Abstract
This research was conducted to understand the influence of foliar applied melatonin (0, 50, 100, 150 and 200 μM) on two Salvia species (Salvia nemorosa L., and Salvia reuterana Boiss) under conditions of water stress. Water stress was applied using a reduced irrigation strategy based on re-watering at 80%, 60% and 40% of the field capacity (FC). Increasing water stress, while significantly enhancing malondialdehyde (MDA), H2O2, electrolyte leakage, oxidized glutathione (GSSG), and total glutathione (GT), reduced glutathione (GSH), catalase (CAT), peroxidase (POD), superoxide dismutase (SOD) and glutathione reductase (GR) activities, which led to a marked reduction in fluorescence (Fv/Fm). Foliar application of melatonin alleviated the oxidative stress by increasing GT, CAT, POD, SOD and GR activities and reducing GSSG. In particular, melatonin heightened GSH content as well as the ratio of GSH/GSSG when compared to non-sprayed water stressed plants. Melatonin-treated plants had significantly lower SOD and POD activities than control plants under drought stress, while the CAT activity was enhanced with the foliar treatment. Essential oil yield of both Salvia species increased with the decrease in irrigation from 80% to 60% FC but diminished with the more severe water deficit (40% FC). Essential oil components of Salvia nemorosa were β- caryophyllene, germacrene- B, spathulenol, and cis- β- farnesene, while (E) - β- ocimene, α- gurjnnene, germacrene-D, hexyl acetate and aromadendrene was the major constituents of Salvia reuterana. When plants were subjected to water deficit, melatonin treatment increased the concentration and composition of the essential oil. In particular, melatonin treatments improved the primary oil components in both species when compared to non-melatonin treated plants. In conclusion, reduced irrigation regimes as well as melatonin treatments resulted in a significant improvement of essential oil production and composition in both Salvia species.
Subject terms: Plant sciences, Plant stress responses
Introduction
Plants growing in arid and semi-arid regions often experience conditions of reduced precipitation and erratic rainfall patterns. Water stress reduces chlorophyll content and consequently, photosynthetic performance1–3. Severe water limitations can cause an imbalance between cellular redox components, where antioxidant defences do not counteract the greater production of reactive oxygen species (ROS). This induces a series of oxidative damages, leading to impaired growth and development4,5 and a reduction in plant fresh and dry weight6. Additionally, high ROS concentrations within particular organelles can oxidize cellular constituents, such as membrane lipids, indicated by an increase in the lipid peroxidation by-product malondialdehyde (MDA), as well as carbohydrates, proteins, and DNA7. Plants exhibit several physiological adaptations to deal with the negative impact of water stress8. In order to cope with oxidative stress, plants activate their antioxidant system and the major enzymes involved are superoxide dismutase (SOD), peroxidise (POD) and catalase (CAT)9,10. Maintenance of higher levels of antioxidants is a pivotal physiological strategy adopted by plants to counteract the negative effects of ROS11.
Salvia species are an important source of secondary metabolites that have relevant benefits to human nutrition and health. Leaves of Salvia plants have been used in traditional medicine for centuries and are also listed in the official pharmacopoeias4. Salvia reuterana and Salvia nemorosa are native Salvia plant species of Iran whose essential oils and chemical components have been studied extensively12,13. Salvia reuterana is mostly cultivated in the central highlands of Iran and possesses neurological, antimicrobial, antioxidant, chemotherapeutic and antidiabetic properties13,14. According to a study conducted by Rajabi et al.14, Salvia reuterana is present in the central regions of Iran with arid and semi-arid climate, while Salvia nemorosa is more likely to be originated from the northern regions of Iran with higher humidity and rainfall. Despite their importance as medicinal plants, Salvia are drought sensitive, and oxidative damages have previously been reported in drought-stressed Salvia plants, which were shown to activate the antioxidant system4.
Melatonin (N-acetyl-5-methoxytryptamine), a derivative of tryptophan, is ubiquitous in living organisms and is reported to have a hormone-like role in several plant species15–17. Melatonin was found to operate as a mammalian hormone and neurotransmitter, however its presence and potential role in plants was only recently documented18–20. In plants, melatonin is involved in multiple physiological processes, including growth and photosynthesis, biological rhythms, rooting and seed germination, and osmoregulation21. Its ability to protection against abiotic and biotic stresses, in particular low temperature22, drought23,24, UV-B25 and heavy metal pollution26 has also been documented. Many investigations have indicated that melatonin could be considered a plant growth regulator, with a mode of action that works in conjunction with other chorismate-derived phytohormones, including indole-3-acetic acid (IAA)27 and salicylic acid28. Melatonin has a pivotal role in the activation of the antioxidant system and induces changes in gene expression of several physiological mechanisms21,29.
Salvia species, important medicinal plants in arid and semi-arid regions, are often at risk of drought stress12–14. Between the two Salvia species reported on here, drought resistance in Salvia reuterana is known to be higher than Salvia nemorosa. In this contest, improvement in drought resistance in both Salvia species has an important practical significance for the agricultural production of medicinal herbs. Currently, there is no report on the potential use of exogenous melatonin in Salvia production. Therefore, the present study was conducted to investigate the impact of foliar melatonin on growth, photosynthetic rate, oxidative stress, and essential oil production under drought stress conditions of two Salvia species. The results would grant new insights into melatonin function in plants and provide a new tool for medicinal plant cultivation to consistently improve yield and quality.
Material and methods
Experimental design and treatments
Salvia species used in this study included Salvia nemorosa L., and Salvia reuterana Boiss. Commercial seedlings (15–20 cm height), purchased from a commercial nursery (Pakan Seed Institute of Isfahan, Iran), were transplanted (two per pot) into plastic pots (11 cm diameter and 8.5 cm height). The experiment was carried under controlled environment conditions in the experimental greenhouse at Isfahan University of Technology (Iran) during 2018. Environmental parameters were 20°–15 °C day-night temperature, 60% relative humidity and 16 h photoperiod with a constant average Photosynthetic Active Radiation (PAR) of 300 µmol m−2 s−1 as previously suggested by Caser et al.30. The study was conducted in a greenhouse with uniform environmental conditions; therefore, the experiment was arranged as a completely randomized design (RCD) with two factors (irrigation and foliar applied melatonin). Each treatment contained nine plant samples of three biological replicates. Each replicate consisted of a pot (three plants/pot). For each measurement, three samples were randomly separated from different parts of the plant. The experimental factors were irrigation treatments at 3 levels; irrigation to 80% field capacity (control), 60% field capacity (mild stress) and 40% field capacity (severe stress) according to the method reported by Sedaghat et al.31. The irrigation treatments were associated with foliar applied melatonin at five levels (0, 50, 100, 150 and 200 μM). Melatonin concentrations were selected based on the experiments reported by Zhang et al.32, in two Salvia species (Salvia nemorosa L., and Salvia reuterana Boiss). The melatonin (Sigma–Aldrich) solutions were prepared according to Li et al.33 by dissolving the solute in ethanol followed by dilution with phosphate buffered saline (PBS) to concentrations of 0, 50, 100, 150 and 200 μM. To improve uniformity of the spray application, for every 100 ml of solution two drops of surfactant (Twin 20) was added to the solution. Melatonin solutions were applied using a manual pump (30 ml per plant), two times per week for 45 days. The control treatment was sprayed with distilled water. The experiment was started after 15 days from the transplanting of the seedlings, after acclimation of the plants to the media and the greenhouse conditions. Salvia plants were grown in sandy loam soil according to Pourmeidani et al.34. To determine the amount of water needed for each irrigation regime, at the beginning of the experiment the soil field capacity (FC) was determined for each pot using the weighing method34. The collected data was used to determine the different amount of water to apply as percentage of the field capacity (FC). Four kg of soil was placed in the oven at 103 °C for 48 hours to determine soil dry weight. Afterwards, the pots were filled with the oven-dried soil and irrigated until water saturation. After 24 hours the pots were weighed every two hours. To avoid evaporation, the pot was covered with a plastic cover. The percentage of water in soil under FC condition was determined by the following equation:
After deducting pot weight and weight of the dry soil, the amount of water stored in the FC condition was determined and irrigation treatments (80, 60 and 40% FC) were calculated accordingly. Therefore, pots were weighed daily based on each treatment and water was added to maintain the specific FC level in each pot. Two weeks after full flowering stage, 120-day old plants were harvested by cutting stems 5 cm above the soil surface and the measurements were made on freshly mature leaves. All the data were subjected to analysis of variance (ANOVA) and means were then separated using the Least Significant Difference (LSD) using SAS (version 8.2; SAS Institute, Cary, NC, USA) and MSTAT-C software35 (Freed and Scott 1989). The heatmap was generated using Heml Heatmap Illustrator Software36.
