Online Abstract
Catalytic carbonyl-olefin metathesis reactions enable direct carbon-carbon bond formation between carbonyl and olefin substrates relying on carbonyl-activation with a suitable Lewis acid. Based on this reaction design principle, efficient protocols for intermolecular carbonyl-olefin metathesis, as well as ring-closing and ring-opening carbonyl-olefin metathesis have been developed.
Keywords: FeCl3, carbonyl-olefin metathesis, Lewis acid catalysis, oxetane
Origin
Routine methodologies for alkene formation rely on direct elimination, stoichiometric carbonyl-olefination, and olefin-olefin metathesis. Carbonyl-olefin metathesis (COM) represents a new, catalytic alternative for direct alkene formation from carbonyl and olefin substrates upon activation with a Lewis acid.
Reaction Mechanism
Metathesis reactions describe chemical transformations involving an exchange of parts of two substrates. Specifically, in carbonyl-olefin metathesis reactions carbonyl and alkene substrates are interchanged directly to form new carbonyl and alkene products. The Lewis acid catalyst (FeCl3 in Figure 2) binds to the carbonyl oxygen of the substrate (1) to enable formation of an oxetane intermediate (4) via a [2+2]-cycloaddition with the alkene substrate. This step proceeds in a concerted, yet asynchronous, fashion in which the carbon-carbon bond is formed to a greater extent than the carbon-oxygen bond in the transition state (3). Subsequent asynchronous, concerted retro-[2+2]-cycloaddition (via transition state 5) results in fragmentation of the oxetane intermediate and formation of the desired alkene as metathesis product (6) and the carbonyl component and byproduct (7). Several metal- and carbocation-based Lewis acids have been shown to catalyze carbonyl-olefin metathesis reactions, however, FeCl3 proved particularly efficient resulting in high yields of up to 99% yield without competing isomerization of the desired alkene products. Based on this design principle, reaction protocols for intermolecular, ring-opening and ring-closing carbonyl-olefin metathesis have been developed.
