Summary
The massive inflow of patients with COVID-19 requiring urgent care has overloaded hospitals in France and impacts the management of other patients. Deferring hospitalization and non-urgent surgeries has become a priority for surgeons today in order to relieve the health care system. It is obviously not simple to reduce emergency surgery without altering the quality of care or leading to a loss of chance for the patient. Acute appendicitis is a very specific situation and the prevalence of this disease leads us to reconsider this particular disease in the context of the COVID-19 crisis. Indeed, while the currently recommended treatment for uncomplicated acute appendicitis is surgical appendectomy, the non-surgical alternative of medical management by antibiotic therapy alone has been widely evaluated by high-quality studies in the literature. Insofar as the main limitation of exclusively medical treatment of uncomplicated acute appendicitis is the risk of recurrent appendicitis, this treatment option represents an alternative of choice to reduce the intra-hospital overload in this context of health crisis. The aim of this work is therefore to provide physicians and surgeons with a practical guide based on a review of the literature on the medical treatment of uncomplicated acute appendicitis in adults, to offer this alternative treatment to the right patients and under good conditions, especially when access to the operating room is limited or impossible.
Keywords: Acute appendicitis, Appendectomy, Antibiotic therapy, COVID-19, Coronavirus
Introduction
The new coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11, 2020 [1] and has put health care systems under new and grave challenges that include major decisions to take in the management of surgical emergencies. The massive influx of patients requiring in-hospital management, some requiring intensive care with respiratory assistance, has congested the working load while threatening the safety of health care for patients without COVID-19. Consequently, the goal of management of these patients is double:
-
•
delay the management of patients who are not absolute emergencies, in order to free-up bed space in the hospitals as well as the health care personnel needed to care for these patients and;
-
•
avoid the loss of chance for these patients in this setting.
Attesting to this conundrum are the combined studies of the gastro-intestinal surgical community, who have elaborated recommendations for the management of gastro-intestinal cancer during this troubled period [2]. While efforts to defer elective surgery seem feasible, the problem is much more difficult for surgical emergencies.
Non-complicated acute appendicitis involves approximately 40,000 patients/year in France [3] and constitutes a specific setting where a non-surgical alternative can be envisioned. In a report presented at the French Society for Gastro-Intestinal Surgery (Société française de chirurgie digestive (SFCD)) meeting in November 2019 (not yet published), surgical appendectomy was recommended in preference to medical treatment by antibiotics alone for the management of non-complicated acute appendicitis, mainly because the risk of recurrent appendicitis was found to range from 16% to 40% at one year after initial treatment [4], [5], [6], [7], [8], [9], [10], [11]. However, the exceptional situation related to COVID-19 has led us to reconsider medical treatment alone for this particularly prevalent disease. However, this therapeutic option requires recognition of a certain number of notions in order to avoid compromising patient safety. Of note, in the current state of our knowledge, there are no arguments indicating that patients with COVID-19 respond differently to antibiotic therapy and therefore, medical treatment of non-complicated acute appendicitis could be an option in patients with or suspected to have the disease.
The goal of this article is to provide medical and surgical physicians with a practical guide based on an analysis of the literature on the medical treatment of non-complicated acute appendicitis in the adult in order to propose this therapeutic alternative to the right patients and under good conditions.
Question 1: To which patients can non-surgical treatment of acute appendicitis be proposed?
This therapeutic option was studied prospectively in the adult by several teams and the results were reported in six randomized clinical trials [4], [5], [6], [7], [8], [10] and two non-randomized prospective studies [9], [11]. Inclusion criteria were very similar between the different studies, i.e., adult patients who had a confirmed diagnosis of non-complicated acute appendicitis.
With respect to patient status, only two studies had a maximum age limit [5], [10]. In the study by Vons et al. [8], old age was not a risk factor of failure. Therefore, advanced age in non-complicated appendicitis is not an argument against medical treatment alone because of the risk of appendicular neoplasm. Data concerning co-morbidities is limited because the majority of patients with non-complicated acute appendicitis were young and did not have associated severe disease, but common sense must prevail before proposing non-surgical treatment to patients with at-risk antecedent history (immunosuppression, patients with mechanical heart valves…).
Obviously, the efficacy of non-surgical treatment in patients with suspected or confirmed COVID-19 has never been assessed. However, initial results of antibiotic therapy for other indications in this subset of patients has not shown any decrease in antibiotic efficacy related to the viral disease. Avoidance of surgery in these patients is important because it reduces the risk of exposing the operating room staff, particularly in case of inadvertent release of pneumoperitoneum during laparoscopy [2]. Moreover, post-operative mortality in COVID-19 positive patients seems to be higher than expected, even for elective surgery where morbidity is usually low [12]. This is another reason why a non-surgical alternative is so important in patients with confirmed or suspected COVID-19 infection.
Several case series have described successful non-operative treatment in the pregnant woman [13], [14], however, in one study on 400 pregnant women treated medically for non-complicated appendicitis, the risk of severe sepsis, septic shock and thromboembolic disease was higher compared to pregnant women who had undergone appendectomy [15]. Thus, medical management of appendicitis in pregnant women carries a higher risk compared to surgery and should be avoided whenever possible.
In general, when antibiotic treatment is prescribed, between 10 and 15% of patients discontinue their treatment prematurely, as soon as their symptoms improve; this obviously decreases the efficacy and safety of this therapeutic option [16], [17]. Therefore, expectations of patient compliance and social environment factors should be taken into account and weighed against the possible consequences of appendectomy for the patient and the health care providers in the specific context of the pandemic.
The only criteria on imaging studies that was statistically significantly associated with failure of medical treatment and progression to a more complicated form of appendicitis in the French study was the presence of appendicular fecalith [8]; this result underscores the importance of avoiding medical treatment alone when a fecalith is found.
