Abstract
Recent experimental study shows that the pre-lithiated MoS2 monolayer exhibits an enhanced electrochemical performance, coulombic efficiency of which is 26% higher than the pristine MoS2 based anode. The underlying mechanism of such significant enhancement, however, has not yet been addressed. By means of density functional theory (DFT) calculations, we systematically investigated the adsorption and diffusion behavior of lithium (Li) atoms on the MS2 (M = Mo, W, V) monolayers. On the pre-lithiated MS2 monolayers, the adsorption energy of extra Li ions are not significantly changed, implying the feasibility of multilayer adsorption. Of importance, the Li diffusion barriers on pre-lithiated MS2 are negligibly small because of the charge accumulation between the diffusing Li ions and the pre-lithiating Li layer. Correspondingly, we report that the pre-lithiation should be a general treatment which can be employed on many transition-metal di-chalcogenides to improve their storage capacities and charge-discharge performance in Li ion batteries. In addition, we propose that the pre-lithiated VS2 may serve as an outstanding anode material in LIBs.
Subject terms: Density functional theory, Materials for energy and catalysis
Introduction
The Lithium-ion battery (LIB) has been regarded as one of the most indispensable and promising devices in the fields of telecommunications, electric automobiles and electric power grids1,2. Today, graphite is widely used as the anode material of commercial LIBs owing to its layered structure, good electric conductance and excellent chemical stabilities3,4. Nevertheless, the maximum specific capacity of lithium ions of graphite (LiC6) is only 372 mA∙h ∙ g−1. As a result, numerous researches have been devoted to the searches of new anode materials with higher energy densities1,5–7. In addition to the specific capacity, columbic efficiency has also been employed to evaluate the performance of electrodes in LIBs. Thus, an ideal anode material, should not only accommodate densely packed Li ions, but also allow for fast Li diffusions to promote the charge-discharge rate1,8–10. In the past decade, a number of two-dimensional (2D) materials, including transition-metal oxides, di-chalcogenides (MO2 and MS2) and BN, have been successfully synthesized11–13. Their electronics properties and potential applications in devices have also been explored and proposed as electrode material for LIBs14–20. Very recently, Yang et al. report that the coulombic efficiency of MoS2 can be significantly improved by the pre-lithiation treatment, in which the MoS2 is on direct contact with lithium foils21. Despite the improved performance of MoS2 upon pre-lithiation, the underlying mechanism however, has not yet been addressed. Herein, systematic Density Functional Theory (DFT) calculations have been conducted to explore (i) the chemical insights of the enhanced performance after pre-lithiathion, and (ii) the effect of pre-lithiathion on other MS2 nanosheets. Our results revealed that the pre-lithiathion allows for multilayer adsorption and fast diffusion of Li ions on the MS2. In addition, pre-lithiathion may serve as a general treatment for improving the performance of MS2 anode in LIB. Last but not least, the VS2 monolayer provides relatively high Li binding strength, negligibly small Li diffusion barriers, and large theoretical capacity comparing with MoS2 and WS2 counterparts. We thus propose that the pre-lithiated VS2 monolayer is an outstanding anode material for LIBs. These results may open up a new avenue for the development of the next-generation high-performance LIBs.
