Abstract
Background and Objectives
Minimizing disability is critical to reduce the costly health care associated with disability and maintain quality of life into old age. We examined the effect sizes of nonpharmacological intervention studies in reducing disability and explored the active ingredients of interventions.
Research Design and Methods
A scoping review was conducted via PubMed, PsycINFO, and CINAHL databases. Thirty-one randomized controlled trials were included. Eight active ingredients were identified by three experts (exercise, problem-solving, cognitive behavioral therapy, environmental modification, education, goal setting, comprehensive geriatric assessment, and cognitive training).
Results
The range of Cohen’s d was –0.85 to 1.76 across 31 studies (included 33 interventions); 67% studies (n = 22) obtained small-to-negative effect sizes (d = –0.85 to 0.18), accounting for 83% participants across studies. Interventions that incorporated exercise, problem-solving, cognitive behavior therapy, and environmental modification were associated with stronger effect sizes. Interventions that incorporated comprehensive geriatric assessment obtained small effect sizes.
Discussion and Implications
Majority of intervention studies found little or no effect in reducing disability for older adults. To optimize the effects of nonpharmacological interventions, we recommend researchers to (i) develop a screening tool for “risk of disability” to inform those who are early on the disability progression, yet not experience any difficulties in activities of daily living and instrumental activities of daily living; (ii) specify the active ingredients embedded in complex interventions to facilitate change in disability; and (iii) select sensitive tools to capture the progression of disability in late life.
Keywords: Intervention efficacy, Active ingredients, Complex interventions
One of three older adults experiences disability. Disability is defined by the inability to sustain independence with basic activities of daily living (ADL) or instrumental activities of daily living (IADL; Kraus, 2017; Ortman, Velkoff, & Hogan, 2014). ADL and IADL disabilities are associated with substantial health care costs; older adults with disability have greater out-of-pocket health care expenditures than older adults without disability (Mitra, Palmer, Kim, Mont, & Groce, 2017). Older adults with disability also experience a lower sense of well-being (Groessl et al., 2007). Effective strategies to minimize disability become critical to reduce costly health care related to disability and sustain quality of life into old age.
Nonpharmacological interventions are promising to reduce disability in late life. They adopt behavioral change techniques, devices, and technologies to facilitate change in health and quality of life (Boutron, Moher, Altman, Schulz, & Ravaud, 2008). They may include complementary and alternative medicine and therapy (e.g., Tai Chi). As such, they often have fewer risks and side effects than pharmacological interventions (Krishnan et al., 2018). Typically, they are developed for older adults with a single medical condition, once disability has emerged. For example, moderate-intensity, supervised exercise programs have been developed to improve function after acute myocardial infarction or cardiac arrest (Boyce et al., 2017; Peixoto et al., 2015). Although these nonpharmacological interventions have demonstrated success in reducing disability, intervening after the emergence of medical conditions is often too late to minimize disability because the acute medical condition already results in newly acquired disability (Capistrant et al., 2014). In addition, after acute medical conditions, these illnesses often have exacerbations or remissions that lead to risks of comorbidity and long-term disability (Brown et al., 2009; Collins et al., 2018).
Instead of focusing on a single medical condition or comorbidity, researchers have adopted various eligibility criteria (e.g., frailty or at-risk of falling) to examine how to minimize disability through nonpharmacological interventions for broader groups of older adults (Ferrucci et al., 2004). Older adults who fit these criteria may have disability that is not caused by an acute medical condition, but they have a higher risk for acquiring more severe disability, as describe by Ferrucci and colleagues. Little research has examined how effective these nonpharmacological interventions are at minimizing disability for older adults that fit these eligibility criteria.
In addition, the active ingredients that drive the efficacy of nonpharmacological interventions are poorly specified and evaluated (Boutron et al., 2008). Active ingredients are the key components that are embedded in the interventions to change outcomes. Nonpharmacological interventions are often complex interventions, as defined by the Medical Research Council (Craig et al., 2008). A complex intervention is composed of more than one active ingredient (Michie, Fixsen, Grimshaw, & Eccles, 2009). These active ingredients can be problem-solving, goal setting, exercise, or comprehensive geriatric assessment (Michie et al., 2013; Wells, Seabrook, Stolee, Borrie, & Knoefel, 2003). The evaluation of active ingredients is critical because they may determine intervention efficacy in reducing disability in late life.
The purpose of this scoping review was to examine the effects of nonpharmacological interventions on disability in community-dwelling older adults participating in randomized controlled trials. The active ingredients of the interventions were also examined. We chose to include studies that recruited community-dwelling older adults to inform the development of future home-based programs. Information gleaned from this review may provide insights into how to optimize the effects of nonpharmacological interventions on disability for older adults.
Methods
Identifying Relevant Studies
We followed the scoping review methodological approach (Arksey & O’Malley, 2005; Moher, Liberati, Tetzlaff, & Altman, 2009). An electronic search was conducted in January 2018 of PubMed, PsycINFO, and CINAHL databases to locate intervention studies (without date restrictions on the publications). The terms used for the literature search were older adults, disability, preclinical disability, activities of daily living, instrumental activities of daily living, intervention, social engagement, social participation, treatment outcome, clinical trial, and clinical study. Terms were paired to search for eligible studies.
Study Selection
We included studies that (i) recruited community-dwelling older adults aged at least 50 years, (ii) examined nonpharmacological interventions via randomized controlled trials (without the use of medication or substances), (iii) measured ADL or IADL disabilities as primary or secondary outcomes, (iv) included at least one follow-up, (v) were written in English, and (vi) had sufficient data to calculate effect sizes. We excluded interventions that (i) focused on a specific diagnosis (e.g., cardiac arrest), (ii) focused on one gender; (iii) were not home based, and (iv) had follow-up longer than 3 years to minimize the influence of follow-up duration on results. Mendeley software (version 1.17) was used to manage the selection process (Mendeley Ltd, 2017). The preferred reporting items for systematic review and meta-analysis guidelines were followed (Supplementary Appendix A; Moher et al., 2009).
A two-level screening process was performed to determine the inclusion eligibility: (i) title and abstract review and (ii) full-text review. One reviewer (C. Y. W) reviewed abstracts and titles. Discrepancies in eligibility were determined through consensus by three authors (J. R., E. R. S., and C. Y. W.).
Charting the Data
Study characteristics (number of participants, age, gender, dosage, intervention session format, measure of disability, and follow-up duration) and the means and standard deviations of disability were extracted for intervention and control groups. Baseline disability was categorized into four levels (negligible, mild, moderate, and severe) using the cutoff points of measures of disability listed in the studies.
Active ingredients of interventions were identified and coded based on the descriptions of interventions within the articles. Three independent authors coded the active ingredients separately (J. R., E. R. S., and C. Y. W.). Discrepancies were discussed and resolved by three authors (J. R., E. R. S., and C. Y. W.). Complex intervention (YES/NO) was characterized by more than one included active ingredient (Craig et al., 2008).
Collating, Summarizing, and Reporting the Results
Effect sizes were computed using standardized mean differences, as known as Cohen’s d. The Cohen’s d was estimated by the differences between the mean changes of disability in the intervention and control groups divided by the pooled standard deviation (SD) of disability at baseline (Feingold, 2009). The effect sizes of the primary outcome were selected if multiple assessments were used to measure disability. The effect size of the longest follow-up was selected if there were multiple follow-ups.
STATA (version 15.0; StataCorp, 2017) and SPSS (version 24.0; IBM Corp., 2013) were used for statistical analyses. For each active ingredient, the heterogeneity of included studies was computed using the I-square statistics (I2; Higgins & Thompson, 2002). The magnitude of heterogeneity was followed by low (30%), moderate (50%), and high (75%). All the analyses were considered significant at the .05 two-tailed α level.
Cohen’s d was used to categorize the four-level magnitude of effect sizes (<0.2 = negligible; 0.2–0.5 = small; 0.5–0.8 = moderate; >0.8 = large; Lakens, 2013). We calculated the proportion of participants within each magnitude of effect size (negligible, small, moderate, and large) across active ingredients. The greater proportion of participants in the studies with moderate-to-large effect sizes, the more influence of an active ingredient on disability. This approach provided a visualization of magnitudes of effect sizes across studies, accounting for study sample sizes. Forest plots were generated for each active ingredient to visualize and synthesize the effect sizes of studies.