Synthesis
The selection of patients eligible for medical treatment for non-complicated acute appendicitis relies on:
-
•
the certainty of the diagnosis of non-complicated acute appendicitis;
-
•
patient characteristics that are compatible with this choice, in particular, absence of major co-morbidities creating a risk for the patient (immunosuppression, mechanical heart valve …). Age in itself is not an exclusion factor;
-
•
to the best of our current knowledge, suspected or confirmed COVID-19 positivity does not constitute a contra-indication to antibiotic therapy alone for non-complicated acute appendicitis. To the contrary, this non-surgical option could avoid increased post-operative mortality as well as exposure of the health care personnel in the operating room in this subgroup of patients;
-
•
non-operative treatment should be avoided in the pregnant woman;
-
•
the expected degree of patient compliance and the social environment once the patient is at home should be considered in the decision;
-
•
the presence of endo-appendicular fecalith is a risk factor for failure and should, whenever possible, lead to prefer surgery in this context.
Question 2: Can one omit imaging in a patient with typical clinical and biological signs?
In the setting of non-surgical treatment, it is essential to be certain of the diagnosis and that appendicitis is not complicated. Effectively, the absence of surgical exploration does not allow to correct the diagnosis in case of error, and the risk would then be to fail to recognize and thereby delay treatment of appendicular peritonitis, for example.
Neither the analysis of clinical signs [18] nor laboratory signs, such as hyperleukocytosis [19] or increased CRP [20] are sufficient for diagnostic certainty. Combination of these data in composite scores can increase the overall performance, but none of the current scores perform well enough to assure a positive or negative diagnosis. The most well known score is that of Alvarado [21]; the sensitivity and specificity of this score were 69% and 77%, respectively, in a recent meta-analysis [22]. Several other scores have been designed, among which one can cite the RIPASA score [23] with a sensitivity and specificity of 97% and 55%, respectively [22] or, the Andersson score that has been reported to correctly class 73% of patients without appendicitis but only 37% of patients with appendicitis [24].
Even though COVID-19 infection is not one of the differential diagnoses with non-complicated acute appendicitis, it is interesting to note that fever and gastro-intestinal signs such as abdominal pain, diarrhea, nausea and vomiting as well as elevated CRP are among the clinical and laboratory signs of COVID-19 infection [25]. In the context of a pandemic, these data might skew the analysis of these clinical and laboratory signs as well as the scores predictive of acute appendicitis.
Therefore, for the reasons mentioned above, imaging is indispensable to confirm the diagnosis when acute appendicitis is suspected. Of eight prospective studies that assessed antibiotic therapy alone for non-complicated appendicitis, only two [5], [11] did not rely on imaging for diagnostic confirmation. Imaging can rely on sonography alone [4], CT scanner alone [8], [10], or, one or the other [6], [7], [9]. The initial rate of failure of medical treatment alone varied little, suggesting that the diagnostic efficacy of CT and sonography are similar when the type of investigation is well adapted to the patient (Table 1 ). Sonography (US) avoids radiation exposure, is less expensive than CT, and avoids tying up the CT scanner, which is needed to manage other COVID patients. Conversely, The limitations of US include unreliable visualization of the appendix in 35–53% of cases [26], reduced performance in women or obese patients [27] and, high operator-dependency [26]. When US is performed under good conditions and the appendix is correctly identified, performance [28] approaches that of standard or low-dose CT scan [29]. In conclusion, US represents an option to relieve restricted availability of the CT scanner in the radiology department when appendicitis is suspected, and the result, while not equivocal, could be sufficient to decide whether the patient can be treated with antibiotics or not. However, although US frees some space in the CT department, it still requires a trained radiologist and eventually, if the information provided is not sufficient, a CT scan will still be needed. In one meta-analysis on 2665 patients, the sensitivity and specificity of abdominal MRI for the diagnosis of acute appendicitis was both 96% [30]. This imaging modality is therefore another possible choice, but from the management strategy viewpoint in the midst of the COVID crisis, MRI takes time, is expensive and availability is limited, and therefore is not a logistical solution.
Table 1.
Initial and late failures of medical treatment of non-complicated acute appendicitis in prospective studies.
| Study | Initial failure rate | Failure at one year after initial success | Treatment modality in case of suspected recurrence | Overall recurrence-free success rate at one year |
|---|---|---|---|---|
| Eriksson, 1995 [4] | 5% | 37% | Appendectomy: 100% Antibiotic therapy: 0% |
60% |
| Styrud, 2006 [5] | 12% | 14% | Appendectomy: 100% Antibiotic therapy: 0% |
76% |
| Hansson, 2009 [6] | 9% | 12% | Appendectomy: 80% Antibiotic therapy: 20% |
78% |
| Turhan, 2009 [7] | 18% | 10% | Appendectomy: 89% Antibiotic therapy: 11% |
75% |
| Vons, 2011 [8] | 12% | 29% | Appendectomy: 100% Antibiotic therapy: 0% |
63% |
| Park, 2014 [9] | 8% | 13% | Appendectomy: 98% Antibiotic therapy: 2% |
84% |
| Salminen, 2015 [10] | 6% | 23% | Appendectomy: 100% Antibiotic therapy: 0% |
73% |
| Allievi, 2017 [11] | 20% | 21% | Appendectomy: 100% Antibiotic therapy: 0% |
63% |
Synthesis
The data provided by clinical examination and laboratory tests are insufficient to propose non-surgical treatment in a patient suspected of non-complicated acute appendicitis because of the high risk of diagnostic error. In the absence of surgical exploration, the consequences of diagnostic error can be of serious concern.
