Abstract
Rotational thromboelastometry (ROTEM) can only detect high-degree hyperfibrinolysis (HF), despite being frequently used in trauma patients. We investigated whether considering FIBTEM HF (the presence of maximal lysis (ML) > 15%) could increase ROTEM-based HF detection’s sensitivity. This observational cohort study was performed at a level 1 trauma centre. Trauma patients with an Injury Severity Score (ISS) > 15 who underwent ROTEM in the emergency department between 2016 and 2017 were included. EXTEM HF was defined as ML > 15% in EXTEM. We compared mortality rates between EXTEM HF, FIBTEM HF, and non-HF patient groups. Overall, 402 patients were included, of whom 45% were men (mean age, 52.5 years; mean ISS, 27). The EXTEM HF (n = 37), FIBTEM HF (n = 132), and non-HF (n = 233) groups had mortality rates of 81.1%, 22.3%, and 10.3%, respectively. The twofold difference in mortality rates between the FIBTEM HF and non-HF groups remained statistically significant after Bonferroni correction (P = 0.01). On multivariable Cox regression analysis, FIBTEM HF was independently associated with in-hospital mortality (adjusted hazard ratio 2.15, 95% confidence interval 1.21–3.84, P = 0.009). Here, trauma patients with FIBTEM HF had significantly higher mortality rates than those without HF. FIBTEM be a valuable diagnostic method to improve HF detection’s sensitivity in trauma patients.
Subject terms: Outcomes research, Risk factors
Introduction
Trauma is a major cause of death in people under the age of 40 years, and bleeding is the leading cause of preventable death in trauma patients1,2. More than 30% of haemorrhagic trauma patients present with coagulopathy at the emergency department (ED). In comparison with patients without coagulopathy, those with coagulopathy have increased risks of multiple organ failure, massive transfusion, and death3–5. Trauma-induced coagulopathy is a complex condition that is affected by various factors, such as haemodilution, hypothermia, fibrinolysis, activated protein C, endothelial changes, and platelet dysfunction5–8.
Hyperfibrinolysis (HF) isa central feature of traumatic coagulopathy and is defined as an abnormal increase in the degree of fibrinolytic activity compared to physiological fibrinolysis9,10. Since HF is associated with high mortality rates and massive bleeding, its early detection is important in the treatment of trauma patients. However, it is difficult to diagnose HF in a clinically relevant time frame11,12. Currently, viscoelastic haemostatic assays (VHAs), such asthromboelastography (TEG) and rotational thromboelastometry (ROTEM),are the only tools used for the detection of HF within a clinically relevant time frame11,13. However, VHA only detects massive fibrinolytic activation and is insensitive in the identification of non-massive HF14.
In the FIBTEM test, the contribution of plateletsto clot formation is inhibited by cytochalasin-D13. Because platelets retain large amounts of plasminogen activator inhibitor and because platelet-rich thrombus is resistant to tissue plasminogen activator (tPA)-mediated fibrinolysis15,16, we hypothesized that the sensitivity of HF detection is improved with the use of the FIBTEM test. This study aimed(a) to compare mortality rates between FIBTEM HF andnon-HF patient groups and (b) to determine whether FIBTEM HF is independently associated with mortality.
Results
Baseline characteristics
During the study period, a total of 426 severe trauma patients (ISS > 15) underwent the ROTEM test. The exclusion criteria were age <15 years (n = 7) and presentation at our hospital 12 hours after trauma occurrence (n = 17). The final study population consisted of 402 patients. The participants’ mean age was 52.5 years, 45% of them were male, and their median ISS was 27. The most commonly observed injury mechanism was traffic accident (58%), followed by falling to a lower level (27%). Seventy-nine patients (19.7%) underwent MT, and the overall in-hospital mortality rate was 20.6%. The baseline characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics, vital signs, and outcomes with EXTEM HF group, FIBTEM HF group, and non-HF group.
