Abstract
The authors investigated the second botulism outbreak to occur in a maximum security prison in Arizona within a 4-month period. Botulism was confirmed in eight men aged 20 to 35 years who reported sharing a single batch of pruno made with potatoes. Initial symptoms included blurred vision, slurred speech, muscle weakness, ptosis, and dysphagia. All patients received heptavalent botulinum antitoxin, seven required mechanical ventilation, and all survived. The median incubation period was 29 hours. Sera from all patients and leftover pruno tested positive for botulinum toxin type A. Botulism should be considered among prisoners with cranial nerve palsies and descending, symmetric flaccid paralysis. Prison-brewed alcohol, particularly when made with potatoes, can be a vehicle for botulism and is associated with outbreaks of botulism in prisons.
Keywords: botulism, botulinum, outbreak, prison
Introduction
Foodborne botulism is a rare, paralytic illness that can result in death. Botulism is caused by ingestion of a neurotoxin produced by the bacterium Clostridium botulinum, a gram-positive, ubiquitous, soildwelling anaerobe that produces botulinum toxin in specific conditions, including low-oxygen, low-acidity, low-sugar, and warm environments (Bell & Kyriakides, 2000). Botulinum toxin, of which seven subtypes exist, A to G, is among the most powerful neurotoxins affecting humans. The toxin prevents acetylcholine release at the neuromuscular junction of voluntary motor and autonomic cholinergic muscle fibers, blocking stimulation of muscle fibers (Shapiro, Hatheway, & Swerdlow, 1998). Intoxication manifests as cranial nerve palsies and a descending, symmetric flaccid paralysis. When not treated with botulinum antitoxin, approximately 5% of illnesses result in death because of respiratory muscle paralysis (Hughes et al., 1981). Supportive treatment forms the mainstay of clinical care, and heptavalent botulinum antitoxin can prevent disease progression if administered early during the clinical course (Centers for Disease Control and Prevention [CDC], 1998).
Approximately 23 cases of foodborne botulism are reported in the United States each year, the majority resulting from incorrectly produced home-canned foods (Sobel, Tucker, Sulka, McLaughlin, & Maslanka, 2004). Outbreaks of foodborne botulism are rare; however, three outbreaks among prisoners were reported during 2004 to 2011. All three outbreaks were associated with the consumption of prison-brewed illicit alcohol, known colloquially as pruno, toilet wine, or hooch (CDC, 2013). Pruno is typically made when ingredients are heated and fermented in a sealed plastic bag or jar for a period of 3 to 5 days; ingredients can include water, fruit, bread, and sugar; and recipes vary depending on geography and inmate access to food items. All prison-related botulism outbreaks, including the three previously reported outbreaks and the August 2012 outbreak in Prison A, have been specifically associated with pruno made with potatoes as a main ingredient. We report results from the investigation of an outbreak of botulism among Prison A inmates during November 2012 associated with the consumption of pruno made with potatoes. A joint investigation with the Arizona Department of Corrections, the CDC (Atlanta, Georgia), the Pinal County Division of Public Health (PCDPH), and the Arizona Department of Health Services (ADHS) sought to confirm the outbreak, determine the source, and institute control and prevention measures.
Methods
Case Definitions
A probable case was defined as signs and symptoms of cranial nerve palsies (e.g., ptosis, double vision, or blurred vision) and weakness, dysphagia, or impaired gag reflex in a Prison A inmate who had consumed pruno from November 23 to December 10, 2012. A confirmed case was a clinically compatible case that was laboratory confirmed by detection of botulinum toxin in serum, stool, or pruno, or isolation of C. botulinum from stool.
Laboratory Testing
Patient sera were tested for botulinum toxin by using mass spectrometry and mouse bioassay before and 24 hours after administration of heptavalent botulinum antitoxin (CDC, 2010). Rectal swabs, gastric aspirates, and stool specimens were tested for botulinum toxin before antitoxin administration. Pruno confiscated from a patient’s cell on November 24, 2012, was tested for C. botulinum by using enzyme-linked immunosorbent assay and polymerase chain reaction. The pruno was tested for botulinum toxin by using mass spectrometry and mouse bioassay. To compare botulinum toxin types from the August and November outbreaks in Prison A, pulsed-field gel electrophoresis (PFGE) using restriction enzymes Sma1 and Xho1 was performed on isolates from stool specimens from inmates hospitalized during the August outbreak and compared with PFGE patterns from inmates hospitalized during the November outbreak. PFGE was also performed on an isolate from a pruno sample discovered in Prison A during the November outbreak.
