Abstract
Background and Objectives
The persistent status of ageism as one of the least acknowledged forms of prejudice may be due in part to an absence of quantifying its costs in economic terms. In this study, we calculated the costs of ageism on health conditions for all persons aged 60 years or older in the United States during 1 year.
Research Design and Materials
The ageism predictors were discrimination aimed at older persons, negative age stereotypes, and negative self-perceptions of aging. Health care costs of ageism were computed by combining analyses of the impact of the predictors with comprehensive health care spending data in 1 year for the eight most-expensive health conditions, among all Americans aged 60 years or older. As a secondary analysis, we computed the number of these health conditions experienced due to ageism.
Results
It was found that the 1-year cost of ageism was $63 billion, or one of every seven dollars spent on the 8 health conditions (15.4%), after adjusting for age and sex as well as removing overlapping costs from the three predictors. Also according to our model, ageism resulted in 17.04 million cases of these health conditions.
Discussion and Implications
This is the first study to identify the economic cost that ageism imposes on health. The findings suggest that a reduction of ageism would not only have a monetary benefit for society, but also have a health benefit for older persons.
Keywords: Ageism, Perceptions of aging, Health, Cost, Age stereotypes
A resolution to create a “legal instrument to promote and protect the rights and dignity of older persons” has been resisted by a majority of United Nations member states, including the United States (United Nations, 2012, 2014, 2017). There might be greater support if there was a recognition of the extent to which ageism, which is targeted by the resolution, imposes economic costs on nations. This was examined for the first time in this study, which focused on the health care costs that are generated by ageism in the United States, whose overall costs surpass those of any other country (Organisation for Economic Co-operation and Development, 2017).
The ageism that we examined in this study fits a conceptual model, stereotype embodiment theory (SET), that is derived from empirical research (Levy, 2009). This theory proposes that observations of the way older persons are treated, and age beliefs are expressed in the culture, tend to be assimilated at a young age and undergo reinforcement over time, often without awareness. Also, according to SET, there are three discrete ageism predictors: age discrimination, defined as detrimental treatment of older persons; negative age stereotypes, defined as the negative beliefs of older persons about older people in general; and negative self-perceptions of aging, defined as the negative beliefs of older persons about their own aging.
Extensive research has shown, consistent with a SET prediction, that the three ageism variables will adversely affect the health outcomes of older persons. These studies, which have been conducted in five continents and are supported by four meta-analyses, provide evidence for the directionality of ageism affecting health (Horton, Baker, Pearce, & Deakin, 2008; Lamont, Swift, & Abrams, 2015; Meisner, 2012; Westerhof et al., 2014). To illustrate, experimental studies have found that when older individuals are randomly assigned to a negative-age-stereotype condition, it impairs health outcomes, such as memory performance and balance, compared to those in a neutral or positive-age-stereotype condition (e.g., Lamont et al., 2015; Levy, 2009; Levy & Leifheit-Limson, 2009; Lee & Lee, 2018). Similarly, an experimental study found that when older individuals were randomly exposed to age discrimination in the form of patronizing speech, they performed significantly worse on a cognitive task than those not exposed to patronizing speech (Hehman & Bugental, 2015).
Also supporting the prediction that the three ageism variables will adversely affect the health outcomes of older persons, longitudinal studies conducted with them in the community have found that ageism variables measured earlier in life predict health outcomes later in old age (e.g., Levy, Ferrucci, Zonderman, Slade, Troncoso, & Resnick, 2016; Westerhof et al., 2014). As an example, a study found that young adults holding more-negative age stereotypes were twice as likely to experience cardiovascular events up to 40 years later than their young adult peers holding more-positive age stereotypes, after adjusting for relevant covariates including family history of cardiovascular disease (Levy, Zonderman, Slade, & Ferrucci, 2009). Similarly, numerous studies have found that perceived age discrimination and negative self-perceptions of aging predict worse health for older persons years later (e.g., Levy, Slade & Kasl, 2002; Marchiondo, Gonzales, & Williams, 2017; Sargent-Cox, Anstey, & Luszcz, 2012).
The directionality of ageism predictors on health finds additional support from studies that have demonstrated age stereotypes tend to be resistant to even extremely stressful events (e.g., Levy, Slade, Chung, & Gill, 2015). Also, age discrimination and self-perceptions of aging tend to be stable over time (see Supplementary Material). Further, the impact of ageism on health is stronger than the reverse association (e.g., Levy et al., 2002; Sargent-Cox et al., 2012; Wurm, Tesch-Römer, & Tomasik, 2007).