Results
Study Characteristics
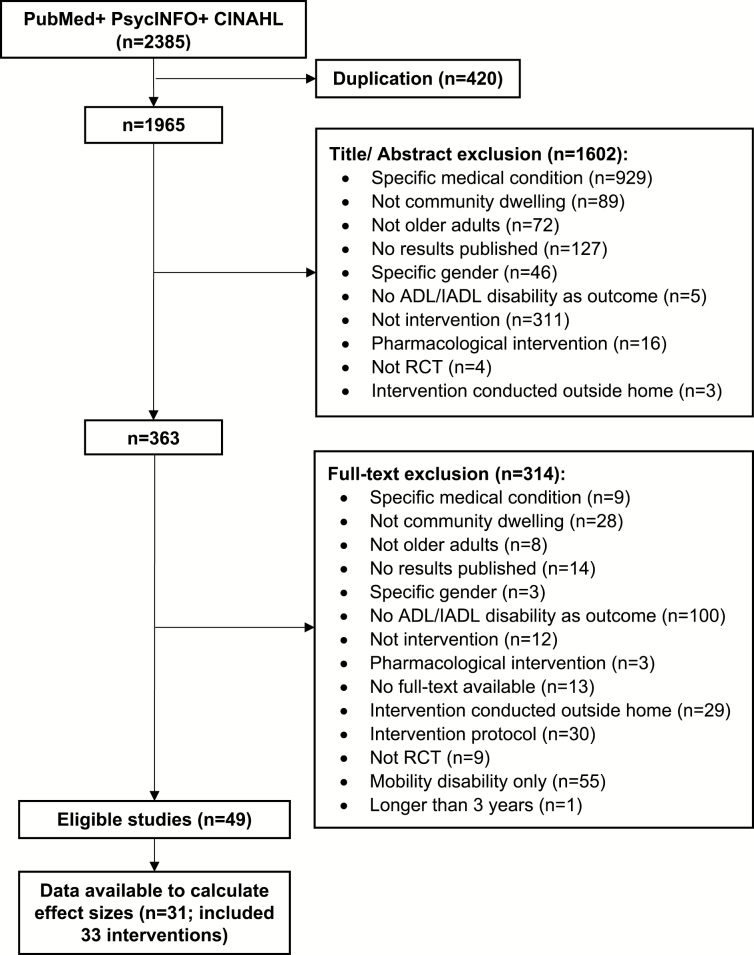
A total of 2,385 articles were identified; 363 articles were reviewed by full text. A total of 49 articles were eligible for this review (Figure 1). Sixteen authors were contacted and requested to provide means and SDs for studies that did not include necessary data. Means and SDs were obtained from two authors. There were 31 studies (included 33 interventions) with sufficient data (i.e., means and SDs) to be included in the analyses (Table 1; Figure 2; Supplementary Appendix B).
Figure 1.
Review diagram.
Table 1.
Included Studies (n = 31)
| Authors, (year) | Population | No. allocated | Mean age (year) | Female (%) | Dosage | Session format | Disability measure | Primary outcome (Yes; No) | Follow-up duration (month) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | |||||||
| Alexopoulos et al. (2011) | Major depressive disorder | 110 | 111 | 72.8 | 73.2 | N/A | N/A | 12 Weekly sessions | Individual | WHODAS | Yes | 9 |
| Binder et al. (2002) | Frailty | 66 | 49 | 83.0 | 83.0 | 52.0 | 53.0 | 1 h/3 times × 12 weeks | Group | FSQ | Yes | 9 |
| Bouman et al. (2008) | Poor health conditions | 139 | 154 | 75.8 | 75.6 | 60.0 | 60.0 | 1–1.5 h/8 sessions | Individual | GARS | Yes | 24 |
| Callahan et al. (2005) | Major depression or dysthymic disorder | 906 | 895 | 71.0 | 71.4 | 64.1 | 65.6 | 6–8 Sessions | Individual | 7 IADL | Yes | 12 |
| Cameron et al. (2013) | Frailty | 108 | 116 | 83.4 | 83.2 | 67.0 | 68.0 | 10 Sessions | Individual | BI | Yes | 12 |
| Chin et al. (2001) | Physical inactivity | 80 | 74 | 77.5 | 78.9 | 73.0 | 70.0 | 45 min/2 times × 17 weeks | Group | 16 ADL | Yes | 3 |
| Clare et al. (2015) | Without dementia or intellectual disability | 22 | 27 | 68.2 | 70.2 | 79.2 | 85.2 | A session and a follow-up call | Individual | FCAS | Yes | 12 |
| 21 | 27 | 67.5 | 70.2 | 95.8 | 85.2 | |||||||
| Clark et al. (1997) | Without dementia | 101 | 202 | N/A | N/A | 64.0 | 65.5 | 2 h per week/9 month | Group+ Individual | FSQ | Yes | 9 |
| Counsell et al. (2007) | Income less than 200% of the federal poverty level | 474 | 477 | 71.8 | 71.6 | 75.5 | 76.5 | 1 Visit and 1 phone call | Individual | 7 ADL + 6 BADL | Yes | 24 |
| Day et al. (2012) | Preclinical disability | 171 | 190 | N/A | N/A | 66.2 | 69.7 | 1 h/2 times × 48 weeks |
Group | LLFDI | Yes | 6 |
| Dorresteijn et al. (2016) | Concerns about falls and associated activity avoidance | 141 | 171 | 78.4 | 78.3 | 68.0 | 72.3 | 3 Sessions; 4 phone calls | Individual | GARS | No | 12 |
| Fairhall et al. (2012) | Frailty | 111 | 121 | 83.4 | 83.2 | 67.0 | 68.0 | 1 h/10 sessions | Individual | LSA-UAB | Yes | 12 |
| Foley et al. (2011) | Discharge from day rehabilitation | 34 | 36 | 78.3 | 79.9 | 79.0 | 81.0 | 1 h/24 sessions | Individual | BI | Yes | 3 |
| Gill et al. (2004) | Frailty | 91 | 91 | 82.8 | 83.5 | 85.0 | 74.0 | 16 Sessions × 6 months | Individual | 8 ADL | Yes | 12 |
| Gitlin et al. (2006) | Difficulty with two IADLs or one ADL | 154 | 146 | 79.5 | 78.5 | 82.5 | 81.1 | 1.5 h/6 sessions | Individual | 6 ADL + 6 IADL | Yes | 12 |
| Haines et al. (2009) | Discharged from hospital | 19 | 34 | 80.9 | 80.5 | 74.0 | 53.0 | 8 Weekly phone calls | Individual | FAI | Yes | 6 |
| Hendriks et al. (2008) | Fall | 123 | 134 | 74.5 | 75.2 | 66.9 | 70.1 | 2 Visits | Individual | FAI | No | 12 |
| Kerse et al. (2010) | Depressive symptoms | 94 | 87 | 81.4 | 80.8 | 63.9 | 53.1 | 1 h/8 sessions | Individual | NEADL | Yes | 12 |
| Kerse et al. (2014) | Participated in primary care practice | 1787 | 1619 | 80.4 | 80.3 | 56.0 | 54.0 | 3 Visits | Individual | NEADL | Yes | 36 |
| King et al. (2012) | Received assistance from the home care | 82 | 82 | 80.5 | 78.4 | 77.4 | 69.9 | At least 5 visits | Individual | NEADL | No | 7 |
| Kono et al. (2012) | Frailty | 105 | 100 | 80.3 | 79.6 | 73.9 | 74.1 | 4 Visits | Individual | BI | Yes | 24 |
| Lannin et al. (2007) | Mild to no cognitive impairments | 5 | 5 | 80.0 | 82.4 | 100 | 60.0 | 55–85 min/1 session | Individual | NEADL | Yes | 3 |
| Liu et al. (2014) | Fall | 64 | 58 | 74.5 | 74.5 | 87.5 | 86.2 | 1.5 h/8 sessions | Group | 5 Social activities | No | 3 |
| Mahoney et al. (2007) | Fall | 130 | 135 | 79.6 | 80.3 | 78.7 | 78.3 | 2 Visits/phone calls | Individual | BI | No | 12 |
| Pighills et al. (2011) | Fall | 87 | 78 | 78.0 | 80.0 | 71.0 | 67.0 | 1 Visit/2 phone calls | Individual | BI | No | 12 |
| 73 | 78 | 79.0 | 80.0 | 62.0 | 67.0 | |||||||
| Rockwood et al. (2003) | Frailty | 85 | 80 | 81.4 | 82.2 | 56.8 | 57.5 | 1–6 Visits/1 follow-up | Individual | Lawton IADL | N/A | 12 |
| Rydwik et al. (2010) | Frailty | 20 | 19 | 83.5 | 82.9 | 47.8 | 69.6 | 1 h/2 times × 12 weeks | Group | FIM | No | 24 |
| Szanton et al. (2011) | Low income; difficulties in 1ADL or 2 IADLs | 20 | 15 | 79.0 | 77.0 | 96.0 | 94.0 | 1 h/10 sessions | Individual | 5 ADL+ 6 IADL | Yes | 6 |
| van Hout et al. (2010) | Frailty | 331 | 330 | 81.3 | 81.5 | 72.2 | 68.8 | 3 Visits/phone contacts | Individual | GARS | Yes | 18 |
| Villareal et al. (2006) | Frail and obese | 17 | 10 | 71.1 | 69.4 | 60.0 | 71.0 | 1.5 h/3 days × 26 weeks | Group | FSQ | Yes | 24 |
| von Bonsdorff et al. (2008) | Sedentary | 310 | 306 | 77.6 | 77.6 | 74.5 | 75.2 | 1 h/every 4 months phone calls | Individual | Lawton IADL | Yes | 24 |
| Total | 6081 | 5952 | 77.9 | 78.4 | 71.8 | 69.7 |
Note: N/A = none applicable; WHODAS = World Health Organization Disability Assessment Schedule; FSQ = Functional Status Questionnaire; GARS = Groningen Activity Restriction Scale; BI = Barthel Index); FCAS = Florida Cognitive Activities Scale; LLFDI = Late-Life Function and Disability Instrument); LSA-UAB = University of Alabama at Birmingham Study of Aging Life-Space Assessment; FAI = Frenchay Activities Index; NEADL = Nottingham Extended Activities of Daily Living Scale; FIM = Functional Independence Measure.