Figure 2.
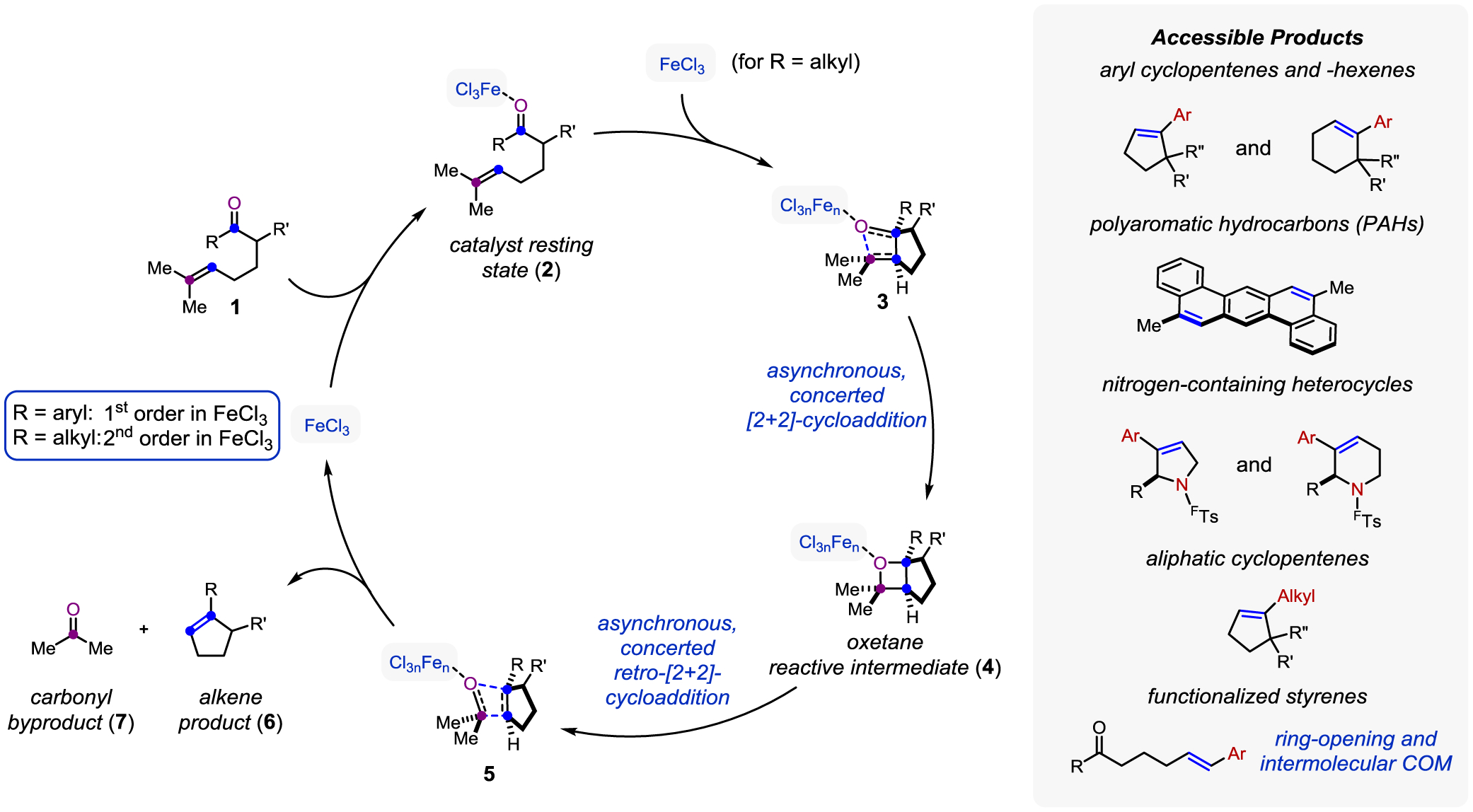
Currently accessible products include aryl cyclopentenes and –hexenes, polyaromatic hydrocarbons, nitrogen containing heterocycles, aliphatic cyclopentenes, as well as functionalized styrenes. Importantly, aryl and alkyl ketone substrates undergo the desired carbonyl-olefin metathesis via distinct reaction mechanisms. Compared to aryl ketones, their aliphatic analogs bind less strongly to the Lewis acid catalyst by ~ 1 kcal/mol and lack the transition state stabilization necessary for oxetane formation. Importantly, reactions of both aryl and alkyl ketones exhibit zero-order dependence in substrate, consistent with the catalyst resting state (2) being FeCl3 bound to carbonyl. However, aryl ketones show first-order dependence in FeCl3 whereas alkyl ketones show second-order dependence in Lewis acid which results in distinct activation modes for the two different substrate classes. When considering aryl ketones, the catalyst resting state in which monomeric FeCl3 is bound to substrate is sufficiently activated to undergo carbonyl-olefin metathesis. In comparison, the activation barrier for alkyl ketones is higher and requires a second equivalent of FeCl3 to bind to form an Fe(III) singly-bridged homodimer for the reaction to proceed. This homobimetallic association of a second equivalent of FeCl3 generates a stronger Lewis acid that functions as a “superelectrophile” that is capable of lowering the energy of the transition state to provide sufficient activation for alkyl ketone substrates.