SET further postulates that each of the ageism predictors exert their influence on health through three pathways: psychological, behavioral, and physiological (e.g., Levy, 2009). A number of studies have provided evidence for these ageism–health pathways (Levy, 2009; Levy & Bavishi, 2018; Levy, Slade, Pietrzak, & Ferrucci, 2018; Palmore, 2015; Westerhof et al., 2014). Supporting the psychological pathway, research has found that negative age stereotypes can exacerbate stress when older participants are randomly assigned to age-stereotype conditions in the laboratory (Levy, Hausdorff, Hencke, & Wei, 2000); and over time, as assessed by the stress biomarkers of cortisol and C-reactive protein (Levy & Bavishi, 2018; Levy, Moffat, Resnick, Slade, & Ferrucci, 2016). On the behavioral level, research has found that negative self-perceptions of aging predict worse health behaviors over time, such as noncompliance with prescribed medications (Kim, Moored, Giasson, & Smith, 2014; Levy & Myers, 2004). On the physiological level, it has been found that negative age stereotypes predict detrimental brain changes decades later, including the accumulation of plaques and tangles and reduction in size of the hippocampus (Levy, Ferrucci, et al., 2016).
Building on this background, the aim of the current study was to identify the health care costs, among eight of the most-expensive health conditions, associated with the three ageism predictors for the total population of older persons in the United States. As a secondary analysis, we computed the number of these health conditions that were experienced due to the ageism predictors.
An advantage of setting up the study with these discrete predictors is that each could be targeted in future interventions. Because research has found psychological processes are amplified when they affect self-concepts (Markus, 1977; Petersen, Stahlberg, & Dauenheimer, 2000), as occurs with self-perceptions of aging and, to a lesser extent, with age stereotypes (Levy, 2009), we predicted that the health care costs of older persons would be greatest for negative self-perceptions of aging, followed by negative age stereotypes, and then by age discrimination.
The present study integrates two fields, which do not customarily interact, by drawing on a set of predictors that are usually examined by social psychologists and an outcome that is usually studied by economists. The latter tend to focus on younger persons and biological factors as well as medical factors, rather than the societal antecedents of these factors (Neuman, Sanders, Russell, Siegel, & Ganiats, 2016). Accordingly, the economic costs of ageism on health had not been previously studied.
Design and Methods
Overall Analytic Plan
To calculate the health care costs of ageism, as well as the number of health-condition cases affected by ageism for all Americans aged 60 years and older, our study combined effect sizes from ageism and health-condition research with the most-recent (2013) comprehensive health care spending data available from the Institute for Health Metrics and Evaluation (IHME, 2018). To increase the number of analyses predicting the impact of ageism on the selected eight health outcomes, we included effect sizes from two sources: our new systematic review of all relevant ageism research and the prospective models we developed using the nationally representative Health and Retirement Study (HRS) (Sonnega & Weir, 2014). To reduce the likelihood that health is affecting ageism rather than ageism is affecting health, within the same cohort we selected measures of ageism at baseline and health conditions assessed at subsequent waves, adjusting for covariates in these models. In summing the health care costs of the ageism predictors, we removed the overlapping contributions. (See Supplementary Material for a description of our method of generating costs for components of ageism, which was developed for this analysis, along with relevant calculations.)
Measures
Predictors: Ageism Variables
The age-discrimination measures that were used in the systematic review studies and the HRS models included the Everyday Discrimination Scale (Williams, Yu, Jackson, & Anderson, 1997), which assessed how often participants experience a set of occurrences based on their age, including “You are treated with less courtesy or respect than other people.” The age-stereotypes measures that were used in the systematic review studies included the Expectations Regarding Aging Survey (Sarkisian, Steers, Hays, & Mangione, 2005), which asks participants to rate items on whether they are true, such as “Forgetfulness is a natural occurrence just from growing old.” The self-perceptions of aging measures used in the systematic review studies and the HRS models included the five-item Attitude Toward Own Aging subscale of the Philadelphia Geriatric Center Morale Scale (Lawton, 1975; Liang & Bollen, 1983), which asks participants how much they agree with five items, including “The older I get the more useless I feel.” (An explanation of how we used the three ageism predictors to dichotomize the population into the high- and low-ageism groups, and generated the prevalence of the high-ageism groups that were used in the cost calculations, can be found in Supplementary Material.)
