Abstract
Follicle-stimulating hormone (FSH), an essential regulator of mammalian fertility, is synthesized by pituitary gonadotrope cells in response to activins. In mice, activins signal via SMAD3, SMAD4, and FOXL2 to regulate transcription of the FSHβ subunit (Fshb) gene. Gonadotrope-specific deletion of Foxl2, alone or in combination with Smad4, renders mice FSH-deficient. Whether human FSHB expression is similarly regulated is not known. Here, we used a combination of transgenic and conditional knockout mouse strains to assess the roles of activins, FOXL2, and SMAD4 in regulation of the human FSHB gene. First, we cultured pituitaries from mice harboring a human FSHB transgene (hFSHB mice) and measured both murine Fshb and human FSHB messenger ribonucleic acid (mRNA) expression in response to exogenous activins or two antagonists of endogenous activin-like signaling (follistatin-288 and SB431542). Both murine Fshb and human FSHB expression were stimulated by activins and reduced by the inhibitors. Next, we analyzed human FSHB expression in hFSHB mice carrying floxed Foxl2 and Smad4 alleles. Cre-mediated ablation of FOXL2 and SMAD4 strongly reduced basal and activin-stimulated murine Fshb and human FSHB expression in cultured pituitaries. Finally, the hFSHB transgene was previously shown to rescue FSH production and fertility in Fshb knockout mice. However, gonadotrope-specific Foxl2/Smad4 knockout females carrying the hFSHB transgene have significantly reduced murine Fshb and human FSHB pituitary mRNA levels and are hypogonadal. Collectively, these data suggest that similar to Fshb regulation in mice, FOXL2 and SMAD4 play essential roles in human FSHB expression.
Keywords: FSH, transgene, gonadotrope, activin, pituitary, FOXL2, SMAD4
Mammalian reproduction is dependent on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secreted by gonadotrope cells of the anterior pituitary gland. FSH is required for ovarian follicle maturation in females and supports quantitatively normal spermatogenesis in males (1). FSH is a dimeric glycoprotein composed of noncovalently linked α and β subunits. Synthesis of the FSHβ subunit (encoded by Fshb in rodents and FSHB in humans) is rate-limiting in production of the hormone (2,3). Fshb transcription is potently stimulated and inhibited, respectively, by activins and inhibins, structurally related ligands of the transforming growth factor beta (TGFβ) superfamily (4). Activins preferentially signal via the activin type IIA receptor (ACVR2A) and the activin type IB or IC receptors (ACVR1B or ACVR1C, respectively) to regulate FSH in vitro (5,6). Upon ligand binding, the type II receptors recruit and trans-phosphorylate the type I receptors, which in turn phosphorylate signaling proteins in the SMAD (homolog of Drosophila mothers against decapentaplegic) family. Although both SMAD2 and SMAD3 are phosphorylated by activin type I receptors, SMAD3 plays a more significant role in Fshb expression in mice (7). Once phosphorylated, SMAD3 complexes with the co-SMAD, SMAD4, in the cytoplasm. The proteins rapidly accumulate in the nucleus where they bind the proximal murine Fshb promoter in combination with the transcription factor forkhead box L2 (FOXL2) (8).
FSH levels are significantly reduced in Acvr2a knockout mice (9), indicating that activins or related TGFβ ligands that use this receptor are essential for FSH production in vivo. Similarly, mice with gonadotrope-specific deletions of Foxl2 or Smad4 have reduced FSH levels in both sexes and, in the case of females, produce smaller litters compared to controls (10-12). Mice lacking both Foxl2 and Smad4 in gonadotropes are FSH-deficient due to a selective loss in Fshb expression; females are sterile (11,12).
Whether the human FSHB gene is similarly regulated by activins, FOXL2, and SMAD4 is not known. Human FSHB promoter-reporters are poorly responsive to activins in immortalized murine gonadotrope-like cells (13-15), and there are currently no human gonadotrope cell lines available (16). In addition, the FOXL2/SMAD cis-regulatory elements identified in the murine Fshb promoter are similar, although not perfectly conserved in the human FSHB promoter (17). Nonetheless, other observations do suggest an important role for activins or related TGFβ ligands in human FSH production. For example, serum FSH levels are reduced in postmenopausal women treated with either the ectodomain of ACVR2A or a monoclonal antibody inhibitor of activin type II receptors (18,19).
To determine whether and how activins regulate human FSHB expression, we employed a mouse model harboring a 10-kb human FSHB transgene, which contains all exons and introns, as well as 4 and 2 kb of 5ʹ and 3ʹ flanking sequence (20). The transgene directs human FSHB expression exclusively to gonadotropes in mice and rescues fertility in Fshb knockouts (21). Importantly, human FSH production is greatly reduced in human FSHB (hFSHB) mice lacking functional activin type IIA receptors (22). Here, we examined transcription factors regulating human FSHB expression by crossing hFSHB transgenics with floxed Foxl2 and Smad4 mice. We then deleted the latter genes using Cre recombinase in vitro and in vivo enabling us to directly assess the roles of FOXL2 and SMAD4 in human FSH synthesis.
Materials and Methods
Reagents
Human recombinant (rh-) activin A (338-AC) and activin B (659-AB-005) were from R&D Systems (Minneapolis, MN, US). RQ1 RNase-Free DNase (M6101), random primers (C1181), MMLV-reverse transcriptase (M1701), and RNasin (N2511) were from Promega (Madison, WI, US). SB431542 (S4317), pancreatin (P3292), actinomycin D (A9514), and collagenase (Type I-C0130) were from Sigma (St. Louis, MO, US). Media 199 (M199; 31100-035), Hanks’ Balanced Salt Solution (HBSS) without calcium/magnesium (14170-112), TRIzol Reagent, and SYBRgreen Supermix for quantitative polymerase chain reaction (qPCR) were from Invitrogen (Burlington, ON, Canada). EvaGreen 2X qPCR MasterMix-S was from Applied Biological Materials Inc. (ABM, Richmond, BC, Canada). Oligonucleotides were purchased from IDT (Coralville, IA, US). Gentamycin (450-135-XL), 100X antibiotic-antimycotic (450-115-EL), and deoxynucleotide triphosphates (dNTPs) were from Wisent (St-Bruno, Quebec, Canada). Follistatin 288 was provided by Dr. Tom Thompson (University of Cincinnati).