| Variable | Overall (n = 402) | Non-HF (n = 233) | FIBTEM HF (n = 132) | EXTEM HF (n = 37) | P value |
|---|---|---|---|---|---|
| Male, n (%) | 183 (45.5) | 100 (42.9) | 61 (46.2) | 22 (59.5) | 0.169 |
| Age (y), mean (SD) | 52.50 (17.95) | 53.26 (17.70) | 51.70 (18.23) | 50.59 (18.79) | 0.592 |
| Injury mechanism, n (%) | |||||
| Traffic accident | 234 (58.2) | 129 (55.4) | 84 (63.6) | 21 (56.8) | 0.307 |
| Falling to a lower level | 110 (27.4) | 63 (27.0) | 36 (27.3) | 11 (29.7) | |
| Penetrating | 8 (2.0) | 7 (3.0) | 0 (0.0) | 1 (2.7) | |
| Others | 50 (12.4) | 34 (14.6) | 12 (9.1) | 4 (10.8) | |
| SBP, mean (SD) | 99.95 (43.01) | 107.35 (35.68) | 101.14 (43.15) | 49.11 (51.07)a,b | <0.001 |
| Heart rate, mean (SD) | 94.96 (29.36) | 97.33 (25.41) | 93.95 (27.82) | 83.65 (49.57) | 0.170 |
| RTS, median [IQR] | 11.00 [9.00, 12.00] | 11.00 [10.00, 12.00] | 11.00 [9.00, 12.00]a | 7.00 [1.00, 8.00]a,b | <0.001 |
| ISS, median [IQR] | 27.00 [22.00, 34.00] | 25.00 [21.00, 30.00] | 27.00 [22.00, 34.25]a | 34.00 [26.00, 41.00]a,b | <0.001 |
| 4 h RBC, median [IQR] | 1.00 [0.00, 4.75] | 1.00 [0.00, 3.00] | 2.00 [0.00, 6.00]a | 10.00 [5.00, 19.00]a,b | <0.001 |
| MT, n (%) | 79 (19.7) | 30 (12.9) | 27 (20.5) | 22 (59.5)a,b | <0.001 |
| In hospital mortality, n (%) | 83 (20.6) | 24 (10.3) | 29 (22.0)a | 30 (81.1)a,b | <0.001 |
HF, hyperfibrinolysis; SD, standard deviation; SBP, systolic blood pressure; RTS, revised trauma score; IQR, interquartile range; ISS, injury severity score; 4 h RBC, packed red blood cell units for 4 hours; MT, massive transfusion.
aP < 0.05 compared to non-HF group, bP < 0.05 compared to FIBTEM HF group.
Comparison of the EXTEM HF, FIBTEM HF, and non-HF groups
The highest proportion of patients belonged to the non-HF group (58.0%), followed by the FIBTEM HF group (32.8%) and the EXTEM HF group (9.2%). All patients in the EXTEM HF group showed FIBTEM ML > 15%. There were no statistical differences in terms of age, sex, or injury mechanism between the groups.
Compared to the non-HF group, the FIBTEM HF and EXTEM HF groups hadhigher median ISSs (25 vs. 27 vs. 34, P < 0.001) and received MT more frequently (12.9% vs. 20.5% vs. 59.5%, P < 0.001). The mortality rate was also significantly different between the groups (P < 0.001). The mortality rate in the EXTEM HF group was the highestand was about eighttimes higher than that in the non-HF group (81.1% vs. 10.3%). The mortality in the FIBTEM HF group was about twotimes higher than that in the non-HF group (Fig. 1); this difference was statistically significant after Bonferronicorrection (22.3 vs. 10.3%, P = 0.01) (Table 1).
Figure 1.
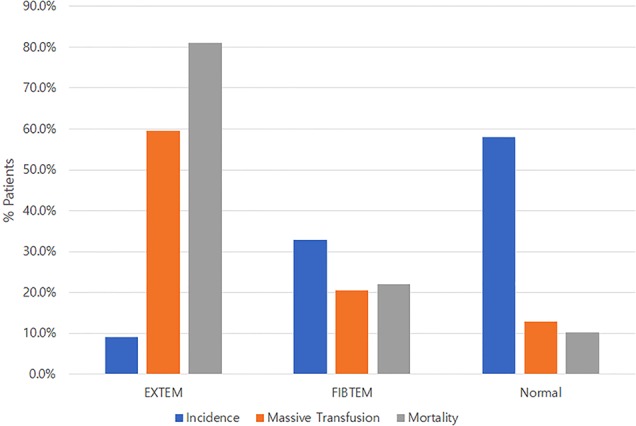
Incidence, massive transfusion, and in-hospital mortality in the EXTEM HF, FIBTEM HF, and non-HF groups. HF: hyperfibrinolysis.