Primary Outcome: Excess Health Care Costs Due to Ageism
We calculated the excess health care spending due to ageism (defined as the extent to which this spending is higher for those in the high-ageism group, compared to those in the low-ageism group) for eight of the 10 most-expensive health conditions in the United States during 2013 (Dieleman et al., 2016). (Of the 10 conditions, two are not applicable to older persons because they include neonatal costs.) The eight health conditions consisted of cardiovascular disease, chronic respiratory disease, musculoskeletal disorders, injuries, diabetes mellitus, treatment of smoking, mental disorders, and non-communicable diseases.
The excess health care spending due to ageism was derived from the following: (a) number of Americans aged 60 years or older in 2013; (b) prevalence of ageism based on percentage of people at the negative end of each of the three predictor groups; (c) effect sizes of the impact of the three predictors on the eight health conditions; (d) prevalence of the eight health conditions in 2013, the most recent year for which health care spending was available; and (e) IHME costs per person of the eight health conditions in 2013. A benefit of the IHME data set is that in presenting health care costs, each dollar spent is only attributed to one health care category (IHME, 2018). (See Supplementary Material for description of the sources of the numbers, and the calculations for excess costs due to ageism that adjusted for age and sex, and which removed the overlapping costs of the three ageism predictors.)
Secondary Outcome: Number of Health Conditions Due to Ageism
To calculate the number of health conditions due to ageism, we determined the number of people in the high-ageism groups, as well as the difference in health condition rates for those in the low- and high-ageism groups; this was done for each of the eight health conditions and for each of the three ageism predictors. (See Supplementary Material for description of the sources of and calculations for these numbers that adjusted for age, sex, and overlapping number of health conditions due to the three ageism predictors.)
Covariates
To assure that we are reporting the two outcomes, the impact of ageism on the health care costs and the number of health conditions caused by ageism, above and beyond the health care costs of age and sex, we adjusted for these variables in two ways. First, in calculating the effect sizes for the impact of ageism on health, based on our systematic review, the statistics were abstracted from published studies with models that analyzed older participants and adjusted for covariates including age and sex. Most (94%) of these studies also adjusted for additional covariates, including demographic and health variables. Further, the ageism–health conditions effect sizes from HRS were based on prospective models that examined only participants who were aged 60 years or older at baseline and adjusted for participants’ age and sex.
The second way that we adjusted for age and sex in modeling excess cost due to ageism and the number of health condition cases due to ageism was by taking into account 5-year increments for all women and for all men for each health condition for all Americans aged 60 years and older in 2013, as listed by IHME. (See Supplementary Material for the example of how we calculated excess health care costs for men aged 60–64 years.) The year 2013 was selected for the demographic information as this was the most recent year available for the IHME health care cost data.
Effect Sizes Generated from Systematic Review of Ageism–Health Conditions
To generate the costs of the three ageism predictors on the eight health conditions, we combined effect sizes from a systematic review of the literature and from our HRS models. For the systematic review, we screened articles that appeared in five databases: PubMed, PsycINFO, Embase, Global Health, and Web of Science. The systematic review followed the Cochrane methodology (Moher, et al., 2009). Study inclusion criteria were: (a) published in peer-reviewed journals, (b) examined at least one of the three ageism predictors as an independent variable and at least one of the eight health conditions as a dependent variable, (c) analyzed information from participants who were at least aged 60 years, and (d) applied statistical approaches to adjust for potential confounding. Meta-analytic techniques combined the effect sizes of studies that examined associations of the same predictors and outcomes. (See description in the section labeled “Combining the Ageism–Health Condition Effect Sizes.”)
We developed search terms for the ageism predictors that are appropriate for each database. Search terms for age discrimination included the following: ageism, age discrimination, ageist beliefs, and ageist behaviors. Search terms for age stereotypes included the following: stereotype, stereotyping, self-stereotyping, and view of aging. Search terms for self-perceptions of aging included the following: self-perceptions of aging, self-concept, attitudes toward own aging, and age satisfaction. This yielded 12,558 articles. After removing duplicate articles, 8,555 articles remained.
Two reviewers then screened the titles and abstracts of these articles based on our inclusion criteria, which led to 193 studies. To increase the likelihood that all relevant articles were captured, three additional steps were undertaken. First, we screened the references of the 193 articles that survived the initial screening. Second, we screened the references of relevant systematic reviews and meta-analyses. Third, we screened other articles written by researchers who were the first or last author on at least two of the 193 articles. This process identified an additional two articles, which gave us a total of 195 articles.