Chlorophyll fluorescence (Fv/Fm) ratio and Electrolyte leakage (EL)
The maximum photochemical efficiency of photosystem II (Fv/Fm) was measured with a fluorometer (Walz, Effeltrich, Germany) after 30 min of leaf dark adaptation. The Fv/Fm ratio was calculated as: Fv/Fm = (Fm- F0)/Fm, where, Fm and F0 represented the maximum and minimum yields of dark-adapted leaves, respectively. Electrolyte leakage (EL) was measured by using a conductivity meter according to Ozden et al.37.
Malondialdehyde (MDA) and Hydrogen peroxide (H2O2) concentrations
The accumulation of MDA because of lipid peroxidation was assessed by the Thiobarbituric acid (TBA) according to Wang et al.27 and was calculated on a fresh weight basis, using the following formula:
H2O2 was assessed spectrophotometrically after the reaction with Potassium iodide (KI), according to the method reported in Velikova and Loreto (2005)38. The content of H2O2 was calculated using a standard curve with known concentrations of hydrogen peroxide.
Glutathione pool estimation
Total glutathione was measured according to the method described by Sahoo et al.39. The fresh leaf samples were centrifuged at 11500 × g for 15 min at 4 °C and the 0.4 mL of the supernatant was then added to 1 mL of 0.5 M potassium phosphate buffered at pH 7.5. Then, 100 µL 2-nitrobenzoic acid (DTNB, 10 mM), 200 µL bovine serum albumin (BSA, 10 mM), 100 µL nicotinamide adenine dinucleotide (NADH, 0.5 mM) were added to the vial and incubated at 37 °C for 15 min. Finally, the mixture was allowed to cool and the change in absorbance at 412 nm was measured against a blank containing 0.4 mL of water. The results were expressed as nmol per gram fresh weight (nmol gFW−1). For the GSSG assay, the GSH was removed by addition of 2-vinylpyridine to the supernatant for 1 h at 25 °C. The extract (100 µL) was mixed with 600 µL reaction buffer (100 mM potassium phosphate buffer containing 5 mM EDTA, pH 7.5), 100 µL of diluted yeast glutathione reductase (GR, 20 U/mL), and 100 µL of 10 mM DNTB. The reaction was initiated by adding 100 µL of 2.5 mM nicotinamide adenine dinucleotide phosphate (NADPH), and after mixing thoroughly, the rate of absorption change at 412 nm was measured spectrophotometrically. The results were expressed as nmol per gram fresh weight (nmol g FW−1). GSH content was calculated by subtracting GSSG from total glutathione.
Enzyme (CAT, POD, SOD and GR) extraction and assay
Fresh foliar tissue (0.2 g) from Salvia seedlings (uppermost leaves) was harvested, weighed, washed with distilled water and then homogenized with a mortar and pestle with 5 ml chilled sodium phosphate buffer (50 mM, pH 7.8). The homogenates were centrifuged at 15,000 g for 15 min at 4 °C. The supernatant was stored at 4 °C and used for CAT, POD, SOD and GR assays. CAT activity was measured by the method of Blume and McClure (1980). CAT activity was expressed as μmol of hydrogen peroxide oxidized per minute per milligram of protein. POD activity was determined spectrophotometrically, by measuring the oxidation of o-dianisidine (3, 3- dimethoxybenzidine) at 460 nm as described by Ranieri et al.40 and expressed as units (μmol of dianisidine oxidized per minute) per mg of protein. SOD activity was estimated by recording the decrease in absorbance of superoxide-nitroblue tetrazolium complex by the enzyme (Cavalcanti et al.)41. A 3 mL of reaction mixture was prepared with 0.1 mL of 13 mM L-methionine, 0.1 mL of 75 µM p- nitroblue terazolium chloride (NBT), 0.1 mL of 100 µM EDTA, 0.1 mL riboflavin (2 µM) in a 1.5 mL of 50 mM potassium phosphate buffer pH 7.8, 50 µL of the enzymatic extract and distilled water. The reaction was started under illumination of fluorescent lamp (30 W) at 25 °C and stopped 5 min later by turning the lamp off. The blue formazane produced by NBT photo-reduction was measured as an increase in absorbance at 560 nm. The control reaction mixture had no enzyme extract (with maximal colour formation). The blank solution had the same complete reaction mixture, but was kept in the dark. One SOD unit was defined as the amount of enzyme required to inhibit 50% of the NBT photo-reduction in comparison with tubes lacking the plant extract and expressed as a unit of enzyme activity per mg of protein. Glutathione reductase (GR) activity was identified by following the rate of NADPH oxidation at 340 nm according to Balabusta et al.42. The assay mixture included 0.5 mM NADPH, 10 mM GSSG, 6.25 mM MgCl2 in 0.1 M phosphate buffer (pH 7.5), and 100 µL of the enzyme extract in the total volume of 400 µL. GR activity was expressed as µmol of NADPH oxidized during 1 min per 1 mg of proteins (µ mol min−1 mg protein−1). Protein content of the extracts was determined according to the method of Bradford (1976)43.
Oil extraction and chemical composition
Extraction and yield calculation of essential oil in Salvia nemorosa L., and Salvia reuterana Boiss., was achieved according to the method of Fernandes et al.44 using 120 days old plants (from seed germination to full bloom stage). Plant tissue collected from the flowering stems of Salvia nemorosa L., and Salvia reuterana Boiss., were dried in a stove at 30 °C until constant weight was obtained, then crushed into small fragments by a grinding machine and stored in a freezer at −20 °C until the beginning of the distillation process. The essential oils were extracted by hydro-distillation in a Clevenger-type apparatus over 3 h in 1.5 L of water. Water was then removed from the essential oil using anhydrous sodium sulphate and then weighed. The essential oil content was determined based on the volume extracted per 100 g of leaf dry biomass (% w/v). The essential oil yield was also determined by multiplying the essential oil content by the leaf dry biomass (g plant−1).
GC/MS conditions and analysis and identification of volatile components
The composition of essential oils of Salvia nemorosa L. and Salvia reuterana Boiss was detected using a gas chromatograph attached to a mass spectrometer (tandem GC-MS), including a Shimadzu GC-9 gas chromatograph equipped with a DB-5 (dimethylsiloxane, 5% phenyl) fused silica column (30 m × 0.25 mm × 0.25 m), with internal diameter of the column 0.25 mm, film thickness of 0.25 µm. Helium was used as the carrier gas at a flow rate of 2 ml/min with a linear velocity of 32 cm/s. The flame ionization detector (FID) temperature was 265 °C and the injector temperature was 250 °C. The percentage of the different compounds was calculated by the area normalization method, without considering response factors. Identification of the components was based on a comparison of their mass spectra (MS) with a computer library or with the authentic compound (standard spectra) and confirmation of compound identities was also obtained using relative retention indices (RI). Moreover, the percentage of each component of the essential oil was calculated by utilizing the peak height as well as the peak area.
