Abstract
The antioxidant mechanism is crucial for resisting oxidative damage induced by drought stress in plants. Different antioxidant mechanisms may contribute to the tolerance of cassava to drought stress, but for a specific genotype, the response is still unknown. The objective of this study was to investigate antioxidant response and physiological changes of four cassava genotypes under water stress conditions, by keeping the soil moisture content as 80% (control), 50% (medium), 20% (severe) of field capacity for a week. Genotypes RS01 and SC124 were keeping higher relative water content (RWC) and relative chlorophyll content (SPAD value) and less affected by oxidative stress than SC205 and GR4 under drought stress. RS01 just showed slight membrane damage and oxidative stress even under severe drought conditions. A principal component analysis showed that cassava plant water status was closely related to the antioxidant mechanism. Antioxidant response in genotypes RS01 and SC124 under drought stress might attribute to the increased accumulation of ascorbate (AsA) and glutathione (GSH) content and higher superoxide dismutase (SOD) and catalase (CAT) activities, which explained by the up-regulation of Mn-SOD and CAT genes. However, Genotypes SC205 and GR4 mainly depended on the accumulation of total phenolics (TP) and increased glutathione reductase (GR) activity, which attribute to the up-regulation of the GR gene. Our findings could provide vital knowledge for refining the tactics of cultivation and molecular breeding with drought avoidance in cassava.
Subject terms: Plant physiology, Plant stress responses
Introduction
Drought stress is commonly induced by rainfall patterns, greenhouse effect and the variations of temperature. It is an important environmental stress factor that limits plant growth, regulation, and distribution1–3. Compared with other abiotic stresses, drought stress exerts more restrictions on crop productivity4, especially on the marginal lands with poor soils and limited water resources. For resource-limited small farmers in these marginal areas, cassava (Manihot esculenta Crantz) is an important staple food crop due to its inherent tolerance to stressful environments5. Because of its starchy roots, cassava is used for starch extraction and as feed resource and feedstock production in China and other Southeast Asian countries6. Drought is one of the main constraints that limit cassava growth and production, particularly during the first three months after planting7. Therefore, it is urgent to understand the mechanisms underlying drought tolerance of cassava at the seedling stage.
Plants have developed defense mechanisms which enable them to adapt and survive under drought condition in their life cycle8. The drought response of plants varies from species and the severity of the drought stress. The mechanisms of cassava resistant to water deficit include stomatal closure, decreased leaf area, the proper maintenance of net photosynthetic rate for prolonged drought, and the ability to explore water from deep soil layers9. The defense strategies against drought environment also vary from different cassava cultivars. During a mild drought period, SC124 cassava cultivar showed a “survival” mode by early stomatal closure and decreased photosynthesis resulting in early growth quiescence, while shedding of older leaves but continuing to grow in Agr7 cassava cultivar10. The strategy of SC124 cultivar is more beneficial for survival under severe drought stress than Agr7.
During a prolonged drought stress condition, reactive oxygen species (ROS) generate excessively and cause oxidative damage11. ROS can damage multiple cellular components such as proteins and lipids, and unlimited disruption will finally lead to cell death12,13. In order to counteract the production of ROS under such conditions, antioxidant defense mechanisms were formed in the long-term evolvement in plants. One vital member of this defense system is enzymatic machinery including superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), glutathione reductase (GR), catalase (CAT), etc. In addition, non-enzymatic antioxidants such as ascorbic acid (AsA), glutathione (GSH), total phenolics (TP) and total flavonoids (TF) also contribute to the alleviation of oxidative damage. The activities of SOD, CAT, and POD in cassava leaves increased to remove superoxide free radicals and control the level of membrane lipid peroxidation during drought stress conditions14. However, mechanisms of non-enzymatic antioxidants in cassava under water deficit are still unknown.
Currently, it is prevalent to study the mechanism of stress response at genetic, physiological and molecular levels15. To have a better understanding of the factors affecting antioxidant regulation, it is important to associate antioxidant enzyme activity and related-gene expression in different genotype species16. However, the studies on response mechanism under drought stress in cassava were limited to the physiological or molecular method only. Taking this into account, the present research was aimed at the elucidation of antioxidant response mechanism under drought in cassava seedlings combining genetic, physiological and molecular approaches.
Results
Relative water content and SPAD values
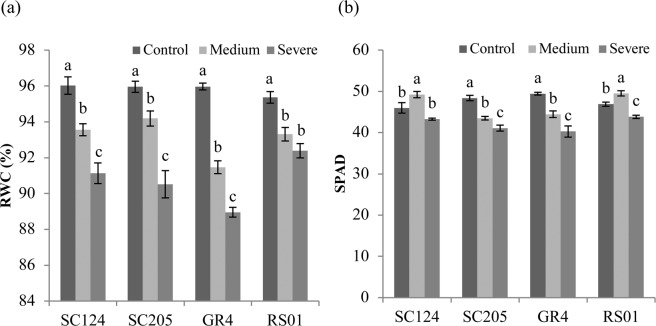
Drought stress caused a significant decline in the RWC of each genotype. The reduction of RWC in GR4 was more obvious than other genotypes (Fig. 1(a)). When severe drought stress occurred, RWC of GR4 was reduced by 7.31%, compared with 5.10%, 5.67% and 3.12% in SC124, SC205, and RS01 respectively. Changes in SPAD values varied in different genotypes (Fig. 1(b)). In genotypes SC205 and RS01, a stress-dependent reduction of SPAD values with increasing drought intensity was observed. In contrast, the SPAD values of SC124 and RS01 shoots increased under medium drought stress while decreased after exposure to severe drought stress. RWC and SPAD of RS01 and SC124 were both higher than that of GR4 and SC205 under severe drought stress. The results of two-way ANOVA revealed the significant differences for both RWC and SPAD levels, regarding treatments, genotypes and their interactions (Table 1).
Figure 1.
Physiological parameters in four cassava genotypes under control, medium, and severe stress. Leaf concentrations of (a) relative water content (RWC), (b) chlorophyll content (SPAD), are shown as means with SD (n = 4). For each genotype, different letters above the bars indicate significant differences between treatments.
Table 1.