Computational Details
The DFT calculations were carried out with the Vienna ab-initio Simulation Package (VASP) code22–25. The projector augmented-wave potentials (PAW)26 and the Perdew–Burke–Ernzerhof (PBE) generalized gradient approximation (GGA) functional25,27 were used to describe the electron–ion interactions and electronic exchange correlations, respectively. The effect of on-site Coulomb interactions on the binding of Li ions on the MoS2, MoSe2, WS2 and WSe2 have been investigated by previous theatrical studies28. It was shown that the binding energy, binding height and diffusion barriers of Li ions are not significantly affected by the on-site Coulomb interactions. Correspondingly, the PBE functional was selected in this work. The conjugate gradient scheme was used to relax all atomic positions and lattice constants until the components of the forces on each atom is of the order of 10−3 eV Å−1. A plane-wave basis set with kinetic energy cutoff is set as 500 eV to ensure the accuracy of the simulation results. The number of K-mesh was (16 × 16 × 1) for the primitive MS2 unit cell and scaled according to the size of the supercells in the total energy and self-consistent-field (SCF) potential calculations. Based on the primitive cell (1 × 1), different supercells including (2 × 1), (2 × 2), (3 × 3), and (4 × 4), hexagonal structures as the ideal models are used to analyze the adsorption of lithium. The corresponding Brillouin zones of the (2 × 1), (2 × 2), (3 × 3), and (4 × 4) supercells are sampled with the Γ-centered k-point grid of 9 × 9 × 1, 8 × 8 × 1, 6 × 6 × 1, and 2 × 2 × 1, respectively. The lattice constants of MoS2 (3.186 Å), WS2 (3.186 Å) and VS2 (3.236 Å) were obtained from our DFT calculations. These lattice constants are in good agreement with the experimental values29–33. A vacuum of 20 Å along the z-axis was applied to prevent interlayer interactions from transnationally periodic images. The Climbing Image Nudged Elastic Band (CI-NEB) method was used to find the saddle points and minimum energy paths between the initial and final states34–36.
Results and Discussion
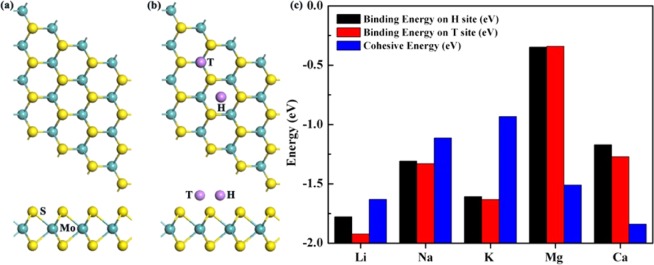
In two-dimensional transition-metal di-chalcogenides, the atomic layer of metal elements are sandwiched between two S layers. As shown in Fig. 1(a), the Mo−S bond length of the 2H-MoS2 is 2.41 Å, and the Mo−S − Mo bond angle is 80.68°, agreeing well the previous theoretical results37. Two binding sites are considered for analyzing the adsorption of Li ions on the MoS2. The top site (T site) is directly above one Mo atom, while the hollow site (H site) is above the center of a hexagon, as shown in Fig. 1(b). We have also examined the other possible adsorption sites (e.g. above the S atom), however, the adsorbed Li ion is observed to move to the neighboring T site after structural relaxation. The binding energy of metal atoms on the MS2 is defined as:
| 1 |
Figure 1.
The top and side views of the optimized structures of (a) a MoS2 monolayer and (b) the top (T) and hollow (H) binding sites of a metal ion adsorbed on the MoS2 monolayer. The Mo atoms, S atoms and the binding sites are represented with green, yellow and purple circles, respectively. (c) The binding energies and metal cohesive energies of Li, Na, K, Mg, Ca on MoS2.
The EnLi-MS2 is the total energy of the coupled structure, in which n Li ions adsorbing on the MS2. ELi is the energy of an isolated Li atom in a vacuum. EMS2 is the energy of an isolated MS2 monolayer. And n is the number of adsorbed Li atoms. According to such definition, a more negative binding energy indicates a more favorable exothermic interaction between MS2 and Li atoms. As shown in Fig. 1, the adsorption of a Li ion at the T site (−1.94 eV) is more stable than that on the H site (−1.78 eV), with a Li-S distance being 2.37 Å, consisting well with previous theoretical studies28,37. In addition to Li ions, the adsorption of other metal elements which possess potential barrier applications have also been calculated. The binding energies of different adsorbing atoms and their corresponding cohesive energies are shown in Fig. 1(c). It can be seen that the binding of Li, Na and K atoms on MoS2 are stronger than the metallic bonds in their bulk structures. This suggests that the MoS2 may also be employed as anode materials for Na and K ion batteries.