Mice
We crossed hFSHB/+ mice inter se to obtain homozygotes hFSHB/hFSHB. hFSHB zygosity was determined by qPCR from genomic deoxyribonucleic acid (DNA) (23) (Table 1). Homozygous hFSHB mice were crossed with mice harboring floxed Foxl2 and/or Smad4 alleles. Ultimately, we generated hFSHB/+;Foxl2fx/fx or hFSHB/+;Foxl2fx/fx;Smad4fx/fx mice for in vitro recombination experiments. Gonadotrope-specific Smad4/Foxl2 knockouts carrying the human FSHB transgene were generated using hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx and GnrhrGRIC/GRIC mice. First, hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx males were crossed with GnrhrGRIC/GRIC females, generating hFSHB/+;Foxl2fx/+;Smad4fx/+;GnrhrGRIC/+ females that were backcrossed with hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx males. All animal work was performed in accordance with institutional and federal guidelines and approved by the McGill University and Goodman Cancer Centre Facility Animal Care Committee (protocol 5204).
Table 1.
Genotyping and qPCR primers
| Genotyping | |
|---|---|
| hFSHB | 5’ --> 3’ |
| Forward | TCCTTTTCTGTTGCTGGAAAGC |
| Reverse | CACCAAGTGGTGTTGATGCTTA |
| Foxl2 | |
| Forward | GGACAGCTTCTGGATGCAGAGCC |
| Reverse | CAGCGGAGGCGACAAAGCGGAGTCGCAGG |
| Smad4 | |
| Forward | GGGCAGCGTAGCATATAAGA |
| Reverse | GACCCAAACGTCACCTTCAG |
| GRIC | |
| Forward | GGACATGTTCAGGGATCGCCAGGC |
| Reverse | GCATAACCAGTGAAACAGCATTGCTG |
| qPCR | |
| Rpl19 | |
| Forward | CGGGAATCCAAGAAGATTGA |
| Reverse | TTCAGCTTGTGGATGTGCTC |
| hFSHB | |
| Forward | ACCACAGACCAGGATGAAGAC |
| Reverse | CCTGGCTGGGTCCTTATACAC |
| mFshb | |
| Forward | GTGCGGGCTACTGCTACACT |
| Reverse | CAGGCAATCTTACGGTCTCG |
| Cga | |
| Forward | TCCCTCAAAAAGTCCAGAGC |
| Reverse | GAAGAGAATGAAGAATATGCAG |
| Lhb | |
| Forward | ACTGTGCCGGCCTGTCAACG |
| Reverse | AGCAGCCGGCAGTACTCGGA |
| Gnrhr | |
| Forward | TTCGCTACCTCCTTTGTCGT |
| Reverse | CACGGGTTTAGGAAAGCAAA |
| Foxl2 | |
| Forward | ACAACACCGGAGAAACCAGAC |
| Reverse | CGTAGAACGGGAACTTGGCTA |
| Smad4 | |
| Forward | TCACAATGAGCTTGCATTCC |
| Reverse | CCATCCACAGTCACAACAGG |
DNA extraction and genotyping
Genomic DNA was extracted from toe biopsies using 0.4 mL of lysis buffer (100 mmol/L Tris-hydrochloride [pH 8.5], 5 mmol/L ethylenediamine tetra-acetate [pH 8.0], 200 mmol/L sodium chloride, 0.2% [v/v] sodium dodecylsulphate, and 100 μg/L proteinase K). Samples were incubated overnight in a water bath at 55°C. After centrifugation at 12,000 rpm for 10 min, the supernatant was collected and mixed by inversion with 0.5 mL isopropanol. Precipitated DNA was collected with a micropipette tip and dissolved in 40 μL of 10 mmol/L Tris (pH 8.0). The presence of the GRIC allele, as well as, wild-type and floxed alleles for Foxl2 and Smad4 were detected by polymerase chain reaction (PCR) using the primers indicated in Table 1. Zygosity determination of the hFSHB transgene was performed by qPCR (Table 1).
Primary pituitary cultures
Primary cultures were performed as previously described (24). Briefly, pituitaries were collected from 10- to 12-week old hFSHB/+;Foxl2fx/fx or hFSHB/+;Foxl2fx/fx;Smad4fx/fx male and female mice in M199 medium supplemented with 10% (v/v) fetal bovine serum (FBS). Pituitaries were washed 3 times in HBSS with 150 µmol/L CaCl2, cut several times with a scalpel, and digested in collagenase (1.5 mg/mL) (Sigma #C-0130; diluted in HBSS with 30 mg/mL BSA, pH 7.4, 40 µL/pituitary) at 37ºC for 2 hours. The suspension was then washed with 3 mL calcium-free HBSS, centrifuged for 5 min at 1000 × g, and resuspended in pancreatin solution (Sigma P3292; 4.5 mg/mL in calcium-free HBSS; 40 µL/pituitary). Pancreatin digestion was performed in a 37ºC water bath with manual agitation for 6 to 8 min. Finally, the cell suspension was washed 3 times in 5 mL M199 media containing 10% (v/v) FBS, and cells were seeded at density of 3 to 3.5 × 105/well in 48-well plates.
Adenoviral transduction and treatment of pituitary cultures
Primary cultures were transduced with adenoviruses as previously described (24). Viral transductions were performed using adenoviruses that express enhanced green fluorescent protein (GFP) or Cre-internal ribosome entry site-enhanced green fluorescent protein (hereafter Cre-expressing adenovirus) (Baylor College of Medicine Vector Development Laboratory, Houston, TX, US) at a multiplicity of infection of 600 in M199 medium containing 10% (v/v) FBS. The following day, virus-containing medium was removed and replaced with medium containing 2% (v/v) FBS with 1 nM activin A, 1 nM activin B, 10 µM SB431542, 200 ng/mL follistatin 288, or dimethyl sulfoxide (DMSO) as vehicle. Treatments were performed for 2, 6, 12, or 24 h in duplicate, and the experiment was repeated 3 times. Cells were harvested, and ribonucleic acid (RNA) was extracted using the total RNA mini kit from Geneaid FroggaBio (RB300, Toronto, ON, Canada).
Quantitative RT-PCR
Pituitaries were isolated from 7- to 8-week-old control (hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx;Gnrhr+/+) and deletion mutant mice (hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx; GnrhrGRIC/+), immediately frozen in liquid nitrogen, and stored at -80°C until analysis. Pituitaries were homogenized in 500 μL TRIzol reagent (15596026; Life Technologies), and total RNA was extracted following the manufacturer’s instructions. RNA concentration was determined by NanoDrop. Two hundred nanograms of RNA from pituitary glands or cultured pituitary cells were reverse transcribed. The resulting complementary DNA was analyzed by qPCR. Relative human FSHB, murine Fshb, Lhb, Gnrhr, Cga, Foxl2, and Smad4 messenger RNA (mRNA) levels (normalized to Rpl19) were determined using the 2−ΔΔCt method as described in (25). Primer sequences are indicated in Table 1.