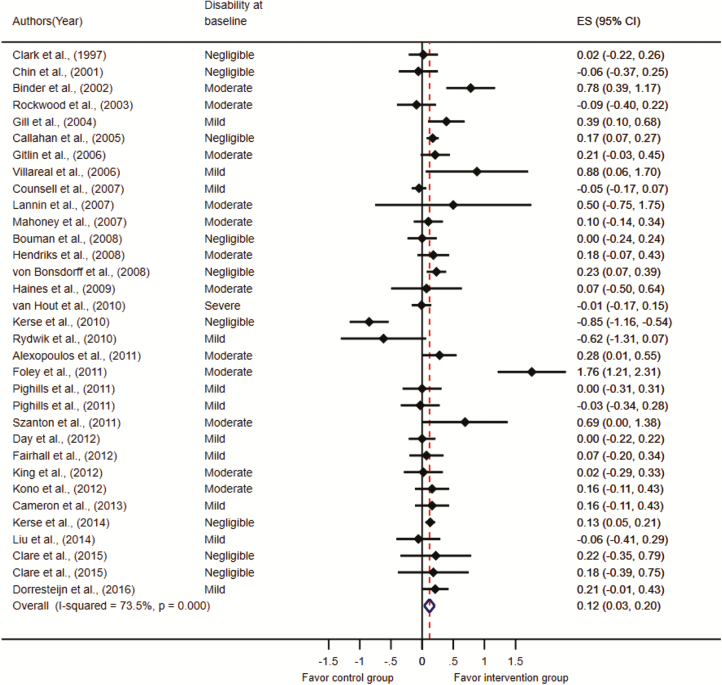
Figure 2.
A forest plot of the effects of 33 interventions on disability.
A quarter of the studies (25.8%) had longer than 12-month follow-up. Nearly three-fourths studies (74.2%) listed disability as their primary outcome. Almost half of the studies (47.2%) examined complex interventions. Most of the studies (80.6%) had individual sessions in the interventions as opposed to group sessions. Intervention dosages varied from 2 to 78 sessions (3 sessions per week for 26 weeks).
Eleven measurements were used to assess disability (Table 1). Several authors developed participant-reported measures of disability (n = 7). Barthel Index (n = 5; Mahoney & Barthel, 1965) and Nottingham Extended Activities of Daily Living Scale (n = 4; Nouri & Lincoln, 1987) were the most frequently used tools to assess ADL and IADL disabilities. Other studies used Groningen Activity Restriction Scale (n = 3; Kempen & Suurmeijer, 1990), Functional Status Questionnaire (n = 3; Jette et al., 1986), Frenchay Activities Index (n = 2; Schuling, de Haan, Limburg, & Groenier, 1993), or Lawton IADL measures (n = 2; Lawton & Brody, 1969).
Participant Characteristics
Studies recruited participants with negligible (n = 9), mild (n = 11), moderate (n = 12), and severe disability (n = 1; Figure 2).
Effects of Intervention Studies
There was a moderate-to-high heterogeneity among the 31 studies (I2= 73.5%, p < .001). Figure 2 illustrates the effect size d ranged from –0.85 to 1.76 across studies, with 9 having statistically significant effect sizes (Alexopoulos et al., 2011; Binder et al., 2002; Callahan et al., 2005; Foley, Hillier, & Barnard, 2011; Gill et al., 2004; Kerse et al., 2014; Szanton et al., 2011; Villareal, Banks, Sinacore, Siener, & Klein, 2006; von Bonsdorff et al., 2008). Of the 31 studies, 22 had negligible effect sizes, accounting for 83% of total participants; 6 studies had mild effect sizes, accounting for 14% of total participants; 3 studies had moderate effect sizes, accounting for 2% of total participants; and 2 studies had large effect sizes, accounting for 1% of total participants. Studies included measures of ADL disability, IADL disability, and ADL-combined IADL disability. These effect size ranged from d = –0.62 to 1.76, d = –0.09 to 0.69, and d = –0.85 to 0.88, respectively.
Effects of Active Ingredients Embedded in the Interventions
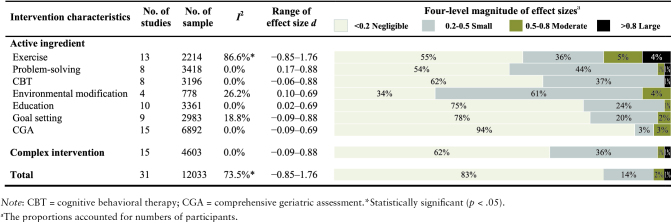
Table 2–3 shows eight active ingredients that were identified and defined as exercise, problem-solving, cognitive behavioral therapy, environmental modification, education, goal setting, comprehensive geriatric assessment, and cognitive training.
Table 2.
Definitions of Active Ingredients
| Active ingredient | Definition | Keywords |
|---|---|---|
| Exercise | Strengthen physical function, body structure, and physiological reserves | Exercise; physical activity; strengthening; walk; physical training; Tai-Chi; balance; mobility; agility; stretching |
| Problem-solving | Identify problems in daily activities, propose solutions to solve the problems, and implement solutions | Problem-solving; action plan; review solutions; identify strategies; refine strategies; design a plan; mutual problem-solving; overcome barriers; propose ways |
| Cognitive behavioral therapy | Discuss and identify patterns of thinking or behaviors. Change distorted thoughts to change behaviors and mood | Cognitive behavioral therapy; cognitive behavioral interventions |
| Environmental modification | Modify environmental factors, such as home, light, rug, and handrail | Environmental modification; eliminate environmental hazards; home modification |
| Education | Deliver, shape, or instruct knowledge on how to perform a behavior or deal with situations | Education; impart knowledge; didactic teaching; educational videotape |
| Goal setting | Identify goals that are relevant to health professionals or participants | Goal setting; preview goals; identify goals; prioritize goals; set realistic goals |
| Comprehensive geriatric assessment | A therapeutic process that incorporated medical, psychosocial or functional assessments to inform care plans for primary care teams and rehabilitation fields | Comprehensive geriatric assessment; multidimensional geriatric instrument; standardized health assessment |
| Cognitive training | Train specific cognitive domains, including processing speed, memory, attention, or reasoning | Cognition training; mathematics; attention memory; visuospatial ability; processing speed; reasoning; visual search skill |
Exercise
Exercise was an active ingredient of 13 interventions in 13 studies (Binder et al., 2002; Cameron et al., 2013; Chin A Paw, de Jong, Schouten, Hiddink, & Kok, 2001; Day et al., 2012; Dorresteijn et al., 2016; Fairhall et al., 2012; Foley et al., 2011; Gill et al., 2004; Gitlin et al., 2006; Haines et al., 2009; Kerse et al., 2010; Rydwik, Frandin, & Akner, 2010; Villareal et al., 2006; Table 3; Supplementary Appendix C). There was high heterogeneity among the 13 studies (I2 = 86.6%, p < .001). The effect size d ranged from –0.85 to 1.76 across studies, with four studies having statistically significant effect sizes (Binder et al., 2002; Foley et al., 2011; Gill et al., 2004; Villareal et al., 2006). Exercise programs focused on aerobic training, resistance training, balance, or Tai-Chi. Foley and colleagues conducted an aerobic exercise program for older adults with musculoskeletal impairments, surgeries, or falls. Results showed a large, statistically significant effect size (d = 1.76). Villareal and colleagues engaged older adults in flexibility, endurance, strength, and balance training. They found a large effect size (d = 0.88; baseline to 26 months). Gill and colleagues trained older adults in bed transfer, indoor, and outdoor mobility. Results demonstrated a small-to-moderate effect size (d = 0.39; baseline to 12 months). There were negative effect sizes in favor of the control groups over time in the studies of Chin and colleagues.