Results and discussion
Analysis of main and interaction effects
Our study evaluated the responses of Salvia nemorosa L. and Salvia reuterana Boiss. to foliar melatonin under reduced irrigation; in conditions of water stress (Fig. 1). The results reported significant main and interactive effects (at 5% and 1% probability levels) of species, water stress, melatonin, species × water stress, species × melatonin, water stress × melatonin and species × water stress × melatonin on electrolyte leakage (EL), essential oil content (EOC), essential oil yield (EOY), oxidized glutathione (GSSG), total glutathione (GT), reduced glutathione (GSH), glutathione reductase (GR), and superoxide dismutase (SOD) (Tables 1 and 2). Fv/Fm, MDA, H2O2, GSH/GSSG, CAT and POD activity showed significant change in response to all main and interactive effects other than the interactive effect of species × water stress × melatonin (Tables 1 and 2). Interactive effect of water stress × melatonin was significant on all the above-mentioned traits at 1% probability levels (Tables 1 and 2). The interactive effect of species × melatonin was regarded non-significant only in terms of MDA accumulation, GR and POD activities (Tables 1 and 2). However, interactive effects of species × melatonin and species × water stress on GSH/GSSG ratio and CAT activity showed no significant differences (Tables 1 and 2). Similar effect of species, melatonin, water stress, and species × water stress significantly triggered different responses in Salvia species were recently reported14,45. The significant main and interactive effects of species, water stress and species × water stress in the Salvia cultivars studied in our experiment were also in line with the results obtained in another Lamiaceae species, Thymus44, further supporting these results.
Figure 1.
Schematic overview of Salvia nemorosa L, (indicated as S1) responses to three irrigation regimes containing: D1, D2 and D3 (80%, 60% and 40% FC, respectively), with melatonin levels including: M1, M2, M3, M4 and M5 (0, 50, 100, 150 and 200 µM, respectively). Horizontal bar = 1 cm.
Table 1.
Mean square values of the analysis of variance of electrolyte leakage (EL%), Fv/Fm ratio, lipid peroxidation indicated by malondialdehyde (MDA) (nmol g−1), H2O2 content (nmole g−1), essential oil content (EOC %) and essential oil yield (EOY g plant−1).
| Source of variants | df | EL | Fv/Fm | MDA | H2O2 | EOC | EOY |
|---|---|---|---|---|---|---|---|
| Species (S) | 1 | 230.40** | 0.03** | 9.29** | 181.62** | 0.35** | 0.08** |
| Drought (D) | 2 | 6910.24** | 0.72** | 260.72** | 10699.09** | 0.52** | 0.13** |
| Melatonin (M) | 4 | 177.52** | 0.02** | 1.92** | 326.68** | 0.24** | 0.06** |
| S × D | 2 | 36.66** | 0.002** | 1.43** | 13.80** | 0.07** | 0.02 |
| S × M | 4 | 3.39** | 0.0006** | 0.02 ns | 2.67** | 0.003* | 0.0008** |
| D × M | 8 | 33.85** | 0.0008** | 0.28** | 37.63** | 0.01** | 0.003** |
| S × D × M | 8 | 3.46** | 0.0002ns | 0.03ns | 1.17ns | 0.003** | 0.0008* |
| Error | 60 | 0.81 | 0.00009 | 0.06 | 0.67 | 0.0008 | 0.0002 |
| CV (%) | 5.20 | 1.62 | 6.31 | 2.30 | 4.21 | 4.19 |
* and **: Significant at the 5% and 1% probability levels, respectively., ns: Non- Significant, according to the LSD multiple range test at P ≤ 0.05. df, degrees of freedom.
Table 2.
Mean square values of the analysis of variance of oxidized glutathione (GSSG) (nmole g−1 FW), total glutathione (GT) (nmole g−1 FW), reduced glutathione (GSH) (nmole g−1 FW), GSH/GSSG, Glutathione reductase (GR) (µmol min−1 mg protein−1), catalase (CAT) activity (U mg−1 protein), peroxidase (POD) activity (U mg−1 protein) and superoxide dismutase (SOD) and activity (U mg−1 protein).
| Source of variants | df | GSSG | GT | GSH | GSH/GSSG | GR | CAT | POD | SOD |
|---|---|---|---|---|---|---|---|---|---|
| Species (S) | 1 | 85.71** | 30731.13** | 36352.05** | 31.75ns | 0.0004** | 0.0004** | 0.004** | 0.007** |
| Drought (D) | 2 | 20875.64** | 521364.43** | 188251.46** | 33110.52** | 140.29** | 0.02** | 0.63** | 0.004** |
| Melatonin (M) | 4 | 281.50** | 40178.05** | 53509.01** | 1092.27** | 3.49** | 0.0008** | 0.01** | 0.001** |
| S × D | 2 | 63.56** | 2517.30** | 3992.04** | 15.44ns | 0.47** | 0.000003 ns | 0.0004* | 0.003** |
| S × M | 4 | 8.28** | 1072.17** | 806.11** | 6.62ns | 0.01 ns | 0.00006 ns | 0.00008 ns | 0.0002** |
| D × M | 8 | 80.54** | 6338.33** | 9166.92** | 345.67** | 0.08** | 0.00008* | 0.003** | 0.0001** |
| S × D × M | 8 | 3.88** | 418.35* | 346.75* | 4.54ns | 0.05* | 0.00005ns | 0.00006ns | 0.0003** |
| Error | 60 | 0.6 | 158.12 | 190.19 | 11.76 | 0.02 | 0.00003 | 0.00008 | 0.000002 |
| CV (%) | 2.98 | 3.80 | 4.95 | 11.62 | 3.42 | 10.87 | 6.23 | 4.74 |
* and **: Significant at the 5% and 1% probability levels, respectively., ns: Non- Significant, according to the LSD multiple range test at P ≤ 0.05. df, degrees of freedom.
