. 1a)7–13. However, these transformations usually require stoichiometric CuII or AgI salts as oxidants. Moreover, the heteroatom-containing directing groups can undergo intramolecular Michael addition and give cyclized products instead of the desired alkenylated products7–13. In 2019, Gevorgyan and coworkers disclosed an elegant radical relay strategy for the alkenylation of alcohols with visible-light-mediated palladium catalysis14. Nonetheless, the requirement of a silicon-based auxiliary lowers the atom economy of this strategy, and the substrate scope is limited to alcohols. On the other hand, auxiliary-free dehydrogenative alkenylation offers practical advantages and has attracted substantial interest (Fig. 1b)15–20. Although much progress has been achieved, the developed systems usually require stoichiometric peroxides and a large excess of the C–H partner (over 40 equivalents of the alkanes), and the substrate scopes are limited to simple hydrocarbons and aromatic aldehydes. In pursuing methods for olefin construction25–27 and photomediated C–H bond functionalization28–31, we aspired to develop an auxiliary-free and oxidant-free strategy for Csp3–H and Csp2(O)–H alkenylation with the C–H substrate as the limiting reagent (Fig. 1c). Importantly, this would allow the late-stage functionalization of advanced synthetic intermediates and pharmaceutical relevant molecules without de novo synthesis32.
Fig. 1. Strategies for the dehydrogenative alkenylation of alkanes and aldehydes with alkenes.
a Auxiliary-assisted alkenylation. b Auxiliary-free alkenylation. c Merging of a hydrogen atom transfer photocatalyst and a cobaloxime catalyst enables site-selective direct alkenylation of C–H substrates.
In this context, we were inspired by direct hydrogen atom transfer (HAT) photocatalysts that could achieve the straightforward activation of C–H bonds33. Of particular interest was the decatungstate anion ([W10O32]4−), a polyoxometalate photocatalyst that has been broadly applied in various functionalizations of alkanes and aldehydes34–39. On the other hand, photocatalytic dehydrogenative cross-coupling reactions with concomitant hydrogen evolution have recently been developed through cooperative photoredox and cobaloxime catalysis, as pioneered by the Wu and Lei groups40–44. Inspired by these studies, we proposed that the combination of decatungstate anion and a cobaloxime catalyst could enable the direct activation and alkenylation of alkanes and aldehydes using alkenes as the feedstocks. We herein report that by incorporating decatungstate catalysis and cobaloxime catalysis, direct alkenylation of alkanes and aldehydes with aryl alkenes can be achieved in the absence of hydrogen acceptors. This strategy features a broad substrate scope, high C–H site selectivity, excellent E selectivity of the alkene products, and the use of the C–H substrate as the limiting reagent. Application of this strategy in late-stage functionalization was demonstrated by the selective alkenylation of natural products and pharmaceutically important molecules.
Results
Proposed reaction mechanism
A hypothesized catalytic cycle is depicted in Fig. 2. Photoexcitation of decatungstate anion 1 would produce triplet excited state 2 after intersystem crossing45. Excited 2 can abstract hydrogen atoms from the alkane or aldehyde and afford carbon-centered radical R• and reduced decatungstate 345. Subsequent addition of radical R• to an alkene would furnish radical intermediate 4, which can be reversibly captured by CoII 5 to form CoIII intermediate 646,47. Photo-irradiation of the alkyl CoIII complex would deliver the alkene product along with a CoIII–H intermediate via a formal β-H elimination process46,47. CoIII–H 7 would react with another proton to release H2 and deliver CoIII 848–50. Finally, a single-electron reduction of CoIII 8 (E1/2 CoIII/CoII = −0.68 V versus Ag/Ag+ in MeCN)50,51 by reduced decatungstate 3 (E1/2 [W10O32]4−/[W10O32]5− = −0.96 V versus Ag/Ag+ in MeCN)52 would regenerate both catalysts. With this hypothesis in mind, we investigated the direct alkenylation of alkanes and aldehydes under various conditions.
Fig. 2. Proposed mechanism for the dehydrogenative alkenylation of alkanes and aldehydes.
The plausible mechanism involves a hydrogen atom transfer process, a single electron transfer process, and cobalt-catalyzed alkene formation and hydrogen evolution.
