Abstract
BACKGROUND:
Mutations in the gamma-glutamyl carboxylase (GGCX), which is required for vitamin K-dependent (VKD) protein activation, can result in vitamin K clotting factor deficiency (VKCFD1). A recent report described a VKCFD1 patient with a homozygous carboxylase mutation that altered splicing and deleted exon 2 (Δ2GGCX). Only Δ2GGCX RNA was observed in the patient.
OBJECTIVES:
Loss of exon 2 deletes carboxylase sequences thought to be important for membrane topology and consequent function. Carboxylase activity is required for life, and we therefore tested whether the Δ2GGCX mutant is active.
METHODS:
HEK 293 cells were edited using CRISPR-Cas9 to eliminate endogenous carboxylase. r-Wild type- and r-Δ2GGCX carboxylases were then expressed and tested for carboxylation of the VKD protein factor IX. A second approach monitored carboxylation biochemically, using r-carboxylases expressed in insect cells that lack endogenous carboxylase.
RESULTS AND CONCLUSIONS:
Δ2GGCX activity was undetectable in both assays, which is strikingly different from low levels of carboxylase activity observed with other VKCFD1 mutants. Similar clotting function between patients with the Δ2GGCX and these mutations must therefore arise from a novel mechanism. Low levels of properly spliced carboxylase RNA that produce full-length protein would not have been observed in the previous study. The results suggest that the splicing defect is incomplete. Δ2GGCX RNA has been detected in normal human liver and has been designated carboxylase isoform 2; however, Δ2GGCX protein was not observed in normal human liver. Lack of activity and protein expression suggest that isoform 2 is not physiologically relevant to normal VKD protein carboxylation.
Keywords: Blood Coagulation Disorders, Inherited, CRISPR-Cas, Gamma-Glutamyl Carboxylase, Vitamin K, VKCFD1
INTRODUCTION
Vitamin K-dependent (VKD) proteins comprise a family whose activities require the conversion of specific clusters of Glu residues to carboxylated Glu (Gla)[1]. This modification is performed by the gamma-glutamyl carboxylase (GGCX), which uses the oxygenation of vitamin K hydroquinone (KH2) to drive Glu carboxylation. KH2 oxygenation results in an inactive vitamin K epoxide (KO) product that must be recycled for continuous VKD protein carboxylation, and the vitamin K oxidoreductase (VKORC1) is responsible for KO to KH2 reduction [2]. This interconversion between the vitamin K forms is referred to as the vitamin K cycle. To date, 16 VKD proteins have been identified and implicated in a broad range of functions that include hemostasis, calcium regulation, growth control, signal transduction and apoptosis [1, 3]. Glu carboxylation is ubiquitous in mammals, with all tissues expressing a subset of VKD proteins, and a single carboxylase modifies all of these proteins. Liver is the major site of synthesis of VKD clotting factors, and elimination of the carboxylase in mice results in perinatal hemorrhaging that is fatal [4]. Lethal hemorrhaging is also observed in VKORC1 null mice [5]. A VKORC1 paralog, VKORC1L1, also reduces KO and supports VKD protein carboxylation in vivo [6], but its physiological role is not well understood. Defining how these individual carboxylation components support VKD protein carboxylation is essential, given the broad biological impact of this family of proteins.
Mutations in GGCX or VKORC1 cause vitamin K clotting factor deficiency (VKCFD) 1 or 2, respectively. VKCFD is rare and associated with severe bleeding that results from reduced carboxylation of VKD clotting factors [7, 8]. In the case of VKCFD1, the mutations result in a carboxylase that retains some activity to support survival. Mutations in GGCX can also result in a second pathology, pseudoxanthoma elasticum-like disease, where the patients have mild bleeding but excessive calcification [9]. The carboxylase is autosomal, and patients with VKCFD1 or PXE-like are either homozygous for the mutation or have two different carboxylase mutations. The carboxylase reaction is complex, with catalysis and regulation involving multiple substrates and cofactors, and how carboxylase mutants disrupt carboxylation to cause disease has only been explained with a few mutants [10–13].
A recent report described a novel mechanism for VKCFD1 in a patient with uniparental disomy of chromosome 2, which contains the carboxylase gene. The patient was homozygous for a carboxylase mutation inherited from the father, who was heterozygous with mutant and wild type carboxylase alleles and did not exhibit VKCFD1. The mutation altered a splicing acceptor sequence that resulted in aberrant splicing that skipped exon 2 (Δ2GGCX). The only carboxylase RNA observed in the patient was Δ2GGCX RNA, which led to the conclusion that the mutant retains activity [14], since some carboxylase activity is required for life. Functional Δ2GGCX would be surprising, as the carboxylase is an integral membrane protein and exon 2 encodes sequences thought to be important for the proper structure of the carboxylase in the membrane [15]. We therefore assessed the activity of the Δ2GGCX enzyme. Biochemical assays that measured carboxylation of a model peptide and the VKD protein factor IX (fIX), as well as cellular analysis of fIX carboxylation, indicated that the mutant was completely inactive. The results suggest that the patient with the Δ2GGCX mutation survives not because the mutant is active but rather due to an incomplete splicing defect that results in some expression of full-length carboxylase.
MATERIALS AND METHODS
Generation of mutant carboxylase deleted in exon 2.