Then, two reviewers read the full text of these 195 articles to examine whether they met all of the inclusion criteria. To avoid counting similar findings twice, when two or more studies that used the same predictor, the same outcome, and the same data set were found, we included the study with the largest sample size. As a quality check, all studies that met inclusion criteria were reviewed by a third reviewer. Any discrepancies were resolved through the following sequence: consulting the original article, discussion, and reaching a consensus among the three reviewers. This led to the final 17 articles. (See Supplementary Material for figure that depicts the flowchart of how we identified the relevant articles and the article list.)
We abstracted information related to the study effect size from the final list of 17 articles that met all inclusion criteria. If there were multiple measures of the predictors, some of which were worded in the positive direction and some in the negative direction, we selected the one that was negative and recorded the statistics that aggregated the most information about the impact of the predictor. Interrater reliability was 94.5. We then conducted random-effects meta-analyses to combine results of studies that examined the same predictors and the same outcomes.
Effect Sizes Generated from HRS Models of Ageism–Health Conditions
To derive ageism–health condition effect sizes from HRS (Sonnega & Weir, 2014), we calculated whether the earliest measurement of age discrimination or self-perceptions of aging, for persons aged 60 or older at baseline, predicted a later measurement of one of the eight health conditions, after adjusting for age and sex. In HRS, age discrimination was assessed in 2006 or 2008, and self-perceptions of aging were assessed in 2008 or 2010. HRS health conditions were assessed in 2012, the closest preceding year to 2013—the year of the most recent IHME spending data. (See Supplementary Material for how the HRS measures were matched to the eight health conditions.) The odds ratios were calculated for participants aged 60 years and older and were adjusted for age and sex.
Combining the Ageism–Health Condition Effect Sizes
Meta-analytic techniques were used to combine ageism effect sizes for studies with the same predictor and health outcome and to combine effect sizes for the same predictor and health outcome when they came from the systematic review and the HRS analyses. Although there was complete effect size data for all of the health conditions for the self-perceptions of aging predictor and for all of the ageism predictors for the most-prevalent health condition, cardiovascular disease, we imputed effect sizes for missing cells. To be conservative, we based the effect size on the smallest effect size with another ageism predictor and the same health condition.
Results
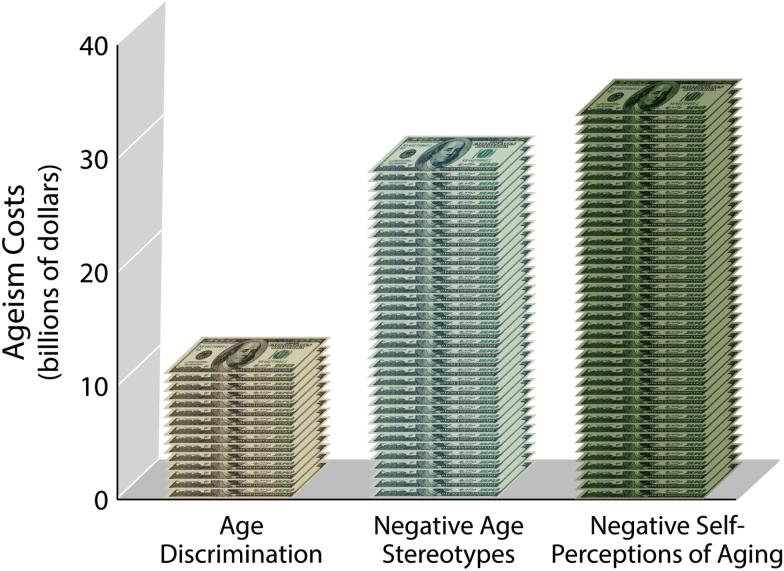
Consistent with our hypothesis, we found that the health care cost of negative self-perceptions of aging exceeded the health care cost of negative age stereotypes, which exceeded the health care cost of age discrimination (see Figure 1). The excess cost was $11.1 billion for age discrimination, $28.5 billion for negative age stereotypes, and $33.7 billion for negative self-perceptions of aging. These costs were adjusted for age and sex.
Figure 1.
Health care costs of age discrimination, negative age stereotypes, and negative self-perceptions of aging in 1 year
The total cost of the ageism predictors, after removing overlap between them, for the eight most-expensive health conditions was $63 billion for 1 year. This equates to 15.4% of overall health care spending for these conditions among those aged 60 years or older in 2013, the most recent year for which health care spending was available.
For each of the predictors and health conditions, the cost per person was significantly higher among those in the high-ageism group, compared to those in the low-ageism group. Further, the prevalence of each health condition was significantly greater for the high- than the low-ageism group (see Table 1).