Reaction optimization
Our investigation began with the alkenylation of cyclooctane with cyclooctane as the limiting reagent and styrene as the alkene partner. After extensive evaluation (Table 1 and Supplementary Tables 1–10), we established that the combination of tetra-n-butylammonium decatungstate (TBADT, 9, 4 mol%), Co(dmgH)(dmgH)2Cl2 (10, 1 mol%), and 2,6-lutidine (L5, 10 mol%) in acetonitrile (0.1 M) at 60 °C were the optimal conditions, affording alkenylated product 11 in 69% isolated yield with exclusive E selectivity (Table 1, entry 1). Reducing the amount of styrene from 10 equiv. to 5 equiv. resulted in a much lower yield (Table 1, entry 2). After careful analysis of the crude product mixture using GC-MS and 1H NMR spectroscopy (see Supplementary Discussion), it was found that cobalt hydride, a key intermediate in the proposed catalytic cycle (Fig. 2), also promoted the oligomerization of styrene53. Due to this competing side reaction, 10 equiv of styrene is needed to achieve a high yield of the desired product. Nonetheless, considering the abundance of alkene feedstocks ($ 0.002/mmol for styrene) and the versatile reactivity of olefins, this method is still highly valuable. Hydrogen gas was produced in 64% yield by GC analysis of the crude product mixture (Supplementary Figs. 3–5), which supported our mechanistic hypothesis. The possibility of styrene or styrene oligomers serving as hydrogen acceptors54 was excluded since byproducts from olefin hydrogenation were not detected (Supplementary Figs. 6–9). During the reaction optimization, we found that the ligand had a large impact on the dehydrogenative alkenylation (Table 1, entries 3–12). Axial ligands on cobaloxime can readily dissociate, and recoordination of these ligands facilitates β-H elimination55,56. Moreover, the electronic and steric properties of axial ligands could dramatically influence the reactivity of cobaloxime complexes56,57. A survey of heterocyclic ligands (Supplementary Table 8) revealed that 2,6-lutidine (L5) was most effective among all the pyridine ligands evaluated, most probably because it provided a suitable steric environment around the cobalt center. In addition, the use of 50 mol% of 2-methylbenzimidazole (L9) was also effective. The use of inorganic bases such as sodium bicarbonate failed to increase reaction yield, confirming that these heterocycles function as ligands rather than bases (Table 1, entry 13 and Supplementary Table 9). No product was detected in the absence of TBADT 9, cobaloxime 10 or light (Table 1, entry 14), demonstrating that all these components are required. It was noted that other direct HAT photocatalysts resulted in very low conversions under the established conditions (Table 1, entry 15).
Table 1.
Selected optimization results.
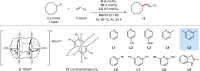 | ||
|---|---|---|
| Entry | Deviation from standard conditionsa | Yield (%)b |
| 1 | None | 70 (69) |
| 2 | 5 equiv. of styrene | 36 |
| 3 | Without L5 | 34 |
| 4 | 1 mol% of L5 | 58 |
| 5 | L1 instead of L5 | 59 |
| 6 | L2 instead of L5 | 62 |
| 7 | L3 instead of L5 | 64 |
| 8 | L4 instead of L5 | 60 |
| 9 | L6 instead of L5 | 58 |
| 10 | L7 instead of L5 | 66 |
| 11 | L8 instead of L5 | 55 |
| 12 | L9 (50 mol%) instead of L5 | 72 |
| 13 | NaHCO3 (10 mol%) instead of L5 | 34 |
| 14 | Without 9 or 10 or light | 0 |
| 15 | Eosin Yc or benzophenone instead of 9 | < 5 |
aReactions were performed under the irradiation of a 24 W 370 nm LED.
bYields were determined from the crude 1H NMR spectra using 1,3,5-trimethoxybenzene as an internal standard. Isolated yields in parentheses. Only E alkenes were generated.
c18 W blue LED was used.