Histology
Ovaries were isolated from 7- to 8-week-old control and deletion mutant females, fixed in 10% formalin (HT501128, Millipore-Sigma) overnight at room temperature and stored in 70% ethanol. Testes isolated from 7- to 8-week-old males were immersed in Bouin’s fixative solution (1120-16, Ricca Chemical Company) overnight, followed by an overnight incubation in 100% ethanol. Finally, the testes were stored in 70% ethanol. Fixed tissues were paraffin embedded, and sections from ovarian or testicular samples were collected and stained with hematoxylin and eosin at the McGill Centre for Bone and Periodontal Research. Images were acquired using an Axiocam 506 color camera on a Zeiss Axio Imager M2 microscope. Images were processed using ZenPro imaging software v2.3 (Zeiss, Canada).
Statistics
Data from control and deletion mutant mice and/or cell cultures were compared with 1-way or 2-way analysis of variance (primary culture treatments), t-test (reproductive organ weights), or unpaired t-test (pituitary gene expression) using GraphPad Prism 8.0. Post-hoc pair-wise comparisons were made with Holm-Sidak corrections. Significance was assessed relative to P < 0.05; n.s. means not statistically significant.
Results
Human FSHB expression is stimulated by activins
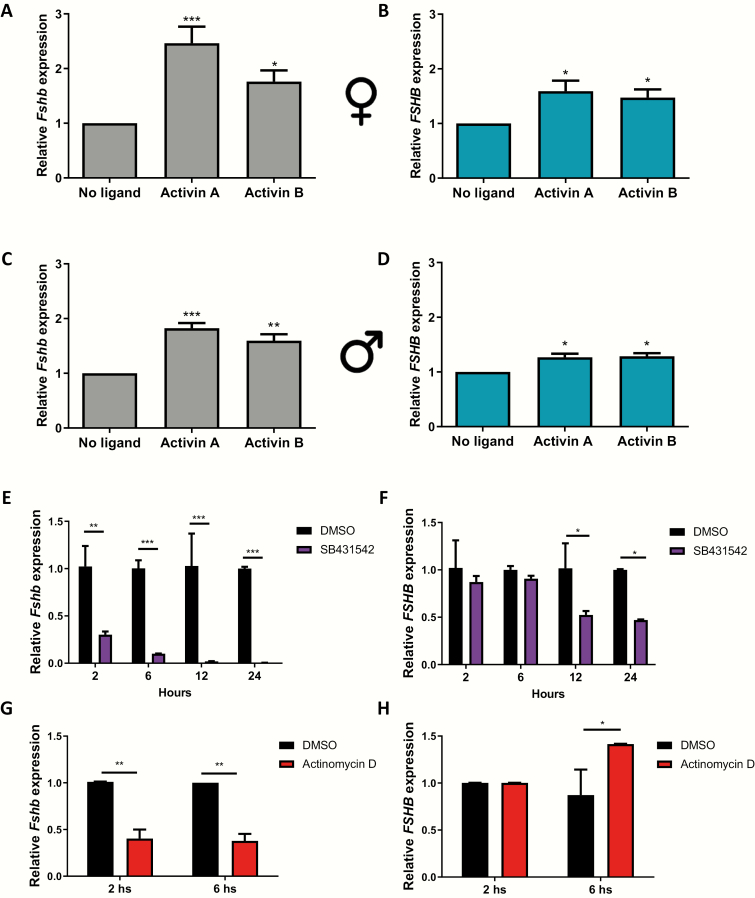
Pituitary cell cultures from hFSHB mice express both murine Fshb (Fshb) and human FSHB (FSHB) mRNAs. Using species-specific qPCR primers, we analyzed how the 2 mRNAs were regulated by exogenous activins in the same experiments. Activin A and B stimulated murine Fshb expression 1.6 to 2.5-fold after 6 h of treatment in female and male pituitary cultures (Fig. 1A and 1C). Exogenous activins also increased human FSHB mRNA levels, although to a lesser extent (1.2 to 1.6-fold) (Fig. 1B and 1D). Next, we treated male cultures with the activin type I receptor inhibitor SB431542 for 2, 6, 12, or 24 hours to block the effects of endogenous activins (or related TGFβ ligands that signal through similar type I receptors). Murine Fshb expression was significantly suppressed after 2 h and abolished by 12 h of inhibitor treatment (Fig. 1E). In contrast, human FSHB mRNA levels were only reduced starting between 6 and 12 h after addition of the inhibitor (Fig. 1F). Treatment of the cultures with the transcription inhibitor, actinomycin D, for 2 or 6 h revealed that human FSHB mRNA was more stable than murine Fshb mRNA in these cultures (Fig. 1G and 1H), perhaps explaining the relative resilience of the former in response to SB431542.
Figure 1.
The human FSHB transgene is activin-regulated in pituitary cultures. Pituitary cells were isolated from adult human FSHB transgenic female (A, B) and male (C, D) mice. Cells were treated for 6 h with 1 nM activin A or activin B. Relative murine Fshb (A and C, black bars) and human FSHB (B and D, aqua bars) mRNA levels were determined by RT-qPCR using species-specific primers and normalized by Rpl19 expression. (E and F) Pituitary cells were isolated from male hFSHB transgenic mice. Cells were treated with DMSO or SB431542 (10 µM) for 2, 6, 12, or 24 h. Murine Fshb (E) and human FSHB (F) mRNA expression were determined as above. (G and I) Pituitary cells were isolated from male hFSHB transgenic mice. Cells were treated with DMSO or actinomycin D (10 µg/mL) for 2 or 6 h. Murine Fshb (G) and human FSHB (I) mRNA expression were determined previously described. Data represent the mean (+standard error of the mean) of 3 independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.005.