Subsequently, the Li storage capacities of MS2 monolayer (M = Mo, W, V) were investigated. A series of Li/MS2 configurations with different stoichiometry of LixMS2 (x = 0.125, 0.222, 0.500, 1.000, and 2.000) were constructed by adding one Li ion on each side of the (4 × 4), (3 × 3), (2 × 2), (2 × 1) and (1 × 1) supercells, respectively. As shown in Fig. 2, the binding energies of Li ions decreases with increasing Li coverages. It is worthy to note that the Li binding energies on VS2 are much larger than on other MS2. When x = 2, full Li coverages are achieved on both sides of MS2. It is seen that the averaged binding energies of Li ions on fully covered VS2, MoS2 and WS2 are −2.58 eV, −1.35 eV and −1.56 eV, respectively. This indicates strong attractive interactions between Li ions and MS2 monolayers at the full coverage.
Figure 2.
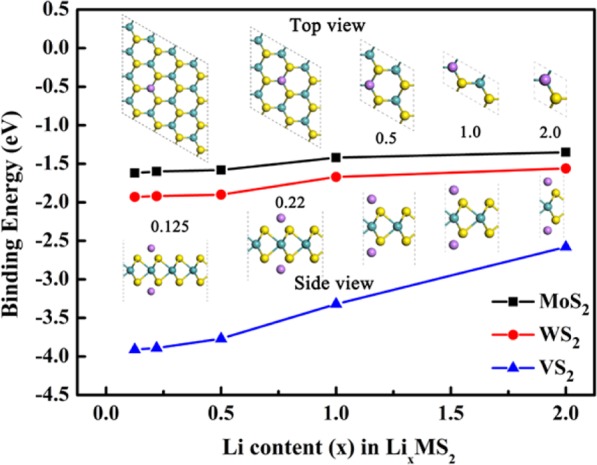
The top and side views of LixMS2 and their averaged Li binding energies on MS2 monolayers.
The Li2MS2 represents the highest Li storage capacity on bare MS2. At this coverage, the theoretical capacity can be calculated with the following equation:
| 2 |
here c is the number of adsorbed cations on a MS2 unit and n is the valence state of fully ionized cations from electrolyte, F is the Faraday constant (26801 mA∙h∙mol−1), and the molar weight of MS2. In this case, c is 2 at the full coverage, and n is 1for Li ions. Correspondingly, for the adsorption capacities are 334.87, 256.49 and 465 mA∙h ∙ g−1 for the pristine MoS2, WS2 and VS2 monolayers, respectively.
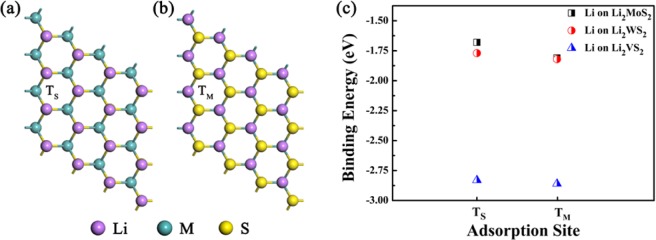
Previous experimental studies show that the pre-lithiated MoS2 monolayer exhibit better performance compared with the pristine MoS221. In order to obtain an in-depth understanding, the adsorption and diffusion of extra Li atoms on the pre-lithiated MS2 are investigated. Firstly, as shown in Fig. 3, two possible pre-lithiated configurations have been considered, the layered Li atoms prefer to adsorb above the TM site of the MS2 monolayer with the binding energies being −1.81 eV (MoS2), −1.82 eV (WS2), and −2.86 eV (VS2), respectively. The corresponding Li-S distances are 2.45 Å (MoS2), 2.51 Å (WS2) and 2.32 Å (VS2).
Figure 3.
The top and side views of the pre-lithiated MS2 monolayer (M = Mo, W, V), with one Li atom adsorbing (a) above the S atoms (TS site) and (b) above each metal atoms (TM site). (c) The binding energies of a full coverage of Li atoms adsorbing on MS2 monolayers at the TS and TM sites.