Δ2GGCX lacking exon 2 was constructed by overlap PCR using a wild type carboxylase cDNA as template [16] and primers A-D (Supplemental Table). The C-terminus contained a C-terminal Ala linker followed by a FLAG tag (AAADYKDDDDK). The PCR product was cloned into the TOPO vector (pCR4-TOPO, Thermo Fisher) and then sequenced to verify the mutation. A BamH I fragment encoding Δ2GGCXflag was then subcloned into pBacPAK8 (Clontech) for expression in SF21 insect cells. Δ2GGCXflag was also subcloned into pCMV6-AC (Origene) for expression in mammalian cells, in parallel with wild type carboxylaseflag. An untagged Δ2GGCX variant was also constructed for expression in mammalian cells. BamH I-EcoR I and EcoR I-BamH I fragments containing the N-terminal exon 2 deletion mutation and C-terminal wild type carboxylase sequences were ligated into the BamHI site of pCEP4 (Thermo Fisher), and the carboxylase cDNA was then sequenced.
Activity assays for Δ2GGCX expressed in SF21 insect cells.
Baculoviruses containing either wild type carboxylaseflag or Δ2GGCXflag were prepared by cotransfection with pBacPAK-6, as previously described [2, 17]. Expression of carboxylaseflag alone used a multiplicity of infection (moi) of 5 to infect SF21 cells, while expression of both carboxylaseflag and fIX used moi’s of 5 and 10, respectively. Microsomes prepared as before [16] were resuspended in 50 mM HEPES pH 7.4, 500 mM NaCl, 10% glycerol, and then solubilized with CHAPS (0.5%) at a protein concentration of 4 mg/ml. Solubilized microsomes underwent centrifugation (105 X g, 4°C, 1 hr), and supernatants were then used in the assays below. Carboxylation of the peptide FLEEL was assayed in a reaction mixture containing 800 mM ammonium sulfate, 50 mM BES pH 6.9, 5 mM DTT, 0.16% CHAPS, 0.16% phosphatidyl choline, 200 μM KH2, 1.3 mM (14C)-CO2, 10 μM factor X propeptide and 2.5 mM FLEEL (Anaspec). Activity monitored (14C)-CO2 incorporation into FLEEL by scintillation counting, as before [2]. Vitamin K epoxidation activity was measured in parallel reactions where nonradioactive CO2 replaced (14C)-CO2. Epoxidation measured KO production by HPLC resolution of vitamin K forms and quantitation by absorbance [18].
FIX carboxylation was assayed using a reaction cocktail as in the peptide assay but without FLEEL and factor X propeptide and with 500 mM NaCl replacing ammonium sulfate. Following the addition of enzyme, aliquots were withdrawn at timed intervals and quenched by the addition of gel loading buffer containing SDS (1%). Samples were boiled and then gel electrophoresed, along with (14C)-BSA standards. Protein was transferred to nitrocellulose membranes, and then quantitated using PhosphorImager analysis to measure (14C)-CO2 incorporation into fIX [19].
Western analysis was performed in parallel with all activity assays to quantitate carboxylase expression. To accentuate the size difference between wild type and Δ2GGCX carboxylases, samples were treated with endoglycosidase H (New England Biolabs). Deglycosylation was according to the manufacturer’s instructions, except that denaturation was at 25°C for 1 hr. Following gel electrophoresis, Western analysis was performed using an antibody against the carboxylase C-terminus (0.4 μg/ml) and goat anti-rabbit secondary antibody conjugated to IR dye 800 CX (0.2 μg/ml, LiCor Biosciences).
Generation of r-fIX 293 cells.
A BamHI fragment containing the fIX cDNA was subcloned into pCMV6-A-puro (Origene), and the plasmid was transfected into 293 cells. Puromycin (2.5 μg/ml) was used to select clones stably-expressing r-fIX, which were screened in a Western with antibody against fIX [19]. Clonal isolates that expressed varying levels of fIX were tested for the extent of carboxylation of secreted fIX using an antibody against Gla (BioMedica Diagnostics), and determining the ratio of signal for anti-Gla versus anti-fIX reactivity. Comparison to a fully-carboxylated fIX standard (Enzyme Research Laboratories) was used to select a clonal isolate with full carboxylation, which was verified in a clotting assay and by Gla quantitation [17].
CRISPR-Cas9 editing in fIX 293 cells to eliminate endogenous carboxylase expression.
Editing efficiency in 293 cells was measured using several different gRNAs, which were chosen from a published database for the human genome [20] or by searching ZiFit for CRISPR-Cas9 sites in GGCX exon 4 and adjacent intron sequences (http:/zifit.partners.org). Primers encoding the target sequence were subcloned into an 8 kB BamHI-BsmBI fragment of pCasGuide (Origene) that contains a cassette for expressing the CRISPR-Cas9 enzyme. After sequencing, plasmids (2.5 μg) were transiently transfected into 293 cells (106 cells) using Mirus Biosciences transfection reagent, along with 50 ng ptd-Tomato-N1 (Clontech) that encodes a fluorescent protein for monitoring transfection efficiency. Cells were incubated for three days and then tested for editing using a T7 assay. Genomic DNA was isolated (DNEasy Blood and Tissue Kit, Qiagen), followed by PCR amplification with primers E and F using the EnGen Mutation Detection Kit (New England Biolabs) as instructed. The PCR products were digested with T7 endonuclease, followed by gel electrophoresis and quantification of editing efficiency (GelDoc EZ Imager, Biorad).