FOXL2 regulates basal and activin-stimulated human FSHB expression
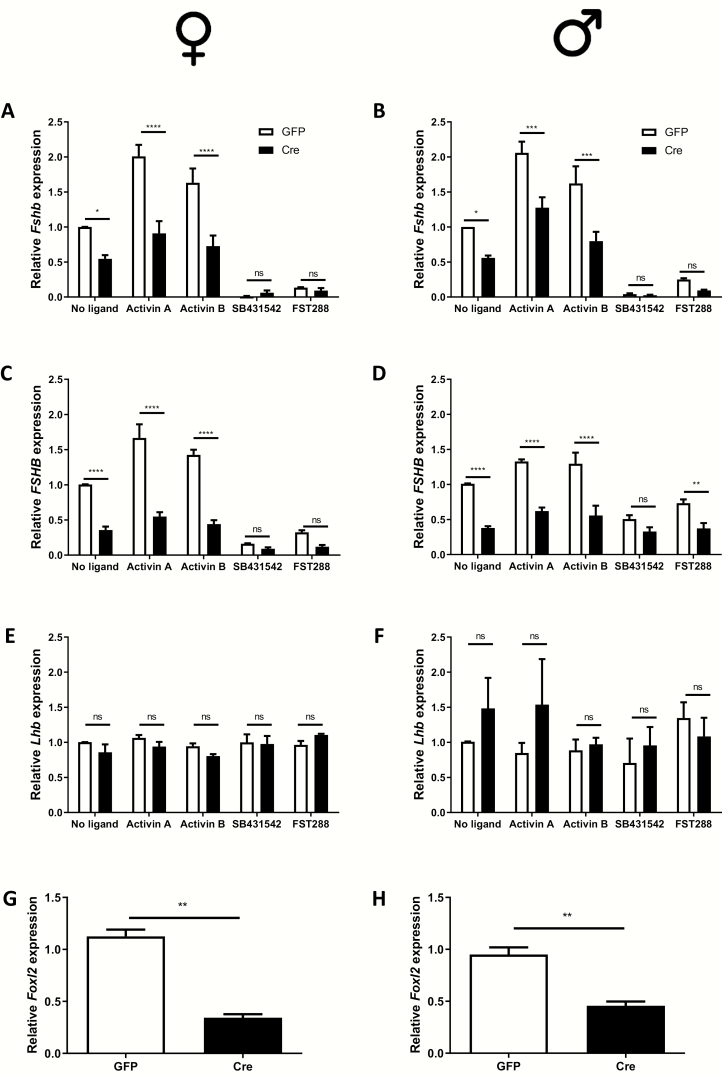
To test the potential role of FOXL2 in human FSHB expression, we generated hFSHB mice carrying floxed alleles for Foxl2. We cultured pituitary cells from these animals and transduced them with a control (GFP) or Cre-expressing adenovirus. Basal and activin-stimulated murine Fshb and human FSHB mRNA levels were significantly reduced in Cre-transduced cells from females (Fig. 2A and 2C) and males (Fig. 2B and 2D). In contrast, murine LHβ subunit (Lhb) expression was not significantly affected by the Cre adenovirus (Fig. 2E and 2F). Foxl2 mRNA levels were depleted by 60% to 70% in cells transduced with the Cre relative to control adenovirus (Fig. 2G and 2H). In the same experiments, we treated other wells with SB431542 (the type I receptor inhibitor) or with the soluble activin bioneutralizing protein follistatin 288 (FST288). Basal murine Fshb mRNA levels were greatly reduced by both inhibitors (Fig. 2A and 2B). Human FSHB (Fig. 2C and 2D), but not murine Lhb (Fig. 2E and 2F), mRNA levels were similarly reduced. Thus, both murine Fshb and human FSHB mRNA expression are dependent on FOXL2 and TGFβ ligands that are follistatin-sensitive and signal through the type I receptors inhibited by SB431542 (ACVR1B, ACVR1C, and/or TGFBR1).
Figure 2.
FOXL2 regulates human FSHB expression in pituitary cultures. Pituitary cells were isolated from adult hFSHB/+;Foxl2fx/fx female (A, C, E) and male (B, D, F) mice. After in vitro recombination with a Cre-expressing adenovirus (Cre; a GFP-expressing virus was used as control), cells were treated with activin A (1 nM), activin B (1 nM), follistatin 288 (FST288, 200 ng/ml), or SB431542 (10 µM) for 6 h. RNA was collected and murine Fshb (A, B), human FSHB (C, D), Lhb (E, F), and Foxl2 (G, H) expression determined by RT-qPCR. Data represent the mean (+standard error of the mean) of 3 independent experiments/sex. *P < 0.05, **P < 0.01, ***P < 0.005, or ****P < 0.001; n.s., not statistically different vs. GFP treatment.
FOXL2 and SMAD4 are essential regulators of basal and activin-induced human FSHB expression
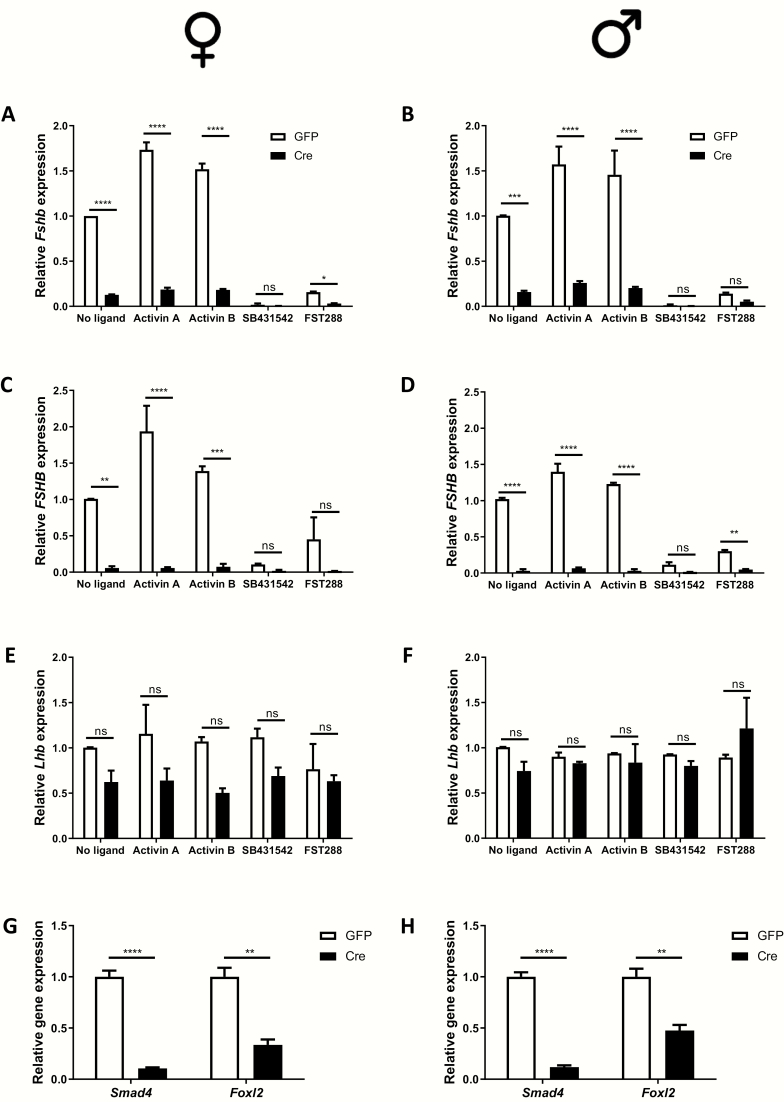
Reduction of Foxl2 expression impaired but did not completely block murine Fshb or human FSHB mRNA expression in cultured pituitaries (Fig. 2). We previously reported that the combination of Foxl2 and Smad4 ablation was sufficient to abolish murine Fshb expression both in vitro and in vivo. Here, we examined the roles of the 2 proteins in human FSHB expression by transducing pituitary cultures from hFSHB/+;Foxl2fx/fx; Smad4fx/fx mice with control or Cre-expressing adenoviruses. In cells transduced with the Cre adenovirus, basal and activin-stimulated murine Fshb and human FSHB expression were almost completely lost in both sexes (Fig. 3A-3D), whereas murine Lhb expression did not change significantly (Fig. 3E and 3F). Depletion of Foxl2 and Smad4 was efficient in these assays (Figs. 3G and 3H). FST288 and SB431542, particularly in combination with the Cre adenovirus, greatly impaired murine Fshb and human FSHB expression (Figs. 3A-D). Thus, both SMAD4 and FOXL2 regulate human FSHB expression.