Drought treatments of the chosen cassava genotypes.
| Soure of variance | DF | RWC | SPAD | RLC | MDA | H2O2 | AsA | GSH | TP | TF | SOD | POD | CAT | APX | GR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype (G) | 3 | 142.49** | 62.21** | 52.44** | 11.35** | 123.26** | 318.49** | 69.92** | 45.35** | 199.92** | 268.61** | 101.80** | 138.90** | 0.63NS | 126.65** |
| Treatment (T) | 2 | 9.02** | 6.99** | 28.97** | 5.44** | 21.26** | 83.55** | 145.92** | 13.25** | 15.50** | 9.44** | 10.64** | 74.79** | 242.68** | 43.63** |
| G × T | 6 | 5.12** | 9.85** | 12.02** | 0.73NS | 10.50** | 48.02** | 54.03** | 5.14** | 8.54** | 32.15** | 17.54** | 26.86** | 47.25** | 25.64** |
Results of two-way ANOVAs for the independent variables ‘genotype’ and ‘treatment’, and the ‘genotype × treatment’ interactions. The measurement included relative water content (RWC), chlorophyll content (SPAD), relative leaf conductivity (RLC), malondialdehyde (MDA), hydrogen peroxide (H2O2), ascorbate (AsA), glutathione (GSH), total phenolics (TP), total flavonoids (TF), specific activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR). DF:Degrees of freedom, NS:non-significant, *significant at P = 0.05, **significant at P = 0.01
Relative leaf electrical conductivity (RLC), malondialdehyde (MDA) and hydrogen peroxide (H2O2) content
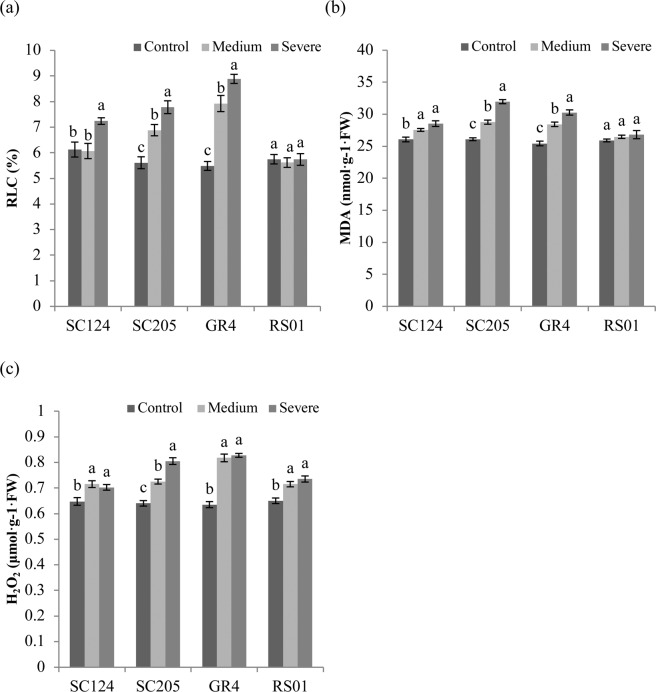
Drought stress has a significant effect on RLC and MDA of all genotype shoots except RS01(Fig. 2(a,b)). In genotypes SC205 and GR4, RLC and MDA levels increased significantly with increasing drought stress. However, unclear correlations with drought intensity were showed in both RLC and MDA levels of SC124. H2O2 accumulations of all genotypes tended to rise under drought stress conditions (Fig. 2(c)). The greatest increase was up to 30.31% in GR4, followed by 25.78%, 13.08% and 10.42% in SC205, RS01, and SC124, respectively. MDA and H2O2 of RS01 and SC124 were both higher than that of GR4 and SC205 under severe drought stress. All the analyzed parameters (RLC, MDA, and H2O2) were significantly affected by treatments, genotypes, and their interactions, except for the interaction between the two factors as for MDA (Table 1).
Figure 2.
Oxidative damage markers in four cassava genotypes under control, medium, and severe stress. Leaf concentrations of (a) relative leaf conductivity (RLC), (b) malondialdehyde (MDA), (c) hydrogen peroxide (H2O2), are shown as means with SD (n = 4). For each genotype, different letters above the bars indicate significant differences between treatments.
Non-enzymatic antioxidants
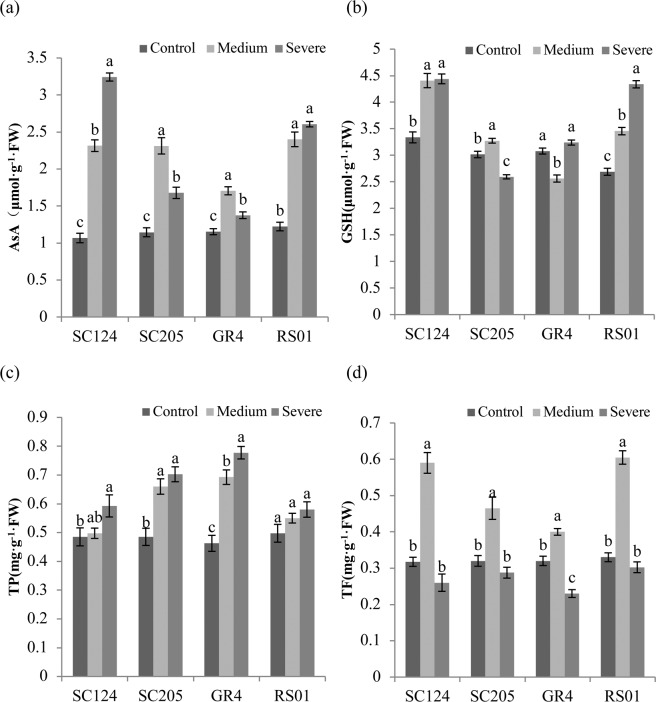
AsA content was similar in the four cassava genotypes under normal condition and increased significantly after exposure to medium drought stress (Fig. 3(a)). The strongest response to drought was showed in the AsA content of SC124, which was induced almost 3-fold under severe drought stress. Change patterns of GSH content varied from different cassava genotypes under water stress (Fig. 3(b)). The strongest drought-induced increase in GSH content was observed in RS01 under severe drought stress. TP content of all genotypes increased in parallel with increasing drought stress with the greatest increase seen in GR4 (68.11%), followed by SC205 (44.85%), SC124 (22.16%) and RS01 (16.58%), respectively (Fig. 3(c)). After exposure to drought stress, all the genotypes showed similar changing patterns of TF content with the maximum level seen under medium drought (Fig. 3(d)). Two-way ANOVA showed that all compounds analyzed (AsA, GSH, TP, and TF) differed significantly in respect of treatments, genotypes and their interactions (Table 1).
Figure 3.