Table 3.
Effect Sizes of Active Ingredients
Problem-solving
Problem-solving was an active ingredient of nine interventions in eight studies (Alexopoulos et al., 2011; Callahan et al., 2005; Clare et al., 2015; Dorresteijn et al., 2016; Gitlin et al., 2006; Szanton et al., 2011; Villareal et al., 2006; von Bonsdorff et al., 2008; Table 3; Supplementary Appendix D). There was no statistically significant heterogeneity among the eight studies (I2 = 0.0%, p = .72). Effect size d ranged from 0.21 to 0.88 across studies, with five studies having statistically significant effect sizes (Alexopoulos et al., 2011; Callahan et al., 2005; Szanton et al., 2011; Villareal et al., 2006; von Bonsdorff et al., 2008). Alexopoulos and colleagues and Callahan and colleagues used problem-solving therapy to reduce disability for older adults with depressive symptoms. Alexopoulos and colleagues found a significant group difference in change in disability (d = 0.28; baseline to 36 weeks), but Callahan and colleagues did not find a significant group difference (d = 0.17; baseline to 12 months). Villareal and colleagues applied problem-solving skills to modify eating habits and lifestyles in older adults who were obese. Results showed a large effect size (d = 0.88; baseline to 6 months). Szanton and colleagues used problem-solving techniques to resolve behavioral and environmental barriers for older adults. They found a moderate effect size (d = 0.69; baseline to 6 months).
Cognitive behavioral therapy
Cognitive behavioral therapy was an active ingredient of eight interventions in seven studies (Alexopoulos et al., 2011; Callahan et al., 2005; Clare et al., 2015; Dorresteijn et al., 2016; Liu & Tsui, 2014; Villareal et al., 2006; von Bonsdorff et al., 2008; Table 3; Supplementary Appendix E). There was no statistically significant heterogeneity among the seven studies (I2 = 0.0%, p = .60). The effect size d ranged from –0.06 to 0.88 across studies, with four studies having statistically significant effect sizes (Alexopoulos et al., 2011; Callahan et al., 2005; Villareal et al., 2006; von Bonsdorff et al., 2008). Two interventions incorporated cognitive behavioral therapy to reduce the fear of falling and promote reengagement in ADL for older adults (Dorresteijn et al., 2016; Liu & Tsui, 2014). Zijlstra and colleagues (2009) and Dorresteijn and colleagues (2016) together developed “A Matter of Balance-Netherlands program” for frail older adults. Results showed that the intervention group had more reductions in disabilities compared with the usual-care control (d = 0.21; baseline to 12 months). Liu and colleagues incorporated cognitive behavioral techniques to reduce the fear of falling. Results showed a negative effect size (d = –0.06). Alexopoulos and colleagues and Callahan and colleagues incorporated cognitive behavioral techniques to change older adults’ distorted thoughts (e.g., outdoor activities will make me fall). Both studies found small-to-moderate effect sizes (d = 0.28; 0.17). Villareal and colleagues recruited frail older adults and adopted cognitive behavioral therapy to change diets, exercise, and functional status. Results showed a high magnitude effect size (d = 0.88).
Environmental modification
Environmental modification was an active ingredient of four interventions in four studies (Gill et al., 2004; Gitlin et al., 2006; Mahoney et al., 2007; Szanton et al., 2011; Table 3; Supplementary Appendix F). There was negligible heterogeneity among the four studies (I2 = 26.2%, p = .25). The effect size d ranged from 0.10 to 0.69 across studies, with two studies having statistically significant effect sizes (Gill et al., 2004; Szanton et al., 2011). Environmental modification usually involved (i) assessing environmental hazards, (ii) removing environmental hazards, (iii) installing equipment. Gitlin and colleagues and Szanton and colleagues designed environmental modification interventions to reduce disability for low-income older adults with mild disabilities. Szanton and colleagues found a significant group difference in change in disability (d = 0.69; baseline to 6 months), but a nonsignificant group difference in the study of Gitlin and colleagues (d = 0.21). Gill and colleagues had physical therapists evaluate the home environment and provide recommendations to remove cords, replace mats, and install adaptive equipment. Results showed a group difference in change in disability (d = 0.39; baseline to 12 months).
Education
Education was an active ingredient of 10 interventions in 10 studies (Callahan et al., 2005; Clark et al., 1997; Dorresteijn et al., 2016; Gill et al., 2004; Gitlin et al., 2006; King, Parsons, Robinson, & Jorgensen, 2012; Lannin et al., 2007; Mahoney et al., 2007; Szanton et al., 2011; von Bonsdorff et al., 2008; Table 3; Supplementary Appendix G). There was no statistically significant heterogeneity among the 10 studies (I2 = 0.0%, p = .52). The effect size d ranged from 0.02 to 0.69 across studies, with four having statistically significant effect sizes (Callahan et al., 2005; Gill et al., 2004; Szanton et al., 2011; von Bonsdorff et al., 2008). Education programs incorporated diverse modalities, including educational videotape, booklet, volunteer lecture, or homework. Three studies provided education on exercise (Gill et al., 2004; Mahoney et al., 2007; von Bonsdorff et al., 2008). Exercise programs of von Bonsdorff and colleagues and Gill and colleagues found small-to-moderate effect sizes (d = 0.23; 0.39), but a nonsignificant effect size was observed in the program of Mahoney and colleagues (d = 0.10). Callahan and colleagues provided educational videotapes for older adults with depressive symptoms. The intervention group reduced more disability than the control group (d = 0.17; baseline to 12 months). Lannin and colleagues educated older adults on safety precautions for performing ADL. Results showed a nonsignificant moderate effect size (d = 0.50; baseline to 3 months). Clark and colleagues applied the didactic teaching method to help older adults select healthy lifestyles. They found no group difference in change in disability (d = 0.02; baseline to 9 months).
Goal setting
Goal setting was an active ingredient of nine interventions in nine studies (Alexopoulos et al., 2011; Callahan et al., 2005; Clare et al., 2015; Dorresteijn et al., 2016; Fairhall et al., 2012; King et al., 2012; Rockwood et al., 2003; Szanton et al., 2011; Villareal et al., 2006; Table 3; Supplementary Appendix H). There was no statistically significant heterogeneity among the nine studies (I2 = 18.8%, p = .28). The effect size d ranged from –0.09 to 0.88 across studies, with four studies having statistically significant effect sizes (Alexopoulos et al., 2011; Callahan et al., 2005; Szanton et al., 2011; Villareal et al., 2006). Goals were described as either client-centered goals (e.g., go shopping myself; Alexopoulos et al., 2011; Callahan et al., 2005; Clare et al., 2015; Dorresteijn et al., 2016; Fairhall et al., 2012; Szanton et al., 2011; Villareal et al., 2006) or practitioner-centered goals (e.g., monitor blood sugar; King et al., 2012; Rockwood et al., 2003) across the nine included interventions. Three of seven client-centered goal setting interventions focused on physical activity goals (Dorresteijn et al., 2016; Fairhall et al., 2012; Villareal et al., 2006). Two of nine interventions used tools to facilitate goal-setting processes (Clare et al., 2015; Rockwood et al., 2003). Clare and colleagues used the Bangor Goal Setting Interview to guide participants in selecting five goals: physical activity, cognitive activity, physical health, diet, and social engagement. Results showed a nonsignificant, small effect size (d = 0.22; baseline to 12 months). Rockwood and colleagues used the Goal Attainment Scale to facilitate goal selection and scaling processes. They found a nonsignificant, negative effect size (d = –0.09; baseline to 3 months).