Significant differenceswere observed in laboratory values and ROTEM parametersacross the groups (Table 2). The EXTEM HF group showed the lowest platelet and fibrinogen values, followed by the FIBTEM HF groupand non-HF group. After performing Bonferroni corrections, the FIBTEM HF group continued to have significantly lower platelet (P = 0.019) and fibrinogen (P < 0.001) values than the non-HF group. The PT INR and aPTTvalues were the highest in the EXTEM HF group, while the FIBTEM HF group showed higher PT INR and aPTTvalues than the non-HF group.
Table 2.
Laboratory value and ROTEM parameter with EXTEM HF group, FIBTEM HF group, and non-HF group.
| Variable | Overall (n = 402) | Non-HF (n = 233) | FIBTEM HF (n = 132) | EXTEM HF (n = 37) | P value |
|---|---|---|---|---|---|
| Fibrinogen, median[IQR] | 193.80 [140.67, 234.60] | 214.00 [179.40, 265.85] | 159.20 [122.17, 203.62]a | 125.70 [76.30, 172.95]a,b | <0.001 |
| PTINR, (median[IQR] | 1.08 [0.99, 1.23] | 1.03 [0.97, 1.15] | 1.11 [1.02, 1.32]a | 1.43 [1.25, 1.84]a,b | <0.001 |
| aPTT, median [IQR] | 30.20 [26.40, 36.75] | 29.10 [25.60, 33.10] | 30.80 [26.75, 39.60]a | 55.40 [39.80, 116.40]a,b | <0.001 |
| Hb, median[IQR] | 12.50 [10.90, 14.20] | 12.60 [11.00, 14.30] | 12.40 [10.88, 14.12] | 12.10 [9.40, 13.80] | 0.141 |
| Platelet, median [IQR] | 212.50 [168.25, 258.25] | 225.00 [178.00, 262.00] | 199.00 [148.75, 251.25]a | 172.00 [103.00, 193.00]a,b | <0.001 |
| pH, median[IQR] | 7.38 [7.29, 7.42] | 7.39 [7.33, 7.43] | 7.36 [7.29, 7.41]a | 7.22 [7.01, 7.32]a,b | <0.001 |
| Base Excess, median[IQR] | −2.80 [−7.30, −0.20] | −2.05 [−6.23, 0.80] | −3.50 [−7.55, −0.85]a | −11.80 [−16.12, −6.75]a,b | <0.001 |
| HCO3, median [IQR] | 22.30 [18.80, 24.80] | 22.70 [19.85, 25.30] | 21.90 [18.55, 24.40] | 17.80 [12.57, 20.02]a,b | <0.001 |
| EXTEM_CT, median [IQR] | 59.00 [52.00, 73.00] | 55.00 [50.00, 64.00] | 64.00 [56.00, 78.00]a | 88.00 [71.00, 135.00]a,b | <0.001 |
| EXTEM_α, median [IQR] | 71.00 [65.00, 75.00] | 72.00 [69.00, 76.00] | 69.00 [62.00, 73.00]a | 60.00 [51.00, 67.00]a,b | <0.001 |
| EXTEM_MCF,median [IQR] | 60.00 [54.00, 63.00] | 62.00 [58.00, 65.00] | 57.00 [51.00, 61.00]a | 40.00 [28.00, 50.00]a,b | <0.001 |
| FIBTEM_CT, median [IQR] | 63.00 [51.00, 92.00] | 57.00 [49.00, 71.75] | 77.00 [55.00, 129.25]a | 102.00 [77.00, 510.00]a,b | <0.001 |
| FIBTEM_α, median [IQR] | 49.00 [0.00, 67.00] | 60.00 [42.00, 69.00] | 0.00 [0.00, 54.50]a | 0.00 [0.00, 16.00]a | <0.001 |
| FIBTEM_MCF (median [IQR]) | 11.00 [7.00, 15.00] | 13.00 [10.00, 17.00] | 9.00 [6.00, 12.00]a | 6.00 [4.00, 9.00]a,b | <0.001 |
| FIBTEM_ML (median [IQR]) | 6.00 [3.00, 9.75] | 100.00 [100.00, 100.00] | 28.00 [20.00, 45.00] a | 100.00 [100.00, 100.00] a,b | <0.001 |
HF, hyperfibrinolysis; IQR, interquartile range; PT_INR, prothrombin time and international normalized ratio; aPTT, activated partial thromboplastin time; Hb, hemoglobin; pH, potential hydrogen; HCO3, bicarbonate.