When non-surgical treatment is envisioned, it is absolutely necessary to confirm the diagnosis by imaging and ensure that the appendicitis is uncomplicated. US, abdominal CT scan or MRI are all acceptable alternatives. In the current health crisis, availability of the various imaging modalities as well as radiologists are important elements to take into account in the choice of which investigation to use.
Question 3: Antibiotic therapy: which administration route, type and duration?
All the prospective studies [4], [5], [6], [7], [9], [10], [11], except that of Vons et al. [8], required that patients receive nothing per mouth and proposed initial intra-venous (IV) administration of antibiotics, followed by oral administration after discharge. In the Vons et al. study, initial treatment was oral without fasting if the patient was not nauseated. As the outcomes reported by Vons et al. appear no worse than that of the other studies (Table 1), oral administration of antibiotics seems reasonable and acceptable as long as the patient is not nauseated and does not vomit. A noninferiority randomized trial (APAC II) is currently underway to compare the efficacy of IV followed by oral administration vs initial oral administration [31].
With regard to the type of antibiotic therapy, each study proposed an empirical protocol based on probabilistic reasoning; none of the protocols were compared between themselves. Table 2 details the different types of antibiotic regimens proposed in each study. In spite of the differences in protocols, the reported efficacy in each study was similar, in particular with regard to the initial failure rate (Table 1). Of note, no one study has compared the efficacy of the various antibiotic therapy regimens. The choice of the best antibiotic therapy is difficult. It seems pertinent to target the bacteria that are present in the appendicular lumen, essentially Escherichia coli, and Bacteroïdes or Streptococcus strains [32]. In reality, the modifications of the appendicular microbiota are much more complex and are not limited to a small list of a few bacteria. One study dedicated to appendicular microbiota highlighted complex modifications in bacterial phyla that prevail during appendicitis with notably a high individual variability [33]. Moreover, the resistance to antibiotic therapy differs somewhat between non-complicated and complicated appendicitis [34]. Parallelly, modifications of the submucosal bacterial infiltration have been reported during appendicitis. Fusobacterium nucleatum appears to be the main bacteria found in the submucosa of patients with acute appendicitis and bacterial infiltration increases with the severity of appendicitis [35].
Table 2.
Antibiotic therapy regimens for medical treatment alone in non-complicated acute appendicitis.
| Study | Antibiotic therapy regimens | Duration of antibiotic therapy | Expected duration of hospital stay |
|---|---|---|---|
| Eriksson, 1995 [4] | IV: Cefotaxime 2 g*BID and Tinidazole 800 mg*QD for two days then Oral: Ofloxacin 200 mg*BID and Tinidazole 500 mg*BID for eight days | 10 days | 2 days |
| Styrud, 2006 [5] | IV: Cefotaxime 2 g*BID and Tinidazole 800 mg*QD for two days then Oral: Ofloxacin 200 mg*BID and Tinidazole 500 mg*BID for ten days | 12 days | 2 days |
| Hansson, 2009 [6] | IV: Cefotaxime 1 g*BID and Metronidazole 1.5 g*QD for one day then Oral: Ciprofloxacine 500 mg*BID and Metronidazole 400 mg*TID for nine days |
10 days | 1 day |
| Turhan, 2009 [7] | IV: Ampicillin 1 g*QID and Gentamycin 160 mg*QD and Metronidazole 500mg*TID for two days then Oral: (Ampicillin + metronidazole) for eight days | 10 days | 2 days |
| Vons, 2011 [8] | Amoxicillin + clavulanic acid 1 g TID if weight < 90 kg and 1 g QID if weight ≥ 90 kg, oral (or IV if nausea) | 8 days | Return to home as soon as possible starting day 1 |
| Park, 2014 [9] | IV: 2nd generation cephalosporin + Metronidazole | 4 days | 2 days |
| Salminen, 2015 [10] | IV: Ertapenem 1 g QD for three days then Oral: Levofloxacin 500 mg QD and Metronidazole 500 mg TID for seven days | 10 days | 3 days |
| Allievi, 2017 [11] | IV: Piperacillin + Tazobactam 4.5 g* QID (variable duration) or IV: Ertapenem 1 g QD for three days or IV: Ceftriaxone 1 g QD + Metronidazole 500 mg* TID (variable duration) then oral: Amoxicillin + clavulanic acid/1 g* TID for five days | 8 days | 3 days |
In the end, it is difficult to know which bacteria to target with probabilistic antibiotic therapy. The only way to resolve the problem would be to compare the efficacy of protocols that have already been assessed (Table 2). Among the different protocols, Salminen et al. [10] and Allievi et al. [11] proposed initiating therapy with ertapenem. This choice is based on the emergence of enterobacteria that produce wide-spectrum beta-lactamases resistant to amoxicillin and third generation cephalosporins [36]. The reported efficacy of this type of antibiotic therapy is not superior to that of other antibiotic therapy protocols (Table 1), but exposes the patient to a selection of bacteria that are resistant to ertapenem [37]. Likewise, one study found that the rate of early failure in patients treated by Piperacillin + Tazobactam [11] was not reduced, while, similar to ertapenem, this antibiotic therapy exposes the patient to an important risk of selection of resistant bacteria.
Neither the choice of antibiotic therapy nor its duration have been compared in the same protocol, and therefore the ideal antibiotic, its duration and the balance between efficacy and emergence of resistance or the risk of complications such as Clostridium difficile colitis is not known. Table 2 shows the duration of treatment that was provided empirically in each of the trials. The differences observed were not enormous, ranging from 8 to 12 days, the only exception being the study of Park et al. [9] where the duration was 4 days.