Non-enzymatic antioxidants in four cassava genotypes under control, medium, and severe stress. Leaf concentrations of (a) ascorbate (AsA), (b) glutathione (GSH), (c) total phenolics (TP), (d) total flavonoids (TF), are shown as means with SD (n = 4). For each genotype, different letters above the bars indicate significant differences between treatments.
Antioxidant enzyme activities
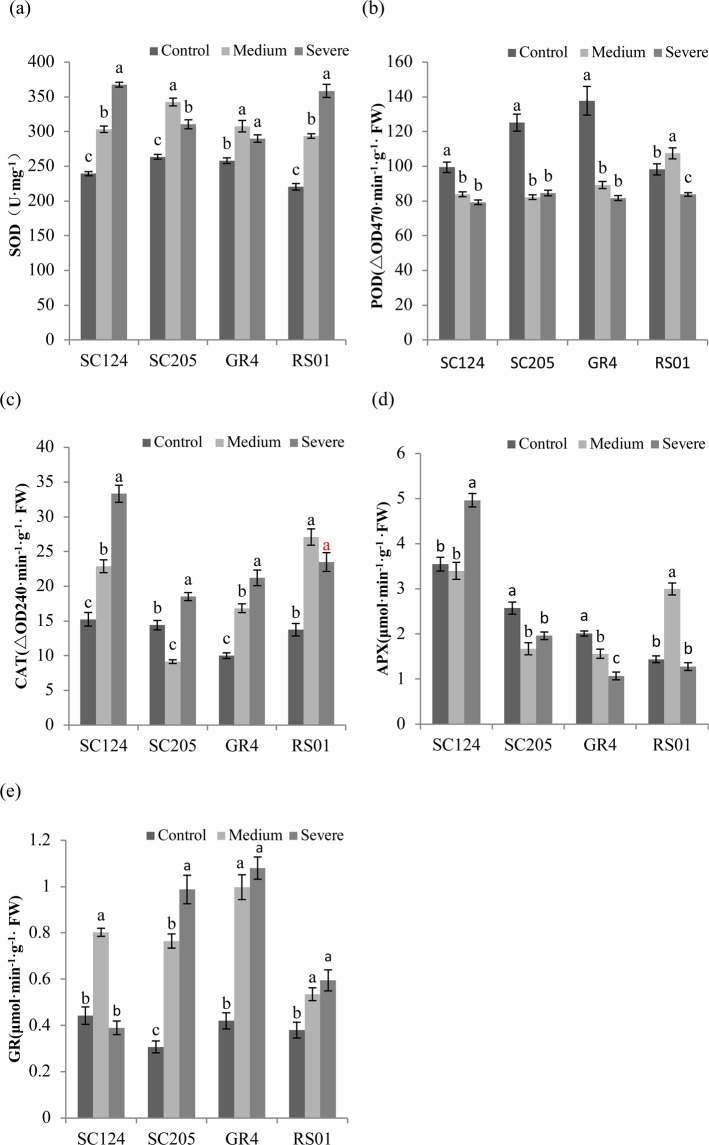
Variation patterns of antioxidant enzyme activities in response to drought stress were altered depending on genotypes and enzymes. SOD activity rose in drought-treated plants of each genotype as compared with control (Fig. 4(a)). This increase was most obvious in RS01 (62.69% of control), followed by SC124 (53.56%), SC205 (17.75%) and GR4 (12.34%), respectively. POD activity tended to decline under drought in each genotype although the trend was not suitable for RS01 where activity increased under medium drought (Fig. 4(b)). CAT activity of each genotype tended to rise under drought stress and the stronger increase was shown in genotypes SC124 and RS01 (Fig. 4(c)). The APX activities of SC205 and GR4 declined in response to drought while an increase of this enzyme activity was seen in RS01 and SC204 induced by medium and severe drought, respectively (Fig. 4(d)). Drought stress caused an increase in GR activity of each genotype and this increase was greater in SC205 and GR4 (Fig. 4(e)). All enzymatic activities were significantly influenced by treatments, genotypes, and their interactions. An exception was for the differences of APX activity between treatments, which was not significant (Table 1).
Figure 4.
The activity of antioxidant enzymes in four cassava genotypes under control, medium, and severe stress. The graphs show specific activity of (a) superoxide dismutase (SOD), (b) peroxidase (POD), (c) catalase (CAT), (d) ascorbate peroxidase (APX), (e) glutathione reductase (GR), as means with SD (n = 4). For each genotype, different letters above the bars indicate significant differences between treatments.
Gene expression in response to drought stress
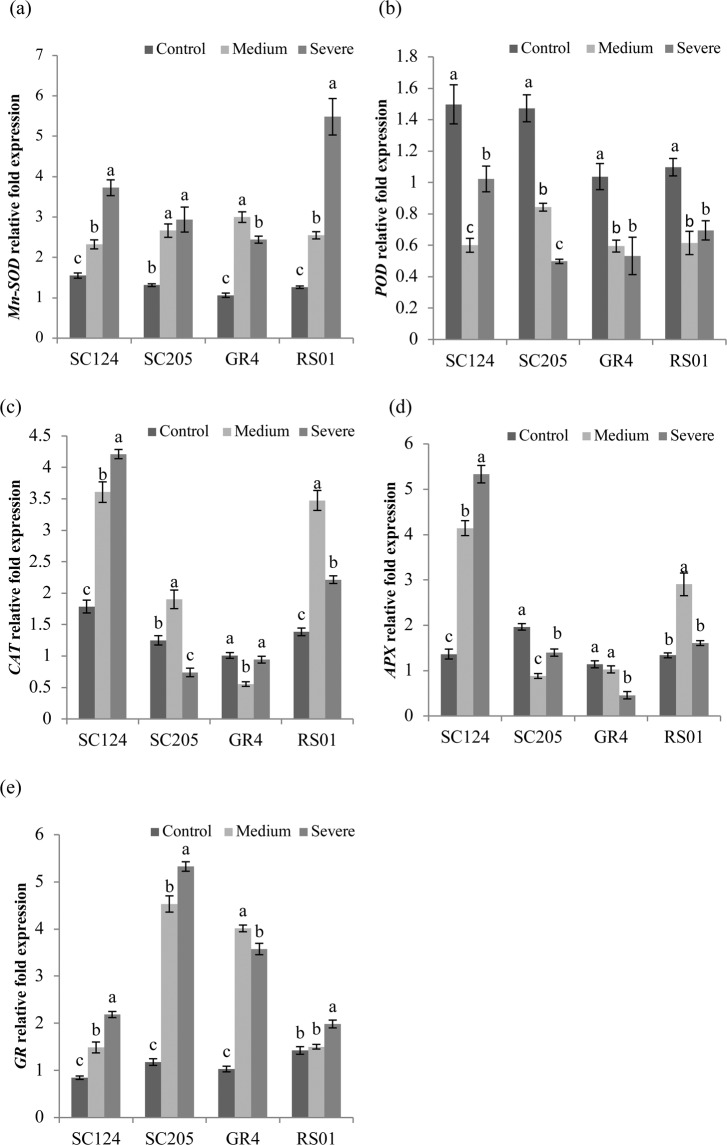
The expression analysis of five genes related to antioxidant enzymes was conducted by using specific primers (Table 2). These genes include Mn-SOD (encoding mitochondrial manganese superoxide dismutase), POD (peroxidase), CAT (catalase), APX (ascorbate peroxidase) and GR (glutathione reductase). In response to water stress, the expression of Mn-SOD of each genotype was upregulated as compared to corresponding control with the highest increase in transcript level seen in RS01(almost up to 6-fold) under severe drought stress (Fig. 5(a)). The expression of Mn-SOD was in line with the SOD activity change. The transcriptional level of POD in all genotypes was inhibited by drought. The expression of POD was consistent with the change in the profile of POD activity only in GR4 (Fig. 5(b)). The expression of CAT tended to be upregulated after exposure to drought in each genotype, except for GR4 and SC205 under medium and severe drought stress, respectively (Fig. 5(c)). The transcript level of CAT going along with the CAT activity change was only observed in SC124 and RS01 genotypes. The expression level of APX was upregulated in genotypes SC124 and RS01 whilst downregulated in SC205 and GR4 (Fig. 5(d)). The trend of changes in the expression of APX was similar to the APX activity variation. Drought stress induced the up-regulation of the GR gene in each genotype (Fig. 5(e)). The changing trend in the transcript level of GR was similar to the GR activity changes in all genotypes except SC124.