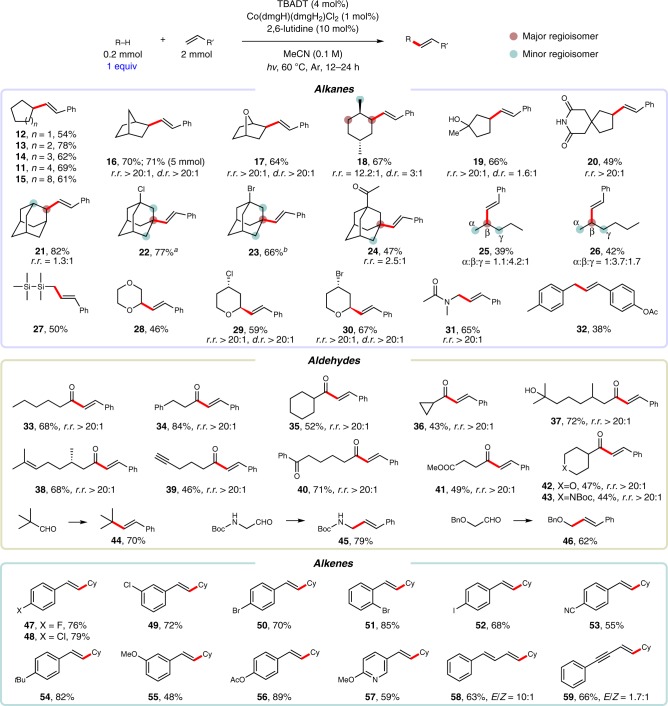
Substrate scope
With the optimized conditions in hand, we set out to examine the scope and site-selectivity of this C-H alkenylation (Fig. 3). Throughout these studies, the C–H substrate was used as the limiting reagent. A wide range of alkanes were smoothly alkenylated in moderate to good yields with exclusive E selectivity (11–32). Cyclic alkanes with ring sizes ranging from 5 to 12 gave alkenylated products 11–15 in good yields (54–78%). The alkenylation reaction was amenable to scale-up, delivering alkene 16 in 71% yield on a 5.0-mmol scale. Interestingly, this dual-catalytic system exhibits a strong preference for the functionalization of sterically accessible and electron-rich C-H sites. For instance, the secondary C–H bonds in norbornane (16), 1,4-epoxycyclohexane (17) and trans-1,4-dimethylcyclohexane (18) were selectively alkenylated, while the sterically hindered tertiary C–H bonds were not reactive despite their relatively lower bond dissociation energies (BDEs)58. Primary C–H bonds, owing to their high BDEs58, were barely functionalized in the presence of secondary C–H bonds (18–19). The steric preference of this transformation is also illustrated by the regiospecific alkenylation of 1-methylcyclopentanol (19) at the remote methylene site. Similarly, the least hindered and most electron-rich methylene site of azaspirodecanedione (20) was selectively alkenylated. The reaction of adamantane (21) furnished two regioisomers (1.3:1) in 82% yield, while the reaction of adamantane derivatives bearing electron-withdrawing groups (22–24) occurred predominantly at the tertiary sites. This unique selectivity observed among adamantane derivatives probably originated from the steric accessibility of the tertiary C–H bond due to its equatorial character59 and the remarkably long life-time of 1-adamantyl radicals compared to 2-adamantyl radicals60. For linear alkanes, pentane (25, α:β:γ 1.1:4.2:1) and hexane (26, α:β:γ 1:3.7:1.7) were preferentially functionalized at the internal secondary positions. Hexamethyldisilane, which possesses one of the strongest Csp3-H bonds (BDE = 101.3 kcal/mol, see Supplementary Discussion), underwent alkenylation to afford product 27 in 50% yield. Activated Csp3–H bonds were also examined. Ethers (28–30), amides (31) and alkylbenzenes (32) were all found to be competent substrates (38–67%). Notably, substituted tetrahydropyrans afforded alkenylation products (29 and 30) with excellent regio- and diastereoselectivity.
Fig. 3. Scope of dehydrogenative alkenylation.
Isolated yields. E/Z > 99:1 unless otherwise noted. a60% selectivity. b67% selectivity. Ac = acetyl, Boc = tert-butyloxycarbonyl, Bn = benzyl, Cy = cyclohexyl.
α,β-Unsaturated ketones are core structures in a wide variety of naturally occurring and man-made molecules that are of synthetic, biological and pharmaceutical importance61. The direct alkenylation of aldehydes represents a highly attractive and sustainable strategy for the synthesis of this important family of compounds. We were excited to find that various primary and secondary aldehydes were effective substrates under the optimal alkenylation conditions, providing α,β-unsaturated ketones in decent yields with exclusive E selectivity (33–43). Notably, excellent site selectivity was observed for aldehyde C–H bonds, while other activated C–H bonds, such as benzylic (34), allylic (38), propargylic (39) and α-heteroatom C–H bonds (42 and 43), were not functionalized. The preference of formyl C–H abstraction by decatungstate anion can be explained by the polar transition state due to the partial positive charge on the carbonyl carbon (Supplementary Fig. 21)62. A tertiary aldehyde (44), 2-tert-butyloxycarbonylamino and 2-benzyloxy acetaldehydes (45 and 46) acted as alkyl radical equivalents and gave decarbonylative alkenylated products exclusively in high yields (62–79%). However, aromatic aldehydes gave poor yields under the established reaction conditions.