Symptomatic treatment alone, without antibiotic therapy or surgery for non-complicated acute appendicitis was assessed in one non-inferiority randomized trial [38]. The inclusion criteria were strict, and, of note, the maximal appendicular diameter was supposed to be between 6 mm and 11 mm. The initial failure rate was nearly the same (7.2% in the group without antibiotic therapy versus 7.4% in the group with antibiotic therapy) (P = 0.957)). Similarly, the difference in failure rates at one year was not statistically significant (20.7% vs. 23.4% for those treated or not with antibiotic therapy, respectively; P = 0.609). In spite of the encouraging results of this trial, such a revolution in the treatment of non-complicated acute appendicitis warrants confirmation by further studies before any authoritative recommendation can be made. This is the goal of the APPAC III trial, currently underway [39].
Synthesis
Antibiotic therapy can be started directly, administered orally if the patient does not have nausea or vomiting. No study has yet compared the different antibiotic therapy protocols to determine which is best.
With regard to the choice and duration of antibiotic therapy, several protocols have been evaluated. Amoxicillin + clavulanic acid 1 g TID for 8 days has the advantage of being simple, inexpensive, can be administered either IV or by mouth, and does not require any modification during the administration period. In case of penicillin allergy, combined antibiotic therapy with a fluoroquinolone and an imidazole (ciprofloxacin 500 mg BID and metronidazole 500 mg TID for 8 days) can be proposed. The efficacy of initial antibiotic therapy by ertapenem or tazocillin has not been shown to be superior and are not recommended because of the risk of resistance.
Symptomatic treatment without any antibiotic therapy has not yet been sufficiently evaluated to be proposed with optimal safety.
Question 4: Can complete ambulatory medical treatment be proposed?
None of the eight prospective trials proposed an exclusive medical treatment for appendicitis [4], [5], [6], [7], [8], [9], [10], [11]. Table 2 lists the minimal durations of hospital stay in each study before the patient was allowed to leave the hospital, the time period ranging between 1 to 3 days. The goal of short hospital stay was to be able to detect early failure of antibiotic therapy, most often related to unrecognized complicated appendicitis, and to avoid surgical delay. Except in the circumstances of the current pandemic health crisis, it does not seem reasonable to propose ambulatory treatment without intra-hospital surveillance because the safety of this option has never been evaluated.
During the COVID-19 pandemic, the choice of antibiotic therapy alone as an alternative to surgical treatment allows to avoid mobilization of operating rooms and teams, post-surgery care and frees up medical personnel for other duties, starting with both physicians and nurses working in intensive care and anesthesiology. Moreover, this avoids any contact between the patient and other potentially contaminated patients in the recovery room. Conversely, this strategy does not reduce the number of beds if medical management is started in hospital. In the study by Vons et al. [8] that allowed patient discharge much earlier than the other trials, the duration of hospital stay in the group undergoing medical treatment alone was not statistically significantly shorter than that of patients undergoing appendectomy (3.96 days vs. 3.04 days, respectively, P = 0.08), although it did show that tendency. As ambulatory appendectomy has been shown to be feasible and safe in selected patients [40], [41], [42], it might be possible to reduce the duration of bed occupancy by proposing ambulatory appendectomy rather than management by antibiotic therapy alone.
However, the relevant question is to know whether ambulatory antibiotic therapy alone in patients diagnosed with non-complicated acute appendicitis in the emergency department is an option to consider if complete saturation of beds for patients with severe forms of COVID-19 makes it impossible to hospitalize appendicitis patients. Although this option has never been evaluated directly, the current emergency situation highlights the need to assess the data in the literature on this topic. In the study by Lefrançois et al. [40], depending on the timing of diagnosis, patients selected for ambulatory appendectomy were allowed to return home with antibiotic therapy (amoxicillin and clavulanic acid) and return the next day for operation. No serious incident was reported in connection with the delay before surgery. A strict selection of these patients according to a Saint-Antoine score ≥ 4 (Table 3 ), based on the probability of successful ambulatory surgery, allows selection of those patients with low risk for complicated appendicitis [40]. The score was validated in a study from another center in 2019 [43]. For these reasons, under the current circumstances, certain patients, selected according to the Saint-Antoine score, could be treated in a completely ambulatory setting as long as they were clearly informed. As well, these patients should have the possibility of contacting the surgical unit without going through the emergency department if the course of events at home becomes unfavorable. It seems preferable that this decision be made by the same surgeon who interrogated and examined the patient to begin with, so that he/she assumes the consequences of this decision, is responsible for the information given to the patient and the management in case of any unfavorable event occurring at home.
Table 3.
Saint-Antoine scale [40].
| Item | Points |
|---|---|
| BMI (body mass index) < 28 kg/m2 | 1 point |
| Leucocyte count < 15,000/μL | 1 point |
| CRP < 3 mg/dL | 1 point |
| No radiological signs of perforation | 1 point |
| Diameter of appendix ≤ 10 mm | 1 point |
Synthesis
Patients with an uneventful course can be discharged from hospital after 24 h of hospitalization for non-complicated acute appendicitis treated by antibiotic therapy alone. The safety of this management policy has been assessed in the literature.
Exclusive ambulatory management has not yet been evaluated in the literature. However, in the current health crisis, this option can be entertained in selected patients, for instance with the help of the Saint-Antoine score (≥ 4).
The decision to manage non-complicated acute appendicitis by antibiotic therapy alone should be taken by a gastrointestinal surgeon to guarantee the safety of this as-yet unvalidated option.
Question 5: What is the risk of initial failure?
Initial failure can be defined as failure of initial antibiotic therapy leading to either change of antibiotics, interventional radiological drainage or surgery. The risk of initial failure of antibiotic therapy alone to treat non-complicated acute appendicitis is relatively low since it ranged from 5 to 20% in the eight prospective studies on the topic [4], [5], [6], [7], [8], [9], [10], [11] (Table 1). The meta-analysis by Findlay et al. [44] found a primary failure rate of 10.2% when evaluating the six randomized studies.