aP < 0.05 compared to non-HF group, bP < 0.05 compared to FIBTEM HF group.
Survival analysis
Kaplan–Meier curves and a log rank test showed that that mortality increased from the non-HF to the FIBTEM HF and EXTEM HF groups (Fig. 2). The results of the univariableand multivariable Cox regression modelsare shown in Table 3. In the multivariable Cox regression model, FIBTEM HF was independently associated with in-hospital mortality after adjustment for age, sex, injury mechanism, fibrinogen, ISS, and platelet count. The adjusted hazard ratio (aHR) indicated significantly elevated in-hospital mortality in the FIBTEM HF group, compared to thatin the non-HF group (aHR 2.153, 95% confidence interval 1.208–3.837, P = 0.009).
Figure 2.
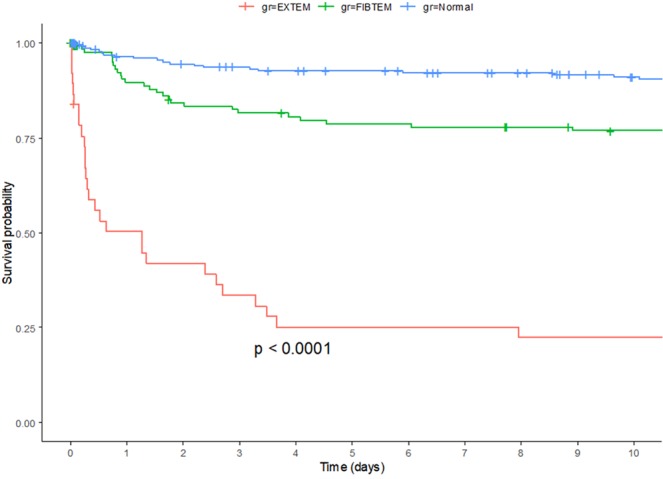
Kaplan–Meier survival analysis in the EXTEM HF, FIBTEM HF, and non-HF groups. HF: hyperfibrinolysis.
Table 3.
Cox regression for in-hospital mortality.
| Variables | Unadjusted HR [95% CI] | Unadjusted p value | Adjusted HR [95% CI] | Adjustedp value |
|---|---|---|---|---|
| HF | ||||
| non-HF | Reference | Reference | ||
| EXTEM | 15.775 [9.163, 27.157] | <0.001 | 8.158 [4.161, 15.997] | <0.001 |
| FIBTEM | 2.423 [1.410, 4.164] | 0.001 | 2.153 [1.208, 3.837] | 0.009 |
| Male gender | 1.426 [0.927, 2.193] | 0.107 | 0.995 [0.627, 1.578] | 0.982 |
| Age | 1.017 [1.004, 1.030] | 0.010 | 1.023 [1.009, 1.038] | 0.001 |
| Injury mechanism | ||||
| penetrating | Reference | Reference | ||
| Traffic accident | 1.080 [0.666, 1.752] | 0.754 | 1.080 [0.666, 1.752] | 0.921 |
| Falling to a lower level | 0.894 [0.439, 1.823] | 0.759 | 1.318 [0.167, 10.405] | 0.793 |
| Others | 0.541 [0.075, 3.923] | 0.543 | 1.039 [0.121, 8.905] | 0.972 |
| SBP | 0.982 [0.977, 0.987] | <0.001 | 0.993 [0.987, 0.999] | 0.016 |
| ISS | 1.054 [1.036, 1.072] | <0.001 | 1.040 [1.018, 1.062] | 0.000 |
| Fibrinogen | 0.994 [0.991, 0.997] | <0.001 | 1.000 [0.998, 1.003] | 0.717 |
| PT_INR | 1.337 [1.235, 1.448] | <0.001 | 1.164 [1.051, 1.289] | 0.003 |
HR, hazard ratio; CI, confidence interval; HF, hyperfibrinolysis; SBP, systolic blood pressure;ISS, injury severity score; PT_INR, prothrombin time and international normalized ratio.