The gRNA (primers G and H, Supplemental Table) giving the highest editing efficiency was subsequently chosen for generating clones lacking the carboxylase. Transiently-transfected cells were plated at a low cell density to isolate well separated clones, which were screened using the T7 assay as above. Clonal isolation did not involve selection of edited clones, and approximately 100 isolates were therefore tested. Clones showing editing were then tested in an activity assay. Cells were lysed in 25 mM sodium phosphate pH 7.9, 25 mM KCl, 20% glycerol, 0.75% CHAPS and then assayed for carboxylation of the peptide FLEEL, as described above but using a reaction time of 18 hr. Lysates were also analyzed in Westerns, using antibodies against the carboxylase or VKORC1 [19, 21]. Candidates clones were then tested for fIX carboxylation by culturing cells in serum free media and monitoring secreted material for fIX expression and carboxylation using antibodies against fIX and Gla, respectively, as before [21]. A suitable clonal isolate was then subjected to sequence analysis. PCR amplification was performed on genomic DNA, and the PCR product was cloned into pCR4-TOPO. Twelve transformants were then sequenced, using primers I and J (Supplemental Table).
Expression of recombinant carboxylases in edited fIX 293 cells.
Edited fIX 293 cells lacking endogenous carboxylase were transfected with r-wild type carboxylaseflag/pCMV6-AC or r-Δ2GGCXflag/pCMV6-AC, and clones were selected using G418 (0.5 mg/ml, Sigma). Clonal isolates were screened in a Western using anti-carboxylase antibody [22]. Cells transfected with r-wild type carboxylaseflag/pCMV6-AC were also screened for the carboxylation of FLEEL peptide. To measure Δ2GGCX activity, clones were monitored for fIX carboxylation and expression using antibodies against Gla and fIX, respectively, along with a fIX standard (Enzyme Research Laboratories).
RESULTS
Biochemical analyses reveal an inactive Δ2GGCX mutant.
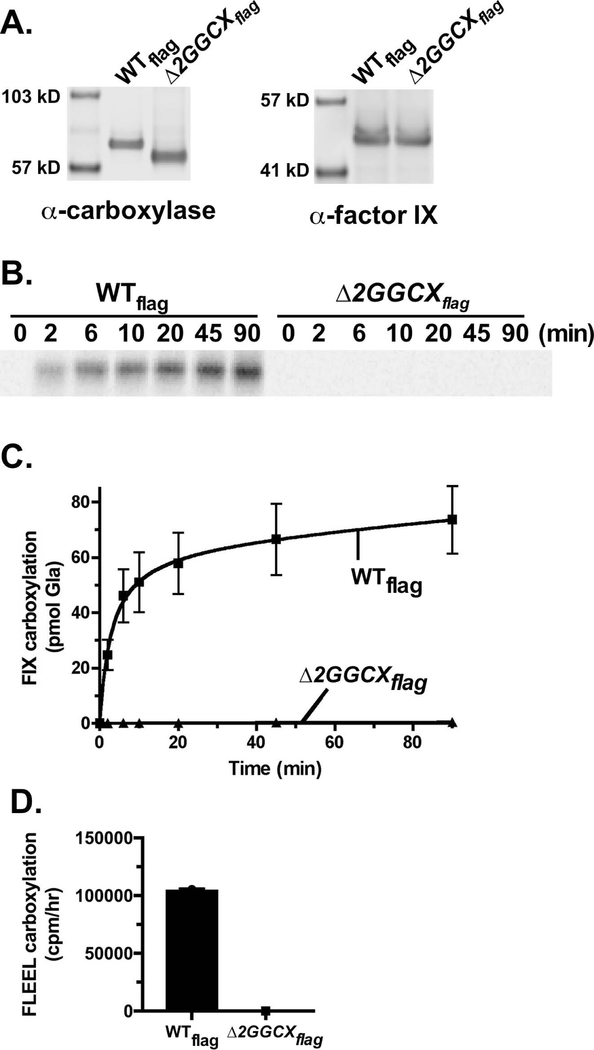
The Δ2GGCX mutant lacking exon 2 was assayed for activity using baculovirus-infected insect cells, which have been valuable for studying carboxylase mutants because the insect cells do not contain endogenous carboxylase but synthesize active enzyme when exogenously introduced. Cells infected with viruses containing wild type- or Δ2GGCXflag cDNAs were analyzed in a Western, after deglycosylation to accentuate the molecular weight difference between the two carboxylases. Δ2GGCX was expressed at a level similar to that of wild type carboxylase, and showed increased gel mobility consistent with the loss of the 56 residues encoded by exon 2 (Fig. 1A). The analysis was performed using membrane preparations (i.e. microsomes) to avoid potential loss of Δ2GGCX activity during purification. The carboxylase is an integral membrane protein [3], and the Western shows that Δ2GGCX is also microsomal.
Figure 1. Loss of exon 2 sequences inactivates the carboxylase.
Insect cell microsomes containing wild type carboxylase (WTflag) or a mutant lacking exon 2 (Δ2GGCXflag) were assayed for expression (A), carboxylation of a Glu substrate (FLEEL) (B), and epoxidation of vitamin K hydroquinone (C).
When tested for activity that measures (14C)-CO2 incorporation into a Glu-containing substrate (FLEEL), the Δ2GGCX mutant was totally inactive, i.e. <0.01% compared with wild type enzyme (Fig. 1B). The carboxylase is also an epoxidase, converting reduced vitamin K to the epoxide form, and this activity was monitored by isolation of vitamin K forms and quantitation using HPLC. No epoxidation (<0.01%) was observed with the Δ2GGCX mutant (Fig. 1C), indicating that the mutant would not support the carboxylation of any Glu-substrate.