Clinical Course and Epidemiologic Study
Chart reviews were conducted to monitor antitoxin and hospital-related complications, neurologic recovery, and neurologic response to antitoxin. Open-ended, conversational interviews were conducted with inmates in Prison A. Patients affected during the August and November outbreak were also interviewed to more fully understand the culture of pruno production and consumption.
Results
Chronology of the Outbreak
On November 24, five inmates sought treatment for symptoms, including blurred vision, ptosis, dizziness, and slurred speech progressing to respiratory distress. The inmates were transferred to an intensive care unit (ICU) and intubated to provide mechanical ventilation. Two additional inmates sought treatment on November 25 for blurred vision, slurred speech, and weakness. These two inmates were also intubated to provide mechanical ventilation upon admission to ICU. The following day, an eighth inmate reported vomiting, blurred vision, and dizziness; this inmate was admitted to ICU, but mechanical ventilation was not required and he was not intubated. All eight patients received heptavalent botulinum antitoxin within 24 hours of hospital admission (Table 1). All eight inmates reported drinking from the same batch of pruno or eating potato pieces from the same pruno mixture on November 23 at approximately 1 p.m.
Table 1.
Demographics and Clinical Characteristics of Patients Receiving Diagnosis of Botulism in Arizona Prison A, November 24 to 26, 2012.
| Patient | Age (years) | Incubation Period (hours) | Mechanical Ventilation (days) | Comorbidities |
|---|---|---|---|---|
| 1 | 24 | 10 | 96 | None |
| 2 | 28 | 17 | 109 | Asthma |
| 3 | 25 | 19 | 86 | None |
| 4 | 25 | 19 | > 159a | Hepatitis C |
| 5 | 20 | 28 | 25 | None |
| 6 | 35 | 41 | 27 | Hepatitis C, GERD |
| 7 | 25 | 41 | 36 | Hepatitis C |
| 8 | 33 | 67 | 0 | Hepatitis C |
Note. All patients were men. All drank from the same batch of prison-brewed illicit alcohol on November 23. GERD = gastroesophageal reflux disease.
Patient still on mechanical ventilation as of May 2, 2013.
Patient Characteristics