The generality of this C–H alkenylation with respect to the alkene component was subsequently investigated. Electron-deficient (47–53) and electron-rich (54–56) styrene derivatives possessing ortho-, meta-, or para-substituents were all found to efficiently provide the alkenylated products in moderate to good yields and with exclusive E selectivity. The 1,2-disubstituted vinyl pyridine product (57) could be obtained in 59% yield. 1,3-Dienes (58) and enynes (59) were also suitable coupling partners, even though the E/Z selectivity was diminished. Importantly, functional groups that are typically sensitive to transition-metal catalysis, such as alkyl bromides (23 and 30), alkenes (38), alkynes (39) and aryl iodides (52), were all well tolerated, allowing for subsequent orthogonal functionalization reactions.
Late-stage selective C–H alkenylation of complex molecules
The potential of this protocol for the late-stage site-selective alkenylation of complex molecules was subsequently investigated. As illustrated in Fig. 4, a number of natural products and derivatives were successfully alkenylated under the optimal conditions. The observed regioselectivities were in accordance with the preferences described above. Camphene, a terminal-olefin-containing natural product, was alkenylated at sterically accessible methylene sites (60, 57%). A moderate yield of alkenylated eucalyptol was obtained (61, 51%) with excellent site selectivity (over 20 to 1) for the less hindered methylene site. This selectivity is remarkable, especially compared to TBADT-catalyzed photo-oxygenation of eucalyptol (2.4 to 1 methylene selectivity)63. Useful efficiencies were observed for the terpenoid (+)-fenchol with 74% regioselectivity for the unactivated and less hindered methylene sites (62, 46%). The steroid trans-androsterone acetate, which contains five tertiary C–H bonds and nine methylene sites, underwent selective alkenylation on the B ring (63, 64%). This selectivity is predictable, as the C–H bonds on the A ring and D ring are electronically deactivated, while the C–H bonds on the C ring are sterically inaccessible. Finally, a complex aldehyde derivative of lithocholic acid was readily functionalized, delivering α,β-unsaturated ketone 64 in 75% yield with excellent selectivity.
Fig. 4. Late-stage alkenylation of natural products and derivatives.
Isolated yields. Standard conditions unless otherwise noted. aOverall yields over three steps: (1) protection: hexamethyldisilazane (0.6 equiv.), ammonium thiocyanate (0.05 equiv.), DCM, 0 °C → RT, 12 h; (2) alkenylation: standard conditions for dehydrogenative alkenylation; (3) deprotection: tetra-n-butylammonium fluoride (1 equiv.), THF, RT, 12 h. DCM = dichloromethane. THF = tetrahydrofuran.
Mechanistic considerations
Although excited decatungstate anions have been applied to abstract electron-rich and sterically accessible C–H bonds64, the excellent sterically controlled selectivity of this C–H alkenylation (e.g., eucalyptol 61) prompted us to investigate the potential role of the cobaloxime catalysts. Control experiments with different ligands on the cobalt catalyst (Supplementary Table 12) suggested that planar dimethylglyoximate ligands gave the cobalt catalyst exceptional steric bulk65 and contributed to the high regioselectivity. In addition, various control experiments have been conducted to further support the proposed mechanism in Fig. 2 (see Supplementary Discussion). The presence of radical intermediates was evidenced by radical trapping experiments using 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO, Supplementary Fig. 10). Light on-off experiments demonstrated that continuous light irradiation was necessary for the transformation (Supplementary Fig. 13). We measured the kinetic isotope effect (KIE) from two parallel reactions and an intermolecular competition experiment, and the KIE values were calculated to be 1.0 and 2.0 respectively (Supplementary Figs. 14–16). These results indicated that C–H cleavage may not be the rate-determining step66. The UV–Vis absorption of the reaction mixture was also monitored. Two absorption bands at 440–500 nm and 550–700 nm were observed after 10 min light irradiation and subsequent exposure to air (Supplementary Figs. 17–18), which indicates the presence of CoII and CoI intermediates respectively48,49.
Discussion
In summary, we disclosed a general strategy for the dehydrogenative alkenylation of alkanes and aliphatic aldehydes with aryl alkenes using the C–H substrates as the limiting reagent. The late-stage functionalization of complex pharmaceutically important molecules was achieved with high levels of site selectivity, and a broad scope of functional groups were tolerated, providing a unique method for olefin synthesis and molecular diversification. The sterically and electronically dictated site selectivity originates from the dual effect of both the decatungstate and cobaloxime catalysts. Our future efforts will be directed toward further expanding the alkene scope and developing strategies to further tune the site-selectivity of the C–H alkenylation.