Discussion
In this study, we categorized trauma patients into the EXTEM HF, FIBTEM HF, and non-HF groups. Although the incidence of EXTEM HF was low (9%), the mortality rate of patients with EXTEM HF was extremely high (81%). Patients with FIBTEM HF accounted for one-third of the study population, and their mortality was more than twice as that of patients in the non-HF group (22% vs. 10%). The FIBTEM HF group underwent MT more commonly and had a worse level of coagulopathy than the non-HF group. In the multivariable Cox regression model, FIBTEM HF was identified as an independent risk factor for in-hospital mortality.
A number of severe trauma patients have disproportionately increased fibrinolytic activity, and HF is an important component of acute traumatic coagulopathy11,12. VHAs, such as TEG and ROTEM, provide general information fromclot formation to clot lysis and are the only tools for the diagnosis of HF within an appropriate time frame17. However, previous studies have shown that VHAs have low sensitivity for HF detection4,14. In 2013, Raza et al.14 classified patients who presented to a major trauma centre into three groups, based on their fibrinolytic activity: a normal group (plasmin-α2 antiplasmin [PAP] < 1,500 µg/L), a moderate group (PAP > 1,500 µg/L and EXTEM ML < 15%), and a severe group (PAP > 1,500 µg/L and EXTEM ML > 15%). More than half of the patients (57%) showed moderate fibrinolytic activity, and their 28-day mortality rate was more than 12times higher than that observed in the normal fibrinolytic activity group. Only 5% of cases with severe fibrinolytic activity could be detected using ROTEM analysis, suggesting that a large number of cases with fibrinolytic activity were undetected by ROTEM,resulting in a noticeably high mortality.
Platelets play a key role in haemostasis, and previous studies have demonstrated that thrombocytopenia and platelet hypofunction are closely related to mortality12,18–21. Plateletsare also related to fibrinolysis, and Franz et al. showed that platelet-rich plasma is more resistant to tPA-induced fibrinolysis than platelet-poor plasma22. Based on these observations, we focused on FIBTEM,in which platelet function is blocked. In our study, similar to previous studies, the prevalence of EXTEM HF was low, but the associated mortality was extremely high14,23. The number of patients with FIBTEM HFwas more than three times higher than the number of patients with EXTEM HF, and the mortality rate was more than twice as high as in the non-HF group. Among the patients with FIBTEM HF, MT was performed more frequently, and such patients had a worse degree of coagulopathy.
Several studies have usedthe FIBTEM test in trauma patients3,24,25. Schöchl et al. reported that FIBTEM MCF and FIBTEM A10 had a high predictive value for MT in trauma patients3. Hagemo et al. showed that a FIBTEM CA5 threshold of ≤9 mm led to the detection of the need for MT in 77.5% of patients24. Although not intended for injury patients, Harr et al. found that FIBTEM detects fibrinolysis faster than INTEM and EXTEM26. However, to the best our knowledge, this is the first study to use FIBTEM HF to improve the sensitivity of HF detection.