Comprehensive geriatric assessment
Comprehensive geriatric assessment was an active ingredient of 15 interventions in 15 studies (Bouman, van Rossum, Ambergen, Kempen, & Knipschild, 2008; Cameron et al., 2013; Counsell et al., 2007; Fairhall et al., 2012; Gill et al., 2004; Hendriks et al., 2008; Kerse et al., 2014; King et al., 2012; Kono et al., 2012; Mahoney et al., 2007; Pighills, Torgerson, Sheldon, Drummond, & Bland, 2011; Rockwood et al., 2003; Szanton et al., 2011; van Hout et al., 2010; Table 3; Supplementary Appendix I). There was negligible heterogeneity among the 15 studies (I2 = 0%, p = .46). The effect size d ranged from –0.09 to 0.69 across studies, with three studies having statistically significant effect sizes (Gill et al., 2004; Kerse et al., 2014; Szanton et al., 2011). The comprehensive geriatric assessment was often defined as a therapeutic process that incorporated medical, psychosocial, or functional assessments to inform care plans for primary care teams and rehabilitation fields (Wells et al., 2003). The underlying premise was that via conducting comprehensive assessments, older adults’ disability can be potentially reduced due to more awareness of health care providers and the development of care plans. Thus, the comprehensive geriatric assessment could be treated as an active ingredient that may lead to behavioral changes. Traditionally, older adults had less involvement in the development of care plans. Kerse and colleagues sent a yearly survey to participants to screen health conditions and triggered referrals to regional geriatrics assessment and rehabilitation services (d = 0.13). Counsell and colleagues used the comprehensive geriatric assessments to coordinate care by sharing results with primary care physicians to inform goal setting and implementation (d = –0.05). Gill and colleagues’ comprehensive geriatric assessment specifically assessed physical impairments and home environmental barriers (d = 0.39; baseline to 12 months). Szanton and colleagues involved participants in the care plans; they not only collected problematic ADL but interviewed environmental barriers that may impede behavioral changes (e.g., floor holes, uneven carpeting, lack of railings, and banisters). Results demonstrated a moderate-to-large effect size (d = 0.69; baseline to 6 months). Pighills and colleagues used the results of comprehensive geriatric assessments to provide recommendations for older adults, instead of primary care teams. They found a negative effect size in favor of the control group (d = –0.03).
Cognitive training
Cognitive training was an active ingredient of five interventions in three studies (Ball et al., 2002; Corbett et al., 2015; Ng et al., 2015). Data were insufficient to calculate effect sizes.
Complex Interventions
Fifteen interventions were complex interventions, as indicated by having more than one active ingredient in the intervention (Table 3; Supplementary Appendix J). There was no statistically significant heterogeneity among the 15 studies (I2 = 0.0%, p = .54). The effect size d ranged from –0.09 to 0.88 across studies, with six studies having statistically significant effect sizes (Alexopoulos et al., 2011; Callahan et al., 2005; Gill et al., 2004; Szanton et al., 2011; Villareal et al., 2006; von Bonsdorff et al., 2008). The number of active ingredients in the complex interventions was as followed: 2 (n = 3; Cameron et al., 2013; Clare et al., 2015; Rockwood et al., 2003); 3 (n = 6; Alexopoulos et al., 2011; Clare et al., 2015; Fairhall et al., 2012; King et al., 2012; Mahoney et al., 2007; von Bonsdorff et al., 2008); 4 (n = 4; Callahan et al., 2005; Gill et al., 2004; Gitlin et al., 2006; Villareal et al., 2006); 5 (n = 2; Dorresteijn et al., 2016; Szanton et al., 2011).
Discussion
This review examined the science related to nonpharmacological intervention studies to reduce disability in community-dwelling older adults. The majority of included studies showed negligible-to-small effect sizes in minimizing disability. Yet, this finding may be argued to be clinically meaningful. For example, a small reduction of disability may slow down the progression toward severe disability and potentially reduce the cost of health care programs because health care expenditures are positively correlated with the severity of disability in aging population (Manton, Gu, & Lamb, 2006). Interventions that included exercise, problem-solving, cognitive behavioral therapy, and environmental modification as active ingredients were associated with stronger effect sizes in reducing disability. We urge caution when interpreting this finding, given that the active ingredients were not mutually exclusive among interventions. The results may not be confirmatory until a systematic review in comparing active ingredients is completed. Altogether, these findings may inform future intervention strategies and priorities to reduce disability in late life.
Negligible-to-Small Effect Sizes Across Studies
There exist several possibilities to explain the negligible-to-small effect sizes across studies. The use of diverse eligibility criteria across studies resulted in a range of disability severity statuses among participants in the studies. Most of the included studies included older adults with negligible-to-moderate disability. As such, the effect sizes may not be as large as those interventions that aimed to reduce disability for older adults who have already developed severe disability. This explanation is based on the assumption that older adults with severe disability may have room to gain greater improvements than those with negligible or mild disability. Another explanation is our use of a conservative approach to calculate effect sizes, which has been suggested to generate conservative estimates (Feingold, 2009). Negligible-to-small effect sizes may result from the psychometric properties of measures of disability. A measurement with low sensitivity to change may fail to detect the true effects of intervention studies (Fok & Henry, 2015). Last, certain control groups (e.g., nutrition interventions) may have had some influence on disability rather than attention control groups, thus reducing the magnitude of effect sizes.
In addition, we found that the ranges of effect sizes varied from studies that focused on ADL, IADL, or ADL + IADL disabilities. Studies that focused on ADL disability had the widest range of effect sizes. Previous studies have suggested that ADL disability is more severe than IADL disability (Leibold, Holm, Raina, Reynolds III, & Rogers, 2014). Thus, studies focused on reducing ADL disability may result in more changes than IADL disability. Future studies that aim to reduce disability may provide the rationale of how interventions may change ADL and IADL disability.
The Effects of Active Ingredients on Disability
Many combinations of active ingredients were found in included interventions. The combination of problem-solving and environmental modification showed promise in reducing disability. This combination echoes current concept about the emergence of disability as a mismatch between personal strengths and environmental demands (World Health Organization, 2002). Problem-solving focuses on building individuals’ problem-solving skills when facing barriers in ADL and IADL (D’Zurilla & Nezu, 2010), whereas environmental modification changed contexture factors to match individuals’ needs (Petersson, Lilja, Hammel, & Kottorp, 2008). The change of both personal and contextual factors helps older adults engage in ADL and IADL and thus, reduce disability.
Exercise showed promise in reducing disability, whereas comprehensive geriatric assessment found little or no effects on disability reduction. Interestingly, exercise and comprehensive geriatric assessment were often the only active ingredients in the interventions, comparing to goal setting and education that were often combined with other active ingredients. This suggests that active ingredients have different applicability and roles within interventions; and intervention effects are not determined by the number of active ingredients. Some active ingredients aim to initiate new behaviors, whereas others aim to maintain preferred behaviors (Wood, Quinn, & Kashy, 2002). How to combine the most effective active ingredients to reduce disability requires iterative case and pilot studies.
The mode of delivery can differ within the same active ingredient. For example, goal setting can be led by practitioners, older adults, or caregivers. In this review, we found that goals were mostly determined by practitioners, instead of older adults or caregivers. Because practitioner-selected goals may not always support an older adult autonomy and a family’s expectation to change behaviors (Locke & Latham, 2002), future studies should separate those three perspectives while examining the effects of goal setting on disability.
Literature on comprehensive geriatric assessment has demonstrated its effectiveness for controlling disease progression and predicting mortality rates in late life (Stuck, Egger, Hammer, Minder, & Beck, 2002). Although our ultimate goal was to reduce disability, the involvement of older adults and family in the care plan processes following the comprehensive geriatric assessment becomes critical to empower older adults and caregivers to drive behavioral changes (Krishnan et al., 2017). However, in our review, some interventions that incorporated comprehensive geriatric assessment did not partner with older adults or their caregivers while making care plans. Thus, the potency of comprehensive geriatric assessment in reducing disability may not be as large as in controlling disease progression and mortality.