Chloroplast membrane integrity and photosynthetic efficiency
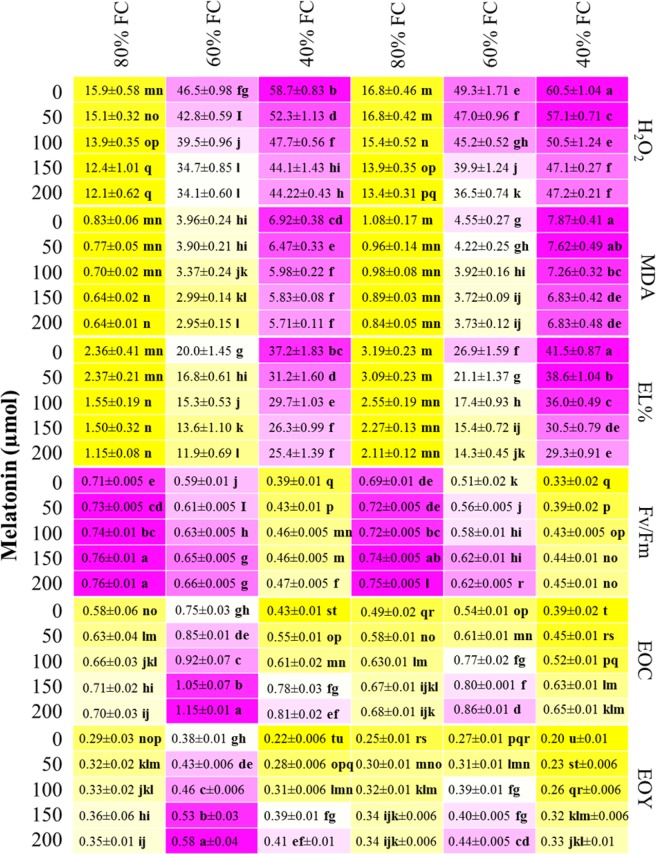
Chloroplasts are a primary location for the generation of ROS in plant cells and they have been reported to be the most sensitive organelles to abiotic stress46–48. The accumulation of ROS causes damage to chloroplast being that the chloroplasts are the major source of activated O2 in plants26. Water stress has been reported to be responsible for generating severe oxidative stress in plant crops, as indicated by an increase in H2O2 and lipid peroxidation levels48,49. This was observed here, where both Salvia species grown under water stress exhibited a significant increase in H2O2 generation (Fig. 2). However, melatonin treatments significantly compensated for oxidative damage caused by the water stress in both Salvia nemorosa and Salvia reuterana (Fig. 2). The protective role of melatonin may be due to its effect in maintaining a steady state of intracellular ROS concentrations, thereby reducing membrane damage from drought stress50. Our results are consistent with those reported in other studies using Cucumis sativus L. seedlings19 and grapevines50 under water and salinity stress. Lipid peroxidation expressed as MDA accumulation is a good representation of the oxidative damage in plants. Therefore, the increased MDA production as a result of water stress (Fig. 2) can be considered as evidence of oxidative plant stress leading to cell production of reactive oxygen species (ROS) such as H2O2 generation51. This was further evidenced by the increase in electrolyte leakage in response to increasing deficit irrigation (Fig. 2). Similar to H2O2, melatonin treatment had a significant effect on mitigating MDA production and EL in both Salvia species (Fig. 2). Meng et al.50 reported that under water stress conditions, grapevines leaves of melatonin-treated cuttings accumulated less MDA and had lower relative electrolytic leakage than cuttings that did not receive any melatonin treatment. As expected, the negative effects of mild and severe water stress (60 and 40% FC, respectively) on the quantum efficiency of Photosystem II (Fv/Fm) were significantly reduced in Salvia reuterana when compared to Salvia nemorosa (Fig. 2). These results are consistent with Huang et al.52 and Ahmad et al.53 who reported that melatonin application alleviated the negative effects of oxidative stress and ROS production. Even at mild water stress (60% FC), a reduced Fv/Fm ratio was recorded in the two species, and a more intense water stress (40% FC) had a further negative effect on this physiological parameter (Fig. 2). However, both Salvia species sprayed with melatonin reported a positive response, with an improved ratio of Fv/Fm under water stress (Fig. 2). In accordance with the results reported by Wang et al.23, melatonin has the potential to maintain high photosynthetic efficiency in plants and our results proved that melatonin application relieved water stress improving the efficiency of Fv/Fm (Fig. 2). In support of our findings, melatonin has been already reported that improves electron transport in chloroplast under different environmental stresses51,54 and consequently photosynthetic efficiency (Fv/Fm). Melatonin has the potential to protect plants from the adverse effects of drought stress by enhancing the ROS scavenging efficiency. It helps in protection of photosynthetic apparatus and reduction of drought induced oxidative stress53. These results demonstrated that foliar application of melatonin improved photosynthetic capacity and reduced the oxidative damage in Salvia species under water stress conditions, and supported the hypothesis that exogenous application of melatonin could effectively enhance stress tolerance in plants54,55.
Figure 2.
Heatmap representation of the interactive effect of drought stress and melatonin application on H2O2 content (nmol g−1), lipid peroxidation indicated by malondialdehyde (MDA) (nmol g−1), electrolyte leakage (EL%), Fv/Fm ratio, essential oil content (EOC %), and essential oil yield (EOY) (g plant−1) of Salvia nemorosa L., and Salvia reuterana Boiss. Three-way ANOVA test: Means followed by the same letter are not significantly different by the LSD Multiple Range test at P ≤ 0.05. Purple and yellow represent increased and decreased values, respectively.
Glutathione pool and enzymatic antioxidant activities
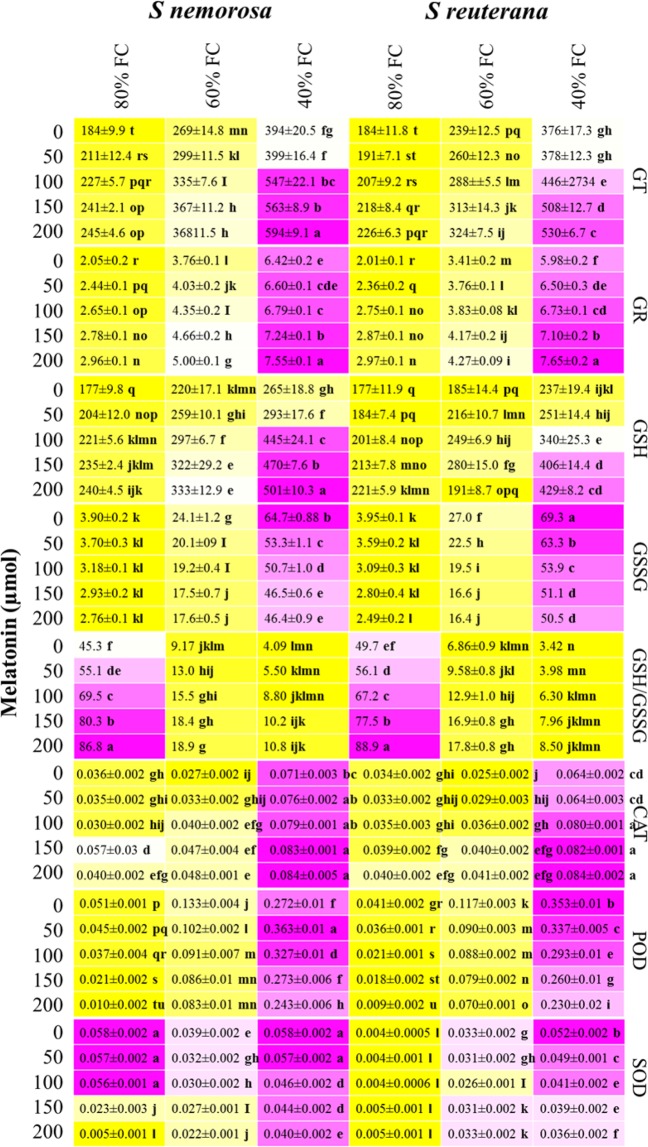
When exposed to oxidative stress, plants can activate their oxygen scavenging systems by increasing the activity of antioxidant enzymes or by mobilization of their non-enzymatic antioxidants, such as reduced glutathione (GSH)42,55. In this study, both non-enzymatic and enzymatic antioxidants were evaluated. Glutathione Reductase (GR) exists primarily in the chloroplast and reduces glutathione disulfide (GSSG) to glutathione (GSH). GSH participates in enzymatic and non-enzymatic H2O2 degradation to maintain a reductive state within the cell39 and ameliorates ROS-induced damage. This was recently shown by Hasanuzzaman et al.48,49 in Brassica napus. Here, increasing drought stress produced higher cell H2O2 concentrations, but was mitigated by increasing melatonin concentration (Fig. 1). In response, GT, GR, and GSH were similarly modulated, reaffirming this the role of this system in ameliorating drought stress. A similar effect of melatonin on total glutathione content was observed also by Wang et al.23 on apple leaves when treated with a foliar melatonin application. Additionally, oxidized glutathione (GSSG) reported a significant increase when both Salvia species were subjected to water stress, but decreased with increasing melatonin (Fig. 3). The GSH/GSSG ratio is considered as an indirect indicator of oxidative stress and damage. When plants are exposed to oxidative stress GSSG is accumulated and the ratio of GSH to GSSG decreases42,55. In our study, mild and severe water stress decreased the GSH/GSSG ratio in both Salvia species, indicative of stress (Fig. 3). However, the increasing ratio of GSH/GSSG in response to exogenously applied melatonin showed the beneficial effect of melatonin in reducing oxidative stress and improving water stress tolerance, in line with the research reported by Galano et al.29 that also showed that foliar application of melatonin in wheat plants exposed to stress conditions elevated total GSH content, as well as significantly enhanced the ratio of GSH/GSSG compared to control plants and stressed plants without melatonin. Similar to glutathione, enzymatic antioxidant activity in plants subjected to increasing water stress regimes was enhanced, with the exception of SOD in S nemorosa. SOD, POD and CAT levels indicate that the main protective enzymes in the enzymatic defence system, can effectively scavenge reactive oxygen species19,20. Jafari et al.14 suggested the higher activity of SOD in medicinal plants was due to their increased resistant to drought stress, suggesting that Salvia nemorosa with higher SOD activity (0.058) could be more water stress tolerant than Salvia reuterana (0.052), as reported in Fig. 3. Additionally, the activity of catalase (CAT) activity increased with higher levels of melatonin application, whereas POD and SOD saw an opposite response in most cases (Fig. 3). Catalase is a member of the haem peroxidase family of enzymes that are distinctly responsible for the dismutation of H2O2 into H2O and O26. Given this, and that POD and SOD have other roles within the redox system, it is understandable how only CAT activity increased similar to that of H2O2. Interestingly, Munne-Bosch et al.4 found that in response to the oxidative burst (H2O2) marking the start of the ripening process in guava, MDA concentration, POD, and SOD activity increased, whereas, GT, GR, GSH, and CAT decreased. These results suggest that CAT operates similarly to GR in response to oxidative stimuli, but are not related with POD and SOD activity, a result seen here in response to the redox-modulator melatonin.