Figure 4 shows the configurations and corresponding binding energies of extra Li atoms adsorbing the pre-lithiated VS2 (Li2VS2) monolayer with various coverages. As seen, the binding energies of the Li ions on Li2VS2 monolayer decreases gradually with the elevation of the related storage ratio (x). On the pre-lithiated VS2, the Li ions used for pre-lithiation are assumed anchored on the VS2 and thus the Li storage capacity is defined as:
| 3 |
maximum theoretical capacity of the Li atoms on the pre-lithiated MS2 (M = Mo, W, V) monolayers were 308.14, 204.70 and 415.67 mA∙h ∙ g−1 respectively. Thus, from the point of the binding energy and the theoretical capacity, Li2VS2 is relatively more suitable for LIBs anode materials for the higher binding energy and theoretical storage capacity.
Figure 4.
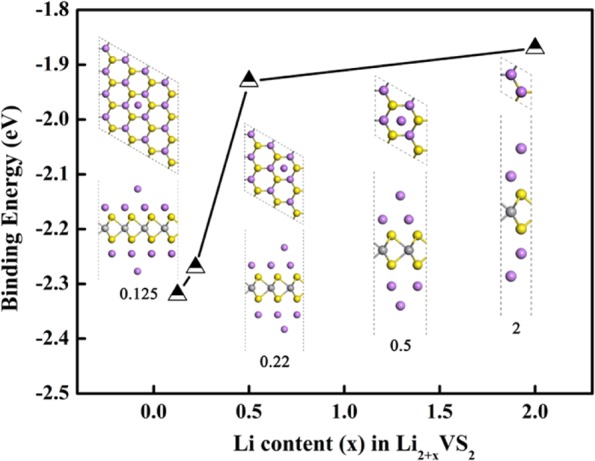
The trends of the binding energies of the Li ion adsorbing on the pre-lithiated VS2 (Li2VS2) with increasing Li coverages.
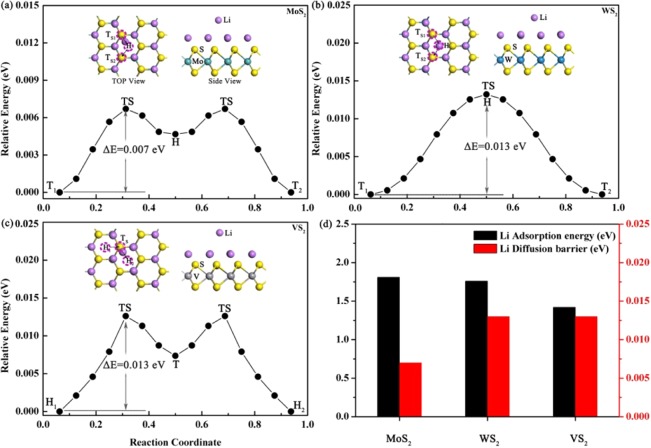
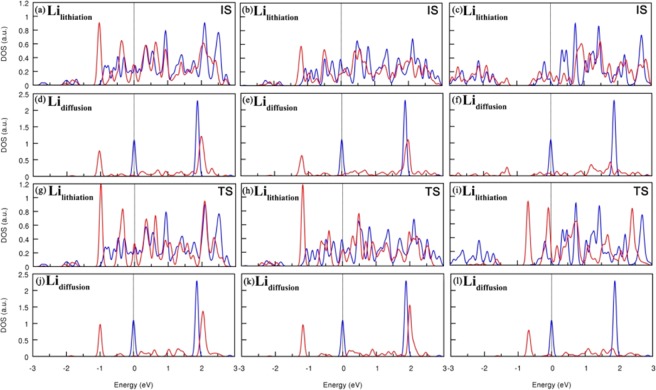
The performance of an electrode material is closely related the mobility of the adsorbed Li ions38. In general, a lower diffusion barrier means a higher diffusion rate39,40. Thus it is necessary to study the diffusion behavior Li ions when the Li2MS2 monolayers are used as the substrates. The migrations of the Li atom among the T site and the H site are studied using the CI-NEB method. The red circles and black arrows in Fig. 5 represent the diffusion pathway of the Li atom from the most stable adsorption site (T site or H site) to the next equivalent stable adsorption site. As seen in Fig. 5(a–c), when Li ions only need to overcome very small energy barriers to diffuse on the pre-lithiated MS2. Taking into account that the Li diffusion barriers in graphite (0.22 eV) and the pristine MS2 (0.22 eV) (M = Mo, W, V) are much higher than those on the he pre-lithiation MS23,28,37,41,42, we can conclude that the pre-lithiation is an effective treatment for MS2 to achieve enhanced charge-discharge rates. The effect of Li diffusion barriers to the charge-discharge rates can be roughly estimated with the Arrhenius equation, D ∝ exp(−Ebarrier/kBT), where Ebarrier and kB are the Li diffusion barrier and Boltzmann constant. T is the temperature43. As can be seen, the diffusion constant increases exponentially with the decreasing diffusion barrier at a constant temperature. Please note that on the three Li2MS2 substrates, the pre-lihtiated VS2 monolayer is the most optimized anode material in terms of high Li binding energy and low diffusion barrier and the high Li adsorption capacity. Figure 6 summarizes the LDOS of the initial states (IS) and transition states (TS) of Li diffusion on Li2MS2 monolayers.