Patients were men (median age 25 years; range: 20 to 35 years) who resided in adjoining pods in Prison A (Figure 1). Two were American Indian and six were Mexican American. The median incubation period was 29 hours (range: 16 to 64 hours); for the seven patients who received mechanical ventilator support, the incubation period was < 48 hours (Table 1).
Figure 1.
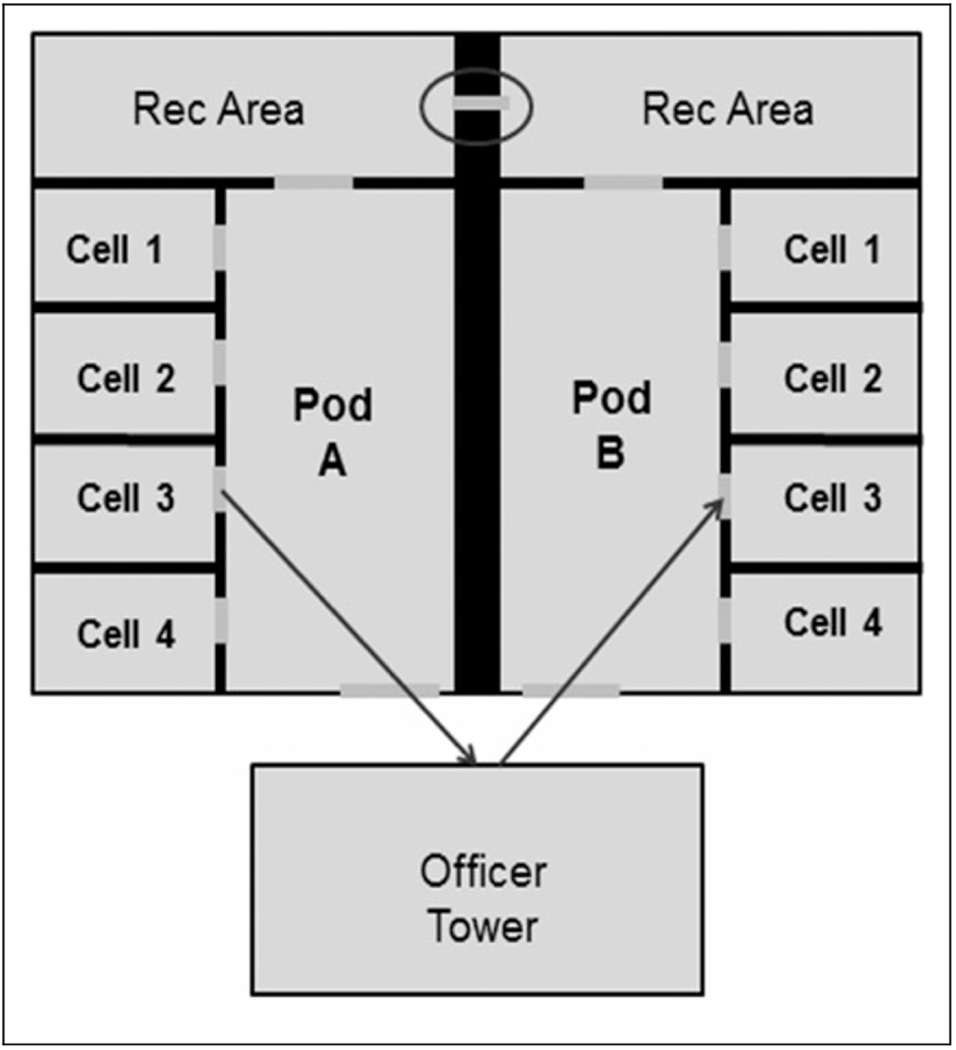
Map of cell area where inmates with diagnosed botulism resided during an outbreak in Arizona Prison A, November 24 to 26, 2012. The arrows indicate the passage of pruno contained in sealed plastic bags under cell doors, as reported by inmates in Prison A. This practice, known as “fishing,” uses walls to bounce pruno packages between cells and pods. The circle indicates drainage holes in the wall of the recreation area through which pruno was passed.
Laboratory Testing
All eight serum samples obtained before antitoxin administration had positive results using both mass spectrometry and mouse bioassay for botulinum toxin type A. All eight serum samples obtained 24 hours after antitoxin administration were negative for toxin. Five rectal swabs were positive for botulinum toxin type A, all seven gastric aspirate samples were negative for toxin, and the stool sample was positive for botulinum toxin type A. The pruno sample yielded C. botulinum and botulinum toxin type A (Table 2). PFGE patterns for botulinum toxin types were indistinguishable within each outbreak (August and November, respectively) but displayed different patterns between the two outbreaks (Figure 2).
Table 2.
Results of Laboratory Testing for Clostridium botulinum and Botulinum Toxin During an Outbreak of Botulism in Arizona Prison A, November 24 to 26, 2012.
| Specimen | Number Submitted | Botulinum Toxin Type A | C. botulinum |
|---|---|---|---|
| Pruno | 1 | Positive | Positive |
| Patient sera | |||
| Pre-antitoxin | 8 | Positive | — |
| Post-antitoxin | 8 | Negative | — |
| Stool | 1 | Positive | Positive |
| Gastric aspirate | 7 | Negative | Negative |
| Rectal swabs | 7 | — | 5 positive |
Note. Serum from all eight patients was tested for botulinum toxin by using mass spectrometry and mouse bioassay before and 24 hours after administration of botulinum antitoxin.
Figure 2.