Figure 3.
Heatmap representation of the interactive effects of drought stress and melatonin application on total glutathione (GT), glutathione reductase (GR), reduced glutathione (GSH), oxidized glutathione (GSSG), GSH/GSSG, catalase (CAT) activity (µ mole min−1 mg protein), peroxidase (POD) activity (µ mole min−1 mg protein), and superoxide dismutase (SOD) activity (µ mole min−1 mg protein) of Salvia nemorosa L., and Salvia reuterana Boiss. Three-way ANOVA test: Means followed by the same letter are not significantly different by the LSD Multiple Range test at P ≤ 0.05. Purple and yellow represent increased and decreased values, respectively.
Oil yield and chemical composition
Mild water stress had a similar impact on both EOC and EOY, with 60% FC having the highest values, and 80% FC, the lowest. Previous research has reported a decrease in essential oil content in different Lamiaceae species under water stress45, while others have shown an increase in essential oil content in Lamiaceae species under drought conditions56. As seen here, cultivar-specific responses to water stress may factor into the differences observed by these studies. Likewise, increasing levels of foliar melatonin application had a positive impact on EOC and EOY in both Salvia species (Fig. 2). Taken together, the highest essential oil content (1.15%) and essential oil yield (0.58%) were recorded with 200 µM melatonin applied on Salvia nemorosa grown under mild water stress (60% FC) conditions (Fig. 2). Despite the clear effect of both water stress and melatonin, the impact of water was greatest. This is displayed by the 60% FC treatment with 0 µM melatonin having a greater EOC and EOY than the other irrigation treatments at 200 µM melatonin for Salvia nemorosa. The composition of essential oil produced under different water stress regimes and melatonin treatments are reported in Tables 3 and 4. Eighteen compounds, accounting for 81.87%-98.87% of the total essential oil content, were identified in Salvia nemorosa (Table 3). The main essential oil components of Salvia nemorosa were β- caryophyllene (37.53–40.13%), germacrene- B (19.83–21.37%), spathulenol (6.37–8.72%), and cis- β- farnesene (5.75–7.83%) (Table 3). Evaluation of essential oil yields of Salvia reuterana also resulted in fourteen components representing 81.34–98.46% of the total essential oil content. Among the characterized compounds, (E) - β- ocimene (35.76–38.82%), α- gurjnnene (16.23–17.66%), germacrene-D (10.7–13.15%), hexyl acetate (6.55–9.21%), and aromadendrene (3.33–5.65%) were the major constituents (Table 4). Those results demonstrated that the highest percentage of major essential oil constituents were observed when both Salvia nemorosa and Salvia reuterana were exposed to mild water stress (60% FC) compared to those that were under severe water stress (40% FC). The various levels of melatonin affected the constituents of essential oils of both Salvia species in different ways. Treatment of Salvia nemorosa with 150 µM of melatonin resulted in the accumulation of β- caryophyllene, Germacrene- B, spathulenol and cis- β- farnesene to a significant extend and more than the other treatments (Table 3), while for Salvia reuterana, 150 µM of melatonin enhanced the accumulation of hexyl acetate and aromadendrene and 200 µM of melatonin caused an increase in (E) - β- Ocimene, α- gurjnnene and germacrene-D more than other treatments (Table 4). Similarly, Sarrou et al.57 also observed that melatonin treated lemon balm (Melissa officinalis) reported an increase in several compounds, in particular nerol, a result similar to our study. Although the role of melatonin in the biosynthesis of essential oil in medicinal plants has not been well understood, one of the mechanisms proposed is related to the similarity in plant function and chemical structure between indole-3-acetic acid (IAA) and melatonin20,24,28, which are both derived from chorismate28. It has been reported that IAA promotes essential oil synthesis in several medicinal plants58,59, and given that melatonin has an auxin-like (IAA) activity, it would be expected that melatonin also promotes the synthesis of essential oils. It has been hypothesized that the increased amount of essential oil of Salvia species in response to foliar melatonin application can be attributed to the potential enhancement of meristematic cells, site of production of several chemical compounds that are fundamental for oil biosynthesis58,59.
Table 3.
Essential oil compound of Salvia nemorosa L., under different irrigation regimes and melatonin treatments.