Figure 5.
The energy profiles of Li diffusion on the pre-lithiated (a) MoS2, (b)WS2, and (c)VS2. (d) The Li binding energy at T site and diffusion barriers.
Figure 6.
The corresponding local densities of states (LDOS) of the initial states (IS) and transition states (TS) of Li diffusion on Li2MS2 monolayers. (a–c) are the LDOS of the pre-lithiated Li atoms with (blue curve) and without (red curve) an additionally adsorbed Li atom. (d–f) are the LDOS of a Li atom in the vacuum (blue curve) and on the Li2MS2 surfaces (red curve) of the IS structures. (g–i) and (j–l) are the LDOS of corresponding Li ions of the transition states.
One of important factors for estimating the performance of LIB anode materials is the electric conductivity. Many pristine MS2 are semiconductors with large band gaps, implying poor electric conductivity44–46. As seen, all Li2MS2 monolayers are conducting materials. More detailed analysis of the LDOS plots shows that when a Li ion adsorbs on the Li2MS2 monolayer, the electronic states of Li ions are more hybridized, indicating that the interactions between the adsorbed Li and pre-lithiating Li layer are chiefly metallic bonding. This is consisted with the previous theoretical investigates47.
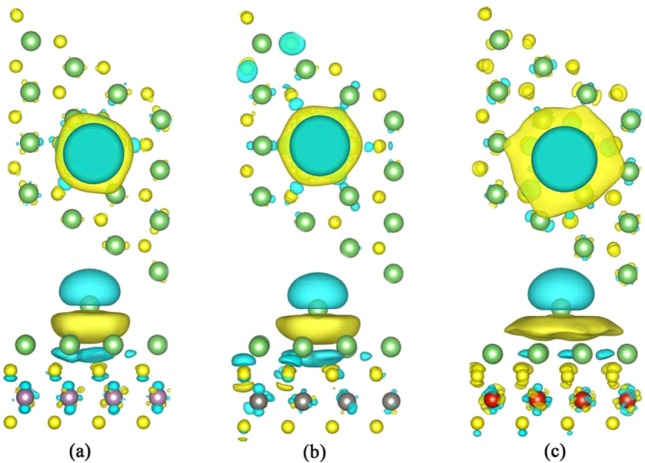
The differential charge densities were calculated in order to identify the bonding characteristics between the diffusing Li ion and the Li2MS2 substrates. As clearly shown in Fig. 7, the electrons are accumulated between the diffusing Li ion and the Li2MS2. In addition, the areas of such charge accumulations expand on three neighing Li ions in the Li2MS2, indicating that the accumulated electrons are delocalized. This agree well with the PDOS analysis that the interactions are metallic bonding. As a result, the migration of the diffusing Li ion does not need to break the Li-Li2MS2 bonds. Correspondingly, the Li diffusion barrier on Li2MS2 should be very small, which is in good consistence with our CI-NEB calculations.
Figure 7.
The top and side views of the differential charge densities of the transition states of the diffusing Li atom on the (a) Li2MoS2, (b) Li2WS2 and (c) Li2VS2 monolayers. The light blue and yellow contours (isosurface = 0.001 e/Å3) represent the charge deletion and charge aggregation, respectively.