Table 1:
Costs of Ageism on Most-Expensive Health Conditions among Older Persons in 1 Yeara
| Effect of Age Discrimination (AD) on Economic Costs | |||||
|---|---|---|---|---|---|
| Cost per person ($) | Prevalence (%) | Excess Cost of High Ageism ($ billions) | |||
| High AD | Low AD | High AD | Low AD | ||
| Cardiovascular Disease | 2,901 | 2,700* | 29.34% | 27.25%* | 3.527 |
| Chronic Respiratory Disease | 1,098 | 962* | 17.06% | 14.93%* | 2.379 |
| Musculoskeletal Disorders | 986 | 864* | 37.14% | 32.63%* | 2.131 |
| Injuries | 700 | 643* | 21.73% | 19.94%* | 0.995 |
| Diabetes Mellitus | 944 | 889* | 30.49% | 28.72%* | 0.960 |
| Mental Disorders | 323 | 270* | 12.14% | 10.30%* | 0.928 |
| Non-communicable Disease | 1,148 | 1,085* | 69.74% | 67.91%* | 1.192 |
| Treatment of Smoking | 0.40 | 0.32* | 11.18% | 8.94%* | 0.001 |
| Effect of Negative Age Stereotypes (AS) on Economic Costs | |||||
| Negative AS | Positive AS | Negative AS | Positive AS | ||
| Cardiovascular Disease | 2,840 | 2,629* | 28.68% | 26.55%* | 7.918 |
| Mental Disorders | 348 | 187* | 12.76% | 7.85%* | 6.050 |
| Chronic Respiratory Disease | 1,052 | 922* | 16.34% | 14.30%* | 4.901 |
| Musculoskeletal Disorders | 945 | 828* | 35.61% | 31.29%* | 4.384 |
| Diabetes Mellitus | 935 | 858* | 30.20% | 27.73%* | 2.875 |
| Injuries | 681 | 626* | 21.13% | 19.40%* | 2.076 |
| Non-communicable Disease | 1,127 | 1,066* | 69.14% | 67.34%* | 0.140 |
| Treatment of Smoking | 0.31 | 0.30* | 10.39% | 8.32%* | .020 |
| Effect of Negative Self-perceptions of Aging (SPA) on Economic Costs | |||||
| Negative SPA | Positive SPA | Negative SPA | Positive SPA | ||
| Cardiovascular Disease | 3,235 | 2,284* | 32.82% | 22.91%* | 13.003 |
| Chronic Respiratory Disease | 1,268 | 737* | 19.71% | 11.40%* | 7.268 |
| Musculoskeletal Disorders | 1,139 | 661* | 43.16% | 24.76%* | 6.539 |
| Diabetes Mellitus | 1,045 | 765* | 33.76% | 24.74%* | 3.828 |
| Mental Disorders | 338 | 232* | 12.58% | 9.07%* | 1.458 |
| Non-communicable Disease | 1,122 | 940* | 73.56% | 63.36%* | 0.874 |
| Injuries | 687 | 632* | 21.32% | 19.58%* | .762 |
| Treatment of Smoking | 0.38 | 0.31* | 10.64% | 8.51%* | .001 |
aBased on population of Americans aged 60 or older in 2013.
*These differences are significant at p < .001 after adjusting for age and sex.
To extend our analysis beyond dollars to human experiences, we also calculated the number of cases of the eight health conditions that were experienced by those aged 60 years or older in the United States during 1 year due to ageism, after removing overlap of predictors. (See Table 2 and Supplementary Material for details on these calculations.) According to our model, there were 17.04 million cases of the health conditions attributable to ageism.
Table 2:
Number of Health-Condition Cases due to Ageism among Older Individuals, According to Model
| Age Discrimination | Negative Age Stereotypes | Negative Self-perceptions of Aging | |
|---|---|---|---|
| Cardiovascular Disease | 366,365 | 799,617 | 1,355,463 |
| Mental Disorders | 322,075 | 1,848,253 | 480,521 |
| Chronic Respiratory Disease | 371,723 | 765,505 | 1,136,724 |
| Musculoskeletal Disorders | 789,516 | 1,625,494 | 2,517,869 |
| Diabetes Mellitus | 309,528 | 929,077 | 1,233,518 |
| Injuries | 311,940 | 650,559 | 238,830 |
| Non-communicable Disease | 319,758 | 677,614 | 1,395,782 |
| Treatment of Smoking | 391,360 | 782,590 | 291,167 |
Discussion
This study helps to give visibility to the damaging results of ageism. Overall, $63 billion, or one in every seven dollars, spent on health care for the eight most-expensive conditions during 1 year in the United States was due to ageism. This is greater than the total amount the United States spent on health care costs of morbid obesity for the same year (Kim & Basu, 2016; Tsai, Williamson, & Glick, 2011).