| Compound | S1D1M1 | S1D1M2 | S1D1M3 | S1D1M4 | S1D1M5 | S1D2M1 | S1D2M2 | S1D2M3 | S1D2M4 | S1D2M5 | S1D3M1 | S1D3M2 | S1D3M3 | S1D3M4 | S1D3M5 | RIa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | TR | TR | TR | TR | TR | 0.04 | 0.04 | 0.06 | 0.08 | TR | TR | TR | TR | TR | TR | 987 |
| Sabinene | 0.21 | 0.19 | 0.27 | 0.29 | 0.28 | 0.56 | 0.59 | 0.64 | 0.74 | 0.81 | TR | 0.04 | 0.06 | 0.06 | 0.06 | 1006 |
| β-Caryophyllene | 37.53 | 37.88 | 37.69 | 38.45 | 38.61 | 39.12 | 39.79 | 39.96 | 40.13 | 39.87 | 36.68 | 37.54 | 38.81 | 38.96 | 39.92 | 1011 |
| α-Terpinene | TR | TR | TR | TR | TR | 1.4 | 1.5 | 1.59 | 0.12 | 0.07 | TR | TR | TR | TR | TR | 1017 |
| Camphor | 0.03 | 0.03 | 0.05 | 0.04 | 0.08 | 0.08 | 0.07 | 0.05 | 0.08 | 0.09 | TR | TR | TR | TR | TR | 1025 |
| Hexyl butyrate | 0.21 | 0.40 | 0.43 | 0.76 | 0.32 | 0.64 | 0.82 | 0.81 | 0.86 | 0.75 | TR | TR | 0.06 | 0.07 | 0.06 | 1033 |
| Borneol | 1.4 | 1.6 | 1.2 | 1.7 | 1.3 | 1.9 | 2.00 | 2.13 | 2.39 | 1.86 | 1.80 | 2.1 | 3.3 | 3.4 | 2.4 | 1039 |
| Pulegone | 1.37 | 1.73 | 1.94 | 1.91 | 1.82 | 1.46 | 1.48 | 1.55 | 1.83 | 2.08 | 1.59 | 1.78 | 2.32 | 2.64 | 1.58 | 1046 |
| β-Thujone | 0.7 | 1.13 | 1.54 | 1.53 | 1.66 | 1.64 | 1.83 | 1.46 | 1.96 | 1.95 | 1.35 | 1.11 | 2.37 | 2.35 | 2.39 | 1050 |
| Nerolidol | 0.78 | 0.85 | 0.84 | 0.85 | 0.89 | 1.21 | 1.42 | 1.47 | 1.51 | 1.88 | 1.22 | 1.31 | 2.27 | 2.33 | 2.32 | 1057 |
| Valencene | 3.23 | 3.65 | 3.54 | 3.63 | 3.68 | 2.91 | 2.98 | 3.17 | 3.27 | 3.58 | 3.09 | 3.57 | 4.84 | 4.95 | 5.93 | 1066 |
| Spathulenol | 6.37 | 7.18 | 7.45 | 7.58 | 7.74 | 8.12 | 8.35 | 8.65 | 8.72 | 8.47 | 5.03 | 6.11 | 7.27 | 7.56 | 7.77 | 1084 |
| cis- β -Farnesene | 5.75 | 5.68 | 5.82 | 5.85 | 5.92 | 6.21 | 6.48 | 6.77 | 6.85 | 6.41 | 5.29 | 6.55 | 7.69 | 7.83 | 7.80 | 1091 |
| Germacrene-B | 19.83 | 19.90 | 20.19 | 20.60 | 20.57 | 20.84 | 20.85 | 21.11 | 21.37 | 20.66 | 17.97 | 18.65 | 19.88 | 20.04 | 19.75 | 1102 |
| Germacrene-D | 1.66 | 1.84 | 2.31 | 2.54 | 2.72 | 2.28 | 2.74 | 2.81 | 2.81 | 2.39 | 1.85 | 1.08 | 2.14 | 2.13 | 2.16 | 1125 |
| (E)-β-Ocimene | 0.14 | 0.53 | 0.45 | 0.62 | 1.31 | 1.26 | 1.23 | 1.56 | 1.71 | 1.67 | 1.11 | 0.89 | TR | TR | TR | 1148 |
| Limonene | 0.21 | 0.17 | 0.75 | 0.94 | 0.85 | 1.16 | 1.19 | 1.26 | 1.66 | 1.85 | 1.49 | 1.23 | 1.00 | 1.00 | 0.56 | 1159 |
| Valencene | 2.45 | 2.69 | 3.15 | 2.94 | 2.87 | 2.91 | 2.15 | 2.55 | 2.78 | 2.91 | 1.42 | 2.71 | 2.44 | 1.84 | 1.93 | 1165 |
| TCC | 81.87 | 85.45 | 87.62 | 90.23 | 90.62 | 93.74 | 95.51 | 97.60 | 98.87 | 97.30 | 79.89 | 84.67 | 94.45 | 95.16 | 94.63 |
S1: Salvia nemorosa L. D1: irrigation at 80% Fc, D2: irrigation at 60% Fc, D3: irrigation at 40% Fc, M1 (0 µM melatonin), M2 (50 µM melatonin). M3 (100 µM melatonin), M4 (150 µM melatonin) and M5 (200 µM melatonin).
aThe data were sorted based on the Retention Index (RI) of components.
TR = trace, less than 0.01%., TCC = Total chemical composition
Table 4.
Essential oil compound of Salvia reuterana Boiss., under different irrigation regimes and melatonin treatments.
| Compound | S2D1M1 | S2D1M2 | S2D1M3 | S2D1M4 | S2D1M5 | S2D2M1 | S2D2M2 | S2D2M3 | S2D2M4 | S2D2M5 | S2D3M1 | S2D3M2 | S2D3M3 | S2D3M4 | S2D3M5 | RIa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Copaene | 0.60 | 1.1 | 0.50 | 0.70 | 0.90 | 1.30 | 1.40 | 1.54 | 1.87 | 1.74 | TR | 0.01 | 0.07 | 0.04 | TR | 961 |
| Hexyl pentanoate | 1.9 | 2.2 | 1.9 | 1.8 | 2.5 | 2.5 | 1.9 | 1.7 | TR | 0.1 | 0.1 | 0.3 | 0.42 | 0.45 | 0.76 | 984 |
| Hexyl acetate | 6.55 | 6.61 | 6.78 | 6.89 | 6.95 | 8.19 | 8.70 | 8.94 | 9.21 | 8.88 | 6.04 | 7.14 | 7.65 | 7.88 | 7.73 | 1006 |
| Isopenthyl isovalerate | 1.83 | 2.32 | 2.12 | 1.74 | 1.71 | 2.45 | 2.8 | 2.11 | 2.73 | 2.05 | 1.21 | 1.64 | 1.61 | 1.57 | 1.57 | 1011 |
| Germacrene-B | 0.33 | 0.43 | 0.55 | 0.64 | 0.68 | 0.58 | 0.74 | 0.59 | 0.68 | 0.89 | TR | TR | TR | 0.07 | TR | 1015 |
| (E)-β-Ocimene | 35.76 | 35.77 | 35.74 | 35.93 | 35.70 | 38.61 | 38.79 | 38.41 | 38.68 | 38.82 | 37.12 | 37.98 | 37.77 | 37.61 | 37.85 | 1023 |
| Longifolene | 0.45 | 0.46 | 0.42 | 0.53 | 0.53 | 0.79 | 1.60 | 1.74 | 1.79 | 1.82 | TR | TR | 0.05 | 0.12 | TR | 1029 |
| β-Eudesmol | TR | TR | 0.09 | 0.32 | 0.26 | 1.42 | 1.43 | 1.56 | 1.78 | 1.88 | TR | TR | TR | 0.07 | TR | 1033 |
| Germacrene-D | 10.7 | 10.10 | 10.53 | 10.58 | 10.16 | 12.65 | 12.79 | 12.12 | 12.46 | 13.15 | 10.15 | 10.15 | 10.37 | 10.58 | 10.77 | 1046 |
| α -Farnesene | 2.43 | 2.57 | 2.76 | 2.89 | 2.95 | 3.20 | 3. 20 | 3.18 | 3.36 | 3.28 | TR | 1.33 | 1.42 | 1.40 | 1.39 | 1051 |
| Hexyl butyrate | 1.23 | 1.28 | 1.45 | 1.67 | 1.82 | 2.42 | 2.64 | 2.86 | 2.97 | 2.88 | 2.09 | 1.07 | 1.69 | 1.59 | 1.64 | 1073 |
| α -Gurjunene | 16.23 | 16.42 | 16.73 | 16.75 | 16.82 | 18.60 | 18.84 | 17.19 | 17.25 | 17.66 | 16.21 | 15.81 | 15.88 | 15.93 | 15.87 | 1080 |
| Aromadendrene | 3.33 | 3.68 | 3.58 | 3.85 | 3.93 | 5.14 | 5.17 | 5.15 | 5.65 | 5.31 | 4.03 | 4.10 | 4.21 | 4.35 | 4.12 | 1087 |
| β -Caryophyllene | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | TR | 1120 |
| TCC | 81.34 | 82.94 | 83.15 | 84.29 | 84.91 | 97.85 | 96.8 | 97.09 | 98.43 | 98.46 | 76.95 | 79.53 | 81.14 | 81.66 | 81.70 |
S2:Salvia reuterana Boiss. D1: irrigation at 80% Fc, D2: irrigation at 60% Fc, D3: irrigation at 40% Fc, M1 (0 µM melatonin), M2 (50 µM melatonin). M3 (100 µM melatonin), M4 (150 µM melatonin) and M5 (200 µM melatonin).
aThe data were sorted based on the Retention Index (RI) of components.