Carboxylation of a full-length VKD protein, fIX, was also tested. The rationale for testing protein carboxylation despite the lack of peptide activity was that we previously found that a carboxylase mutant that was inactive when expressed alone had activity when stabilized in a VKD protein-carboxylase complex [16]. Δ2GGCX and wild type carboxylases were each coexpressed with fIX in insect cells (Fig. 2A), and (14C)-CO2 incorporation into fIX was monitored by gel electrophoresis and PhosphorImager analysis. Δ2GGCX activity was undetectable in this assay (Fig. 2B, C). Peptide carboxylation was also assessed to determine whether stabilization of Δ2GGCX in a complex with VKD protein conferred activity. No activity was observed (Fig. 2D). The mutant therefore appeared to be totally inactive, prompting subsequent analyses in mammalian cells.
Figure 2. The Δ2GGCX mutant is inactive for fIX carboxylation in biochemical analyses.
(A) Microsomes from insect cells coinfected with fIX and wild type (WTflag) or Δ2GGCXflag carboxylase were analyzed in Westerns for the expression of each protein. (B, C) (14C)-CO2 incorporation into fIX was monitored by subjecting the reaction products to gel electrophoresis, followed by PhosphorImager analysis. (D) Peptide carboxylation was also assessed to determine whether fIX stabilization of Δ2GGCXflag impacted activity.
Editing mammalian cells to eliminate carboxylase activity.
CRISPR-Cas9 editing was performed in 293 cells, which were targeted because they have been widely used to study VKD protein carboxylation. Carboxylase sequences upstream of the codon for Lys218 were targeted, as this residue is absolutely required for carboxylase activity (Fig. 3A)[23]. Several gRNAs subcloned into a vector (pCasGuide) that also encodes CRISPR-Cas9 were first tested for editing efficiency. 293 cells expressing fIX were transiently transfected, followed by PCR amplification of the target site and digestion with T7 that cleaves mismatched DNA (Fig. 3B, C). The gRNA with the highest editing efficiency was then retransfected into fIX 293 cells, and individual clones were screened using the T7 assay. Candidate clonal isolates were subsequently analyzed by sequencing, which revealed three copies of the carboxylase. An isolate was identified that had frameshift mutations in all three alleles (Supplemental Fig. 1). An activity assay measuring (14C)-CO2 incorporation into the FLEEL substrate showed that the edited cells lacked carboxylase activity (Fig. 3D). Carboxylase expression in the edited cells was undetectable, while the expression of VKORC1 that is also required for carboxylation was unaffected (Fig. 3E).
Figure 3. Generation of HEK293 cells lacking endogenous carboxylase.
(A) The carboxylase gene was edited by CRISPR-Cas9 upstream of sequences encoding Lys218 that is required for activity [23]. (B, C) Indels were identified by T7 digestion of PCR products encompassing the targeted site, and sequence analysis revealed frameshift mutations in a clonal isolate chosen for further analysis (Supplemental Fig. 1). (D-E) Two edited clones, one of which was the sequenced clone, lacked carboxylase activity (D) and expression (E) but contained normal levels of the vitamin K epoxide reductase (VKORC1). (F-G) Carboxylation and expression of fIX were monitored in Westerns with antibodies against Gla or fIX, respectively. Samples were first deglycosylated with PNGase F, according to the manufacturer’s instructions, to separate fIX from a background band. Panel F shows the specificity of the antibodies, and panel G shows that fIX carboxylation was eliminated in edited 293 cells. The brackets indicate bands quantitated in the analysis, and the entire blots in panels F and G are shown in Supplemental Fig. 4. The differences in gel migration for carboxylated and uncarboxylated fIX are due to post-translational modifications, some of which are impacted by carboxylation (e.g. glycosylation, [29, 30]).
FIX carboxylation was then tested by using a Western-based assay that monitors fIX carboxylation and expression using anti-Gla and anti-fIX antibodies, respectively. The anti-Gla antibody recognizes Gla but not Glu [24] and the anti-fIX antibody that detects both carboxylated and uncarboxylated fIX is used for normalization (Fig. 3F) [19]. Prior to gel analysis, the samples were treated with PNGase F in order to separate fIX from a background band. Digestion generates multiple bands, all of which are quantitated (indicated by brackets in Fig. 3G). When this assay was applied to monitoring unedited progenitor versus edited fIX 293 cells, carboxylation was only observed in the progenitor cells (Fig. 3G).
We next attempted to restore carboxylase activity in edited fIX 293 cells by transient transfection with r-wild type carboxylase. This approach revealed an issue with using transient transfection to express r-carboxylase. While Western analysis revealed similar levels of transiently-transfected r-carboxylase to that of endogenous carboxylase, r-carboxylase expression suppressed fIX carboxylation and expression (Supplemental Fig. 2A–C). The results are most likely due to the fact that transient transfection gives varying levels of expression in individual cells. FACS analysis showed a large range of expression between individual cells in the transient transfection (Supplemental Fig. 2D–F). This mixed population of cells would include cells expressing high levels of carboxylase, which impairs fIX expression and carboxylation ([25], and Supplemental Fig. 3). As the mixed cell population would confound analysis, we subsequently used stable transfection to generate cells with more homogenous expression of the carboxylase, where cells with expression levels closer to that of endogenous carboxylase could be studied.
Edited cells were stably-transfected with r-wild type carboxylaseflag in pCMV6-AC, which encodes resistance to neomycin. Clones isolated after selection with G418 were screened by a Western and then a peptide carboxylase assay to identify an isolate with an expression level similar to that of endogenous carboxylase in the progenitor fIX 293 cell line. Multiple clones were identified, and a representative isolate was used in subsequent analysis (Fig. 4A, B). When tested for fIX carboxylation, the isolate gave levels identical with that of the progenitor fIX 293 cells (Fig. 4C).
Figure 4. Restoration of carboxylase activity in edited fIX 293 cells.