Figure 3.
FOXL2 and SMAD4 are required for human FSHB expression in pituitary cultures. Pituitary cells were isolated from adult hFSHB/+;Foxl2fx/fx; Smad4fx/fx female (A, C, E, G) and male (B, D, F, H) mice. After in vitro recombination with a Cre-expressing adenovirus (Cre; a GFP-expressing virus was used as control), cells were treated with activin A (1 nM), activin B (1 nM), FST288 (200 ng/mL), or SB431542 (10 µM) for 6 h. RNA was collected and murine Fshb (A, B), human FSHB (C, D), and Lhb (E, F), and Foxl2 and Smad4 (G, H) expression were determined by RT-qPCR. Data represent the mean (+standard deviation) of 2 independent experiments/sex. *P < 0.05, **P < 0.01, ***P < 0.005, or ****P < 0.001; n.s., not statistically significant vs. GFP treatment.
Human FSHB expression depends on FOXL2 and SMAD4 in vivo
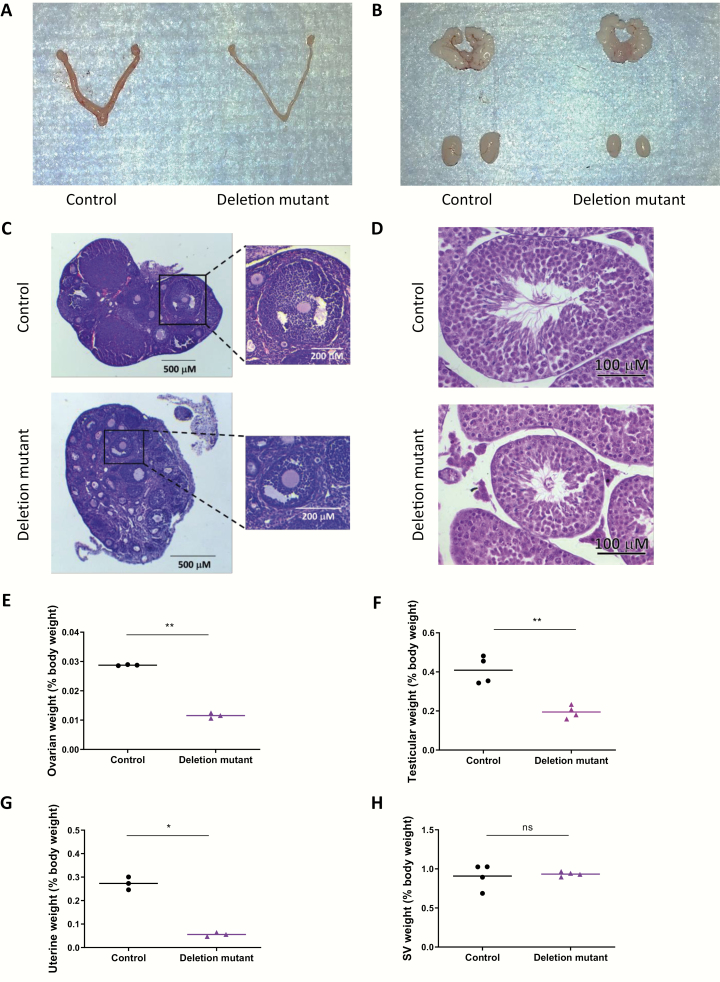
Gonadotrope-specific Foxl2/Smad4 knockout mice have similar phenotypes to Fshb knockouts (11); males and females are FSH-deficient and hypogonadal, and females are sterile (1). In Fshb null mice, these phenotypes are rescued by the human FSHB transgene (21). If, however, human FSHB expression depends on FOXL2 and SMAD4, then hFSHB should not be able to rescue reproductive phenotypes in gonadotrope-specific Foxl2/Smad4 knockouts. Indeed, ovarian and uterine weights were significantly reduced in gonadotrope-specific Foxl2/Smad4 knockout females carrying the hFSHB transgene (deletion mutant) relative to hFSHB transgenics with floxed Foxl2/Smad4 alleles, but without the Cre allele (control mice) (Fig. 4A, 4E, and, 4G). In deletion mutant females, folliculogenesis was arrested at the early antral stage, which is characteristic of mice with FSH deficiency (Fig. 4C). In males, testes weights were similarly reduced in deletion mutant relative to control mice (Fig. 4B and 4F). As seen in tissue sections, seminiferous tubule diameter was reduced in deletion mutants compared to controls (Fig. 4D). Seminal vesicle weights did not differ between genotypes (Figs. 4B and 4H).
Figure 4.