Conclusions
In conclusion, the adsorption of Li ions on the surface of the pristine/pre-lithiated MS2 monolayer (Li2MS2, M = Mo, W, V) are systematically investigated. Our calculations showed that the optimal adsorption sites of Li ions on the pristine MS2 is the on-top site of the metal atoms. A pre-lithiating Li layer is formed when all the on-top sites are occupied by a Li ion. The pre-lithiation of MS2 (M = W and V) will enhance the adsorption and diffusion of Li ions. Although the Li binding energy on the clean MS2 and the pre-lithiation are not significantly different, the Li diffusion barriers on the pre-lithiated MS2 are much less than those on the clean MS2, implying a fast charge-discharge property. In particular, we report that the pre-lithiated VS2 is a very promising anode materials in the Li ion barriers, due to strong Li binding interactions and negligibly small Li diffusion barriers on the Li2VS2. Thus, this work not only interprets the in-depth working principles of the reported pre-lithiation for MoS2, but also propose that the pre-lithiated VS2 may serve as one of the best anode materials in LIBs.
Acknowledgements
The work is supported by the National Key R&D Program of China (Grants No. 2017YFB0701600 and 2017YFA0204800), National Natural Science Foundation of China (Grant No. 21771134, 51761145013, 21673149), the Collaborative Innovation Center of Suzhou Nano Science & Technology, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the 111 Project and the Joint International Research Laboratory of Carbon-Based Functional Materials and Devices.
Author contributions
H.L. and Y.L. conceived the main idea. T.L., Z. J., D.L., C.D. and L.W. performed all the calculation work. All authors analyzed the results and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Haiping Lin, Email: hplin@suda.edu.cn.
Youyong Li, Email: yyli@suda.edu.cn.
References
- 1.Tarascon JM, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature. 2001;414:359–367. doi: 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Wang Z, Chen L, Huang X. Research on Advanced Materials for Li-ion Batteries. Advanced Materials. 2009;21:4593–4607. doi: 10.1002/adma.200901710. [DOI] [Google Scholar]
- 3.Persson K, et al. Lithium Diffusion in Graphitic Carbon. Journal of Physical Chemistry Letters. 2010;1:1176–1180. doi: 10.1021/jz100188d. [DOI] [Google Scholar]
- 4.Winter M, Besenhard JO, Spahr ME, Novák P. Insertion Electrode Materials for Rechargeable Lithium Batteries. Advanced Materials. 1998;10:725–763. doi: 10.1002/(SICI)1521-4095(199807)10:10<725::AID-ADMA725>3.0.CO;2-Z. [DOI] [Google Scholar]
- 5.Nishi Y. Lithium ion secondary batteries; past 10 years and the future. Journal of Power Sources. 2001;100:101–106. doi: 10.1016/S0378-7753(01)00887-4. [DOI] [Google Scholar]
- 6.Kganyago K, Ngoepe P. Structural and electronic properties of lithium intercalated graphite LiC6. Physical Review B. 2003;68:205111. doi: 10.1103/PhysRevB.68.205111. [DOI] [Google Scholar]
- 7.Valencia F, Romero AH, Ancilotto F, Silvestrelli PL. Lithium Adsorption on Graphite from Density Functional Theory Calculations. The Journal of Physical Chemistry B. 2006;110:14832–14841. doi: 10.1021/jp062126+. [DOI] [PubMed] [Google Scholar]
- 8.Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D. Challenges in the development of advanced Li-ion batteries: a review. Energy Environmental Science. 2011;4:3243–3262. doi: 10.1039/c1ee01598b. [DOI] [Google Scholar]
- 9.Sides CR, Martin CR. Nanostructured Electrodes and the Low-Temperature Performance of Li-Ion Batteries. Advanced Materials. 2005;17:125–128. doi: 10.1002/adma.200400517. [DOI] [Google Scholar]
- 10.Winter M, Brodd RJ. What are batteries, fuel cells, and supercapacitors? (vol 104, pg 4245, 2003) Chemical Reviews. 2005;105:1021–1021. doi: 10.1021/cr040110e. [DOI] [PubMed] [Google Scholar]
- 11.Nicolosi, V., Chhowalla, M., Kanatzidis, M. G., Strano, M. S. & Coleman, J. N. Liquid Exfoliation of Layered Materials. Science340 (2013).