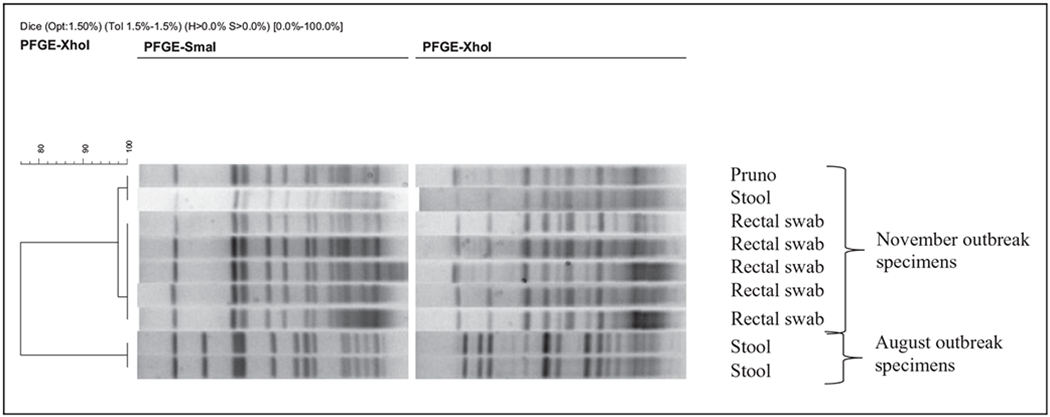
Clostridium botulinum pulsed-field gel electrophoresis (PFGE) results from patient and pruno samples from August and November 2012 botulism outbreaks in Arizona Prison A.
Clinical Course and Epidemiologic Study
Among the seven patients who required intubation, all received tracheostomies and percutaneous endoscopic gastrostomies 11 to 14 days after admission. These patients were transferred to longterm care facilities 2 to 3 weeks after initial hospitalization. The patient who was the last to experience symptoms and was not intubated experienced the least severe symptoms of botulism, and he was discharged back to Prison A 5 days after initial hospitalization.
No evidence of serum sickness or other adverse reactions to antitoxin were identified. However, all seven patients who were intubated experienced fevers (≥ 100.4°F) attributed to ventilator-associated pneumonias; culture of bronchoalveolar lavage specimens isolated bacterial opportunistic pathogens, including methicillin-resistant Staphylococcus aureus, methicillin-sensitive Staphylococcus aureus, Enterobacter aerogenes, Serratia marcescens, Citrobacter koseri, Klebsiella pneumonia, and Escherichia coli.
One patient experienced cholecystitis on November 28, and five patients had elevated lipase levels during the 2 weeks after initial hospitalization. Cholecystitis and lipase abnormalities were not attributed to the antitoxin and were likely because of excessive alcohol intake by patients during the days preceding hospitalization (Table 1).
Prison A is a maximum security facility housing 1,050 inmates. Inmates are confined to cells for 24 hours/day, 4 days/week; showers and 2-hour periods of recreation in an enclosed, outdoor recreation area occur 3 times/week. Inmates do not have contact with other prisoners besides their cellmate. All meals are consumed inside the inmate’s cell.
To make pruno, interviewed inmates reported using potatoes, sugar, bread, water, and fresh and canned fruit. The mixture was fermented in jars or plastic bags used for trash and could be heated by placing in hot water or near warm parts of their body. Pruno was more frequently consumed around holidays. Thanksgiving was the occasion that prompted the production of this pruno batch. Inmates reported sharing the pruno, gratis, between cells by sliding sealed bags of pruno under the space below their cell doors. Two patients were housed in a separate, adjoining pod to the other six inmates, and postulation was that pruno was shared between pods through circular holes (approximately 1 cm in diameter) of a drainage grate in the concrete wall of the recreation area (Figure 1). Hollowed-out coaxial cables, available to inmates through the prison commissary for use with televisions, were attached to the nozzles of prison-issued water bottles, fed through the holes in the grate, and collected on the other side of the wall (Figure 3).
Figure 3.