Gonadotrope-specific Foxl2/Smad4 knockout mice carrying the hFSHB transgene are hypogonadal. Representative images of reproductive organs from (A) female and (B) male control (hFSHB/hFSHB;Foxl2fx/fx;Smad4fx/fx;Gnrhr+/+) and deletion mutant (hFSHB/hFSHB;Foxl2fx/fx; Smad4fx/fx;GnrhrGRIC/+) mice. Ovarian (C) and testicular (D) histology in 7- to 8-week-old control and deletion mutant mice. Individual follicles from the 2 genotypes are shown at higher magnification at the right in (C). Ovarian (E), testicular (F), uterine (G), and seminal vesicle (H) weights (normalized by body weight) are plotted individually. Means are shown as horizontal lines. *P < 0.05, **P < 0.01, n.s., not statistically significant vs. control mice by unpaired t-test.
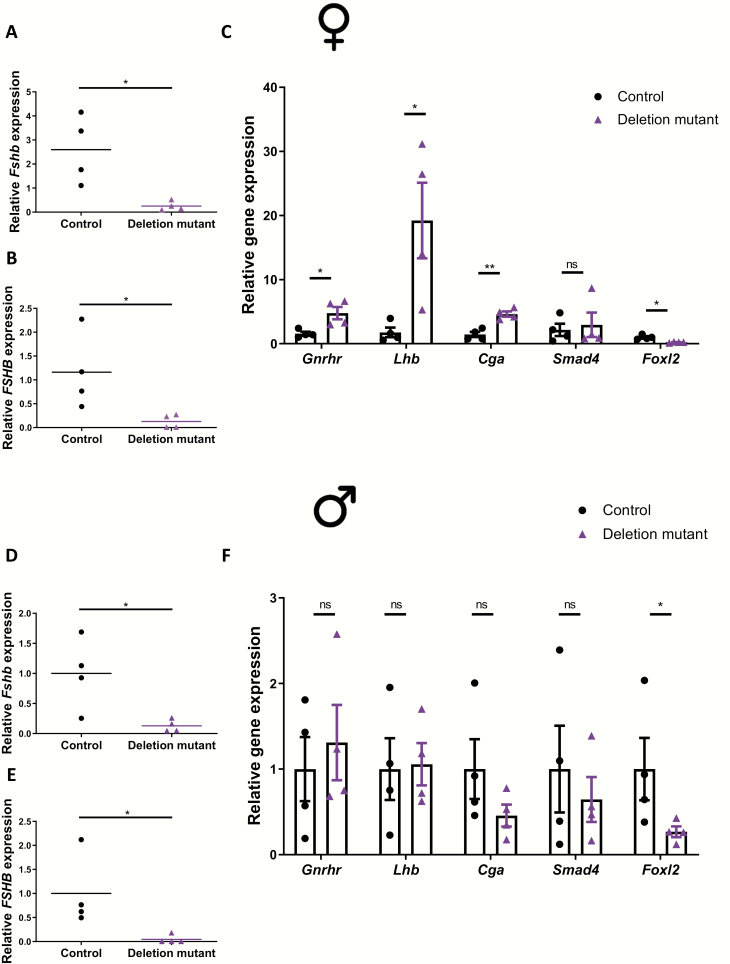
We next examined pituitary gene expression in these animals. Murine Fshb (Figs. 5A and 5D), human FSHB (Fig. 5B and 5E), and Foxl2 (Fig. 5C and 5F) mRNA levels were significantly reduced in deletion mutant compared to control mice. Lhb, Cga, and Gnrhr mRNA levels were significantly upregulated in pituitaries of deletion mutant females compared to controls (Fig. 5C). Lhb, Cga, and Gnrhr mRNA levels did not differ between genotypes in males, although there were nonsignificant trends for reduced Cga and increased Gnrhr expression in deletion mutant mice (Fig. 5F). Smad4 expression did not differ between genotypes in either sex, but it should be noted that Smad4 is expressed in all pituitary lineages (26,27) and the knockout was specific to gonadotropes (5%-10% of all pituitary cells; Fig. 5C and 5F). Collectively, the data show that mice with deletions of Foxl2 and Smad4 in their gonadotropes are severely compromised in their ability to express murine Fshb or human FSHB in vivo.
Figure 5.
Human FSHB expression is significantly reduced in pituitaries of Foxl2/Smad4 conditional knockout mice. Pituitary murine Fshb (A and D); human FSHB (B and E); and Gnrhr, Lhb, Cga, Smad4, and Foxl2 (C and F) expression in 7- to 8-week-old female and male control and deletion mutant mice. mRNA expression was assessed for the indicated genes by RT-qPCR; n = 4/group. *P < 0.05 and **P < 0.01; n.s., not statistically different vs. control mice by unpaired t-test.
Discussion
Although previous studies implicated activins or related TGFβ ligands in the regulation of human FSH synthesis (18,19,22), the underlying mechanisms were not delineated. This stems largely from 2 important factors: (i) there are no human gonadotrope cell lines available, and (ii) human FSHB promoter-reporters are not induced by activins in the murine gonadotrope-like LβT2 cell line. The latter may derive from the absence of important regulatory sequences that lie outside the proximal promoter used in previous studies. Indeed, human FSHB expression in pituitaries of transgenic mice requires both the proximal promoter and sequences flanking the terminal third exon (28). We therefore cloned 3ʹ flanking sequence downstream of luciferase in a human FSHB promoter-reporter, but this too failed to confer activin responsiveness (data not shown). To overcome these previous roadblocks, here, we took advantage of mice harboring a 10 kb human FSHB transgene, which express FSHB in murine gonadotropes in an activin type IIA receptor-dependent manner in vivo (21,22). The data indicate that human FSHB, like murine Fshb, expression is dependent on activin-like signaling, FOXL2, and SMAD4.