Methods
General procedure for dehydrogenative alkenylation of alkanes and aldehydes with alkenes
To a 10 mL oven-dried Schlenk tube equipped with a magnetic stir bar was added the corresponding C–H nucleophile (0.2 mmol, 1.0 equiv.), alkene (2.0 mmol, 10 equiv.), TBADT (26.6 mg, 0.008 mmol, 4 mol%), Co(dmgH)(dmgH2)Cl2 (0.7 mg, 0.002 mmol, 1 mol%), 2,6-lutidine (2.1 mg, 0.02 mmol, 10 mol%) and dry acetonitrile (2 mL). The resulting mixture was cooled to 0 oC using an ice-water bath, and bubbled with argon balloon for 10 min (if the C–H nucleophile was volatile, it was added after argon bubbling). After that, the reaction was placed under a 370 nm LED (2.5 meter strips, 24 W), stirred and irradiated under argon atmosphere. The temperature was maintained at 60 °C using a water bath. The reaction mixture was removed from light and quenched by stirring open to air for 5 minutes. The solvent was removed on a rotary evaporator under reduced pressure and the residue was subjected to column chromatography isolation over silica gel or preparative thin layer chromatography to obtain the corresponding product.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are grateful for the financial support provided by the National University of Singapore and the Ministry of Education (MOE) of Singapore (MOE2017-T2-2-081), NUS (Suzhou) Research Institute and National Natural Science Foundation of China (Grant No. 21702142, 21771135, 21871205). We thank Mr. Tang Jie (National University of Singapore) for the help on quantitative analysis of generated hydrogen gas.
Author contributions
H.C. discovered and developed the reaction. H.C., Y.K., and J.W. conceived and designed the investigations. X.S. conducted density functional theory (DFT) calculations. H.C., K.L.W., B.B.T., and J.M.C.K. performed the experiments. H.C., X.L., and J.W. wrote the manuscript.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Yang Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15878-6.
References
- 1.Smith, M. B. & March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure 6th edn. (John Wiley & Sons, Inc., 2007).
- 2.Le Bras J, Muzart J. Intermolecular dehydrogenative Heck reactions. Chem. Rev. 2011;111:1170–1214. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi J, Yamaguchi AD, Itami K. C-H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2012;51:8960–9009. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 4.Fujiwara Y, Moritani I, Danno S, Asano R, Teranishi S. Aromatic substitution of olefins. VI. Arylation of olefins with palladium(II) acetate. J. Am. Chem. Soc. 1969;91:7166–7169. doi: 10.1021/ja01053a047. [DOI] [PubMed] [Google Scholar]
- 5.Jia C, Kitamura T, Fujiwara Y. Catalytic functionalization of arenes and alkanes via C-H bond activation. Acc. Chem. Res. 2001;34:633–639. doi: 10.1021/ar000209h. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Lu W. Towards ideal synthesis: alkenylation of aryl C-H bonds by a Fujiwara-Moritani reaction. Chem. Eur. J. 2014;20:634–642. doi: 10.1002/chem.201303670. [DOI] [PubMed] [Google Scholar]
- 7.Wasa M, Engle KM, Yu J-Q. Pd(II)-catalyzed olefination of sp3 C-H bonds. J. Am. Chem. Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stowers KJ, Fortner KC, Sanford MS. Aerobic Pd-catalyzed sp3 C-H olefination: a route to both N-heterocyclic scaffolds and alkenes. J. Am. Chem. Soc. 2011;133:6541–6544. doi: 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Chen G, Feng C-G, Gong W, Yu J-Q. Ligand-enabled γ-C-H olefination and carbonylation: construction of β-quaternary carbon centers. J. Am. Chem. Soc. 2014;136:5267–5270. doi: 10.1021/ja501689j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, et al. Ligand-controlled C(sp3)-H arylation and olefination in synthesis of unnatural chiral α-amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, He J, Liu T, Yu J-Q. Ligand-enabled γ-C(sp3)-H olefination of amines: en route to pyrrolidines. J. Am. Chem. Soc. 2016;138:2055–2059. doi: 10.1021/jacs.5b13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoef B, Pastine SJ, Sames D. Cross-coupling of sp3 C-H bonds and alkenes: catalytic cyclization of alkene-amide substrates. J. Am. Chem. Soc. 2004;126:6556–6557. doi: 10.1021/ja049111e. [DOI] [PubMed] [Google Scholar]
- 13.Shi Z, Schröder N, Glorius F. Rhodium(III)-catalyzed dehydrogenative Heck reaction of salicylaldehydes. Angew. Chem. Int. Ed. 2012;51:8092–8096. doi: 10.1002/anie.201203224. [DOI] [PubMed] [Google Scholar]