Table 2.
Primers used for analyzing expression levels of genes related to enzyme activities.
| Gene name | Primers |
|---|---|
| Mn-SOD |
F: CCCAGCATCATACCACATAGA R: GAGATCAGGGAGCGAGAAAGT |
| POD |
F: CTCCGCGATGCTGTCCACAAG R: ACGACACCGTCTCGCCTTCCT |
| CAT | F: GTGGTTCCTGGGATTCACTATTC R:AGGCAGCATCTTGTAGTTGGGT |
| APX |
F: AACTTACGACGTGAAGACGAACA R: AACAACACCAGCGAGCTGATAG |
| GR |
F: CGATGATGAAATGAGGGCAGTG R: GGGTCCGACCAGTAGCAAAGAG |
| Actin |
F:CGATGGTCGTACAACTGGTAT R: ATCCTCGAATCCAGACACTGT |
Figure 5.
Antioxidant enzyme-related genes expression in four cassava genotypes under control, medium, and severe stress. The graphs show specific gene of (a) mitochondrial manganese superoxide dismutase (Mn-SOD), (b) peroxidase (POD), (c) catalase (CAT), (d) ascorbate peroxidase (APX), (e) glutathione reductase (GR), as means with SD (n = 4). For each genotype, different letters above the bars indicate significant differences between treatments.
Principal component analysis (PCA)
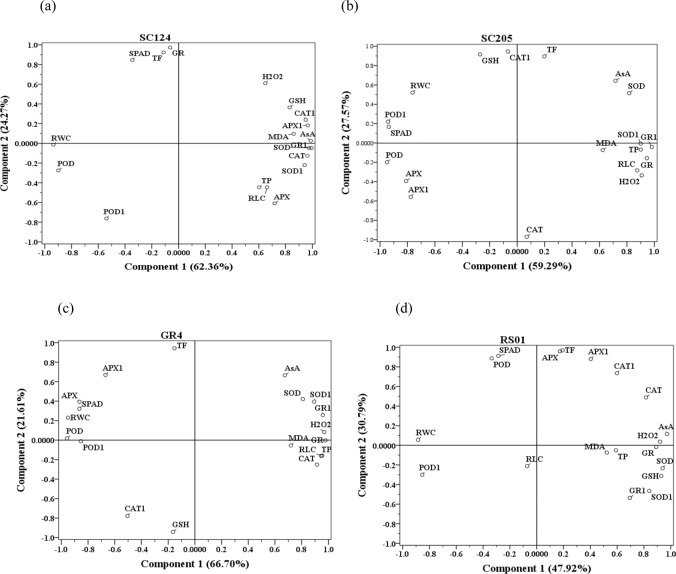
For each genotype, PCA was performed to investigate correlation existed between physiological, biochemical and transcriptional indexes. Besides, PCA also showed the relationship between leaf water status and antioxidant response mechanism in four cassava genotypes. In genotype SC124, the first and second component explained 62.36% and 24.27% of the data variability, respectively (Fig. 6(a)). According to Pearson correlation coefficients, RCW in SC124 correlated significantly with antioxidant contents(AsA, GSH, and TP), enzymatic activities(SOD, POD, CAT, and APX), gene expression(Mn-SOD, CAT, APX1, and GR). For genotype SC205, the first and second components explained 59.29% and 27.57% of the variance, respectively (Fig. 6(b)). RCW in SC205 correlated significantly with TP content, enzymatic activities(POD, CAT, and GR), gene expression(Mn-SOD, POD, CAT, and GR).In genotype GR4, 66.70% of the variability was explained by the first component whilst 21.60% by the second component (Fig. 6(c)). RCW in GR4 correlated significantly with TP content, all the enzymatic activities, gene expression (Mn-SOD, POD, APX, and GR). In genotype RS01, 47.92% and 30.79% of variance were explained by the first and second components, respectively (Fig. 6(d)). RWC in RS01correlated significantly with antioxidant contents (AsA, GSH, and TP), enzymatic activities (SOD, CAT, and GR), gene expression (Mn-SOD, POD, and GR). These results suggested that the water status of cassava seedling was closely related to antioxidant response and these relations varied from different genotypes.
Figure 6.
Principal component analysis (PCA). Site score plots of the studied variables in the drought stress treatments, for the four cassava genotypes, SC124 (a), SC205 (b), GR4 (c) and RS01 (d). PCA s included these variables: relative water content (RWC), chlorophyll content (SPAD), relative leaf conductivity (RLC), malondialdehyde (MDA), hydrogen peroxide (H2O2), ascorbate (AsA), glutathione (GSH), total phenolics (TP), total flavonoids (TF), specific activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and related gene expression of mitochondrial manganese superoxide dismutase (SOD1), peroxidase (POD1), catalase (CAT1), ascorbate peroxidase (APX1), glutathione reductase (GR1).