It is presently unclear whether FOXL2 and SMAD4 regulate murine Fshb and human FSHB expression through conserved and/or distinct mechanisms. In mice, a proximal composite SMAD (underlined)/FOXL2 (italicized) cis-element, 5′-GTCTGTCTAAACA-3′, is critical for activin, FOXL2, and SMAD regulation of Fshb promoter activity (29,30). This composite element is not perfectly conserved in humans, as the G in the first position of the SMAD binding site in mice is A (bolded) in humans, 5′-GTCTATCTAAACA-3′. There is a perfect SMAD binding element (GTCT) immediately 5′ in both species, but the position of this site relative to the conserved FOXL2 binding element may account for the reduced activin responsiveness of human FSHB promoter-reporters. This suggests that FOXL2 and SMAD4 may act at additional or alternative sites to regulate the human FSHB gene. Sequence analysis of the 3′flanking sequence of the human FSHB transgene reveals one candidate FOXL2 binding site at + 4264 bp from the transcription start site (TSS) and 8 candidate SMAD cis-elements at +4426, +4456, +4502, +4592, +5072, +5350, +5378, +5693, and +5771 from TSS. Chromatin immunoprecipitation-PCR or chromatin immunoprecipitation-sequencing for FOXL2 and SMAD4 in hFSHB transgenic mice could prove valuable in determining whether these or other elements bind these transcription factors (28-30). We previously reported that FOXL2 regulates the porcine Fshb promoter through a unique FOXL2/SMAD composite cis-element (31), so it is not unprecedented for FOXL2 and SMADs to regulate Fshb/FSHB in a species-specific fashion. Nevertheless, our data support a common and essential role for these proteins in FSH production in mice, pigs, humans, and likely other mammalian species, even if they mediate their actions in a species-specific fashion.
In our analyses, we focused on the effects of FOXL2 ablation alone or in combination with SMAD4. We did not examine the effects of SMAD4 deletion by itself. Although this analysis could prove useful in future experiments, it is notable that FOXL2 and FOXL2/SMAD4 loss produced similar reductions in murine Fshb and human FSHB expression. That is, FOXL2 ablation led to a partial reduction in mRNA levels, whereas both transcripts were essentially absent in the pituitaries of FOXL2/SMAD4 double knockouts, both in vitro and in vivo. We previously reported that gonadotrope-specific SMAD4 ablation causes a significant, but incomplete reduction of Fshb expression in mice (11). The residual Fshb in these animals is explained by partial compensation by FOXL2 (as replicated here) and/or by SMAD3 (7,10). Therefore, it seems highly likely that SMAD4 ablation alone would similarly lead to reduced but not absent human FSHB expression. Again, this can be addressed in future studies, perhaps in combination with the interrogation of SMAD3′s role in human FSHB synthesis.
While we observed similarities in the regulation of murine Fshb and human FSHB mRNA expression in these studies, there were also notable differences. First, exogenous activins more potently stimulated increases in murine than human transcript levels. Second, the type I receptor inhibitor (SB431542) and follistatin appeared to more potently suppress murine Fshb relative to human FSHB. Ablation of the activin type IIA receptor is associated with the almost complete loss of human FSH production in pituitaries of transgenic mice (22). Therefore, it does not appear that the human gene is less dependent on activin-like signaling. Rather, in the context of the in vitro experiments reported here, the relatively weaker effects of activins and the inhibitors may derive from the time points examined and the greater stability of human FSHB relative to murine Fshb mRNA. Loss of endogenous activin-like signaling (SB431542, FST288) or a block in transcription (actinomycin D) leads to the almost immediate loss of murine Fshb. This indicates that Fshb expression in mice depends on active and persistent signaling by activins or related TGFβ ligands. As human FSHB mRNA may be more stable, the effects of antagonizing the activin-like signaling pathway takes a longer time to manifest. In addition, the lower induction of human FSHB by activins may reflect species differences in the underlying mechanisms of FOXL2/SMAD action, as previously discussed.
Mechanisms underlying differences in murine Fshb and human FSHB mRNA stability have not yet been delineated. However, differential regulation of their respective 3ʹ UTRs by microRNAs (miRNAs) may play a role. Three miRNAs were recently implicated in turnover of rat Fshb mRNA (32-34). Moreover, gonadotropin synthesis is dysregulated in mice lacking DICER, an endoribonuclease involved in miRNA processing, in gonadotropes (35). TargetScan (36) analysis identified 22 miRNAs that potentially target both the Fshb/FSHB 3′UTRs in mice and humans. An additional 300 to 500 miRNAs could differentially regulate the murine Fshb and human FSHB subunits, respectively (data not shown). It is also possible that the apparent mRNA stability differences could derive from copy number differences between the endogenous murine Fshb and transgenic human FSHB. Nevertheless, the effects of actinomycin D suggest, although do not prove, that there may be species differences in mRNA stability. These differences may be physiologically relevant, as FSH is rapidly produced and lost on the morning of estrus at the time of the secondary FSH surge in rodents (timeframe of hours), while increases and decreases in FSH are more gradual during the follicular phase of the human menstrual cycle (timeframe of days) (37-40).
In conclusion, both murine Fshb and human FSHB mRNA levels are regulated by activins (or related TGFβ ligands), and both are dependent on FOXL2 and SMAD4 for their expression. Based on sequence differences between the promoters of the 2 species, these transcription factors may regulate the genes in the 2 species in distinct manners. Human FSHB mRNA appears to be more stable than murine Fshb mRNA, which may contribute to species differences in the dynamics of FSH secretion in females across their reproductive cycles. Finally, it must be noted that the results here were obtained in the context of murine gonadotropes. However, it seems likely that the mechanisms of human FSHB regulation we discerned will be translatable to actual human gonadotropes. As noted, FSH production in humans depends on activin-like signaling (18,19). Moreover, the human FSHB transgene is inhibited by androgens in these mice whereas murine Fshb mRNA expression is enhanced (41,42). Therefore, species-specific regulatory mechanisms appear to be intact in murine gonadotropes. Ultimately, however, development of a human gonadotrope cell line will be required to fully understand regulation of the human FSHB in a homologous cell context.