Tranexamic acid (TXA) is a synthetic lysine analoguethat prevents the conversion of plasminogen to plasmin. It is used in the only evidence-based method available to treat HF11. However, there is a controversy regarding indications for TXA administration. Recent studies have focused on hypofibrinolysis (fibrinolytic shutdown, SD), demonstrating that SD is the most common fibrinolytic state in severe trauma patients and that the mortality rate in such patients is higher than that in patients in a physiologic fibrinolytic state27,28. There is concern surrounding the increases in mortality rate and frequency of thrombotic complications when TXA is administered to patients with SD who are already in a hypofibrinolytic state18,29. Furthermore, unlike the CRASH trial, in which the use of TXA was emphasized, opposing results were observed in clinical research studies,in which the use of TXA enhanced the occurrence of thrombotic events30–32. We believe that FIBTEM HF may have potential for use in selective TXA administration; however, additional research on the topic is needed.
Our study had several limitations. First, owingto its single-centredesign, generalizationof the results is difficult. As we only included severe trauma patients (ISS > 15), the applicability of FIBTEM HF in patients with a less severe condition is uncertain. Further multicentreinvestigations are needed to demonstrate the clinical value of FIBTEM HF. Second, there are several laboratoryvalues that can be used to measure fibrinolysis, such as euglobulin clot lysis time, plasmin-antiplasmin complex level, and plasminogen activator inhibitor-1 level. Although these tests are time-consuming and of limited availability in most trauma centres, the extent of fibrinolysis can be assessed better when analysed in combination with VHA.
In conclusion, although previous studies have emphasized the utility of VHA in the detection of HF in trauma patients, VHA can only detect high fibrinolytic activity. In the current study, we focused on FIBTEM HF and observed that mortality rates and coagulopathy were worse among patients with FIBTEM HF than among patients without HF. We suggest that FIBTEM may be used as a method of improving the sensitivity of HF detection in severe trauma patients.
Materials and Methods
Institutional Review Board of our hospital approved this study and also waived the need for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Study design and setting
This retrospective, observational, single-centre study included patients who presented to the trauma centre of a 1400-bed university-affiliated hospital in Pusan, Korea. Our trauma centre serves as a level 1 trauma centre for patients in Busan City and Gyeongsangnam-do Province. Almost 1,000 severe trauma patients (Injury Severity Score [ISS] > 15) present to our trauma centre annually.
Participants
From January 2016 to December 2017, all injured patients who presented to the trauma centre’s ED with an ISS > 15 and who underwent the ROTEM test were included. The exclusion criteria were (a) age <15 years, (b) presentation at the hospital ≥12 hours after trauma occurrence, and (c) presence of a burn injury.
Data collection and variables
We extracted data from the Korea Trauma Database (KTDB) and electronic medical records. The KTDB was created by the Korean Ministry of Health and Welfare in 2013 for the purpose of collecting information on trauma patients nationwide33. The collected data included data of age, sex, vital signs at trauma centre presentation, injury mechanism, revised trauma score at initial trauma centre presentation, transfusion of packed red blood cell within the first 4 and 24 hours of ED admission, ISS, massive transfusion (MT), and in-hospital mortality. Laboratory (fibrinogen, prothrombin time international normalized ratio [PT INR], activated partial thromboplastin time [aPTT], haemoglobin, platelet, pH, base excess, HCO3) and ROTEM data were also collected. Blood for ROTEM analysis and laboratory test was drawn within 15 minutes of initial ED presentation. Our primary outcome was in-hospital mortality, while the secondary outcome was MT. We defined MT as the transfusion of ≥10 U of packed red blood cells within 24 hours34.
Viscoelastic testing
Viscoelastic haemostatic analysis was performed using ROTEM delta (TEM international GmbH, Munich, Germany). We used three ROTEM tests in the current study: EXTEM, FIBTEM, and APTEM. In the EXTEM test, clot formation is activated by a tissue factor. In the FIBTEM test, a platelet inhibitor (cytochalasin-D) is added, and the platelet contribution to clot formation is removed. The APTEM test includes a plasmin inhibitor, which is an antifibrinolytic agent and inhibits fibrinolysis. For each ROTEM test, the following data were collected: clotting time, clot formation time, maximum clot firmness (MCF), alpha angle, and maximum lysis (ML). We divided the patients into three groups: the non-HF group, EXTEM HF group, and FIBTEM HF group. We defined HF as the presence of EXTEM ML ≥ 15% and normal APTEM ML11,33,35. FIBTEM HF was defined as FIBTEM ML ≥ 15% and normal APTEM ML.