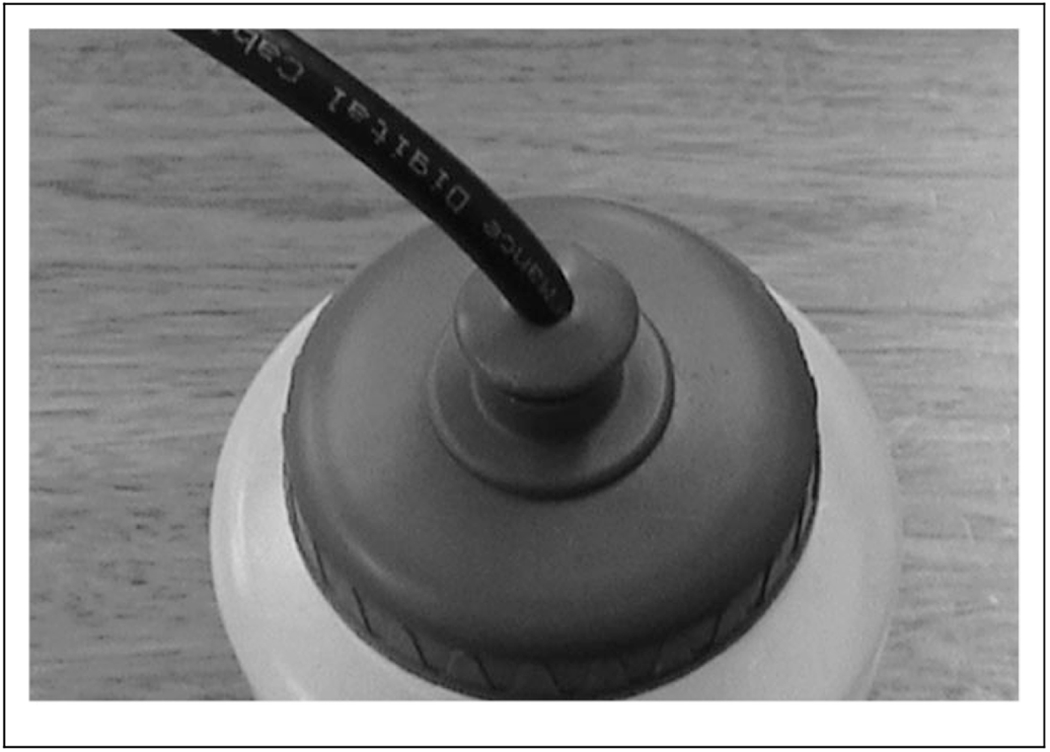
Hollowed-out coaxial cable inserted into prison-issued water bottle. This apparatus was probably used by inmates to pass prison-brewed illicit alcohol through holes in a drainage grate between two pods in the inmate recreation area, Arizona Prison A, November 2012.
Public Health Response
Botulinum antitoxin was released by CDC in response to the requests by ADHS. Antitoxin was released from CDC quarantine stations. ADHS and PCDPH produced and delivered inmate and correctional staff education regarding botulism, including presentations and bilingual educational fliers vetted by inmates. Prison A banned baked potatoes and sugar from inmate meals.
Discussion
During this outbreak of botulism in a maximum security prison in Arizona, eight inmates were treated with botulinum antitoxin after consuming prison-brewed illicit alcohol, referred to as pruno, made with potatoes. This was the second outbreak of botulism in Prison A within a 4-month period; earlier the same year, four Prison A inmates were hospitalized and treated with antitoxin for botulism. One of the earlier patients received tracheal intubation for mechanical ventilation, and one other inmate continues to suffer long-term neurologic sequelae, including tremors and reported emotional and mental health disturbances. Molecular epidemiology via PFGE revealed that samples from the August and November outbreaks had different patterns, indicating the outbreaks were unrelated.
Pruno is produced and consumed in prisons across the world. During 2004 to 2012, five outbreaks of botulism related to pruno made with potatoes were reported in prisons in the United States, including two outbreaks in Prison A. During 2004, four inmates of a California prison were hospitalized with pruno-related botulism, two of whom required intubation for mechanical ventilation. One inmate of a California prison was hospitalized with botulism and intubated during 2005 (Vugia et al., 2009). An outbreak of botulism related to pruno occurred in a Utah maximum security prison during 2011. Eight inmates were hospitalized, and three of those patients received tracheal intubation for mechanical ventilation (CDC, 2012). Potatoes are a suspected source of C. botulinum when introduced to the pruno mixture (anaerobic, low-sugar, and low-acid conditions) and fermented at a warm temperature, which is an environment conducive to botulinum toxin production.
During this investigation, heptavalent botulinum antitoxin was an investigational new drug. It was licensed during March 2013 and replaces the use of licensed botulinum antitoxin B and investigational botulinum antitoxin E (CDC, 2010). It is the only botulinum antitoxin available in the United States for noninfant botulism. Because the antitoxin contains equine-derived antibody, serum sickness could theoretically occur shortly after administration, even though the product is despeciated. The eight patients treated with antitoxin during this outbreak did not suffer serum sickness or any other adverse events associated with antitoxin. The seven intubated patients experienced fevers that were likely because of ventilator-associated pneumonias, and all recovered from the infections with antibiotic therapy. One patient experienced cholecystitis, and five patients had transiently elevated lipases, which returned to normal in < 1 week.