Further, according to our model, 17.04 million cases of the health conditions are due to ageism. This means that even a 10% reduction in the prevalence of ageism could lead to 1.7 million fewer cases of the health conditions. The goal of reducing age discrimination seems plausible because laboratory and field research showed that negative age stereotypes and negative self-perceptions of aging can be made significantly more positive with intervention (Levy, 2009; Levy, Pilver, Chung, & Slade, 2014). A systematic review of interventions among students found that 88% of studies successfully reduced ageism (Chonody, 2015).
In the current study, the health condition that showed the highest excess cost among the three predictors of ageism was cardiovascular disease. This is in accord with research that found exposure to negative age stereotypes leads to heightened cardiovascular stress among older persons and predicts their risk of experiencing cardiovascular events (Levy, Hausdorff, Hencke, & Wei. 2000; Levy et al., 2009); along with the cost of treating cardiovascular conditions.
Strengths of the model we used to generate the costs and prevalence of ageism include the following: (a) basing it on ageism–health effect sizes derived from prospective studies that measured ageism years before the health outcomes; (b) adjusting for covariates, including baseline health, age, and sex, in the studies that generated the ageism–health effect sizes, the health-outcome costs, and the prevalence estimates; (c) removing overlapping costs and overlapping prevalence estimates of the three ageism predictors; (d) including participants from the nationally representative HRS to generate the ageism–health effect sizes; and (e) incorporating the health costs of all Americans aged 60 years or older to generate the excess ageism costs.
There are a number of ways that the costs-of-ageism calculations in this study are conservative. For not only are the eight health conditions a small sampling, but within several of the health-condition subcategories there are no ageism studies available to draw on for the analyses (e.g., vision impairment within noncommunicable diseases); in these cases, models assumed no cost of ageism. Also, we did not include the secondary costs that are associated with health conditions, such as lost hours of employment for those with the health conditions as well as their caregivers. Moreover, ageism examples are found in numerous domains besides health (Butler, 2010; Harris, Krygsman, Waschenko, & Laliberte Rudman, 2018; Neumark, Burn, & Button, 2016; Palmore, 2015).
Several of the predictor–outcome associations that were found in this study have not been previously reported. Among them are the association of age discrimination and negative self-perceptions of aging with diabetes mellitus and with musculoskeletal disorders.
The results of this study make a strong case for interventions. A comprehensive approach would involve addressing the societal sources of injurious images about and behaviors toward the old. An intervention of this type would require a large-scale campaign. Given the magnitude of the health care costs resulting from ageism, even if such an intervention had a limited impact on ageism, its potential could be substantial—not only financially, but also by enhancing the lives of older persons.
Funding
This research was supported by a grant from the National Institute on Aging (U01AG032284) to Becca R. Levy.
Supplementary Material
Acknowledgements
We thank Mark Saba for his creation of the tables and figure.
Conflict of Interest
None reported.