Importance
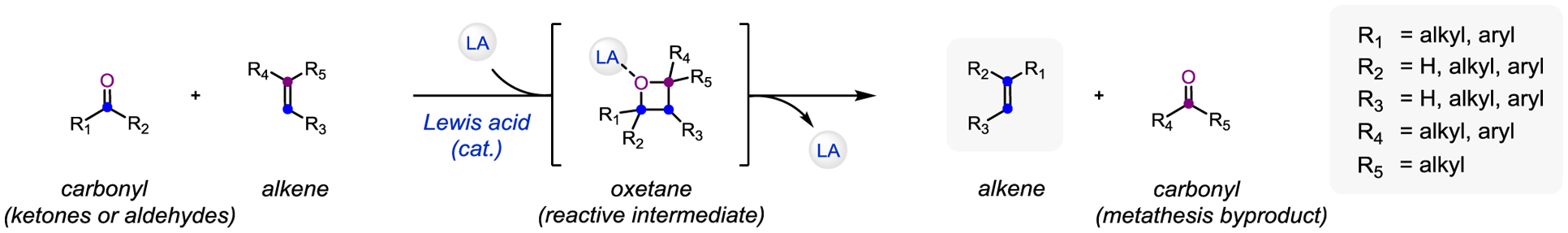
The mechanism for Lewis acid-catalyzed carbonyl-olefin metathesis fundamentally differs from the previously established state of the art reaction protocol. Rather than requiring a full equivalent of air-sensitive molybdenum alkylidene, it is instead based on the in-situ formation of oxetanes as reactive intermediates via [2+2]-cycloaddition of a carbonyl and olefin upon activation with a Lewis acid catalyst. This distinct intermediate no longer limits the process to metal alkylidenes as reagents, and enables catalytic turnover relying on cheap and environmentally benign Lewis acids. As a result, this method can be applied to the scalable synthesis of various classes of useful molecular scaffolds.
Figure 1.
Literature
- 1).Ludwig JR; Zimmerman PM; Gianino JB; Schindler CS * Iron(III)-catalyzed carbonyl-olefin metathesis. Nature 2016, 533, 374–379. 10.1038/nature17432 [DOI] [PubMed] [Google Scholar]
- 2).Ma L; Li W; Xi H; Bai X; Ma E; Yan X; Li Z FeCl3-Catalyzed Ring-Closing Carbonyl–Olefin Metathesis. Angew. Chem. Int. Ed 2016, 55, 10410–10413. 10.1002/anie.201604349 [DOI] [PubMed] [Google Scholar]
- 3).Naidu VR; Bah J; Franzén J Direct Organocatalytic Oxo-Metathesis, a trans-Selective Carbocation-Catalyzed Olefination of Aldehydes. Eur. J. Org. Chem 2015, 8, 1834–1839. 10.1002/ejoc.201403651 [DOI] [Google Scholar]
- 4).Ludwig JR; Phan S; McAtee CC; Zimmerman PM; Devery JJ III; Schindler CS * Mechanistic Investigations of the Iron(III)-Catalyzed Carbonyl-Olefin Metathesis Reaction. J. Am. Chem. Soc 2017, 139, 10832–10842 10.1021/jacs.7b05641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).McAtee CC; Riehl PS; Schindler CS * Polycyclic Aromatic Hydrocarbons via Iron(III)-Catalyzed Carbonyl-Olefin Metathesis. J. Am. Chem. Soc 2017, 139, 2960–2963. 10.1021/jacs.7b01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Albright H; Riehl PS; McAtee CC; Reid JP; Ludwig JR; Karp L; Zimmerman PM; Sigman MS Schindler CS * Catalytic Carbonyl-Olefin Metathesis of Aliphatic Ketones: Iron(III) Homo-Dimers as Lewis Acidic Superelectrophiles. J. Am. Chem. Soc. Just Accepted Manuscript 10.1021/jacs.8b11840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Groso EJ; Golonka AN; Harding RA; Alexander B; Sodano TM; Schindler CS * 3-Aryl-2,5-Dihydropyrroles via Catalytic Carbonyl-Olefin Metathesis. ACS Catalysis 2018, 8, 2006–2011. 10.1021/acscatal.7b03769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Albright HR; Alexander BW; Vonesh H; Schindler CS * GaCl3-Catalyzed Ring-Opening Carbonyl-Olefin Metathesis. Org. Lett 2018, 20, 4954–4958 10.1021/acs.orglett.8b02086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Becker MR; Rykaczewski K; Ludwig JR; Schindler CS * Carbonyl-Olefin Metathesis for the Synthesis of Cyclic Olefins. Org. Synth 2018, 95, 472 10.15227/orgsyn.095.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Fu GC; Grubbs RH Synthesis of cycloalkenes via alkylidene-mediated olefin metathesis and carbonyl olefination. J. Am. Chem. Soc 1993, 115, 3800–3801. 10.1021/ja00062a066 [DOI] [Google Scholar]