- 14.Chuentragool P, et al. Aliphatic radical relay Heck reaction at unactivated C(sp3)-H sites of alcohols. Angew. Chem. Int. Ed. 2019;58:1794–1798. doi: 10.1002/anie.201812398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Wei Y. Copper catalyzed direct alkenylation of simple alkanes with styrenes. Chem. Sci. 2014;5:2379–2382. doi: 10.1039/c4sc00093e. [DOI] [Google Scholar]
- 16.Liu D, Liu C, Li H, Lei A. Copper-catalysed oxidative C-H/C-H coupling between olefins and simple ethers. Chem. Commun. 2014;50:3623–3626. doi: 10.1039/c4cc00867g. [DOI] [PubMed] [Google Scholar]
- 17.Gu H, Wang C. Rhenium-catalyzed dehydrogenative olefination of C(sp3)-H bonds with hypervalent iodine(III) reagents. Org. Biomol. Chem. 2015;13:5880–5884. doi: 10.1039/C5OB00619H. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Liu C, Yuan J, Lei A. Copper-catalyzed oxidative coupling of alkenes with aldehydes: direct access to α,β-unsaturated ketones. Angew. Chem. Int. Ed. 2013;52:2256–2259. doi: 10.1002/anie.201208920. [DOI] [PubMed] [Google Scholar]
- 19.Song C-X, et al. Direct functionalization of benzylic C-Hs with vinyl acetates via Fe-catalysis. Chem. Commun. 2009;40:6002–6004. doi: 10.1039/b911031c. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, Bohle DS, Li C. Cu-catalyzed cross-dehydrogenative coupling: a versatile strategy for C-C bond formations via the oxidative activation of sp3 C-H bonds. Proc. Natl Acad. Sci. USA. 2006;103:8928–8933. doi: 10.1073/pnas.0601687103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jazzar, R. et al. Functionalization of organic molecules by transition-metal-catalyzed C(sp3)-H activation. Chem. Eur. J. 16, 2654–2672 (2010). [DOI] [PubMed]
- 22.Patai, S. The Chemistry of theMetal-Carbon Bond (ed. Stille, J. K.) Vol. 2 (Wiley: New York, 1985).
- 23.Garralda, M. A. Aldehyde C-H activation with late transition metal organometallic compounds. Formation and reactivity of acyl hydrido complexes. Dalton Trans. 3635–3645 (2009). [DOI] [PubMed]
- 24.Newhouse T, Baran PS. If C-H bonds could talk: selective C-H bond oxidation. Angew. Chem. Int. Ed. 2011;50:3362–3374. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue F, et al. Reaction discovery using acetylene gas as the chemical feedstock accelerated by the “stop-flow” micro-tubing reactor system. Chem. Sci. 2017;8:3623–3627. doi: 10.1039/C7SC00408G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng H-P, et al. Photoinduced nickel-catalyzed chemo- and regioselective hydroalkylation of internal alkynes with ether and amide α‑hetero C(sp3)-H bonds. J. Am. Chem. Soc. 2017;139:13579–13584. doi: 10.1021/jacs.7b08158. [DOI] [PubMed] [Google Scholar]
- 27.Cao H, et al. Photo-induced decarboxylative Heck-type coupling of unactivated aliphatic acids and terminal alkenes in the absence of sacrificial hydrogen acceptors. J. Am. Chem. Soc. 2018;140:16360–16367. doi: 10.1021/jacs.8b11218. [DOI] [PubMed] [Google Scholar]
- 28.Zhou R, Liu H, Tao H, Yu X, Wu J. Metal-free direct alkylation of unfunctionalized allylic/benzylic sp3 C-H bonds via photoredox induced radical cation deprotonation. Chem. Sci. 2017;8:4654–4659. doi: 10.1039/C7SC00953D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan X-Z, et al. Eosin Y as a direct hydrogen-atom transfer photocatalyst for the functionalization of C-H bonds. Angew. Chem. Int. Ed. 2018;57:8514–8518. doi: 10.1002/anie.201803220. [DOI] [PubMed] [Google Scholar]
- 30.Deng H-P, Zhou Q, Wu J. Microtubing-reactor-assisted aliphatic C-H functionalization with HCl as a hydrogen-atom-transfer catalyst precursor in conjunction with an organic photoredox catalyst. Angew. Chem. Int. Ed. 2018;57:12661–12665. doi: 10.1002/anie.201804844. [DOI] [PubMed] [Google Scholar]
- 31.Kuang Y, et al. Asymmetric synthesis of 1,4-dicarbonyl compounds from aldehydes by hydrogen atom transfer photocatalysis and chiral Lewis acid catalysis. Angew. Chem. Int. Ed. 2019;58:16859–16863. doi: 10.1002/anie.201910414. [DOI] [PubMed] [Google Scholar]
- 32.Cernak T, et al. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016;45:546–576. doi: 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]
- 33.Capaldo, L. & Ravelli, D. Hydrogen atom transfer (HAT): a versatile strategy for substrate activation in photocatalyzed organic synthesis. Eur. J. Org. Chem. 2056–2071 (2017). [DOI] [PMC free article] [PubMed]