- 12.Geim AK, Grigorieva IV. Van der Waals heterostructures. Nature. 2013;499:419–425. doi: 10.1038/nature12385. [DOI] [PubMed] [Google Scholar]
- 13.Koski KJ, Cui Y. The New Skinny in Two-Dimensional Nanomaterials. ACS Nano. 2013;7:3739–3743. doi: 10.1021/nn4022422. [DOI] [PubMed] [Google Scholar]
- 14.Wang QH, Kalantar-Zadeh K, Kis A, Coleman JN, Strano MS. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nature Nanotechnology. 2012;7:699–712. doi: 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Connell JW. Advances in 2D boron nitride nanostructures: nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale. 2012;4:6908–6939. doi: 10.1039/c2nr32201c. [DOI] [PubMed] [Google Scholar]
- 16.Seo J-w, et al. Two-Dimensional Nanosheet Crystals. Angewandte Chemie-International Edition. 2007;46:8828–8831. doi: 10.1002/anie.200703175. [DOI] [PubMed] [Google Scholar]
- 17.Hwang H, Kim H, Cho J. MoS2 Nanoplates Consisting of Disordered Graphene-like Layers for High Rate Lithium Battery Anode Materials. Nano Letters. 2011;11:4826–4830. doi: 10.1021/nl202675f. [DOI] [PubMed] [Google Scholar]
- 18.Ding S, Zhang D, Chen JS, Lou XW. Facile synthesis of hierarchical MoS2 microspheres composed of few-layered nanosheets and their lithium storage properties. Nanoscale. 2012;4:95–98. doi: 10.1039/C1NR11552A. [DOI] [PubMed] [Google Scholar]
- 19.Du G, et al. Superior stability and high capacity of restacked molybdenum disulfide as anode material for lithium ion batteries. Chemical Communications. 2010;46:1106–1108. doi: 10.1039/B920277C. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, et al. Highly Ordered Mesoporous MoS2 with Expanded Spacing of the (002) Crystal Plane for Ultrafast Lithium Ion Storage. Advanced Energy Materials. 2012;2:970–975. doi: 10.1002/aenm.201200087. [DOI] [Google Scholar]
- 21.Wang Y, et al. Pre-lithiation of onion-like carbon/MoS2 nano-urchin anodes for high-performance rechargeable lithium ion batteries. Nanoscale. 2014;6:8884–8890. doi: 10.1039/C4NR01553C. [DOI] [PubMed] [Google Scholar]
- 22.Kresse G, Hafner J. Ab initio molecular dynamics for liquid metals. Physical Review B. 1993;47:558–561. doi: 10.1103/PhysRevB.47.558. [DOI] [PubMed] [Google Scholar]
- 23.Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B. 1999;59:1758–1775. doi: 10.1103/PhysRevB.59.1758. [DOI] [Google Scholar]
- 24.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical Review B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 25.Kresse G, Furthmuller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Computational Materials Science. 1996;6:15–50. doi: 10.1016/0927-0256(96)00008-0. [DOI] [Google Scholar]
- 26.Blöchl PE. Projector augmented-wave method. Physical Review B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 27.Perdew JP, Burke K, Ernzerhof M. Generalized Gradient Approximation Made Simple. Physical Review Letters. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 28.Jing Y, Zhou Z, Cabrera CR, Chen Z. Metallic VS2 Monolayer: A Promising 2D Anode Material for Lithium Ion Batteries. Journal of Physical Chemistry C. 2013;117:25409–25413. doi: 10.1021/jp410969u. [DOI] [Google Scholar]
- 29.Komsa H-P, Krasheninnikov AV. Effects of confinement and environment on the electronic structure and exciton binding energy of MoS2 from first principles. Physical Review B. 2012;86:241201. doi: 10.1103/PhysRevB.86.241201. [DOI] [Google Scholar]
- 30.Zhuang, H. L. L., Singh, A. K. & Hennig, R. G. Computational discovery of single-layer III-V materials. Physical Review B87 (2013).