References
- Butler R. N. (2010). The longevity revolution: The benefits and challenges of living a long life. New York: Public Affairs. [Google Scholar]
- Chonody J. M. (2015). Addressing ageism in students: A systematic review of the pedagogical intervention research. Educational Gerontology, 41, 859–887. doi:10.1080/03601277.2015.1059139 [Google Scholar]
- Dieleman J. L., Baral R., Birger M., Bui A. L., Bulchis A., Chapin A., … Murray C. J (2016). US spending on personal health care and public health, 1996-2013. JAMA, 316, 2627–2646. doi:10.1001/jama.2016.16885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K., Krygsman S., Waschenko J., & Laliberte Rudman D (2018). Ageism and the older worker: A scoping review. The Gerontologist, 58, e1–e14. doi:10.1093/geront/gnw194 [DOI] [PubMed] [Google Scholar]
- Hehman J. A. & Bugental D. B (2015). Responses to patronizing communication and factors that attenuate those responses. Psychology and Aging, 30, 552–560. doi:10.1037/pag0000041 [DOI] [PubMed] [Google Scholar]
- Horton S., Baker J., Pearce G. W., & Deakin J. M (2008). On the malleability of performance: Implications for seniors. Journal of Applied Gerontology, 27, 446–465. doi:10.1177/0733464808315291 [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME) (2018). Tracking personal health care spending in the US. Seattle, WA: IHME, University of Washington; Retrieved from http://vizhub.healthdata.org/dex. Accessed March 10, 2018. [Google Scholar]
- Kim D. D. & Basu A (2016). Estimating the medical care costs of obesity in the United States: Systematic review, meta-analysis, and empirical analysis. Value in Health, 19, 602–613. doi:10.1016/j.jval.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Kim E. S., Moored K. D., Giasson H. L., & Smith J (2014). Satisfaction with aging and use of preventive health services. Preventive Medicine, 69, 176–180. doi:10.1016/j.ypmed.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont R. A., Swift H. J., & Abrams D (2015). A review and meta-analysis of age-based stereotype threat: Negative stereotypes, not facts, do the damage. Psychology and Aging, 30, 180–193. doi:10.1037/a0038586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. P. (1975). The Philadelphia geriatric center morale scale: A revision. Journal of Gerontology, 30, 85–89. doi:10.1093/geronj/30.1.85 [DOI] [PubMed] [Google Scholar]
- Lee K., & Lee H (2018). Priming effects of age stereotypes on memory of older adults in Korea. Asian Journal of Social Psychology. Advance online publication. doi:10.1111/ajsp.12343 [Google Scholar]
- Levy B. (2009). Stereotype embodiment: A psychosocial approach to aging. Current Directions in Psychological Science, 18, 332–336. doi:10.1111/j.1467-8721.2009.01662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R. & Bavishi A (2018). Survival advantage mechanism: Inflammation as a mediator of positive self-perceptions of aging on longevity. Journal of Gerontology B: Psychological Sciences and Social Sciences, 73, 409–412. doi:10.1093/geronb/gbw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Ferrucci L., Zonderman A. B., Slade M. D., Troncoso J., & Resnick S. M (2016). A culture-brain link: Negative age stereotypes predict Alzheimer’s disease biomarkers. Psychology and Aging, 31, 82–88. doi:10.1037/pag0000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Hausdorff J. M., Hencke R., & Wei J. Y (2000). Reducing cardiovascular stress with positive self-stereotypes of aging. Journal of Gerontology B: Psychological Science and Social Science, 55, 205–213. doi:10.1093/geronb/55. 4.P205 [DOI] [PubMed] [Google Scholar]
- Levy B. R. & Leifheit-Limson E (2009). The stereotype-matching effect: Greater influence on functioning when age stereotypes correspond to outcomes. Psychology and Aging, 24, 230–233. doi:10.1037/a0014563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Moffat S., Resnick S. M., Slade M. D., & Ferrucci L (2016). Buffer against cumulative stress: Positive age self-stereotypes predict lower cortisol across 30 years. Journal of Gerontopsychology and Geriatric Psychiatry, 29, 141–146. doi:10.1024/1662–9647/a000149 [Google Scholar]
- Levy B. R. & Myers L. M (2004). Preventive health behaviors influenced by self-perceptions of aging. Preventive Medicine, 39, 625–629. doi:10.1016/j.ypmed.2004.02.029 [DOI] [PubMed] [Google Scholar]
- Levy B. R., Pilver C., Chung P. H., & Slade M. D (2014). Subliminal strengthening: Improving older individuals’ physical function over time with an implicit-age-stereotype intervention. Psychological Science, 25, 2127–2135. doi:10.1177/0956797614551970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Slade M. D., Chung P. H., & Gill T. M (2015). Resiliency over time of elders’ age stereotypes after encountering stressful events. Journal of Gerontology B: Psychological Sciences and Social Sciences, 70, 886–890. doi:10.1093/geronb/gbu082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Slade M. D., & Kasl S. V (2002). Longitudinal benefit of positive self-perceptions of aging on functional health. Journal of Gerontology B: Psychological Science and Social Science, 57, 409–417. doi:10.1093/geronb/57.5.P409 [DOI] [PubMed] [Google Scholar]