TR = trace, less than 0.01%., TCC = Total chemical composition
Hierarchical cluster analysis
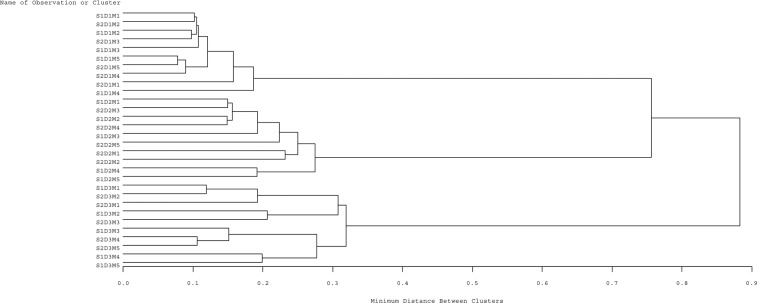
Cluster analysis of the combined treatments (foliar applied melatonin and irrigation regimes) is reported in Fig. 4, based on similarity. The cluster analysis was conducted in relation to enzymatic and non-enzymatic antioxidant properties and essential oil yield of the two Salvia species, which divided the 30 treatment combinations into 3 major clusters. Cluster I includes different foliar melatonin treatments for both Salvia nemorosa and Salvia reuterana under the normal irrigation regime (D1). Cluster II contains Salvia nemorosa and Salvia reuterana and foliar application of melatonin under mild drought stress (D2). Cluster III shows the foliar treatments of melatonin for Salvia nemorosa and Salvia reuterana grown under severe water stress (D3). These results demonstrated that the effect of water irrigation regimes was more prominent in modifying the physiological responses of the two Salvia species, followed as second level of importance by the impact of the foliar applied melatonin and lastly as third level of importance the genetic diversity of the two species of Salvia utilized in the research (Fig. 4). The cluster analysis suggests that irrigation is the best tool for controlling essential oil production in Salvia, but that the effect of melatonin was also great enough to induce similar responses to two Salvia cultivars differing in their response to water stress.
Figure 4.
Hierarchical cluster analysis of exogenous melatonin in two Salvia species under reduced irrigation regimes, based on enzymatic and non-enzymatic antioxidant properties and essential oil yield. S1: Salvia nemorosa L., and S2: Salvia reuterana Boiss. D1: irrigation at 80% Fc, D2: irrigation at 60% FC, D3: irrigation at 40% Fc, M1 (0 µM melatonin), M2 (50 µM melatonin). M3 (100 µM melatonin), M4 (150 µM melatonin) and M5 (200 µM melatonin).
Conclusion
The treatment of two Salvia cultivars with melatonin improved their antioxidant defence system against drought stress. This consequently enabled plants to increase their production of essential oil, especially under mild drought stress conditions. A particular effect of melatonin was observed on CAT activity compared with other antioxidant enzymes, which warrants further research in order to understand their role in increasing essential oil production in Salvia plants. Irrigation had the greatest impact on essential oil production in two Salvia cultivars, however, melatonin also had a significant added effect.
Acknowledgements
We would like to thank the College of Agriculture, Isfahan University of Technology for the financial support to conduct this study and the help of several colleagues during the experimental work.
Author contributions
S.S.B. designed the experiment and was responsible for data acquisition and analysis. S.B.B., J.W. and P.S. participated to the interpretation of the work and all authors contributed to the critical revision of the manuscript and approved the final version submitted for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Osmond B, Badger M, Maxwell K, Björkman O, Leegood R. Too many photons: photorespiration, photoinhibition and photooxidation. Trends Plant Sci. 1997;2:119–121. doi: 10.1016/S1360-1385(97)80981-8. [DOI] [Google Scholar]
- 2.Din J, Khan SU, Ali I, Gurmani AR. Physiological and agronomic response of canola varieties to drought stress. J. Anim. Plant Sci. 2011;21:78–82. [Google Scholar]
- 3.Sadak MS, Abdalla AM, Elhamid EMA, Ezzo MI. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull Natl Res Cent. 2020;44:18. doi: 10.1186/s42269-020-0275-7. [DOI] [Google Scholar]
- 4.Munne-Bosch S, Mueller M, Schwarz K, Alegre L. Diterpenes and antioxidative protection in drought-stressed Salvia officinalis plants. J. Plant Physiol. 2001;158:1431–1437. doi: 10.1078/0176-1617-00578. [DOI] [Google Scholar]
- 5.Lobell DB, Schlenker W, Costa-Roberts J. Climate trends and global crop production since 1980. Science. 2011;333:616–620. doi: 10.1126/science.1204531. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, et al. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015;66(3):647–656. doi: 10.1093/jxb/eru336. [DOI] [PubMed] [Google Scholar]
- 7.Gill S, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Duan B, et al. Interactions between drought stress, ABA and genotypes in Picea asperata. J. Exp. Bot. 2007;58:3025–3036. doi: 10.1093/jxb/erm160. [DOI] [PubMed] [Google Scholar]
- 9.Farooq M, Aziz T, Basra SMA, Cheema MA, Rehman H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008;194:161–168. doi: 10.1111/j.1439-037X.2008.00300.x. [DOI] [Google Scholar]
- 10.Farooq M, Irfan M, Aziz T, Ahmad I, Cheema SA. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013;199:12–22. doi: 10.1111/j.1439-037X.2012.00521.x. [DOI] [Google Scholar]
- 11.Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzyme in growing rice seedling. Plant Growth Regul. 2005;46:209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- 12.Mirza M, Sefidkon F. Essential oil composition of two Salvia species from Iran, Salvia nemorosa L. and Salvia reuterana Boiss. Flavour Frag. J. 1999;14:230–232. doi: 10.1002/(SICI)1099-1026(199907/08)14:4<230::AID-FFJ816>3.0.CO;2-L. [DOI] [Google Scholar]
- 13.Rajabi Z, Ebrahimi M, Farajpour M, Mirza M, Ramshini H. Compositions and yield variation of essential oils among and within nine Salvia species from various areas of Iran. Ind. Crop Prod. 2014;61:233–239. doi: 10.1016/j.indcrop.2014.06.038. [DOI] [Google Scholar]
- 14.Jafari E, Andalib S, Abed A, Rafieian-Kopaei M, Vaseghi G. Neuroprotective, antimicrobial, antioxidant, chemotherapeutic, and antidiabetic properties of Salvia Reuterana: A mini review. Avicenna J. Phytomed. 2014;5(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Hardeland H. Melatonin in plants–diversity of levels and multiplicity of functions. Front. Plant Sci. 2016;7:198. doi: 10.3389/fpls.2016.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardeland R, et al. Melatonin - a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Nawaz MA, et al. Melatonin: current status and future perspectives in plant science. Front. Plant Sci. 2016;6:1230. doi: 10.3389/fpls.2015.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnao MB, Hernández-Ruiz J. Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014;19:789–797. doi: 10.1016/j.tplants.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang LY, Liu JL, Wang WX, Sun Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumbers under salinity-induced stress. Photosynthetica. 2016;54(1):19–27. doi: 10.1007/s11099-015-0140-3. [DOI] [Google Scholar]
- 20.Wang Q, et al. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016;7:1882. doi: 10.3389/fpls.2016.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan DX, et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013;54(2):127–138. doi: 10.1111/jpi.12026. [DOI] [PubMed] [Google Scholar]
- 22.Lei XY, Zhu RY, Zhang GY, Dai YR. Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J. Pineal Res. 2004;36(2):126–131. doi: 10.1046/j.1600-079X.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, et al. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013;54(3):292–302. doi: 10.1111/jpi.12017. [DOI] [PubMed] [Google Scholar]