Discussion
As an important environmental limitation, drought has become a rising concern due to its harm to the development and productivity of crop plants17,18. Cassava is a major staple food to resource-limited people in marginal areas because of its ability to survive and produce in such poor land with infrequent rainfall and low fertility10,17,19. Although cassava is considered as a drought-tolerant crop, its root yield is easily threatened by water stress, especially under serious condition. Therefore, it is critical to screen drought-tolerant cassava germplasm and one of the modern breeding strategies is screening resources for tolerance to severe water deficit during the early period7. The present study by using four cassava genotypes can provide a fundamental basis for the identification of drought-tolerant germplasm resources.
Drought stress causes tissue water loss, which results in leaf wilting20. As a key factor for estimating plant drought tolerance, RWC is reduced by water deficit and the reduction varies from different genotypes21. A similar result was observed in this study. The reduction of RWC under drought was more pronounced in GR4, which showed that the water status in this genotype was more sensitive to drought stress than other genotypes. Water deficit also initiates the degradation of chlorophyll, lead to a decrease of photosynthetic product and eventually inhibit plant growth22. Chlorophyll content (SPAD) was frequently used to evaluate plant drought tolerance due to its high correlation with crop yield23–25. In the present study, reduction of chlorophyll content (SPAD) induced by drought was strongest in genotype GR4 while minimum in RS01, which is in accordance with previous findings of decreased levels of chlorophyll under drought stress in different plant species26,27.
RLC changes can reflect the degree of membrane damage in response to drought. Water deficit increased RLC in genotypes GR4, SC205 and SC124. However, no significant effect of drought on RLC was found in RS01, which showed that membrane injuries of this genotype were slight. MDA is usually used as a reliable marker to judge oxidative stress28. Drought stress significantly increased the MDA content of genotypes SC205 and GR4 whilst had no effect on RS01. Therefore, for RS01, the oxidative stress induced by drought was slight at the seedling stage. H2O2, as one crucial member of ROS induced by drought, increased significantly in the drought-affected cassava seedlings and this increase was stronger in genotypes SC205 and GR4 than RS01 and SC124. In a previous report, Wang et al.29 showed that the H2O2 concentration of apple plants was enhanced under drought stress and the greater increase was observed in the sensitive species, which is consistent with our findings.
Non-enzymatic mechanism plays a vital role in contracting oxidative stress and improving plant drought-tolerance30. Increased AsA and GSH content were induced by water deficiency in rapeseed seedlings31. Similarly, drought stress also caused an increase of AsA and GSH content in cassava seedlings except for unclear variation of GSH content in genotypes SC205 and GR4. Greater increases in both AsA and GSH content were observed in genotypes SC124 and RS01, which showed that both genotypes have a more efficient system for the biosynthesis of these antioxidants. In this experiment, drought stress also enhanced TP content in cassava seedlings. This result is supported by previous findings32. Genotypes SC205 and GR4 showed a stronger increase in TP content, which may be one key defense mechanism against oxidative stress induced by drought in these two genotypes. TF content significantly increased under medium drought whilst sharply declined after exposure to severe drought. This rapid consumption of TF compounds may be responsible for constraining the accumulation of H2O2 by cell33.
There is little doubt that antioxidant enzymes are another crucial member of defense mechanisms against oxidative damage induced by drought34. SOD, as the first frontline defense against oxidative injury, catalyzes the dismutation of O2− and generates H2O2, which is converted to H2O and O2 by CAT35. In this experiment, SOD and CAT activities were activated by water stress in all four cassava genotypes and the rate of increment was higher in RS01 and SC124. These results are supported by the previous report of higher SOD and CAT activities in drought-stressed caper seedlings36. Water deficit significantly depressed POD activity in all genotypes and the stronger depression was observed in genotypes GR4 and SC205, which is in line with the findings of a previous study37. Research showed that POD activity is related to the water retention of leaves38. Thus, the smaller reduction of RWC induced by drought in genotypes RS01 and SC124 may be attributed to the lower decrease of POD activity as compared with GR4 and SC205. APX activity has been reported to activate in bean cultivars, while, in some cases, is unchanged even declined in specific species39. For genotypes SC124 and RS01 in this study, an increase in APX activity was detected in some drought-stressed plants while unchanged in other water-deficient plants. Interesting, this enzyme activity was depressed by water stress in both genotypes SC205 and GR4. These results indicated that the changing trend of APX activity under drought was mainly depended on cassava genotypes. The scavenging of H2O2 mostly attributed to the activating of APX activity40. Therefore, the activation of APX activity in genotypes RS01 and SC124 may be responsible for the lower level of H2O2 under water deficit stress as compared to GR4 and SC205. The function of enzyme GR is catalyzing glutathione disulfide to GSH21. GR activity enhanced in all four genotypes after exposure to drought and the greater increase was observed in SC205 and GR4, which corroborated the results of previous reports36,41,42.
Genotypic difference in drought tolerance is one reason for the different ability to activate antioxidant defense in plants under severe drought43. The different trends of these non-enzymatic and enzymatic antioxidants in four cassava genotypes displayed distinct regulation mechanisms under drought-induced oxidative stress. For genotypes RS01 and SC124, this regulatory mechanism might be mostly attributed to the accumulation of AsA and GSH content and increased activities of SOD and CAT. However, SC205 and GR4 might depend on the accumulation of TP and increased GR activity to resist oxidative damage.
Drought stress can trigger a series of plant regulation, not only including the physiological and biochemical response but also containing the regulation of gene expression16. Studying the relationship between gene expression and stress tolerance can provide reliable information on understanding antioxidant gene activation44. In the present study, the expression of Mn-SOD in all four genotypes was upregulated and was in accordance with the SOD activity change. The highest increase in transcript level was observed in RS01 under severe drought, which showed that Mn-SOD might play an essential role in response to water deficit. These results are in line with the previous reports16,45,46. The transcriptional level of POD was inhibited by drought and was not consistent with the changes of POD activity in all genotypes except for GR4, which revealed that enzyme activity changes were regulated by the post-transcriptional level which in part might result in enzyme inactivation or degradation. In a previous study, Uzilday et al.47 found that CAT gene expression was correlated with CAT activity in cleome Espinosa whilst this correlation was not showed in Cleome gynandra. A similar result was observed in this experiment with the transcript level of CAT going along with the CAT activity only in genotypes SC124 and RS01. In general, the expression levels of APX and GR were correlated with APX and GR activity, respectively.