(A-B) Carboxylase expression and activity were monitored in edited fIX 293 cells stably-transfected with r-carboxylaseflag/pCMV6-AC. An isolate was identified that had levels similar to endogenous carboxylase in the progenitor unedited fIX 293 cell line. (C) FIX secreted from cells expressing r-wild type carboxylase showed the same level of carboxylation as in the progenitor line. The brackets indicate bands quantitated for fIX carboxylation (anti-Gla) or expression (anti-fIX), and the entire blots for both gels are shown in Supplemental Fig. 5.
Δ2GGCX is inactive for cellular fIX carboxylation.
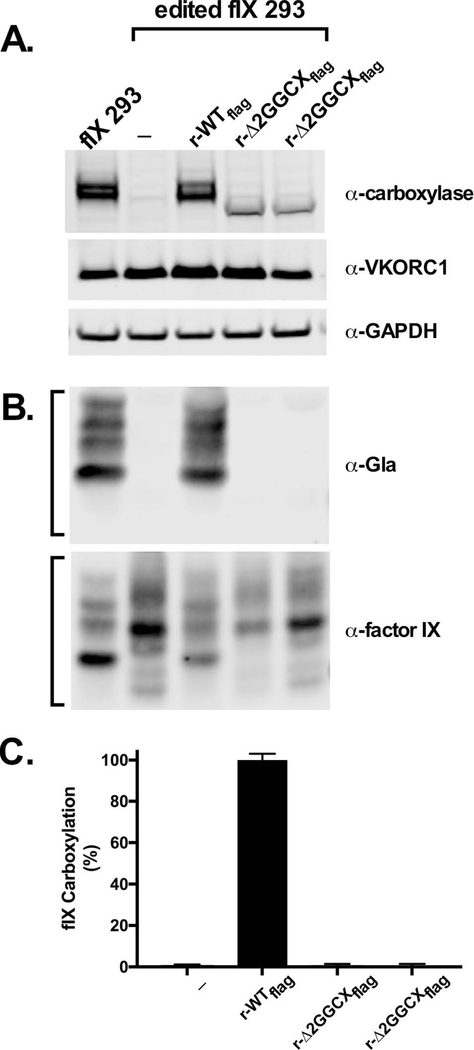
Edited fIX 293 cells lacking endogenous carboxylase were stably transfected with r-Δ2GGCXflag, and several dozen clonal isolates were screened for expression. The mutant was expressed at lower levels than r-wild type carboxylase, and isolates that gave the highest level (Fig. 5A) were chosen for subsequent analysis. Cells were incubated with vitamin K, and carboxylation and fIX secretion were monitored in Westerns using antibodies against Gla or fIX, respectively. While fIX was secreted from the r-Δ2GGCX expressing cells, carboxylated protein was not observed (Fig. 5B, C). Undetectable carboxylation by r-Δ2GGCX represented at least a 500-fold decrease in activity compared to the r-wild type carboxylase cells, based on the sensitivity of the assay and normalization for fIX expression. Differences in carboxylase carboxylation were also apparent, as only the cells expressing wild type carboxylase showed the shift in gel migration that results from carboxylation (Fig. 5A)[22]. Epoxidation activity was also monitored by isolating intracellular vitamin K and then performing HPLC to quantitate the production of KO by the carboxylase. The r-Δ2GGCX mutant showed no activity in this assay (data not shown).
Figure 5. Δ2GGCX is inactive for fIX carboxylation in 293 cells.
(A) Edited fIX 293 cells expressing wild type (r-WTflag) or exon 2-deleted (r-Δ2GGCXflag) carboxylase were analyzed in a Western for carboxylase and VKORC1 levels. The upper band in the fIX 293 and r-WT lanes is due to carboxylase carboxylation that retards gel mobility [22]. (B, C) FIX carboxylation and secretion were quantitated (bracketed bands) using antibodies against Gla and fIX, respectively. The entire blots are shown in Supplemental Fig. 6.
Δ2GGCX protein expression is not observed in human tissue or cells.
The study on the patient with the Δ2GGCX mutation [14] revealed Δ2GGCX RNA in normal controls as well as the patient, and the results showing that Δ2GGCX is inactive prompted us to test whether the Δ2GGCX RNA correlated with protein expression. Blood cells were initially analyzed, as this source revealed Δ2GGCX RNA in the patient with the mutation [14]. Unfortunately, carboxylase expression was extremely low in these cells (data not shown), which precluded analysis. Human liver was also analyzed, because Δ2GGCX RNA was detected in the liver of a healthy subject in the same study [14]. The previously reported ratio of wild type versus Δ2GGCX RNA in liver was ~2:1 [14], and similar levels were observed in this study (Fig. 6A). In contrast, Western analysis performed in parallel with wild type- and Δ2GGCX carboxylase controls only revealed the wild type carboxylase form (Fig. 6A). Δ2GGCX RNA has also been reported in several human hepatocellular carcinoma cell lines [26]. One line, HepG2 cells, was chosen for analysis because the level of Δ2GGCX RNA was relatively high in these cells (~2:1 Δ2GGCX:wild type carboxylase RNA)[26] and because the cell line could be obtained from the same source (ATCC) used in the previous study. While similar levels of Δ2GGCX RNA were detected to that previously reported, Δ2GGCX protein expression was undetectable (Fig. 6B).
Figure 6. Δ2GGCX protein is not observed in human liver or HepG2 cells.