The Effects of Complex Interventions on Disability
The magnitudes of the effect sizes were not as large as we expected among studies that examined complex interventions. This may be due to a variety of possible reasons. First, complex interventions are “built up from more than one active ingredient, which may act both independently and interdependently. (p.455) (Campbell et al., 2007)” A clear understanding of how active ingredients work with each other is a critical step. However, the effects of active ingredients were rarely compared or even described in the complex interventions. For example, does the combination of goal setting with exercise reduce more disability than the combination of goal setting with problem-solving? The unclarity of how active ingredients interact with each other has impeded the design of optimal and efficacious interventions. Second, complex interventions might be inadequately applied (insufficient dosages) in an inappropriate environment (homes vs community settings; Campbell et al., 2007; Craig et al., 2008).
Strengths and Limitations
This study provided valuable insights. First, this review included studies that recruited older adults from negligible-to-severe disability, but this disability was not caused by acute medical conditions. The strategy provided a way to capture older adults at-risk for severe disability. Second, the active ingredients in driving the efficacy of nonpharmacological interventions were explored. This information is critical to inform future intervention development and priorities. Third, disability was selected as the outcome, which is one of the top priorities to reduce the costly health care related to disability and sustain quality of life into old age.
The findings should be interpreted cautiously. First, the quality of studies was not evaluated, which may influence the potency of evidence. Second, research has shown that, often times, the active ingredients of nonpharmacological interventions were not well described in the contents of articles (Abraham & Michie, 2008). This limitation may impede the finding. Third, the adherence rates of interventions were not evaluated, which may influence the reported effects. The study dosage may influence the effects of interventions, especially to make behavioral changes. Last, there was considerable heterogeneity across 31 studies. These variances might contribute to eligibility criteria used in the studies, quality of interventions, and the selection of outcome measures.
Future Directions
Reducing disability among community-dwelling older adults relies on a clear understanding of problematic areas in their ADL and IADL. By comprehensively understanding these day-to-day activities, researchers can investigate the barriers older adults confront and further inform those who are at high risk of further disability.
Specifying and evaluating active ingredients in influencing intervention efficacy can provide valuable insights into why an intervention fails or succeeds, and how it can be optimized. For example, the multiphase optimization strategy identifies active ingredients within interventions and optimizes dosages of each active ingredient to refine complex interventions (Collins, Murphy, & Strecher, 2007). Future studies may adopt systematic protocols and methodologies to refine nonpharmacological interventions “prior to” the implementation phases. This, in turn, would help replication studies and support evidence-based practice.
The measurements that assess disability should obtain sensitivity to capture the change in older adults with minimal disability. Early decline in disability is usually silent and fluctuating. An assessment that captures change in disability for older adults must assess the quality of their performance in ADL and IADL (Freedman et al., 2014).
In summary, nonpharmacological interventions involved many interacting active ingredients in mitigating disability for older adults. Future studies should specify and evaluate active ingredients within nonpharmacological interventions to optimize effects on disability in late life. This review identified several research directions to reduce disability into old age.
Funding
This work was supported by the National Center for Advancing Translational Sciences [KL2 TR000146 to J.R.]. The funding source had no role in the study’s design, conduct, and article’s development and publication.
Conflict of Interest
We have no conflict of interest to declare.
Author’s Contributions
C. Y. W designed the study, collected and analyzed the data, and wrote the article. J. R., E. R. S., and C. Y. W. reviewed, revised, and edited the manuscript. B. F., J. K., and L. T. reviewed and edited the article. J. R., E. R. S., L. T., and C. Y. W. discussed and reviewed the data analyses. C. Y. W. is the guarantor of this work and takes responsibility for the integrity of the data, the accuracy of the data analyses, and the decision to submit and publish the article.
Supplementary Material
References
- Abraham C., & Michie S (2008). A taxonomy of behavior change techniques used in interventions. Health Psychology, 27, 379–387. doi:10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- Alexopoulos G. S., Raue P. J., Kiosses D. N., Mackin R. S., Kanellopoulos D., McCulloch C., & Areán P. A (2011). Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Archives of General Psychiatry, 68, 33–41. doi:10.1001/archgenpsychiatry.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey H., & O’Malley L (2005). Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology, 8, 19–32. doi:10.1080/1364557032000119616 [Google Scholar]
- Ball K., Berch D. B., Helmers K. F., Jobe J. B., Leveck M. D., Marsiske M., … Willis S. L.; Advanced Cognitive Training for Independent and Vital Elderly Study Group (2002). Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA, 288, 2271–2281. doi:10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E. F., Schechtman K. B., Ehsani A. A., Steger-May K., Brown M., Sinacore D. R.,…Holloszy J. O (2002). Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. Journal of the American Geriatrics Society, 50, 1921–1928. doi:10.1046/j.1532-5415.2002.50601.x [DOI] [PubMed] [Google Scholar]
- Bouman A., van Rossum E., Ambergen T., Kempen G., & Knipschild P (2008). Effects of a home visiting program for older people with poor health status: a randomized, clinical trial in The Netherlands. Journal of the American Geriatrics Society, 56, 397–404. doi:10.1111/j.1532-5415.2007.01565.x [DOI] [PubMed] [Google Scholar]
- Boutron I., Moher D., Altman D. G., Schulz K. F., & Ravaud P.; CONSORT Group (2008). Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Annals of Internal Medicine, 148, 295–309. doi:10.7326/0003-4819-148-4-200802190-00008 [DOI] [PubMed] [Google Scholar]
- Boyce L. W., Reinders C. C., Volker G., Los E., van Exel H. J., Vliet Vlieland T. P. M., & Goossens P. H (2017). Out-of-hospital cardiac arrest survivors with cognitive impairments have lower exercise capacity. Resuscitation, 115, 90–95. doi:10.1016/j.resuscitation.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Brown C. J., Roth D. L., Allman R. M., Sawyer P., Ritchie C. S., & Roseman J. M (2009). Trajectories of life-space mobility after hospitalization. Annals of Internal Medicine, 150, 372–378. doi:10.7326/0003-4819-150-6-200903170-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan C. M., Kroenke K., Counsell S. R., Hendrie H. C., Perkins A. J., Katon W., … Unützer J.; IMPACT Investigators (2005). Treatment of depression improves physical functioning in older adults. Journal of the American Geriatrics Society, 53, 367–373. doi:10.1111/j.1532-5415.2005.53151.x [DOI] [PubMed] [Google Scholar]
- Cameron I. D., Fairhall N., Langron C., Lockwood K., Monaghan N., Aggar C., … Kurrle S. E (2013). A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Medicine, 11, 65. doi:10.1186/1741-7015-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N. C., Murray E., Darbyshire J., Emery J., Farmer A., Griffiths F., … Kinmonth A. L (2007). Designing and evaluating complex interventions to improve health care. BMJ (Clinical Research Ed.), 334, 455–459. doi:10.1136/bmj.39108.379965.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capistrant B. D., Mejia N. I., Liu S. Y., Wang Q., & Glymour M. M (2014). The disability burden associated with stroke emerges before stroke onset and differentially affects blacks: results from the health and retirement study cohort. Journals of Gerontology. Series A, Biological sciences and medical sciences, 69, 860–870. doi:10.1093/gerona/glt191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A Paw M. J., de Jong N., Schouten E. G., Hiddink G. J., & Kok F. J (2001). Physical exercise and/or enriched foods for functional improvement in frail, independently living elderly: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 82, 811–817. doi:10.1053/apmr.2001.23278 [DOI] [PubMed] [Google Scholar]