Conclusions
The cassava genotypes RS01 and SC124 were keeping higher RWC and relative chlorophyll content and less affected by oxidative stress at the seedling stage under drought stress. RS01 just showed slight membrane injuries and oxidative stress even under severe drought conditions. The water status of cassava plants was closely related to the antioxidant response. Different regulation mechanisms in the four genotypes in response to oxidative damage were shown by the different trends of antioxidant compounds and enzymes. The mechanism in genotypes RS01 and SC124 might mostly attribute to the increased accumulation of AsA and GSH content and higher SOD and CAT activities, which explained by the up-regulation of Mn-SOD and CAT genes. However, genotypes SC205 and GR4 might depend on the accumulation of TP and increased GR activity, which attributed to the up-regulation of GR gene.
Materials and Methods
Materials
Four cassava genotypes were used for the current study, viz., SC124, SC205, GR4, and RS01. These genotypes are widely planted in China.
Experiment design and sampling
The present experiment was conducted in the glasshouse at Guangxi University (GXU). The stem segments of four genotypes were planted into plastic pots (21 cm × 21 cm) on 30th of March 2018. Each pot was filled with the equal potting mixture (soil: sand: ballast at 2:1:1(v/v/v), respectively) before planting. All cassava shoots were well watered with 0.5 L of water every two days before the application of drought treatments. Dehydration stress treatment was imposed after 50 days of planting, at three different levels, i.e., 80% of field capacity (FC) (control), 50% of FC (medium) and 20% of FC (severe). Five replications were maintained for each genotype and treatment of drought stress. The soil water content was monitored using soil moisture measurement (SU-LPC, Beijing) on a daily basis. The third and fourth fully expanded leaves from each plant were collected after 7 days of treatment. All samples were frozen immediately in liquid nitrogen and then stored at −80 °C until this experiment was finished. Fresh leaf samples were also collected for analyzing the moisture content and the relative electrical conductivity.
The physiological parameters analysis
RWC measurement
The RWC was measured according to Barrs and Weatherley48. Fresh leaf samples (0.1 g, FW) were soaked for 24 h in deionized water and the turgor weight (TW) was calculated. The samples subsequently were dried at 80 °C to a constant dry weight (DW). The RWC was measured by using the following equation: Leaf RWC (%) = (FW-DW)/(TW-DW) × 100
SPAD values
chlorophyll meter (SPAD-502, Minolta, Japan) was used to determine the SPAD values of functional leaves, which can reflect the relative chlorophyll content. The fourth leaf of each plant was chosen for the determination of SPAD values.
RLC measurement
The RLC was measured as described by Chen et al.49. Leaf samples (1.0 g) were cut into about 50 mm2 before weighted. The samples then were incubated in 30 mL deionized water for 2 h at room temperature and kept in vacuum for 20 minutes. A conductivity meter (FE30/EL30, Shanghai) was used to measure electrical conductivity (I1). The samples subsequently were kept in a boiling water bath for 20 min. After the solution cooled to room temperature, the electrical conductivity (I2) was recorded and the RLC was calculated according to the equation: RLC (%) = I1/I2 × 100
MDA content
Leaf samples (0.5 g) were homogenized in 5 mL trichloroacetic acid (TCA, 0.1%). The homogenate was centrifuged at 11000 × g for 20 min. The supernatant was used for measuring the MDA and H2O2 content. MDA content was assayed by the method of Chu et al.50. The supernatant (2 mL) was added to 2 mL of 20% TCA containing 0.6% of the thiobarbituric acid (TBA). The solution was boiled for 30 min and then centrifuged at 4 000 × g for 5 min after cooling. The absorbance of the mixture was measured at 450 nm, 532 nm, and 600 nm. MDA content was estimated according to the following equation:
H2O2 content
H2O2 content was assayed following the method of Alexieva et al.51. The mixture contained supernatant (0.5 mL), 100 mM potassium phosphate buffer (0.5 mL) and 1 M KI (2 mL). Absorbance at 390 nm was measured after developing the mixture in darkness for 1 h. H2O2 content was calculated according to a standard curve.
Non-enzymatic antioxidants
Leaf samples (0.2 g) were homogenated with 6% meta-phosphoric acid containing 1 mM ethylenediaminetetraacetic acid (EDTA). The homogenate was centrifugated at 11000 × g for 20 min, and the supernatant was used for AsA and GSH content analysis.
AsA content was determined spectrophotometrically at 265 nm and calculated on a standard curve according to the method of Huang et al.52. GSH content was assayed as described by Hasanuzzaman and Fujita31. The reaction was measured at 412 nm and GSH content was calculated on the standard curve with a known concentration of GSH.
Leaf samples (1.0 g) were extracted in hydrochloric acid: methanol (v:v = 1:100). Total phenolic (TP) content was determined according to Blainski et al.53 with gallic acid used as a standard. The absorbance of the reaction was measured at 760 nm and TP content was calculated according to the standard concentration.
Total flavonoids (TF) were measured as described by Jia et al.54. Leaf samples (1.0 g) were cut into small pieces and extracted with 100 ml distilled water in a soxhlet extractor for one hour. The absorbance at 510 nm was recorded using catechin as standard.
Antioxidant enzyme activities
Leaf sample (0.2 g) were ground in 5 mL ice-cold phosphate buffer (50 mM, pH 7.8) containing 0.1 mM EDTA and 1% polyvinylpy. The homogenate was centrifuged at 11000 × g for 20 min at 4°C and the supernatant was used for analyzing the activities of SOD, POD, CAT, APX, and GR. The SOD activity was estimated adopting the nitroblue tetrazolium (NBT) method following Chu et al.50. 50 mM phosphate buffer (pH 7.8), 13.0 mM methionine, 10 µM nitroblue tetrazolium (NBT), 0.1 mM EDTA, 0.1 mM riboflavin and 50 µL of enzyme extraction were mixed and the absorbance was recorded at 560 nm. One unit of SOD was defined as the amount of enzyme needed to restrain 50% of NBT. The POD activity was assayed according to the rate of guaiacol oxidation at 470 nm for 3 min49. The reaction mixture included 50 mM phosphate buffer (pH 7.0), 28 mM guaiacol, 5 mM H2O2, and 50 µL of enzyme extraction. The CAT activity was determined following the method of Aebi55. The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 12.5 mM H2O2 and 50 µL of enzyme extraction. The decrease in absorbance was read at 240 nm for 3 min and the activity of CAT was calculated based on the rate of H2O2 consumption. APX activity was assayed according to oxidation of AsA at 290 nm for 1 min56. The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM AsA, 0.1 mM H2O2, and 100 µL of enzyme extraction. GR activity was measured following the method described by Hasanuzzaman and Fujita31. The reaction mixture included 0.1 M potassium phosphate buffer (pH 7.0), 1 mM EDTA, 0.2 mM NADPH, 1 mM GSSG, and 100 µL of enzyme extraction. The decreased absorbance was recorded at 340 nm for 1 min. The activity of GR was calculated basing on the rate of NADPH consumption.