(A) Human liver (NovusBio) was tested for Δ2GGCX RNA and protein. RT-PCR revealed two bands, which were confirmed as Δ2GGCX (388 bp) or wild type GGCX (559 bp) RNA by sequence analysis of the PCR products (Supplemental Fig. 7). Fluorescently labeled Cy5 primers were also used, which allowed quantification of the levels of RNA. Western analysis was performed using antibody against the C-terminus of the carboxylase [22]. Controls were endogenous carboxylase from 293 cells (WT 293) and r-Δ2GGCX, which was untagged to avoid the impact of a tag on gel mobility. (B) HepG2 cells were tested for RNA and protein expression as in part A. Carboxylase carboxylation that retards mobility [22] was observed in HepG2 but not 293 cells due to culturing in different media. Specifically, HepG2 cells were cultured in regular serum that contains some vitamin K, while 293 cells were grown in charcoal-treated serum lacking vitamin K.
DISCUSSION
This study analyzed the consequence of a homozygous Δ2GGCX mutation in a patient with VKCFD1 [14], where aberrant splicing deleted exon 2 that encodes residues 15–71. Our studies show that this deletion inactivates the carboxylase. Biochemical analyses were performed on a Glu-containing peptide and on full-length fIX using insect cells that lack endogenous carboxylase, and activity was undetectable in both cases (Figs. 1, 2). Cellular fIX carboxylation was also tested, using a mammalian 293 cell line that was first edited by CRISPR-Cas9 to eliminate endogenous carboxylase activity. Reintroduction of r-wild type carboxylase into the cells gave robust fIX carboxylation, while none was detected in cells expressing r-Δ2GGCX (Fig. 5). Vitamin K epoxidation drives Glu carboxylation, and this activity was not observed with the Δ2GGCX mutant (Fig. 1). Therefore, all VKD proteins would be expected to show the undetectable carboxylation that was observed with fIX.
Undetectable carboxylase activity with the Δ2GGCX mutant is strikingly different from what has been reported with mutations in other patients with VKCFD1. In assays similar to those used in this study, carboxylase activities of 3–8% have been observed [10, 12, 13]. In contrast, Δ2GGCX mutant activity was at least two orders of magnitude lower (Figs. 1, 2), based on the sensitivity of the assay. The 3–8% levels of carboxylase function in the other patients with VKCFD1 result in fIX clotting activities of 7–90% [10, 27, 28], and the patient homozygous for the Δ2GGCX mutation has 9% activity [14]. However, Δ2GGCX-mediated fIX carboxylation was undetectable in cells (Fig. 5).
Inactive Δ2GGCX could be due to disruption of the normal topology of the carboxylase in membrane. The carboxylase is a polytopic membrane protein, i.e. having multiple membrane-spanning sequences, and several different algorithms predict that the first membrane-associated sequence is largely deleted in the Δ2GGCX mutant (Fig. 7). Polytopic proteins lack a leader sequence, and the first transmembrane sequence is important both for membrane insertion and for determining the orientation of the protein in the membrane. Isolation of the carboxylase showed that it is associated with membranes (Figs. 1, 2, 5). However, if the exon 2 deletion alters proper carboxylase insertion into the membrane, then functional regions of the carboxylase may not be correctly aligned to yield activity. The results with the Δ2GGCX mutant underscore the importance of understanding the membrane-associated structure of the carboxylase. Different algorithms predict variable lengths and numbers of membrane-spanning sequences (Fig. 7). In addition, the carboxylase may be similar to other membrane proteins that have monotopic membrane sequences [3]. These sequences do not span the bilayer but instead run parallel to the membrane and allow interaction between substrates in or outside of the membrane (such as vitamin K and VKD proteins). A monotopic sequence would change the relative orientation of the transmembrane sequences. Carboxylase topology has only been assessed in one study [15], and further work will be of interest for defining the effect of membrane association on function.
Figure 7. The GGCX exon 2 deletion encompasses a predicted membrane domain.
The black box shows residues lost in the exon 2 deletion, and brackets above the carboxylase protein indicate functional regions [1, 31]. Grey boxes represent potential membrane-embedded sequences based on the predictions produced by the TOPCONS server (topcons.cbr.su.se) on May 3, 2018 and by a previous algorithm (TOPPRED [15]).
The observations that the Δ2GGCX mutant is inactive (Figs. 1, 2, 5) but the patient has clotting activities similar to that of other VKCFD1 patients [10, 27, 28] strongly suggests that the splicing defect is incomplete, and that a small amount of properly spliced mRNA is expressed in the patient to support VKD clotting function. A small amount of full-length carboxylase mRNA would have been missed in the previous analysis of the patient [14], but would be sufficient to produce some wild type carboxylase to yield carboxylase activity comparable to that of other VKCFD1 patients. We propose that VKD clotting activities are due to full-length carboxylase rather than the Δ2GGCX mutant. Coagulation was unaffected by vitamin K administration in the patient with the Δ2GGCX mutation, which contrasts other VKCFD1 patients and which is consistent with the proposal. Thus, an incomplete splicing defect would produce wild type GGCX at low levels, and vitamin K binding may already be saturated and would therefore not be affected by higher levels from vitamin K administration. In addition, while mutant carboxylases may show defective vitamin K interactions that respond to higher levels of vitamin K, the same would not necessarily be expected for wild type GGCX. A partial splicing defect has also been suggested for a different mutation in the carboxylase [11].