- Levy B. R., Slade M. D., Pietrzak R. H., & Ferrucci L (2018). Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One, 13, e0191004. doi:10.1371/journal.pone.0191004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R., Zonderman A. B., Slade M. D., & Ferrucci L (2009). Age stereotypes held earlier in life predict cardiovascular events in later life. Psychological Science, 20, 296–298. doi:10.1111/j.1467-9280.2009.02298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J. & Bollen K. A (1983). The structure of the Philadelphia geriatric center morale scale: A reinterpretation. Journal of Gerontology, 38, 181–189. doi:10.1093/geronj/38.2.181 [DOI] [PubMed] [Google Scholar]
- Marchiondo L. A., Gonzales E., & Williams L. J (2017). Trajectories of perceived workplace age discrimination and long-term associations with mental, self-rated, and occupational health. [Epub ahead of print]. Journal of Gerontology B: Psychological Sciences and Social Sciences. doi:10.1093/geronb/gbx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H. (1977). Self-schemata and processing information about the self. Journal of Personality and Social Psychology, 35, 63–78. doi:10.1037/0022-3514.35.2.63 [Google Scholar]
- Meisner B. A. (2012). A meta-analysis of positive and negative age stereotype priming effects on behavior among older adults. Journal of Gerontology B: Psychological Sciences and Social Sciences, 67, 13–17. doi:10.1093/geronb/gbr062 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G, & the PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269, W64. doi:10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Neuman P. J., Sanders G. D., Russell L. B., Siegel J. E., & Ganiats T. G (Eds.). (2016). Cost effectiveness in health and medicine. New York, NY: Oxford University Press. [Google Scholar]
- Neumark D. A., Burn I., & Button P (2016). Experimental age discrimination evidence and the Heckman critique. American Economic Review, 106, 303–308. doi:10.1257/aer.p20161008 [Google Scholar]
- Organisation for Economic Co-operation and Development (2017). Health at a glance 2017: OECD indicators. Paris: OECD Publishing. doi:10.1787/health_glance-2017-en [Google Scholar]
- Palmore E. (2015). Ageism comes of age. Journal of Gerontology B: Psychological Sciences and Social Sciences, 70, 873–875. doi:10.1093/geronb/gbv079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L. E., Stahlberg D., & Dauenheimer D (2000). Effects of self-schema elaboration on affective and cognitive reactions to self-relevant information. Genetic, Social, and General Psychology Monographs, 126, 25–42. [PubMed] [Google Scholar]
- Sargent-Cox K. A., Anstey K. J., & Luszcz M. A (2012). The relationship between change in self-perceptions of aging and physical functioning in older adults. Psychology and Aging, 27, 750–760. doi:10.1037/a0027578 [DOI] [PubMed] [Google Scholar]
- Sarkisian C. A., Steers W. N., Hays R. D., & Mangione C. M (2005). Development of the 12-item expectations regarding aging survey. The Gerontologist, 45, 240–248. doi:10.1093/geront/45.2.240 [DOI] [PubMed] [Google Scholar]
- Sonnega A. & Weir D. R (2014). The Health and Retirement Study: A public data resource for research on aging. Open Health Data, 2, e7. doi:10.5334/ohd.am [Google Scholar]
- Tsai A. G., Williamson D. F., & Glick H. A (2011). Direct medical cost of overweight and obesity in the USA: A quantitative systematic review. Obesity Reviews, 12, 50–61. doi:10.1111/j.1467-789X.2009.00708.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations (2012). General assembly resolution 67/139, Towards a comprehensive and integral international legal instrument to promote and protect the rights and dignity of older persons, A/RES/67/139 Retrieved from undocs.org/A/RES/67/139. Accessed May 15, 2017.
- United Nations (2014). Open-ended working group on ageing, US statement, delivered by Kathy Greenlee: Existing international framework on the human rights of older persons and identification of existing gaps at the international level. Retrieved from https://social.un.org/ageing-working-group/documents/fifth/United%20States.pdf. Accessed May 15, 2018.
- United Nations (2017). Open-ended working group on aging, US statement, delivered by Mr. Martin Garcia Moritan Retrieved from https://social.un.org/ageing-working-group/documents/eighth/Inputs%20Member%20States/UnitedStates.pdf. Accessed May 15, 2018.
- Westerhof G. J., Miche M., Brothers A. F., Barrett A. E., Diehl M., Montepare J. M., … Wurm S (2014). The influence of subjective aging on health and longevity: A meta-analysis of longitudinal data. Psychology and Aging, 29, 793–802. doi:10.1037/a0038016 [DOI] [PubMed] [Google Scholar]
- Williams D. R., Yu Y., Jackson J. S., & Anderson N. B (1997). Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology, 2, 335–351. doi:10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- Wurm S., Tesch-Römer C., & Tomasik M. J (2007). Longitudinal findings on aging-related cognitions, control beliefs, and health in later life. Journal of Gerontology B: Psychological Sciences and Social Sciences, 62, P156–P164. doi:10.1093/geronb/62.3.P156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.