Quantitative Real-Time PCR (qRT-PCR)
RNA prep Pure Plant Kit (Huayueyang, Beijing) was used for extracting total RNA of cassava seedling. The expression of Mn-SOD, POD, CAT, APX and GR genes was analyzed by the qRT-PCR. The cassava Actin gene was selected as the internal control. The gene-specific primers designed in this experiment were verified according to the melting curve and agarose gel electrophoresis (Table 2). All qRT-PCR experiments were performed with AceQ qPCR SYBR® Green Master Mix (Vazyme, Nanjing) on a Bio-Rad CFX96TM real-time instrument (Bio-Rad, USA). The relative expression of all genes with four replicates for each sample was calculated using 2−ΔCt method57.
Statistical analysis
SPSS (IBM SPSS Statistics 24.0) was used to analyze data, which was shown as the mean ± SE of four replicates and were tested by one-way ANOVA. The differences between the treatments were conducted by Duncan test (P < 0.05). The effect of genotype, treatment and their interaction were tested by a two-way ANOVA. In addition, PCA was used to correlate all data measured in this study, independently for each cassava genotype.
Acknowledgements
This work was supported by the Natural Science Foundation of Guangxi Zhuang Autonomous Region (2010GXNSFD013025), Guangxi Scientific and Technological Project (GKG1222014, GKG14121005-2-1) and National Natural Science Foundation of China (U1033004-09), State Key Laboratory of Protection and Utilization of Subtropical Agricultural Biological Resources Project (SKLCOSA-b201609) and (SKLCUSA-b201704), State Key Laboratory of Protection and Utilization of Subtropical Agricultural Biological Resources Independent Project (SKLCUSA-a201802), Guangxi Science and Technology Planning Projection (AB18221127) and Innovation Project of Guangxi Graduate Education (YCBZ2018013).
Author contributions
X.L. initiated and designed the experiment, Y.Z., J.Y., and J.Y. performed the experiments and collected the data, Y.Z. analyzed the data and wrote the manuscript, X.L. and G.N. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fang YJ, Xiong LZ. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol. Life Sci. 2015;72:673–689. doi: 10.1007/s00018-014-1767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjum SA, et al. Growth and developmental responses of crop plants under drought stress: a review. Zemdirbyste-Agriculture. 2017;104:267–276. doi: 10.13080/z-a.2017.104.034. [DOI] [Google Scholar]
- 3.Caser M, et al. Ecophysiological and phytochemical responses of Salvia sinaloensis Fern. to drought stress. Plant. Growth Regul. 2018;84:383–394. doi: 10.1007/s10725-017-0349-1. [DOI] [Google Scholar]
- 4.Kirigwi FM, et al. Evaluation of selection strategies for wheat adaptation across water regimes. Euphytica. 2004;135:361–371. doi: 10.1023/B:EUPH.0000013375.66104.04. [DOI] [Google Scholar]
- 5.El-Sharkawy MA. Cassava biology and physiology. Plant. Mol. Biol. 2004;56:481–501. doi: 10.1007/s11103-005-2270-7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TLT, Gheewala SH, Garivait S. Full chain energy analysis of fuel ethanol from cassava in Thailand. Env. Sci. Technol. 2007;41:4135–4142. doi: 10.1021/es0620641. [DOI] [PubMed] [Google Scholar]
- 7.Okogbenin E, et al. Phenotypic approaches to drought in cassava: review. Front. Physio. 2013;l4:93. doi: 10.3389/fphys.2013.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho MHCD. Drought stress and reactive oxygen species. Plant. Signal. Behav. 2008;3:156–165. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tafur SMD, El-Sharkawy MA, Cadavid LF. Response of cassava (Manihot esculenta Crantz) to water stress and fertilization. Photosynthetica. 1997;34:233–239. doi: 10.1023/A:1006892607834. [DOI] [Google Scholar]
- 10.Zhao PJ, et al. Analysis of different strategies adapted by two cassava cultivars in response to drought stress: ensuring survival or continuing growth. J. Exp. Bot. 2015;66:1477–1488. doi: 10.1093/jxb/eru507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. N. Phytol. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant. Sci. 2002;7:405–410. doi: 10.1016/S1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 13.Abogadallah GM. Antioxidative defense under salt stress. Plant. Signal. Behav. 2010;5:369–374. doi: 10.4161/psb.5.4.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu LL, et al. Physiological investigation and transcriptome analysis of polyethylene glycol (PEG)-induced dehydration stress in cassava. Int. J. Mol. Sci. 2016;17:283. doi: 10.3390/ijms17030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munns R. Genes and salt tolerance: bring them together. N. Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheoran S, et al. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat(Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 2015;177:1282–1298. doi: 10.1007/s12010-015-1813-x. [DOI] [PubMed] [Google Scholar]
- 17.Putpeerawit P, Sojikul P, Thitamade S, Narangajavana J. Genome-wide analysis of aquaporin gene family and their responses to water-deficit stress conditions in cassava. Plant. Physiol. Bioch. 2017;121:118–127. doi: 10.1016/j.plaphy.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Sedaghat M, Tahmasebi-Sarvestani Z, Emam Y, Mokhtassi-Bidgoli A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant. Physiol. Bioch. 2017;119:59–69. doi: 10.1016/j.plaphy.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Turyagyenda LF, et al. Physiological and molecular characterization of drought responses and identification of candidate tolerance genes in cassava. AoB Plants. 2013;5:plt007. doi: 10.1093/aobpla/plt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Zhou G, Shimizu H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009;60:3737–3749. doi: 10.1093/jxb/erp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defense and methylglyoxal detoxification systems. AoB Plants. 2015;7:plv069. doi: 10.1093/aobpla/plv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L, et al. Ecophysiological responses of calcicole cyclobalanopsis glauca (Thunb.) oerst. to drought stress and calcium supply. Forest. 2018;11:667. doi: 10.3390/f9110667. [DOI] [Google Scholar]
- 23.Songsri P, et al. Heritability of drought resistance traits and correlation of drought resistance and agronomic traits in peanut. Crop. Sci. 2008;6:2245–2253. doi: 10.2135/cropsci2008.04.0228. [DOI] [Google Scholar]