Our results showing that Δ2GGCX is inactive raise the question of whether the mutant has physiological relevance in vivo. The study on the Δ2GGCX patient showed that Δ2GGCX RNA is present not only in the patient but also in normal controls [14]. Protein was not assessed, and we therefore tested both protein and RNA expression in human liver. This analysis revealed significant amounts of Δ2GGCX RNA but undetectable expression of Δ2GGCX protein (Fig. 6A). The results could be due to expression of immature RNA that is not translated. Δ2GGCX RNA has also been reported in a study on human hepatoma carcinoma cells [26]. We analyzed a cell line (HepG2 cells) obtained from the same source used in the previous study, which revealed similar levels of Δ2GGCX RNA to what had been reported (~20%, [26]). However, Δ2GGCX protein was undetectable (Fig. 6B). The previous study stated that Δ2GGCX protein expression was 20% that of wild type GGCX, but without data to support that statement. Two different antibodies were used that were specific to each form. The antibody sensitivities were not indicated, and so the relative expression of Δ2GGCX versus wild type GGCX cannot be determined. We used an antibody that recognized both Δ2GGCX and wild type GGCX, and the signal over background indicated that Δ2GGCX protein was expressed at levels <2% that of wild type GGCX. Trace levels of expression raise the question of whether Δ2GGCX suppresses prothrombin carboxylation, as previously proposed [26]. r-Δ2GGCX overexpression was also tested in that study and found to be associated with decreased prothrombin carboxylation. However, Δ2GGCX overexpression used transient transfection, an approach that we showed decreases VKD protein carboxylation even with r-wild type carboxylase (Supplemental Figs. 2 and 3). These considerations indicate the need for more work to determine whether Δ2GGCX is physiologically relevant to cancer.
Supplementary Material
ESSENTIALS.
A carboxylase mutation that impairs splicing to delete exon 2 sequences was previously reported.
We found that the mutant was inactive for vitamin K-dependent (VKD) protein carboxylation.
An incomplete splicing defect likely accounts for VKD clotting activity observed in the patient.
The results indicate the importance of proper carboxylase embedment in the membrane for function.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Bharani Thangavelu for performing some of the initial Δ2GGCX cloning. This work was funded by NIH RO1 HL055666 and NIA RO1 AG051601.
ADDENDUM MAR, KWH, HZ, KWR and KLB designed the experiments, which were performed by MAR, KWH and HZ. MAR, KLB and KWR wrote the manuscript, and KLB and KWR obtained funding for the research.
Footnotes
Conflict of Interest Disclosure. MAR, KWH, HZ, KWR and KLB declare that they have no conflicts of interest.
REFERENCES
- 1.Berkner KL. Vitamin K-dependent carboxylation. Vitam Horm. 2008; 78: 131–56. [DOI] [PubMed] [Google Scholar]
- 2.Rishavy MA, Hallgren KW, Wilson LA, Usubalieva A, Runge KW, Berkner KL. The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation. J Biol Chem. 2013; 288: 31556–66. 10.1074/jbc.M113.497297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berkner KL. The vitamin K-dependent carboxylase. Annu Rev Nutr. 2005; 25: 127–49. [DOI] [PubMed] [Google Scholar]
- 4.Zhu A, Sun H, Raymond RM Jr., Furie BC, Furie B, Bronstein M, Kaufman RJ, Westrick R, Ginsburg D. Fatal hemorrhage in mice lacking {gamma}-glutamyl carboxylase. Blood. 2007; 109: 5270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spohn G, Kleinridders A, Wunderlich FT, Watzka M, Zaucke F, Blumbach K, Geisen C, Seifried E, Muller C, Paulsson M, Bruning JC, Oldenburg J. VKORC1 deficiency in mice causes early postnatal lethality due to severe bleeding. Thromb Haemost. 2009; 101: 1044–50. [PubMed] [Google Scholar]
- 6.Lacombe J, Rishavy MA, Berkner KL, Ferron M. VKOR paralog VKORC1L1 supports vitamin K-dependent protein carboxylation in vivo. JCI Insight. 2018; 3 10.1172/jci.insight.96501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watzka M, Geisen C, Scheer M, Wieland R, Wiegering V, Dorner T, Laws HJ, Gumruk F, Hanalioglu S, Unal S, Albayrak D, Oldenburg J. Bleeding and non-bleeding phenotypes in patients with GGCX gene mutations. Thromb Res. 2014; 134: 856–65. 10.1016/j.thromres.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Ginsburg D. Familial multiple coagulation factor deficiencies: new biologic insight from rare genetic bleeding disorders. J Thromb Haemost. 2004; 2: 1564–72. 10.1111/j.1538-7836.2004.00857.x. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Jiang Q, Pfendner E, Varadi A, Uitto J. Pseudoxanthoma elasticum: clinical phenotypes, molecular genetics and putative pathomechanisms. Exp Dermatol. 2009; 18: 1–11. 10.1111/j.1600-0625.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darghouth D, Hallgren KW, Shtofman RL, Mrad A, Gharbi Y, Maherzi A, Kastally R, LeRicousse S, Berkner KL, Rosa J-P. Compound heterozygosity of novel missense mutations in the gamma-carboxylase gene causes hereditary combined vitamin K-dependent coagulation factor deficiency. Blood. 2006; 108: 1925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin DY, Vermeer C, Stafford DW, Tie JK. Splice-Site Mutation of Exon 3 Deletion in the Gamma-Glutamyl Carboxylase Gene Causes Inactivation of the Enzyme. J Invest Dermatol. 2016; 136: 2314–7. 10.1016/j.jid.2016.05.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutucumarana VP, Stafford DW, Stanley TB, Jin DY, Solera J, Brenner B, Azerad R, Wu SM. Expression and characterization of the naturally occurring mutation L394R in human gamma-glutamyl carboxylase. J Biol Chem. 2000; 275: 32572–7. [DOI] [PubMed] [Google Scholar]