- 24.Zhang N, et al. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.) J. Pineal Res. 2013;54(1):15–23. doi: 10.1111/j.1600-079X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 25.Afreen F, Zobayed SM, Kozai T. Melatonin in Glycyrrhiza uralensis: response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006;41:108–115. doi: 10.1111/j.1600-079X.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 26.Xu SC, et al. Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J Pineal Res. 2010;49(1):86–94. doi: 10.1111/j.1600-079X.2010.00770.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Zeng B, Sun Z, Zhu C. Relationship between proline and Hg+2 – induced oxidative stress in tolerant rice mutant. Archives of Environmental Contamination and Toxicology. 2009;56:723–731. doi: 10.1007/s00244-008-9226-2. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Llorca M, Muñoz P, Müller M, Munné-Bosch S. Biosynthesis, Metabolism and Function of Auxin, Salicylic Acid and Melatonin in Climacteric and Non-climacteric Fruits. Front. Plant Sci. 2019;10:136. doi: 10.3389/fpls.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J. Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 30.Caser M, et al. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern., to drought stress. Plant Growth Regul. 2018;48(2):383–394. doi: 10.1007/s10725-017-0349-1. [DOI] [Google Scholar]
- 31.Sedaghat M, Tahmasebi Sarvestani Z, Emam Y, Mokhtassi Bidgoli A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017;119,:59–69. doi: 10.1016/j.plaphy.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YP, Yang SJ, Chen YY. Effects of melatonin on photosynthetic performance and antioxidants in melon during cold and recovery. Biologia Plantarum. 2017;61:571–578. doi: 10.1007/s10535-017-0717-8. [DOI] [Google Scholar]
- 33.Li H, et al. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017;8:295. doi: 10.3389/fpls.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pourmeidani A, Jafari AA, Mirza M. Studying drought tolerance in Thymus kotschyanus accessions for cultivation in dry land farming and low efficient grassland. J. Rangel. Sci. 2017;7(4):331–340. [Google Scholar]
- 35.Freed, R.D. & Scott, E. MSTAT–C; A Software Package for the Design, Management, and Analysis of Agronomic Experiments. Michigan State University, USA (1989).
- 36.Deng W, Wang Y, Liu Z, Cheng H, Xue Y. HemI: A Toolkit for Illustrating Heatmaps. PLoS One. 2014;9(11):e111988. doi: 10.1371/journal.pone.0111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozden M, Demirel U, Kahraman A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009;119:163–168. doi: 10.1016/j.scienta.2008.07.031. [DOI] [Google Scholar]
- 38.Velikova V, Loreto F. On the relationship between isoprene emission and thermo tolerance in Phragmites ausrralis leaves exposed to high temperatures and during the recovery from a heat stress. Plant, Cell Environ. 2005;28:318–327. doi: 10.1111/j.1365-3040.2004.01314.x. [DOI] [Google Scholar]
- 39.Sahoo S, Awasthi JP, Sunkar R, Panda SK. Determining glutathione levels in plants. Methods Mol. Biol. 2017;1631:273–277. doi: 10.1007/978-1-4939-7136-7_16. [DOI] [PubMed] [Google Scholar]
- 40.Ranieri A, Petacco F, Castagna A, Soldatini GF. Redox state and peroxidase system in sunflower plants exposed to ozone. Plant Sci. 2000;159:159–168. doi: 10.1016/S0168-9452(00)00352-6. [DOI] [PubMed] [Google Scholar]
- 41.Cavalcanti FR, Oliveira JT, Martins-miranda AAS, Viegas RA, Silveira JAG. Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol. 2004;163:563–571. doi: 10.1111/j.1469-8137.2004.01139.x. [DOI] [PubMed] [Google Scholar]
- 42.Marta B, Szafranska K, Posmyk MM. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus l.) germinated under chilling stress. Front Plant Sci. 2016;7:575. doi: 10.3389/fpls.2016.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradford MM. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes VF, et al. Light intensity on growth, leaf micromorphology and essential oil production of Ocimum gratissimum. Braz. J. Pharmacognosy. 2013;23(3):419–424. doi: 10.1590/S0102-695X2013005000041. [DOI] [Google Scholar]
- 45.Kabiri R, et al. Foliar application of melatonin induces tolerance to drought stress in Moldavian balm plants (Dracocephalum moldavica) through regulating the antioxidant system. Folia Hort. 2018;30(1):155–167. doi: 10.2478/fhort-2018-0016. [DOI] [Google Scholar]
- 46.Krause GH, Santarius KA. Relative thermo stability of the chloroplast envelope. Planta. 1975;127:285–299. doi: 10.1007/BF00380726. [DOI] [PubMed] [Google Scholar]
- 47.Sun W, Montagu MV, Verbruggen N. Small heat shock proteins and stress tolerance in plants. Biochim Biophys Acta. 2002;1577:1–9. doi: 10.1016/S0167-4781(02)00417-7. [DOI] [PubMed] [Google Scholar]
- 48.Hasanuzzaman M, Fujita M. Selenium pretreatment up-regulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011;143:1758–1776. doi: 10.1007/s12011-011-8998-9. [DOI] [PubMed] [Google Scholar]
- 49.Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants. 2017;23(2):249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng JF, et al. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014;57:200–212. doi: 10.1111/jpi.12159. [DOI] [PubMed] [Google Scholar]
- 51.Yang XL, et al. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica. 2018;56(3):884–892. doi: 10.1007/s11099-017-0748-6. [DOI] [Google Scholar]
- 52.Huang B, et al. Exogenous Melatonin Alleviates Oxidative Damages and Protects Photosystem II in Maize Seedlings Under Drought Stress. Front. Plant Sci. 2019;10:677. doi: 10.3389/fpls.2019.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahmad S, et al. Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. Peer J. 2019;7:e7793. doi: 10.7717/peerj.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma A, Zheng B. Melatonin mediated regulation of drought stress: physiological and molecular aspects. Plants. 2019;8:190. doi: 10.3390/plants8070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez C, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079X.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 56.Askary M, Behdani MA, Parsa S, Mohmoodi S, Jamialahmadi M. Water stress and manure application affect the quantity and quality of essential oil of Thymus daenensis and Thymus vulgaris. Ind. Crop Prod. 2018;111:336–344. doi: 10.1016/j.indcrop.2017.09.056. [DOI] [Google Scholar]
- 57.Sarrou E, Chatzopoulou P, Dimassi-Theriou K, Therios L, Koularmani A. Effect of melatonin, salicylic acid and gibberellic acid on leaf essential oil and other secondary metabolites of bitter orange young seedlings. J. Essent. Oil Res. 2015;27(6):487–496. doi: 10.1080/10412905.2015.1064485. [DOI] [Google Scholar]
- 58.da Silva S, et al. Essential oil composition of Melissa officinalis L. in vitro produced under the influence of growth regulators. J. Braz. Chem. Soc. 2005;16(6):1387–1390. doi: 10.1590/S0103-50532005000800014. [DOI] [Google Scholar]
- 59.Hazzoumi Z, Moustakime Y, Amrani Joutei K. Effect of gibberellic acid (GA), indole acetic acid (IAA) and benzylaminopurine (BAP) on the synthesis of essential oils and the isomerization of methyl chavicol and trans-anethole in Ocimum gratissimum L. SpringerPlus. 2014;3:321. doi: 10.1186/2193-1801-3-321. [DOI] [PMC free article] [PubMed] [Google Scholar]