Patients should be given this information when this therapeutic option is chosen. This is all the more important when strict ambulatory treatment is decided because, a priori, 1 patient out of 10 will require early change in management for failure of this therapeutic strategy. Moreover, it is important to set up follow-up for these patients whether by telephone or by teleconsultation, to ensure that the initial treatment was effective. Likewise, the management of early failure should be anticipated, and the patient should be informed as to what should do in this situation and in particular whether he/she should go the emergency department or, ideally, contact his/her surgeon directly.
Synthesis
The risk of primary failure of non-surgical management of non-complicated acute appendicitis varies between 5 and 20%. The patient should be informed of this. Moreover, adequate follow-up is necessary to integrate this low but not negligible risk into the overall therapeutic management plan.
Question 6: What is the risk of recurrence after initial successful medical treatment?
The one-year success rate is defined as recovery without complications related to recurrent appendicitis or to initial antibiotic therapy at one year. In the eight prospective trials, this rate ranged from 60% to 84% [4], [5], [6], [7], [8], [9], [10], [11] (Table 1). Thereafter, the risk of recurrence beyond one year after initial treatment is far from negligible. Salminen et al. reported that the cumulative risk of recurrence requiring appendectomy was 27.3% at one year, 35.2% at three years and 39.1% at five years after initial antibiotic therapy alone [45] confirming that the risk of recurrence persists at least five years after initial antibiotic therapy.
This result is the principal limitation of non-surgical treatment of non-complicated acute appendicitis and explains the SFCD recommendations that call for initial surgical treatment. The COVID-19 crisis in our hospitals, which has necessitated freeing the operating rooms, beds and health care personnel in order to care for patients with life-threatening short-term prognosis, justifies taking the risk of late recurrence by treating acute appendicitis with antibiotic therapy alone. The clinician who chooses this alterative therapeutic should be fully aware of the risk of recurrence at distance.
Synthesis
The initial success rate ranges from 60% to 84% des patients. The risk of late recurrence persists well beyond one year since the failure rate was 35.2% at 3 years and 39.1% at 5 years in the one study that reported outcomes beyond one year.
Question 7: Should routine interval appendectomy be performed?
There are two reasons to raise the question of interval appendectomy after medical treatment alone: on one side, the risk of recurrence of acute appendicitis, on the other, the risk of appendicular neoplasm in the differential diagnosis or as the initial cause of appendicitis.
With regard to the risk of late recurrence of appendicitis, none of the eight prospective studies proposed routine interval appendectomy after initial conservative management. The goal of these studies was to evaluate the definitive success of non-surgical management of non-complicated acute appendicitis in the adult. The risk of late recurrence was far from negligible several years after initial treatment; nonetheless more than half of the patients in the trial reported by Salminen et al. did not have a recurrence after five year follow-up [45].
In parallel to the risk of recurrence of acute appendicitis is that of appendicular neoplasm; the older the patient, the greater this risk [46]. However, the overall risk of appendicular tumor is low, around 1% [47]. Moreover, the risk is higher in complicated forms of appendicitis, and in particular, appendicular abscess, than in non-complicated appendicitis [48]. Reassuringly, no tumor was found in the five-year follow-up in the Salminen trial, and in particular in the group of patients initially allocated to medical treatment who eventually underwent appendectomy [45]. In the « initial appendectomy» group of this randomized trial, four patients had neoplasms in the operative specimen (1 polyp and 3 neuroendocrine tumors). It is difficult to determine whether the difference between the two groups « Antibiotic therapy » and « Appendectomy » was due simply to chance or resulted from low aggressivity of the completely asymptomatic neuroendocrine tumors in the « Antibiotic therapy » group. Notwithstanding, there is currently no argument strong enough to recommend routine interval appendectomy in order to detect any tumoral lesion that had escaped detection on imaging. As well, routine follow-up colonoscopy has not been evaluated and therefore, until proven otherwise, this strategy cannot be proposed.
Synthesis
In the absence of signs of clinical recurrence of appendicitis and if imaging raises no question of the existence of appendicular neoplasm, there is no reason to propose routine interval appendectomy after non-complicated acute appendicitis in the adult following successful medical treatment.
Question 8: If appendectomy is considered, what adaptations should be proposed in the context of the COVID-19 pandemic?
Management of non-complicated acute appendicitis in the adult should be adapted to each health care structure according to local capacities. Medical treatment, as described above in questions 1 to 7, represents an extreme option in case of non-accessibility to the operating room, non-availability of the necessary OR staff or the fact that all the post-interventional beds are occupied by COVID-19 positive patients creating an increased risk of contamination of the patient who must undergo surgery. However, before proposing antibiotic therapy alone, it is important to evaluate the capacity for surgery in this setting.
Of note, the optimal surgical treatment plan, in particular by strictly ambulatory management, could lead to substantial savings in resources. The safety of ambulatory laparoscopic appendectomy has been shown [40], [43]. A Saint-Antoine score ≥ 4 should guarantee an adequate selection of patients eligible for ambulatory appendectomy [40]. Duration of hospital stay can be less than 24 h or even 12 h and the occupation time of the operating room is short. This surgical option should be carefully considered before concluding in favor of antibiotic therapy alone.