- Clare L., Nelis S. M., Jones I. R., Hindle J. V., Thom J. M., Nixon J. A., … Whitaker C. J (2015). The Agewell trial: A pilot randomised controlled trial of a behaviour change intervention to promote healthy ageing and reduce risk of dementia in later life. BMC Psychiatry, 15, 25. doi:10.1186/s12888-015-0402-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark F., Azen S. P., Zemke R., Jackson J., Carlson M., Mandel D., … Lipson L (1997). Occupational therapy for independent-living older adults. A randomized controlled trial. JAMA, 278, 1321–1326. doi:10.1001/jama.1997.03550160041036 [PubMed] [Google Scholar]
- Collins D. M., Downer B., Kumar A., Krishnan S., Li C. Y., Markides K. S., & Karmarkar A. M (2018). Impact of multiple chronic conditions on activity limitations among older Mexican-American care recipients. Preventing Chronic Disease, 15, E51. doi:10.5888/pcd15.170358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. M., Murphy S. A., & Strecher V (2007). The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. American Journal of Preventive Medicine, 32, S112–S118. doi:10.1016/j.amepre.2007.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett A., Owen A., Hampshire A., Grahn J., Stenton R., Dajani S., … Ballard C (2015). The effect of an online cognitive training package in healthy older adults: An online randomized controlled trial. Journal of the American Medical Directors Association, 16, 990–997. doi:10.1016/j.jamda.2015.06.014 [DOI] [PubMed] [Google Scholar]
- Counsell S. R., Callahan C. M., Clark D. O., Tu W., Buttar A. B., Stump T. E., & Ricketts G. D (2007). Geriatric care management for low-income seniors: a randomized controlled trial. JAMA, 298, 2623–2633. doi:10.1001/jama.298.22.2623 [DOI] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., & Petticrew M.; Medical Research Council Guidance (2008). Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ (Clinical Research Ed.), 337, a1655. doi:10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Zurilla T. J., & Nezu A. M (2010). Problem-solving therapy. In K. S. Dobson. (Ed.), Handbook of cognitive-behavioral therapies (pp. 197–225). New York City: Guilford Press. [Google Scholar]
- Day L., Hill K. D., Jolley D., Cicuttini F., Flicker L., & Segal L (2012). Impact of tai chi on impairment, functional limitation, and disability among preclinically disabled older people: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 93, 1400–1407. doi:10.1016/j.apmr.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Dorresteijn T. A. C., Zijlstra G. A. R., Ambergen A. W., Delbaere K., Vlaeyen J. W. S., & Kempen G. I. J. M (2016). Effectiveness of a home-based cognitive behavioral program to manage concerns about falls in community-dwelling, frail older people: Results of a randomized controlled trial. BMC Geriatrics, 16, 1–11. doi:10.1186/s12877-015-0177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhall N., Sherrington C., Kurrle S. E., Lord S. R., Lockwood K., & Cameron I. D (2012). Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Medicine, 10, 120. doi:10.1186/1741-7015-10-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A. (2009). Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychological Methods, 14, 43–53. doi:10.1037/a0014699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Guralnik J. M., Studenski S., Fried L. P., Cutler G. B. Jr, & Walston J. D.; Interventions on Frailty Working Group (2004). Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. Journal of the American Geriatrics Society, 52, 625–634. doi:10.1111/j.1532-5415.2004.52174.x [DOI] [PubMed] [Google Scholar]
- Fok C. C., & Henry D (2015). Increasing the sensitivity of measures to change. Prevention Science, 16, 978–986. doi:10.1007/s11121-015-0545-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley A., Hillier S., & Barnard R (2011). Effectiveness of once-weekly gym-based exercise programmes for older adults post discharge from day rehabilitation: a randomised controlled trial. British Journal of Sports Medicine, 45, 978–986. doi:10.1136/bjsm.2009.063966 [DOI] [PubMed] [Google Scholar]
- Freedman V. A., Kasper J. D., Spillman B. C., Agree E. M., Mor V., Wallace R. B., & Wolf D. A (2014). Behavioral adaptation and late-life disability: a new spectrum for assessing public health impacts. American Journal of Public Health, 104, e88–e94. doi:10.2105/AJPH.2013.301687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. M., Baker D. I., Gottschalk M., Peduzzi P. N., Allore H., & Van Ness P. H (2004). A prehabilitation program for the prevention of functional decline: effect on higher-level physical function. Archives of Physical Medicine and Rehabilitation, 85, 1043–1049. doi:10.1016/j.apmr.2003.10.021 [DOI] [PubMed] [Google Scholar]
- Gitlin L. N., Winter L., Dennis M. P., Corcoran M., Schinfeld S., & Hauck W. W (2006). A randomized trial of a multicomponent home intervention to reduce functional difficulties in older adults. Journal of the American Geriatrics Society, 54, 809–816. doi:10.1111/j.1532-5415.2006.00703.x [DOI] [PubMed] [Google Scholar]
- Groessl E. J., Kaplan R. M., Rejeski W. J., Katula J. A., King A. C., Frierson G., … Pahor M (2007). Health-related quality of life in older adults at risk for disability. American Journal of Preventive Medicine, 33, 214–218. doi:10.1016/j.amepre.2007.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines T. P., Russell T., Brauer S. G., Erwin S., Lane P., Urry S., … Condie P (2009). Effectiveness of a video-based exercise programme to reduce falls and improve health-related quality of life among older adults discharged from hospital: A pilot randomized controlled trial. Clinical Rehabilitation, 23, 973–985. doi:10.1177/0269215509338998 [DOI] [PubMed] [Google Scholar]
- Hendriks M. R. C., Evers S. M. A. A., Bleijlevens M. H. C., van Haastregt J. C. M., Crebolder H. F. J. M., & van Eijk J. T. M (2008). Cost-effectiveness of a multidisciplinary fall prevention program in community-dwelling elderly people: A randomized controlled trial (ISRCTN 64716113). International Journal of Technology Assessment in Health Care, 24, 193–202. doi:10.1017/S0266462308080276 [DOI] [PubMed] [Google Scholar]
- Higgins J. P. T., & Thompson S. G (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21, 1539– 1558. doi:10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- IBM Corp (2013). IBM SPSS statistics for Windows. Armonk, NY: IBM Corp. [Google Scholar]
- Jette A. M., Davies A. R., Cleary P. D., Calkins D. R., Rubenstein L. V., Fink A., … Delbanco T. L (1986). The Functional Status Questionnaire: reliability and validity when used in primary care. Journal of General Internal Medicine, 1, 143–149. doi:10.1007/BF02596437 [DOI] [PubMed] [Google Scholar]
- Kempen G. I. J. M., & Suurmeijer T. P. B. M (1990). The development of a hierarchical polychotomous ADL-IADL scale for noninstitutionalized elders. The Gerontologist, 30, 497–502. doi:10.1093/geront/30.4.497 [DOI] [PubMed] [Google Scholar]
- Kerse N., Hayman K. J., Moyes S. A., Peri K., Robinson E., Dowell A., … Arroll B (2010). Home-based activity program for older people with depressive symptoms: DeLLITE–a randomized controlled trial. Annals of Family Medicine, 8, 214–223. doi:10.1370/afm.1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerse N., McLean C., Moyes S. A., Peri K., Ng T., Wilkinson-Meyers L., … Connolly M (2014). The cluster-randomized BRIGHT trial: Proactive case finding for community-dwelling older adults. Annals of Family Medicine, 12, 514–524. doi:10.1370/afm.1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. I., Parsons M., Robinson E., & Jörgensen D (2012). Assessing the impact of a restorative home care service in New Zealand: a cluster randomised controlled trial. Health and Social Care in the Community, 20, 365–374. doi:10.1111/j.1365-2524.2011.01039.x [DOI] [PubMed] [Google Scholar]
- Kono A., Kanaya Y., Fujita T., Tsumura C., Kondo T., Kushiyama K., & Rubenstein L. Z (2012). Effects of a preventive home visit program in ambulatory frail older people: a randomized controlled trial. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 67, 302–309. doi:10.1093/gerona/glr176 [DOI] [PubMed] [Google Scholar]
- Kraus L. (2017). 2016 Disability statistics annual report. Durham, NH: University of New Hampshire. [Google Scholar]