- 24.Khadem SA, et al. Effect of animal manure and superabsorbent polymer on corn leaf relative water content, cell membrane stability and leaf chlorophyll content under dry condition. Aust. J. Crop. Sci. 2010;8:642–647. [Google Scholar]
- 25.Rolando JL, et al. Leaf greenness as a drought tolerance related trait in potato (Solanum tuberosum L.) Env. Exp. Bot. 2015;110:27–35. doi: 10.1016/j.envexpbot.2014.09.006. [DOI] [Google Scholar]
- 26.Fotovat. R, Valizadeh M, Toorchi M. Association between water-use efficiency components and total chlorophyll content (SPAD) in wheat (Triticum aestivum L.) under well-watered and drought stress conditions. J. Food Agric. Environ. 2007;5:225–227. [Google Scholar]
- 27.Farhat N, et al. Recovery aptitude of the halophyte Cakile maritima upon water deficit stress release is sustained by extensive modulation of the leaf proteome. Ecotox Env. Safe. 2019;179:198–211. doi: 10.1016/j.ecoenv.2019.04.072. [DOI] [PubMed] [Google Scholar]
- 28.Del Rio LA, et al. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem. Soc. T. 1996;24:434–438. doi: 10.1042/bst0240434. [DOI] [PubMed] [Google Scholar]
- 29.Wang SC, et al. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant. Physiol. Bioch. 2012;51:81–89. doi: 10.1016/j.plaphy.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Dubey RS. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant. Growth Regul. 2005;46:209–221. doi: 10.1007/s10725-005-0002-2. [DOI] [Google Scholar]
- 31.Hasanuzzaman M, Fujita M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhance d tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011;143:1758–1776. doi: 10.1007/s12011-011-8998-9. [DOI] [PubMed] [Google Scholar]
- 32.Al Hassan M, et al. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants. 2017;9:plx009. doi: 10.1093/aobpla/plx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellegrini E, et al. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total. Environ. 2018;647:390–399. doi: 10.1016/j.scitotenv.2018.07.413. [DOI] [PubMed] [Google Scholar]
- 34.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Bioch. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Casas P, Klessig DF. A salicylic acid-binding activity and a salicylic acid-inhibitable catalase activity are present in a variety of plant species. Plant. Physiol. 1994;1006:1675–1679. doi: 10.1104/pp.106.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozkur O, Ozdemir F, Bor M, Turkan I. Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Env. Exp. Bot. 2009;3:487–492. doi: 10.1016/j.envexpbot.2009.04.003. [DOI] [Google Scholar]
- 37.Tian ZG, et al. Antioxidant mechanism and lipid peroxidation patterns in leaves and petals of marigold in response to drought stress. Hortic. Env. Biote. 2012;53:183–192. doi: 10.1007/s13580-012-0069-4. [DOI] [Google Scholar]
- 38.Mercado JA, et al. Changes in the water binding characteristics of the cell walls from transgenic Nicotiana tabacum leaves with enhanced levels of peroxidase activity. Physiol. Plantarum. 2004;122:504–512. doi: 10.1111/j.1399-3054.2004.00429.x. [DOI] [Google Scholar]
- 39.Saglam A, Saruhan N, Terzi R, Kadioglu A. The relations between antioxidant enzymes and chlorophyll fluorescence parameters in common bean cultivars differing in sensitivity to drought stress. Russian J. Plant. Physiol. 2011;1:60–68. doi: 10.1134/S102144371101016X. [DOI] [Google Scholar]
- 40.Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant. Physiol. 2011;155:12–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alam MM, Nahar K, Hasanuzzaman M, Fujita M. Exogenous jasmonic acid modulates the physiology, antioxidant defense, and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant. Biotechnol. Rep. 2014;3:279–293. doi: 10.1007/s11816-014-0321-8. [DOI] [Google Scholar]
- 42.Ma YH, Ma FW, Wang YH, Zhang JK. The responses of the enzymes related with ascorbate-glutathione cycle during drought stress in apple leaves. Acta Physiol. Plant. 2011;1:173–180. doi: 10.1007/s11738-010-0535-5. [DOI] [Google Scholar]
- 43.Khanna-Chopra R, Selote DS. Acclimation to drought stress generates oxidative stress tolerance in drought-resistant than -susceptible wheat cultivar under field conditions. Env. Exp. Bot. 2007;60:276–283. doi: 10.1016/j.envexpbot.2006.11.004. [DOI] [Google Scholar]
- 44.Lin KH, Huang HC, Lin CY. Cloning, expression and physiological analysis of broccoli catalase gene and Chinese cabbage ascorbate peroxidase gene under heat stress. Plant. Cell Rep. 2010;6:575–593. doi: 10.1007/s00299-010-0846-4. [DOI] [PubMed] [Google Scholar]
- 45.Baek KH, Skinner DZ. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant. Sci. 2003;165:1221–1227. doi: 10.1016/S0168-9452(03)00329-7. [DOI] [Google Scholar]
- 46.Wang X, et al. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J. Plant. Physiol. 2005;162:465–472. doi: 10.1016/j.jplph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Uzilday B, et al. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra and Cleome spinosa under drought stress. Plant. science: an. Int. J. Exp. plant. Biol. 2012;182:59–70. doi: 10.1016/j.plantsci.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Barrs HD, Weatherley PE. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aus J. Biol. Sci. 1962;15:413–428. doi: 10.1071/BI9620413. [DOI] [Google Scholar]
- 49.Chen ZF, et al. Abscisic acid and brassinolide combined application synergistically enhance drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hort. 2018;228:1–9. doi: 10.1016/j.scienta.2017.10.004. [DOI] [Google Scholar]
- 50.Chu XT, et al. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus mutants Griseb. S Afr. J. Bot. 2016;104:21–29. doi: 10.1016/j.sajb.2015.10.001. [DOI] [Google Scholar]
- 51.Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant. Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 52.Huang C, et al. Increase sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005;56:3041–3049. doi: 10.1093/jxb/eri301. [DOI] [PubMed] [Google Scholar]
- 53.Blainski A, Lopes GC, Mello LCPD. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852–6865. doi: 10.3390/molecules18066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia ZS, Tang MC, Wu JM. The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 55.Aebi H. Catalase in vitro. Method. Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 56.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell Physeol. 1981;22:867–880. [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]