- 13.Soute BA, Jin DY, Spronk HM, Mutucumarana VP, Lin PJ, Hackeng TM, Stafford DW, Vermeer C. Characteristics of recombinant W501S mutated human gamma-glutamyl carboxylase. J Thromb Haemost. 2004; 2: 597–604. 10.1111/j.1538-7836.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 14.Dasi MA, Gonzalez-Conejero R, Izquierdo S, Padilla J, Garcia JL, Garcia-Barbera N, Argiles B, de la Morena-Barrio ME, Hernandez-Sanchez JM, Hernandez-Rivas JM, Vicente V, Corral J. Uniparental disomy causes deficiencies of vitamin K-dependent proteins. J Thromb Haemost. 2016; 14: 2410–8. 10.1111/jth.13517. [DOI] [PubMed] [Google Scholar]
- 15.Tie J, Wu SM, Jin D, Nicchitta CV, Stafford DW. A topological study of the human gamma-glutamyl carboxylase. Blood. 2000; 96: 973–8. [PubMed] [Google Scholar]
- 16.Rishavy MA, Pudota BN, Hallgren KW, Qian W, Yakubenko AV, Song JH, Runge KW, Berkner KL. A new model for vitamin K-dependent carboxylation: the catalytic base that deprotonates vitamin K hydroquinone is not Cys but an activated amine. Proc Natl Acad Sci U S A. 2004; 101: 13732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berkner KL, McNally BA. Purification of vitamin K-dependent carboxylase from cultured cells. Methods Enzymol. 1997; 282: 313–33. [DOI] [PubMed] [Google Scholar]
- 18.Rishavy MA, Usubalieva A, Hallgren KW, Berkner KL. Novel insight into the mechanism of the vitamin K oxidoreductase (VKOR): electron relay through Cys43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein carboxylation. J Biol Chem. 2011; 286: 7267–78. 10.1074/jbc.M110.172213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallgren KW, Qian W, Yakubenko AV, Runge KW, Berkner KL. r-VKORC1 expression in factor IX BHK cells increases the extent of factor IX carboxylation but is limited by saturation of another carboxylation component or by a shift in the rate-limiting step. Biochemistry. 2006; 45: 5587–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014; 343: 80–4. 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rishavy MA, Hallgren KW, Wilson L, Singh S, Runge KW, Berkner KL. Warfarin alters vitamin K metabolism: a surprising mechanism of VKORC1 uncoupling necessitates an additional reductase. Blood. 2018. 10.1182/blood-2017-09-804666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkner KL, Pudota BN. Vitamin K-dependent carboxylation of the carboxylase. Proc Natl Acad Sci USA. 1998; 95: 466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rishavy MA, Hallgren KW, Yakubenko AV, Shtofman RL, Runge KW, Berkner KL. Bronsted analysis reveals Lys218 as the carboxylase active site base that deprotonates vitamin K hydroquinone to initiate vitamin K-dependent protein carboxylation. Biochemistry. 2006; 45: 13239–48. [DOI] [PubMed] [Google Scholar]
- 24.Brown MA, Stenberg LM, Persson U, Stenflo J. Identification and purification of vitamin K-dependent proteins and peptides with monoclonal antibodies specific for gamma -carboxyglutamyl (Gla) residues. J Biol Chem. 2000; 275: 19795–802. [DOI] [PubMed] [Google Scholar]
- 25.Hallgren KW, Hommema EL, McNally BA, Berkner KL. Carboxylase overexpression impairs factor IX secretion: implications for the release of vitamin K-dependent proteins. Biochemistry. 2002; 41: 15045–55. [DOI] [PubMed] [Google Scholar]
- 26.Ueda N, Shiraha H, Fujikawa T, Takaoka N, Nakanishi Y, Suzuki M, Matsuo N, Tanaka S, Nishina S, Uemura M, Takaki A, Shiratori Y, Yamamoto K. Exon 2 deletion splice variant of gamma-glutamyl carboxylase causes des-gamma-carboxy prothrombin production in hepatocellular carcinoma cell lines. Mol Oncol. 2008; 2: 241–9. 10.1016/j.molonc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenner B, Sanchez-Vega B, Wu SM, Lanir N, Stafford DW, Solera J. A missense mutation in gamma-glutamyl carboxylase gene causes combined deficiency of all vitamin K-dependent blood coagulation factors. Blood. 1998; 92: 4554–9. [PubMed] [Google Scholar]
- 28.Spronk HM, Farah RA, Buchanan GR, Vermeer C, Soute BA. Novel mutation in the gamma-glutamyl carboxylase gene resulting in congenital combined deficiency of all vitamin K-dependent blood coagulation factors. Blood. 2000; 96: 3650–2. [PubMed] [Google Scholar]
- 29.McClure DB, Walls JD, Grinnell BW. Post-translational processing events in the secretion pathway of human protein C, a complex vitamin K-dependent antithrombotic factor. J Biol Chem. 1992; 267: 19710–7. [PubMed] [Google Scholar]
- 30.Yan SC, Razzano P, Chao YB, Walls JD, Berg DT, McClure DB, Grinnell BW. Characterization and novel purification of recombinant human protein C from three mammalian cell lines. Biotechnology (NY). 1990; 8: 655–61. [DOI] [PubMed] [Google Scholar]
- 31.Hallgren KW, Zhang D, Kinter M, Willard B, Berkner KL. Methylation of gamma-carboxylated Glu (Gla) allows detection by liquid chromatography-mass spectrometry and the identification of Gla residues in the gamma-glutamyl carboxylase. J Proteome Res. 2013; 12: 2365–74. 10.1021/pr3003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.