- Krishnan S., Anderson D., Chan S., Kim S., Reistetter T., Sood P., … Heyn P. C (2018). Overview of common complementary and integrative approaches to managing chronic pain: A guide for patients with chronic pain. Archives of Physical Medicine and Rehabilitation, 99, 2393–2394. doi:10.1016/J.APMR.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Krishnan S., Pappadis M. R., Weller S. C., Stearnes M., Kumar A., Ottenbacher K. J., & Reistetter T. A (2017). Needs of stroke survivors as perceived by their caregivers: A scoping review. American Journal of Physical Medicine and Rehabilitation, 96, 487–505. doi:10.1097/PHM.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. (2013). Calculating and reporting effect sizes to facilitate cumulative science:A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. doi:10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannin N. A., Clemson L., McCluskey A., Lin C. W., Cameron I. D., & Barras S (2007). Feasibility and results of a randomised pilot-study of pre-discharge occupational therapy home visits. BMC Health Services Research, 7, 42. doi:10.1186/1472-6963-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. P., & Brody E. M (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9, 179–186. doi:10.1093/geront/9.3_Part_1.179 [PubMed] [Google Scholar]
- Leibold M. L., Holm M. B., Raina K. D., Reynolds C. F. 3rd, & Rogers J. C (2014). Activities and adaptation in late-life depression: A qualitative study. American Journal of Occupational Therapy, 68, 570–577. doi:10.5014/ajot.2014.011130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. W., & Tsui C. M (2014). A randomized trial comparing Tai Chi with and without cognitive-behavioral intervention (CBI) to reduce fear of falling in community-dwelling elderly people. Archives of Gerontology and Geriatrics, 59, 317–325. doi:10.1016/j.archger.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Locke E. A., & Latham G. P (2002). Building a practically useful theory of goal setting and task motivation. A 35-year odyssey. American Psychologist, 57, 705–717. doi:10.1037/0003-066X.57.9.705 [DOI] [PubMed] [Google Scholar]
- Mahoney F. I., & Barthel D. W (1965). Functional evaluation: The Barthel Index. Maryland State Medical Journal, 14, 61–65. [PubMed] [Google Scholar]
- Mahoney J. E., Shea T. A., Przybelski R., Jaros L., Gangnon R., Cech S., & Schwalbe A (2007). Kenosha County falls prevention study: A randomized, controlled trial of an intermediate-intensity, community-based multifactorial falls intervention. Journal of the American Geriatrics Society, 55, 489–498. doi:10.1111/j.1532-5415.2007.01144.x [DOI] [PubMed] [Google Scholar]
- Manton K. G., Gu X., & Lamb V. L (2006). Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proceedings of the National Academy of Sciences of the United States of America, 103, 18374–18379. doi:10.1073/pnas.0608483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendeley Ltd (2017). Mendeley; London: Mendeley Ltd. [Google Scholar]
- Michie S., Fixsen D., Grimshaw J. M., & Eccles M. P (2009). Specifying and reporting complex behaviour change interventions: The need for a scientific method. Implementation Science: IS, 4, 40. doi:10.1186/1748-5908-4-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W., … Wood C. E (2013). The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine, 46, 81–95. doi:10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- Mitra S., Palmer M., Kim H., Mont D., & Groce N (2017). Extra costs of living with a disability: A review and agenda for research. Disability and Health Journal, 10, 475–484. doi:10.1016/j.dhjo.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., & Altman D. G.; PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–9, W64. doi:10.1371/journal.pmed.1000097 [DOI] [PubMed] [Google Scholar]
- Ng T. P., Feng L., Nyunt M. S., Feng L., Niti M., Tan B. Y., … Yap K. B (2015). Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: A randomized controlled trial. American Journal of Medicine, 128, 1225–1236.e1. doi:10.1016/j.amjmed.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Nouri F., & Lincoln N (1987). An extended activities of daily living scale for stroke patients. Clinical Rehabilitation, 1, 301–305. doi:10.1177/026921558700100409 [Google Scholar]
- Ortman J. M., Velkoff V. A., & Hogan H (2014). An aging nation: The older population in the United States. Suitland, MD: United States Census Bureau, Economics and Statistics Administration, US Department of Commerce. [Google Scholar]
- Peixoto T. C., Begot I., Bolzan D. W., Machado L., Reis M. S., Papa V., … Guizilini S (2015). Early exercise-based rehabilitation improves health-related quality of life and functional capacity after acute myocardial infarction: A randomized controlled trial. Canadian Journal of Cardiology, 31, 308–313. doi:10.1016/j.cjca.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Petersson I., Lilja M., Hammel J., & Kottorp A (2008). Impact of home modification services on ability in everyday life for people ageing with disabilities. Journal of Rehabilitation Medicine, 40, 253–260. doi:10.2340/16501977-0160 [DOI] [PubMed] [Google Scholar]
- Pighills A. C., Torgerson D. J., Sheldon T. A., Drummond A. E., & Bland J. M (2011). Environmental assessment and modification to prevent falls in older people. Journal of the American Geriatrics Society, 59, 26–33. doi:10.1111/j.1532-5415.2010.03221.x [DOI] [PubMed] [Google Scholar]
- Rockwood K., Howlett S., Stadnyk K., Carver D., Powell C., & Stolee P (2003). Responsiveness of goal attainment scaling in a randomized controlled trial of comprehensive geriatric assessment. Journal of Clinical Epidemiology, 56, 736–743. doi:10.1016/S0895-4356(03)00132-X [DOI] [PubMed] [Google Scholar]
- Rydwik E., Frändin K., & Akner G (2010). Effects of a physical training and nutritional intervention program in frail elderly people regarding habitual physical activity level and activities of daily living—A randomized controlled pilot study. Archives of Gerontology and Geriatrics, 51, 283–289. doi:10.1016/j.archger.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Schuling J., de Haan R., Limburg M., & Groenier K. H (1993). The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke, 24, 1173–1177. doi:10.1161/01.STR.24.8.1173 [DOI] [PubMed] [Google Scholar]
- StataCorp (2017). STATA Statistical Software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Stuck A. E., Egger M., Hammer A., Minder C. E., & Beck J. C (2002). Home visits to prevent nursing home admission and functional decline in elderly people: Systematic review and meta-regression analysis. JAMA, 287, 1022–1028. [DOI] [PubMed] [Google Scholar]
- Szanton S. L., Thorpe R. J., Boyd C., Tanner E. K., Leff B., Agree E., … Gitlin L. N (2011). Community aging in place, advancing better living for elders: A bio-behavioral-environmental intervention to improve function and health-related quality of life in disabled older adults. Journal of the American Geriatrics Society, 59, 2314–2320. doi:10.1111/j.1532-5415.2011.03698.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hout H. P. J., Jansen A. P. D., van Marwijk H. W. J., Pronk M., Frijters D. F., & Nijpels G (2010). Prevention of adverse health trajectories in a vulnerable elderly population through nurse home visits: A randomized controlled trial [ISRCTN05358495]. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65, 734–742. doi:10.1093/gerona/glq037 [DOI] [PubMed] [Google Scholar]
- Villareal D. T., Banks M., Sinacore D. R., Siener C., & Klein S (2006). Effect of weight loss and exercise on frailty in obese older adults. Archives of Internal Medicine, 166, 860–866. doi:10.1001/archinte.166.8.860 [DOI] [PubMed] [Google Scholar]
- von Bonsdorff M. B., Leinonen R., Kujala U. M., Heikkinen E., Törmäkangas T., Hirvensalo M., … Rantanen T (2008). Effect of physical activity counseling on disability in older people: A 2-year randomized controlled trial. Journal of the American Geriatrics Society, 56, 2188–2194. doi:10.1111/j.1532-5415.2008.02000.x [DOI] [PubMed] [Google Scholar]
- Wells J. L., Seabrook J. A., Stolee P., Borrie M. J., & Knoefel F (2003). State of the art in geriatric rehabilitation. Part I: Review of frailty and comprehensive geriatric assessment. Archives of Physical Medicine and Rehabilitation, 84, 890–897. doi:10.1016/S0003-9993(02)04929-8 [DOI] [PubMed] [Google Scholar]
- Wood W., Quinn J. M., & Kashy D. A (2002). Habits in everyday life: Thought, emotion, and action. Journal of Personality and Social Psychology, 83, 1281–1297. doi:10.1037/0022-3514.83.6.1281 [PubMed] [Google Scholar]
- World Health Organization (2002). Towards a common language for functioning, disability and health (ICF). Geneva: WHO. [Google Scholar]
- Zijlstra G. A., van Haastregt J. C., Ambergen T., van Rossum E., van Eijk J. T., Tennstedt S. L., & Kempen G. I (2009). Effects of a multicomponent cognitive behavioral group intervention on fear of falling and activity avoidance in community-dwelling older adults: Results of a randomized controlled trial. Journal of the American Geriatrics Society, 57, 2020–2028. doi:10.1111/j.1532-5415.2009.02489.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.