Recovery from botulism requires nerve regeneration, a process that can take months to years (Shapiro et al., 1998). During a previous community outbreak, patients reported persistent symptoms, including shortness of breath and dysphagia for ≥ 9 months after symptom onset (Mann, Martin, Hoffman, & Marrazzo, 1981). Severity of clinical illness has also been associated with longer symptom persistence (CDC, 2010; Vugia et al., 2009). Among six Prison A inmates with diagnosed botulism during the August (n = 4 total cases) and November (n = 8 total cases) outbreaks and who were available for interview, as of May 5, 2013, all report persistent symptoms, including weakness, tremors, dry mouth, dysphagia, blurred vision, and emotional disturbances.
During the November outbreak, the patient who experienced the least severe symptoms and was not intubated reported drinking less pruno than the other seven patients. Accurately determining a dose–response association is impossible in this outbreak, but the possibility exists that consuming a lesser amount of pruno resulted in the longer incubation period and milder symptoms. Inmates with the shortest incubation periods were hospitalized the longest, indicating that more severely affected persons might be more likely to present earlier and have longer recovery periods than those with longer incubation periods.
Prison A did not enact any control measures after the first outbreak of botulism during August 2012, when four inmates were hospitalized and treated with antitoxin. After the second outbreak, local public health officials were invited to provide education regarding signs, symptoms, and prevention of foodborne botulism to both inmates and correctional staff. Additionally, the prison banned potatoes and sugar from inmate meals in an attempt to prevent future pruno-associated botulism outbreaks. Pruno production is likely to continue in correctional settings. Inmate and correctional staff education about botulism might help to prevent pruno-associated botulism outbreaks. Prompt recognition and early treatment with botulinum antitoxin can prevent fatalities and longterm sequelae associated with botulism.
Acknowledgments
We would like to thank Agam K. Rao, Jamae Morris, Carolina Luquez, Janet Dykes, and Tara Anderson at the Centers for Disease Control and Prevention, Atlanta, Georgia, and staff at Mountain Vista Medical Center and the Arizona Department of Corrections for their work with this investigation.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centers for Disease Control and Prevention, Arizona Department of Health Services, and Pinal County Public Health Services.
Footnotes
Declaration of Conflicting Interests
The authors disclosed no conflicts of interest with respect to research, authorship, or publication of this article. For information about JCHC’s disclosure policy, please see the Self-Study Exam.
References
- Bell C, & Kyriakides A (2000). Clostridium botulinum: A practical approach to the organism and its control in foods. Oxford, England: Blackwell Science. [Google Scholar]
- Centers for Disease Control and Prevention. (1998). Botulism in the United States, 1899–1996. Retrieved from http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/botulism.pdf
- Centers for Disease Control and Prevention. (2010). Investigational heptavalent botulinum antitoxin (HBAT) to replace licensed botulinum antitoxin AB and investigational botulinum antitoxin E. Morbidity and Mortality Weekly Report, 59, 299. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2012). Notes from the field: Botulism from drinking prison-made illicit alcohol—Utah, 2011. Morbidity and Mortality Weekly Report, 61, 782–784. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). Notes from the field: Botulism from drinking prison-made illicit alcohol—Arizona, 2012. Morbidity and Mortality Weekly Report, 62, 88. [PMC free article] [PubMed] [Google Scholar]
- Hughes JM, Blumenthal JR, Merson MH, Lombard GL, Dowell VR Jr., & Gangarosa EJ (1981).Clinical features of types A and B food-borne botulism. Annals of Internal Medicine, 95, 442–445. [DOI] [PubMed] [Google Scholar]
- Mann JM, Martin S, Hoffman R, & Marrazzo S (1981). Patient recovery from type A botulism: Morbidity assessment following a large outbreak. American Journal of Public Health, 71, 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RL, Hatheway C, & Swerdlow DL (1998). Botulism in the United States: A clinical and epidemiologic review. Annals of Internal Medicine, 129, 221–228. [DOI] [PubMed] [Google Scholar]
- Sobel J, Tucker N, Sulka A, McLaughlin J, & Maslanka S (2004). Foodborne botulism in the United States, 1990–2000. Emerging Infectious Diseases, 10, 1606–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vugia DJ, Mase SR, Cole B, Stiles J, Rosenberg J, Velasquez L, … Inami G (2009). Botulism from drinking pruno. Emerging Infectious Diseases, 15, 69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