Statistical analysis
For the continuous variables, normality was determined by a histogram and skewness >2. Normally distributed continuous variables were summarized in terms of means and standard deviations. Non-normally distributed continuous variables were summarized in terms of medians and interquartile ranges. We compared continuous variables using one-way analysis of variance or a Kruskal-Wallis test with a Bonferroni correction. Categorical variables were summarized in terms of counts and percentages and were compared using the Wilcoxon rank-sum test with Bonferroni correction. In-hospital survival was evaluated using Kaplan–Meier analysis, and a log rank test was performed to compare differences. Cox proportional hazards models were used to assess the independent effect of FIBTEM HF on in-hospital mortality. Baseline characteristics (sex, age, and injury mechanism) and previously suggested risk factors (PT INR, fibrinogen, and ISS) were included as covariates in the multivariable Cox regression model6,36–38. All statistical analyses were performed using R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). P values were two-sided, and a p value lower than 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Pusan National University Hospital (1805-019-067). The need for written informed consent was waived as the data were analysed retrospectively and anonymously.
Acknowledgements
This work was supported by Department of Biostatistics, Clinical Trial Centre, Biomedical Research Institute, Pusan National University Hospital.
Author contributions
I.J.W.: study design, writing, data collection, data interpretation, critical revision. S.W.P.: data analysis, data interpretation. B.K.B.: critical revision, study design. S.H.L.: critical revision, study design. H.J.C.: literature search, critical revision. S.J.P.: literature search, critical revision. T.Y.A.: critical revision. T.S.G.: data analysis. M.J.L.:data analysis, data interpretation. S.R.Y.: critical revision, study design, data analysis, data interpretation.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunt H, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst. Rev. 2015;2:CD010438. doi: 10.1002/14651858.CD010438.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossaint R, et al. The European guideline on management of major bleeding and coagulopathy following trauma. Crit. Care. 2016;20:100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schöchl H, et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit. Care. 2015;15:R265. doi: 10.1186/cc10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas JC, et al. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41:514–521. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 5.Frith D, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J. Thromb. Haemost. 2010;8:1919–1925. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, Christie SA. Coagulopathy of trauma. Crit. Care Clin. 2017;33:101–118. doi: 10.1016/j.ccc.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Meledeo MA, et al. Acute traumatic coagulopathy: the elephant in a room of blind scientists. J. Trauma Acute Care Surg. 2017;82:S33–S40. doi: 10.1097/TA.0000000000001431. [DOI] [PubMed] [Google Scholar]
- 8.Brohi K. Trauma induced coagulopathy. J. Royal Army Med. Corps. 2009;155:320–322. doi: 10.1136/jramc-155-04-15. [DOI] [PubMed] [Google Scholar]
- 9.Hunt BJ, Segal H. Hyperfibrinolysis. J. Clin. Pathol. 1996;49:958. doi: 10.1136/jcp.49.12.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brohi K, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J. Trauma. 2008;64:1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 11.Gall LS, Brohi K, Davenport RA. Diagnosis and treatment of hyperfibrinolysis in trauma (a European perspective) Semin. Thromb. Hemost. 2017;43:224–234. doi: 10.1055/s-0036-1598001. [DOI] [PubMed] [Google Scholar]