- 31.Zhu, Z. Y., Cheng, Y. C. & Schwingenschloegl, U. Giant spin-orbit-induced spin splitting in two-dimensional transition-metal dichalcogenide semiconductors. Physical Review B84 (2011).
- 32.Liao, J., Sa, B., Zhou, J., Ahuja, R. & Sun, Z. Design of High-Efficiency Visible-Light Photocatalysts for Water Splitting: MoS2/AlN(GaN) Heterostructures. Journal of Physical Chemistry C118 (2014).
- 33.Debbichi, L., Eriksson, O. & Lebegue, S. Electronic structure of two-dimensional transition metal dichalcogenide bilayers from ab initio theory. Physical Review B89 (2014).
- 34.Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. Journal of Chemical Physics. 2000;113:9901–9904. doi: 10.1063/1.1329672. [DOI] [Google Scholar]
- 35.Olsen RA, Kroes GJ, Henkelman G, Arnaldsson A, Jónsson H. Comparison of methods for finding saddle points without knowledge of the final states. Journal of Chemical Physics. 2004;121:9776–9792. doi: 10.1063/1.1809574. [DOI] [PubMed] [Google Scholar]
- 36.Henkelman G, Jónsson H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. Journal of Chemical Physics. 2000;113:9978–9985. doi: 10.1063/1.1323224. [DOI] [Google Scholar]
- 37.Wang D, Liu L-M, Zhao S-J, Hu Z-Y, Liu H. Potential Application of Metal Dichalcogenides Double-Layered Heterostructures as Anode Materials for Li-Ion Batteries. Journal of Physical Chemistry C. 2016;120:4779–4788. doi: 10.1021/acs.jpcc.5b11677. [DOI] [Google Scholar]
- 38.Juan, H. et al. First-Principles Calculation of Lithium Adsorption and Diffusion on Silicene. Chinese Physics Letters30, 17103–17106(17104) (2013).
- 39.Kang B, Ceder G. Battery materials for ultrafast charging and discharging. Nature. 2009;458:190–193. doi: 10.1038/nature07853. [DOI] [PubMed] [Google Scholar]
- 40.Vineyard GH. Frequency factors and isotope effects in solid state rate processes. Journal of Physics and Chemistry of Solids. 1957;3:121–127. doi: 10.1016/0022-3697(57)90059-8. [DOI] [Google Scholar]
- 41.Persson, K., Hinuma, Y., Meng, Y. S., Van der Ven, A. & Ceder, G. Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Physical Review B82 (2010).
- 42.Ling, C. & Mizuno, F. Capture Lithium in αMnO2: Insights from First Principles. Chemistry of Materials24 (2012).
- 43.Li H, et al. MoS2/Graphene Hybrid Nanoflowers with Enhanced Electrochemical Performances as Anode for Lithium-Ion Batteries. Journal of Physical Chemistry C. 2015;119:7959–7968. doi: 10.1021/acs.jpcc.5b00890. [DOI] [Google Scholar]
- 44.Li Y, Zhou Z, Zhang S, Chen Z. MoS2 Nanoribbons: High Stability and Unusual Electronic and Magnetic Properties. Journal of the American Chemical Society. 2008;130:16739–16744. doi: 10.1021/ja805545x. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Wu D, Zhou Z, Cabrera CR, Chen Z. Enhanced Li Adsorption and Diffusion on MoS2 Zigzag Nanoribbons by Edge Effects: A Computational Study. The Journal of Physical Chemistry Letters. 2012;3:2221–2227. doi: 10.1021/jz300792n. [DOI] [PubMed] [Google Scholar]
- 46.Mak KF, Lee C, Hone J, Shan J, Heinz TF. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Physical Review Letters. 2010;105:136805. doi: 10.1103/PhysRevLett.105.136805. [DOI] [PubMed] [Google Scholar]
- 47.Xie Y, et al. Prediction and Characterization of MXene Nanosheet Anodes for Non-Lithium-Ion Batteries. Acs Nano. 2014;8:9606–9615. doi: 10.1021/nn503921j. [DOI] [PubMed] [Google Scholar]