Once surgery is considered, it is important to think about the surgical approach. While laparoscopy is generally preferred under normal circumstances [49], the Mac Burney incision should be reconsidered in this exceptional time. Effectively, the potential of aerosol-borne viruses released by laparoscopy in a COVID-19 positive patient exposes the OR personnel to the risk of contamination [2], although there are no specific data on this topic in the literature at the present time. This leads us to propose either antibiotic therapy alone or open appendectomy via laparotomy for patients who are COVID-19 positive. It is difficult to conclude on the best approach for patients who do not present any signs of COVID-19 infection, i.e., asymptomatic carriers or those in the incubation phase. A precautionary principle would be to routinely proscribe laparoscopy during the pandemic. Routine pre-operative testing for viral infection before appendectomy does not seem to be practical at present because of limited access to the tests, the delay before obtaining results which is not instantaneous, and the non-negligible risk of false negatives [50]. Chest CT scan can be helpful in the diagnosis of coronavirus infection, but again, its performance is not optimal [50] and probably not very good in asymptomatic carriers.
While imaging (US, CT, MRI) to confirm the diagnosis of non-complicated acute appendicitis was recommended before antibiotic therapy, it is likewise recommended before surgical treatment via laparotomy. Imaging by CT scan or MRI is preferable because it allows the surgeon to best choose the incision in case of atypical localization of the appendix. The goal is to perform as short an incision as possible (ideally < 3 cm) to increase the success of ambulatory management of McBurney appendectomy.
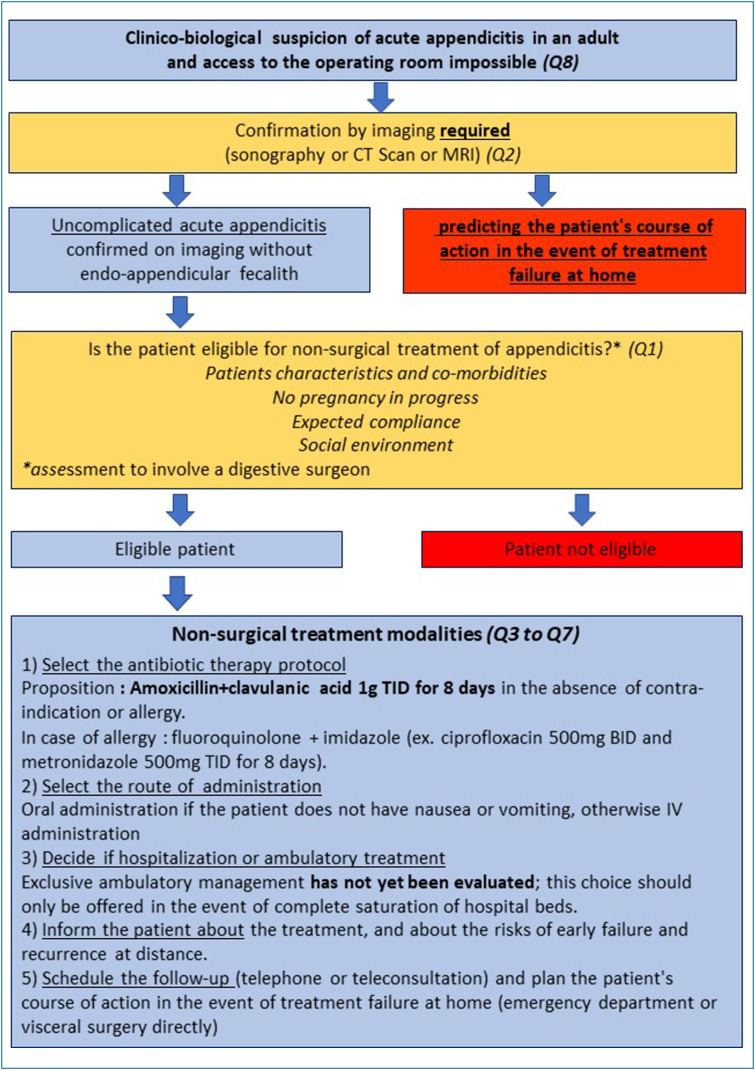
Based on the available data we propose the algorithm in Fig. 1 for the non-surgical management of patients with non-complicated acute appendicitis during this particular period of hospital saturation caused by the COVID-19 pandemic. Medical treatment is therefore a possible alternative that is immediately applicable to care for the multitude of patients with the diagnosis of non-complicated acute appendicitis when access to the operating room is impossible. Thanks to this study, we hope that this therapeutic alternative can be proposed with safety and in full knowledge of the facts by the surgical community, geared to the necessities of each health care facility involved. Any effort to reduce the afflux of patients, even if small, should not be under-estimated because the stakes for patients requiring in-hospital care for a severe form of COVID-19 infection are vital.
Figure 1.