- 12.Gall LS, Davenport RA. Fibrinolysis and antifibrinolytic treatment in the trauma patient. Curr. Opin. Anesthesiol. 2018;31:227–233. doi: 10.1097/ACO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 13.Longstaff C. Measuring fibrinolysis: from research to routine diagnostic assays. J. Thromb. Haemost. 2018;16:652–662. doi: 10.1111/jth.13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raza I, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J. Thromb. Haemost. 2013;11:307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 15.Brogren H, Wallmark K, Deinum J, Karlsson L, Jern S. Platelets retain high levels of active plasminogen activator inhibitor 1. Plos One. 2011;6:e26762. doi: 10.1371/journal.pone.0026762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fay WP, Eitzman DT, Shapiro AD, Madison EL, Ginsburg D. Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and -independent mechanisms. Blood. 1994;83:351–356. doi: 10.1182/blood.V83.2.351.351. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, et al. Viscoelastic Testing in Trauma Consensus Panel 2014 Consensus conference on viscoelastic test-based transfusion guidelines for early trauma resuscitation: report of the panel. J. Trauma Acute Care Surg. 2015;78:1220–1229. doi: 10.1097/TA.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 18.Walsh M, et al. Fibrinolysis in trauma: “myth,” “reality,” or “something in between”. Semin. Thromb. Hemost. 2017;43:200–212. doi: 10.1055/s-0036-1597900. [DOI] [PubMed] [Google Scholar]
- 19.Brown LM, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J. Trauma. 2011;71:S337–S342. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutcher ME, et al. Characterization of platelet dysfunction after trauma. J. Trauma Acute Care Surg. 2012;73:13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon C, et al. Platelet function following trauma. Thromb. Haemost. 2011;106:322–330. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 22.Franz RC. ROTEM analysis: a significant advance in the field of rotational thrombelastography. S. Afr. J. Surg. 2009;47:2–6. [PubMed] [Google Scholar]
- 23.Tauber H, et al. Prevalence and impact of abnormal ROTEM® assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br. J. Anaesth. 2011;107:378–387. doi: 10.1093/bja/aer158. [DOI] [PubMed] [Google Scholar]
- 24.Hagemo JS, et al. Detection of acute traumatic coagulopathy and massive transfusion requirements by means of rotational thromboelastometry: an international prospective validation study. Crit. Care. 2015;19:97. doi: 10.1186/s13054-015-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harr JN, et al. Viscoelastic hemostatic fibrinogen assays detect fibrinolysis early. Eur. J. Trauma Emerg. Surg. 2015;41:49–56. doi: 10.1007/s00068-014-0400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer MA, et al. Fibrinogen in trauma, an evaluation of thrombelastography and rotational thromboelastometry fibrinogen assays. J. Surg. Res. 2015;194:581–590. doi: 10.1016/j.jss.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Moore HB, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J. Am. Coll. Surg. 2016;222:347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore HB, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gall LS, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann. Surg. 2019;269:1184–1191. doi: 10.1097/SLA.0000000000002733. [DOI] [PubMed] [Google Scholar]
- 30.Myers SP, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J. Trauma Acute Care Surg. 2019;86:20–27. doi: 10.1097/TA.0000000000002061. [DOI] [PubMed] [Google Scholar]
- 31.Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. West Indian Med. J. 2010;59:612–624. [PubMed] [Google Scholar]
- 32.Johnston LR, Rodriguez CJ, Elster EA, Bradley MJ. Evaluation of military use of tranexamic acid and associated thromboembolic events. JAMA Sur. 2018;153:169–175. doi: 10.1001/jamasurg.2017.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theusinger OM, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth. Analg. 2011;113:1003–1012. doi: 10.1213/ANE.0b013e31822e183f. [DOI] [PubMed] [Google Scholar]
- 34.Cantle PM, Cotton BA. Prediction of massive transfusion in trauma. Crit. Care Clin. 2017;33:71–84. doi: 10.1016/j.ccc.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Tauber H, et al. Prevalence and impact of abnormal ROTEM(R) assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study. Br. J. Anaesth. 2011;107:378–387. doi: 10.1093/bja/aer158. [DOI] [PubMed] [Google Scholar]
- 36.Winearls J, et al. Fibrinogen in traumatic haemorrhage: a narrative review. Injury. 2017;48:230–242. doi: 10.1016/j.injury.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Champion HR, Sacco WJ, Hunt TK. Trauma severity scoring to predict mortality. World J. Surg. 1983;7:4–11. doi: 10.1007/BF01655906. [DOI] [PubMed] [Google Scholar]
- 38.Wang, I. J. et al. Pre-hospital modified shock index for prediction of massive transfusion and mortality in trauma patients. Am. J. Emerg. Med. 10.1016/j.ajem.2019.01.056 (2019). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