Acknowledgments
Financial Summary: L.O. was supported by a Postdoctoral Fellowship in Reproductive Health from Ferring Pharmaceuticals, and G.S. was supported by a Doctoral Research Award from the Canadian Institutes of Health Research (CIHR-152308). This work was supported by Canadian Institute of Health Research (CIHR) Grants MOP-133394 and PJT-162343, and Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant 2015–05178 (to D.J.B.), and National Institutes of Health (NIH) grants AG029531, AG056046, and The Makowski Endowment (to T.R.K.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201-204. [DOI] [PubMed] [Google Scholar]
- 2. Gharib SD, Roy A, Wierman ME, Chin WW. Isolation and characterization of the gene encoding the beta-subunit of rat follicle-stimulating hormone. Dna. 1989;8(5):339-349. [DOI] [PubMed] [Google Scholar]
- 3. Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15(3):378-389. [DOI] [PubMed] [Google Scholar]
- 4. Ying SY. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988;9(2):267-293. [DOI] [PubMed] [Google Scholar]
- 5. Persson U, Izumi H, Souchelnytskyi S, et al. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434(1-2):83-87. [DOI] [PubMed] [Google Scholar]
- 6. Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol. 2006;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6): 2301-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685-700. [DOI] [PubMed] [Google Scholar]
- 9. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356-360. [DOI] [PubMed] [Google Scholar]
- 10. Tran S, Zhou X, Lafleur C, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. Faseb J. 2014;28(8):3396-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Schang G, Wang Y, et al. Conditional deletion of FOXL2 and SMAD4 in gonadotropes of adult mice causes isolated FSH deficiency. Endocrinology. 2018;159(7):2641-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone beta subunit transcription by activin A. J Mol Endocrinol. 2006;36(1):201-220. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Fortin J, Lamba P, et al. Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone beta-promoter activity. Endocrinology. 2008;149(11):5577-5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghochani Y, Saini JK, Mellon PL, Thackray VG. FOXL2 is involved in the synergy between activin and progestins on the follicle-stimulating hormone β-subunit promoter. Endocrinology. 2012;153(4):2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ooi GT, Tawadros N, Escalona RM. Pituitary cell lines and their endocrine applications. Mol Cell Endocrinol. 2004;228(1-2):1-21. [DOI] [PubMed] [Google Scholar]
- 17. Bernard DJ, Fortin J, Wang Y, Lamba P. Mechanisms of FSH synthesis: what we know, what we don’t, and why you should care. Fertil Steril. 2010;93(8):2465-2485. [DOI] [PubMed] [Google Scholar]
- 18. Ruckle J, Jacobs M, Kramer W, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744-752. [DOI] [PubMed] [Google Scholar]
- 19. Sherman ML, Borgstein NG, Mook L, et al. Multiple-dose, safety, pharmacokinetic, and pharmacodynamic study of sotatercept (ActRIIA-IgG1), a novel erythropoietic agent, in healthy postmenopausal women. J Clin Pharmacol. 2013;53(11):1121-1130. [DOI] [PubMed] [Google Scholar]
- 20. Kumar TR, Fairchild-Huntress V, Low MJ. Gonadotrope-specific expression of the human follicle-stimulating hormone beta-subunit gene in pituitaries of transgenic mice. Mol Endocrinol. 1992;6(1):81-90. [DOI] [PubMed] [Google Scholar]
- 21. Kumar TR, Low MJ, Matzuk MM. Genetic rescue of follicle-stimulating hormone beta-deficient mice. Endocrinology. 1998;139(7):3289-3295. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Larson M, Jablonka-Shariff A, et al. Redirecting intracellular trafficking and the secretion pattern of FSH dramatically enhances ovarian function in mice. Proc Natl Acad Sci U S A. 2014;111(15):5735-5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakurai T, Kamiyoshi A, Watanabe S, Sato M, Shindo T. Rapid zygosity determination in mice by SYBR Green real-time genomic PCR of a crude DNA solution. Transgenic Res. 2008;17(1):149-155. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Fortin J, Ongaro L, et al. Betaglycan (TGFBR3) functions as an inhibin A, but not inhibin B, coreceptor in pituitary gonadotrope cells in mice. Endocrinology. 2018;159(12):4077-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho CC, Bernard DJ. Bone morphogenetic protein 2 acts via inhibitor of DNA binding proteins to synergistically regulate follicle-stimulating hormone beta transcription with activin A. Endocrinology. 2010;151(7):3445-3453. [DOI] [PubMed] [Google Scholar]
- 26. Cheung LYM, George AS, McGee SR, et al. Single-Cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fletcher PA, Smiljanic K, Maso Prévide R, et al. Cell Type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne). 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar TR, Schuff KG, Nusser KD, Low MJ. Gonadotroph-specific expression of the human follicle stimulating hormone beta gene in transgenic mice. Mol Cell Endocrinol. 2006;247(1-2): 103-115. [DOI] [PubMed] [Google Scholar]
- 29. Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol. 2011;25(7):1170-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corpuz PS, Lindaman LL, Mellon PL, Coss D. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone beta-subunit genes. Mol Endocrinol. 2010;24(5):1037-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamba P, Wang Y, Tran S, et al. Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2. Endocrinology. 2010;151(11):5456-5467. [DOI] [PubMed] [Google Scholar]
- 32. Han DX, Sun XL, Wang CJ, et al. Differentially expressed lncRNA-m433s1 regulates FSH secretion by functioning as a miRNA sponge in male rat anterior pituitary cells. Biol Reprod. 2019;101(2):416-425. [DOI] [PubMed] [Google Scholar]
- 33. Han DX, Sun XL, Xu MQ, et al. Roles of differential expression of microRNA-21-3p and microRNA-433 in FSH regulation in rat anterior pituitary cells. Oncotarget. 2017;8(22):36553-36565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han DX, Xiao Y, Wang CJ, et al. Regulation of FSH expression by differentially expressed miR-186-5p in rat anterior adenohypophyseal cells. Plos One. 2018;13(3):e0194300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H, Graham I, Hastings R, et al. Gonadotrope-specific deletion of Dicer results in severely suppressed gonadotropins and fertility defects. J Biol Chem. 2015;290(5):2699-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riffo-Campos AL, Riquelme I, Brebi-Mieville P. Tools for sequence-based miRNA target prediction: what to choose? Int J Mol Sci. 2016;17(12):E1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE. Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab. 2003;88(4):1766-1771. [DOI] [PubMed] [Google Scholar]
- 38. Welt CK, Martin KA, Taylor AE, et al. Frequency modulation of follicle-stimulating hormone (FSH) during the luteal-follicular transition: evidence for FSH control of inhibin B in normal women. J Clin Endocrinol Metab. 1997;82(8):2645-2652. [DOI] [PubMed] [Google Scholar]
- 39. Kovacic N, Parlow AF. Alterations in serum FSH-LH ratios in relation to the estrous cycle, pseudopregnancy, and gonadectomy in the mouse. Endocrinology. 1972;91(4):910-915. [DOI] [PubMed] [Google Scholar]
- 40. Murr SM, Geschwind II, Bradford GE. Plasma LH and FSH during different oestrous cycle conditions in mice. J Reprod Fertil. 1973;32(2):221-230. [DOI] [PubMed] [Google Scholar]
- 41. Kumar TR, Low MJ. Gonadal steroid hormone regulation of human and mouse follicle stimulating hormone beta-subunit gene expression in vivo. Mol Endocrinol. 1993;7(7):898-906. [DOI] [PubMed] [Google Scholar]
- 42. McDonald R, Sadler C, Kumar TR. Gain-of-function genetic models to study FSH action. Front Endocrinol (Lausanne). 2019;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]