Algorithm for non-surgical management of non-complicated acute appendicitis in case of hospital saturation. Q1 to Q8 refer to the questions 1 to 8 treated in the manuscript.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuech J.J., Gangloff A., Di Fiore F. Strategy for the practice of digestive and oncological surgery during the Covid-19 epidemic. J Visc Surg. 2020 doi: 10.1016/j.jviscsurg.2020.03.008. [S1878-7886(20)30070-9; Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vons C., Brami M. Épidémiologie descriptive des appendicites en France : faut-il revoir la physiopathologie des appendicites aiguës ? Bull Acad Natle Med. 2017;201(1–2–3):339–357. [séance du 14 février 2017] [Google Scholar]
- 4.Eriksson S., Granström L. Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis. Br J Surg. 1995;82:166–169. doi: 10.1002/bjs.1800820207. [DOI] [PubMed] [Google Scholar]
- 5.Styrud J., Eriksson S., Nilsson I. Appendectomy versus antibiotic treatment in acute appendicitis. A prospective multicenter randomized controlled trial. World J Surg. 2006;30:1033–1037. doi: 10.1007/s00268-005-0304-6. [DOI] [PubMed] [Google Scholar]
- 6.Hansson J., Körner U., Khorram-Manesh A., Solberg A., Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96:473–481. doi: 10.1002/bjs.6482. [DOI] [PubMed] [Google Scholar]
- 7.Turhan A.N., Kapan S., Kütükçü E., Yiğitbaş H., Hatipoğlu S., Aygün E. Comparison of operative and non operative management of acute appendicitis. Ulus Travma Acil Cerrahi Derg. 2009;15:459–462. [PubMed] [Google Scholar]
- 8.Vons C., Barry C., Maitre S. Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:1573–1579. doi: 10.1016/S0140-6736(11)60410-8. [DOI] [PubMed] [Google Scholar]
- 9.Park H.-C., Kim M.J., Lee B.H. The outcome of antibiotic therapy for uncomplicated appendicitis with diameters ≤ 10 mm. Int J Surg. 2014;12:897–900. doi: 10.1016/j.ijsu.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Salminen P., Paajanen H., Rautio T. Antibiotic Therapy vs. Appendectomy for Treatment of Uncomplicated Acute Appendicitis: The APPAC Randomized Clinical Trial. JAMA. 2015;313:2340–2348. doi: 10.1001/jama.2015.6154. [DOI] [PubMed] [Google Scholar]
- 11.Allievi N., Harbi A., Ceresoli M. Acute Appendicitis: Still a Surgical Disease? Results from a Propensity Score-Based Outcome Analysis of Conservative Versus Surgical Management from a Prospective Database. World J Surg. 2017;41:2697–2705. doi: 10.1007/s00268-017-4094-4. [DOI] [PubMed] [Google Scholar]
- 12.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 Outbreak and Surgical Practice: Unexpected Fatality in Peri-operative Period. Ann Surg. 2020 doi: 10.1097/SLA.0000000000003925. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yefet E., Romano S., Chazan B., Nachum Z. Successful treatment of acute uncomplicated appendicitis in pregnancy with intravenous antibiotics. Eur J Obstet Gynecol Reprod Biol. 2013;169:121–122. doi: 10.1016/j.ejogrb.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Joo J.I., Park H.-C., Kim M.J., Lee B.H. Outcomes of Antibiotic Therapy for Uncomplicated Appendicitis in Pregnancy. Am J Med. 2017;130:1467–1469. doi: 10.1016/j.amjmed.2017.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi N., Patenaude V., Abenhaim H.A. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509–1514. doi: 10.1111/1471-0528.12736. [DOI] [PubMed] [Google Scholar]
- 16.Raupach-Rosin H., Rübsamen N., Schütte G., Raschpichler G., Chaw P.S., Mikolajczyk R. Knowledge on Antibiotic Use, Self-Reported Adherence to Antibiotic Intake, and Knowledge on Multi-Drug Resistant Pathogens–Results of a Population-Based Survey in Lower Saxony, Germany. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grosso G., Marventano S., Ferranti R., Mistretta A. Pattern of antibiotic use in the community: non-adherence and self-prescription rates in an Italian urban population. Mol Med Rep. 2012;5:1305–1310. doi: 10.3892/mmr.2012.818. [DOI] [PubMed] [Google Scholar]
- 18.Wagner J.M., McKinney W.P., Carpenter J.L. Does this patient have appendicitis? JAMA. 1996;276:1589–1594. [PubMed] [Google Scholar]
- 19.Andersson R.E.B. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28–37. doi: 10.1002/bjs.4464. [DOI] [PubMed] [Google Scholar]
- 20.Shogilev D.J., Duus N., Odom S.R., Shapiro N.I. Diagnosing appendicitis: evidence-based review of the diagnostic approach in 2014. West J Emerg Med. 2014;15:859–871. doi: 10.5811/westjem.2014.9.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557–564. doi: 10.1016/s0196-0644(86)80993-3. [DOI] [PubMed] [Google Scholar]
- 22.Frountzas M., Stergios K., Kopsini D., Schizas D., Kontzoglou K., Toutouzas K. Alvarado or RIPASA score for diagnosis of acute appendicitis? A meta-analysis of randomized trials. Int J Surg. 2018;56:307–314. doi: 10.1016/j.ijsu.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Chong C.F., Adi M.I.W., Thien A. Development of the RIPASA score: a new appendicitis scoring system for the diagnosis of acute appendicitis. Singapore Med J. 2010;51:220–225. [PubMed] [Google Scholar]
- 24.Andersson M., Andersson R.E. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843–1849. doi: 10.1007/s00268-008-9649-y. [DOI] [PubMed] [Google Scholar]
- 25.Li L.-Q., Huang T., Wang Y.-Q. 2019 novel coronavirus patients’ clinical characteristics, discharge rate and fatality rate of meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart J.K., Olcott E.W., Jeffrey R.B. Sonography for appendicitis: nonvisualization of the appendix is an indication for active clinical observation rather than direct referral for computed tomography. J Clin Ultrasound. 2012;40:455–461. doi: 10.1002/jcu.21928. [DOI] [PubMed] [Google Scholar]