- 34.Tzirakis MD, Lykakis IN, Orfanopoulos M. Decatungstate as an efficient photocatalyst in organic chemistry. Chem. Soc. Rev. 2009;38:2609–2621. doi: 10.1039/b812100c. [DOI] [PubMed] [Google Scholar]
- 35.Ravelli D, Protti S, Fagnoni M. Decatungstate anion for photocatalyzed “window ledge” reactions. Acc. Chem. Res. 2016;49:2232–2242. doi: 10.1021/acs.accounts.6b00339. [DOI] [PubMed] [Google Scholar]
- 36.West JG, Huang D, Sorensen EJ. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 2015;6:10093. doi: 10.1038/ncomms10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halperin SD, et al. Convenient photocatalytic fluorination of unactivated C-H bonds. Angew. Chem. Int. Ed. 2014;53:4690–4693. doi: 10.1002/anie.201400420. [DOI] [PubMed] [Google Scholar]
- 38.Perry IB, et al. Direct arylation of strong aliphatic C-H bonds. Nature. 2018;560:70–75. doi: 10.1038/s41586-018-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esposti S, Dondi D, Fagnoni M, Albini A. Acylation of electrophilic olefins through decatungstate-photocatalyzed activation of aldehydes. Angew. Chem. Int. Ed. 2007;46:2531–2534. doi: 10.1002/anie.200604820. [DOI] [PubMed] [Google Scholar]
- 40.Chen B, Wu L-Z, Tung C-H. Photocatalytic activation of less reactive bonds and their functionalization via hydrogen-evolution cross-couplings. Acc. Chem. Res. 2018;51:2512–2523. doi: 10.1021/acs.accounts.8b00267. [DOI] [PubMed] [Google Scholar]
- 41.Tang S, Zeng L, Lei A. Oxidative R1-H/R2-H cross-coupling with hydrogen evolution. J. Am. Chem. Soc. 2018;140:13128–13135. doi: 10.1021/jacs.8b07327. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Gao X, Lv Z, Abdelilah T, Lei A. Recent advances in oxidative R1‑H/R2‑H cross-coupling with hydrogen evolution via photo-/electrochemistry. Chem. Rev. 2019;119:6769–6787. doi: 10.1021/acs.chemrev.9b00045. [DOI] [PubMed] [Google Scholar]
- 43.Yu W-L, et al. Dehydrogenative silylation of alkenes for the synthesis of substituted allylsilanes by photoredox, hydrogen-atom transfer, and cobalt catalysis. Angew. Chem. Int. Ed. 2019;58:10941–10945. doi: 10.1002/anie.201904707. [DOI] [PubMed] [Google Scholar]
- 44.Tian W-F, et al. Visible-light photoredox-catalyzed decarboxylative alkylation of heteroarenes using carboxylic acids with hydrogen release. Org. Lett. 2019;21:6930–6935. doi: 10.1021/acs.orglett.9b02539. [DOI] [PubMed] [Google Scholar]
- 45.De Waele V, Poizat O, Fagnoni M, Bagno A, Ravelli D. Unraveling the key features of the reactive state of decatungstate anion in hydrogen atom transfer (HAT) photocatalysis. ACS Catal. 2016;6:7174–7182. doi: 10.1021/acscatal.6b01984. [DOI] [Google Scholar]
- 46.Branchaud BP, Choi YL. Effect of a remote ligand substituent on premature β-H elimination in a cobaloxime-mediated radical alkyl-alkenyl cross coupling. Tetrahedron Lett. 1988;29:6037–6038. doi: 10.1016/S0040-4039(00)82258-3. [DOI] [Google Scholar]
- 47.Garr CD, Finke RG. Radical cage effects in adocobinamide (axial-base-off coenzyme B12): A simple method for trapping [Ado••CoII] radical pairs, a new β-H elimination product from the radical pair and measurement of an unprecedentedly large cage-recombination efficiency factor, Fc ≥ 0.94. J. Am. Chem. Soc. 1992;114:10440–10445. doi: 10.1021/ja00052a045. [DOI] [Google Scholar]
- 48.Du P, Knowles K, Eisenberg R. A homogeneous system for the photogeneration of hydrogen from water based on a platinum(II) terpyridyl acetylide chromophore and a molecular cobalt catalyst. J. Am. Chem. Soc. 2008;130:12576–12577. doi: 10.1021/ja804650g. [DOI] [PubMed] [Google Scholar]
- 49.Lazarides T, et al. Making hydrogen from water using a homogeneous system without noble metals. J. Am. Chem. Soc. 2009;131:9192–9194. doi: 10.1021/ja903044n. [DOI] [PubMed] [Google Scholar]
- 50.Dempsey JL, Brunschwig BS, Winkler JR, Gray HB. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 2009;42:1995–2004. doi: 10.1021/ar900253e. [DOI] [PubMed] [Google Scholar]
- 51.Du P, et al. Visible light-driven hydrogen production from water catalyzed by molecular cobaloxime catalysts. Inorg. Chem. 2009;48:4952–4962. doi: 10.1021/ic900389z. [DOI] [PubMed] [Google Scholar]
- 52.Yamase, T., Takabayashi, N. & Kaji, M. Solution photochemistry of tetrakis(tetrabutylammonium) decatungstate(VI) and catalytic hydrogen evolution from alcohols. J. Chem. Soc., Dalton Trans. 793–799 (1984).