- 27.Lourenco P., Brown J., Leipsic J., Hague C. The current utility of ultrasound in the diagnosis of acute appendicitis. Clin Imaging. 2016;40:944–948. doi: 10.1016/j.clinimag.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Carroll P.J., Gibson D., El-Faedy O. Surgeon-performed ultrasound at the bedside for the detection of appendicitis and gallstones: systematic review and meta-analysis. Am J Surg. 2013;205:102–108. doi: 10.1016/j.amjsurg.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Yun S.J., Ryu C.-W., Choi N.Y., Kim H.C., Oh J.Y., Yang D.M. Comparison of Low- and Standard-Dose CT for the Diagnosis of Acute Appendicitis: A Meta-Analysis. AJR Am J Roentgenol. 2017;208:W198–W207. doi: 10.2214/AJR.16.17274. [DOI] [PubMed] [Google Scholar]
- 30.Duke E., Kalb B., Arif-Tiwari H. A Systematic Review and Meta-Analysis of Diagnostic Performance of MRI for Evaluation of Acute Appendicitis. AJR Am J Roentgenol. 2016;206:508–517. doi: 10.2214/AJR.15.14544. [DOI] [PubMed] [Google Scholar]
- 31.Haijanen J., Sippola S., Grönroos J. Optimising the antibiotic treatment of uncomplicated acute appendicitis: a protocol for a multicentre randomised clinical trial (APPAC II trial) BMC Surg. 2018;18:117. doi: 10.1186/s12893-018-0451-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts J.P. Quantitative bacterial flora of acute appendicitis. Arch Dis Child. 1988;63:536–540. doi: 10.1136/adc.63.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guinane C.M., Tadrous A., Fouhy F. Microbial Composition of Human Appendices from Patients following Appendectomy. MBio. 2013;4 doi: 10.1128/mBio.00366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Marín A., Pérez-López M., Martínez-Guerrero E., Rodríguez-Cazalla L., Compañ-Rosique A. Microbiologic Analysis of Complicated and Uncomplicated Acute Appendicitis. Surg Infect (Larchmt) 2018;19:83–86. doi: 10.1089/sur.2017.210. [DOI] [PubMed] [Google Scholar]
- 35.Swidsinski A., Dörffel Y., Loening-Baucke V. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut. 2011;60:34–40. doi: 10.1136/gut.2009.191320. [DOI] [PubMed] [Google Scholar]
- 36.Pitout J.D.D., Laupland K.B. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado N., Castro B., Berrio I., Manjarrés M., Robledo C., Robledo J. Ertapenem resistance in two tertiary-care hospitals: Microbiology, epidemiology, and risk factors. Enferm Infecc Microbiol Clin. 2017;35:511–515. doi: 10.1016/j.eimc.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Park H.C., Kim M.J., Lee B.H. Randomized clinical trial of antibiotic therapy for uncomplicated appendicitis. Br J Surg. 2017;104:1785–1790. doi: 10.1002/bjs.10660. [DOI] [PubMed] [Google Scholar]
- 39.Sippola S., Grönroos J., Sallinen V. A randomised placebo-controlled double-blind multicentre trial comparing antibiotic therapy with placebo in the treatment of uncomplicated acute appendicitis: APPAC III trial study protocol. BMJ Open. 2018;8:e023623. doi: 10.1136/bmjopen-2018-023623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefrancois M., Lefevre J.H., Chafai N. Management of Acute Appendicitis in Ambulatory Surgery: Is It Possible? How to Select Patients? Ann Surg. 2015;261:1167–1172. doi: 10.1097/SLA.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 41.Friedlander D.F., Krimphove M.J., Cole A.P. Where Is the Value in Ambulatory Versus Inpatient Surgery? Ann Surg. 2019 doi: 10.1097/SLA.0000000000003578. [DOI] [PubMed] [Google Scholar]
- 42.Trejo-Avila M., Cárdenas-Lailson E., Valenzuela-Salazar C., Herrera-Esquivel J., Moreno-Portillo M. Ambulatory versus conventional laparoscopic appendectomy: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:1359–1368. doi: 10.1007/s00384-019-03341-y. [DOI] [PubMed] [Google Scholar]
- 43.Sabbagh C., Masseline L., Grelpois G., Ntouba A., Dembinski J., Regimbeau J.-M. Management of Uncomplicated Acute Appendicitis as Day Case Surgery: Can Outcomes of a Prospective Study Be Reproduced in Real Life? J Am Coll Surg. 2019;229:277–285. doi: 10.1016/j.jamcollsurg.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Findlay J.M., Kafsi J.E., Hammer C., Gilmour J., Gillies R.S., Maynard N.D. Nonoperative Management of Appendicitis in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Coll Surg. 2016;223 doi: 10.1016/j.jamcollsurg.2016.09.005. [814-824.e2] [DOI] [PubMed] [Google Scholar]
- 45.Salminen P., Tuominen R., Paajanen H. Five-Year Follow-up of Antibiotic Therapy for Uncomplicated Acute Appendicitis in the APPAC Randomized Clinical Trial. JAMA. 2018;320:1259–1265. doi: 10.1001/jama.2018.13201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunner M., Lapins P., Langheinrich M. Risk factors for appendiceal neoplasm and malignancy among patients with acute appendicitis. Int J Colorectal Dis. 2020;35:157–163. doi: 10.1007/s00384-019-03453-5. [DOI] [PubMed] [Google Scholar]
- 47.Lietzén E., Grönroos J.M., Mecklin J.-P. Appendiceal neoplasm risk associated with complicated acute appendicitis-a population based study. Int J Colorectal Dis. 2019;34:39–46. doi: 10.1007/s00384-018-3156-x. [DOI] [PubMed] [Google Scholar]
- 48.Mällinen J., Rautio T., Grönroos J. Risk of Appendiceal Neoplasm in Periappendicular Abscess in Patients Treated With Interval Appendectomy vs Follow-up With Magnetic Resonance Imaging: 1-Year Outcomes of the Peri-Appendicitis Acuta Randomized Clinical Trial. JAMA Surg. 2019;154:200–207. doi: 10.1001/jamasurg.2018.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiwari M.M., Reynoso J.F., Tsang A.W., Oleynikov D. Comparison of outcomes of laparoscopic and open appendectomy in management of uncomplicated and complicated appendicitis. Ann Surg. 2011;254:927–932. doi: 10.1097/SLA.0b013e31822aa8ea. [DOI] [PubMed] [Google Scholar]
- 50.Ai T., Yang Z., Hou H. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]