- 53.Debuigne A, et al. Overview of cobalt-mediated radical polymerization: Roots, state of the art and future prospects. Prog. Polym. Sci. 2009;34:211–239. doi: 10.1016/j.progpolymsci.2008.11.003. [DOI] [Google Scholar]
- 54.Wang G-Z, Shang R, Fu Y. Irradiation-induced palladium-catalyzed decarboxylative Heck reaction of aliphatic N-(acyloxy)phthalimides at room temperature. Org. Lett. 2018;20:888–891. doi: 10.1021/acs.orglett.8b00023. [DOI] [PubMed] [Google Scholar]
- 55.Schrauzer GN, Grate JH. Sterically induced, spontaneous Co-C bond homolysis and β-elimination reactions of primary and secondary organocobalamins. J. Am. Chem. Soc. 1981;103:541–546. doi: 10.1021/ja00393a009. [DOI] [Google Scholar]
- 56.Schrauzer GN, Lee L-P, Sibert JW. Alkylcobalamins and alkylcobaloximes. Electronic structure, spectra, and mechanism of photodealkylation. J. Am. Chem. Soc. 1970;92:2997–3005. doi: 10.1021/ja00713a012. [DOI] [PubMed] [Google Scholar]
- 57.Panagiotopoulos A, Ladomenou K, Sun D, Artero V, Coutsolelos AG. Photochemical hydrogen production and cobaloximes: the influence of the cobalt axial N-ligand on the system stability. Dalton Trans. 2016;45:6732–6738. doi: 10.1039/C5DT04502A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo, Y.-R. Comprehensive Handbook of Chemical BondEnergies (CRC Press: Boca Raton, FL, 2007).
- 59.Tabushi I, Hamuro J, Oda R. Free-radical substitution on adamantane. J. Am. Chem. Soc. 1967;89:7127–7129. doi: 10.1021/ja01002a058. [DOI] [Google Scholar]
- 60.Tabushi I, Aoyama Y, Kojo S, Hamuro J, Yoshida Z. Free-radical halogenation of adamantane. Selectivity and relative lifetime of 1-and 2-adamantyl radicals. J. Am. Chem. Soc. 1972;94:1177–1183. doi: 10.1021/ja00759a024. [DOI] [Google Scholar]
- 61.Tanaka, T., Kawase, M. & Tani, S. Urease inhibitory activity of simple α,β-unsaturated ketones. Life Sci. 73, 2985–2990 (2003). [DOI] [PubMed]
- 62.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. doi: 10.1039/a804291h. [DOI] [Google Scholar]
- 63.Zakrzewski J, Giannotti C. Photo-oxygenation of 1,8-cineole by molecular oxygen catalysed by (Bu4N)4W10O32. J. Photochem. Photobiol. A: Chem. 1992;63:173–177. doi: 10.1016/1010-6030(92)85133-F. [DOI] [Google Scholar]
- 64.Ravelli D, et al. Site-selective C-H functionalization by decatungstate anion photocatalysis: Synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 2018;8:701–713. doi: 10.1021/acscatal.7b03354. [DOI] [Google Scholar]
- 65.Nishikubo, Y. & Branchaud, B. P. Cobaloxime π-cation steric and stereoelectronic effects: The amazing effect of a single methyl group adjacent to the site of reaction. J. Am. Chem. Soc. 121, 10924–10927 (1999).
- 66.Simmons EM, Hartwig JF. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012;51:3066–3072. doi: 10.1002/anie.201107334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.