Abstract
Immune checkpoint inhibitors (ICI) have rapidly altered the treatment landscape for multiple tumor types, providing unprecedented survival in some patients. Despite the characteristic durability of response to ICI, unfortunately many patients with initial response will later develop acquired resistance. The current understanding of mechanisms of acquired resistance to ICIs is remarkably limited, perhaps restraining effective development of next-generation immunotherapies. Here, we examine the barriers to progress and emerging clinical reports interrogating acquired resistance with the goal to facilitate efforts to overcome acquired resistance to ICIs in the future.
Keywords: Acquired resistance, ICIs, Immunotherapy
Introduction
The advent of immune checkpoint inhibitors (ICIs) has rapidly transformed the treatment paradigm for multiple cancer types. Over the last decade, starting from the initial approval of CTLA-4 inhibitors in metastatic melanoma in 2011, PD-(L)1 inhibitors are now a routine part of treatment for more than 20 different indications. The treatment of patients with lung cancer is just one stark example of this paradigm shift: beginning from the first phase I study of PD-1 blockade where just one of six patients with NSCLC experienced response in a single target lesion (but not overall achieving partial response) to today, where nearly all patients diagnosed with metastatic lung cancer receive PD-(L)1 blockade as a component of treatment (Brahmer et al., 2010; Gandhi et al., 2018; Reck et al., 2016).
This remarkable shift has been driven in large part by unprecedented durability that is characteristic of response to ICIs. Responses can last years, if not indefinitely, even without continuous treatment (Antonia et al., 2019; Bernard-Tessier et al., 2018; Fradet et al., 2019; Garon et al., 2019; Gettinger et al., 2018a; Hamid et al., 2019; Larkin et al., 2019; Motzer et al., 2015; Schadendorf et al., 2017; Topalian et al., 2019). However, sometimes lost in the understandable enthusiasm related to the clinical impact of ICIs is the reality that the vast majority of patients do not experience benefit from ICIs. The response rate to single agent PD-1 blockade in unselected patients ranges ~40%−70 in some diseases (e.g. melanoma, Merkel cell, Hodgkin’s lymphoma, MSI high tumors) (Armand et al., 2018; Chen et al., 2017; Hamid et al., 2019; Larkin et al., 2019; Le et al., 2017; Nghiem et al., 2019) while the response rates in most other approved diseases is limited to 10–25% (Antonia et al., 2019; Balar et al., 2018; Fradet et al., 2019; Mok et al., 2019; Motzer et al., 2015). Furthermore, even among patients who initially respond to ICIs, disease progression can eventually develop. In short, only a minority of patients achieve the long-term, durable response that is the transcendent feature of ICIs, while resistance is the unfortunate experience for most patients.
Resistance to ICIs may be initially classified into two broad categories: 1) primary resistance, generally referring to patients who do not respond at all and instead progress, quickly or eventually, with ICIs, and 2) acquired resistance, which refers to patients who have a period of initial response to therapy followed ultimately by clinical and/or radiologic progression of disease. To confront the challenge of primary resistance, substantial effort has been spent on combination strategies, often with empiric orthogonal therapies, to broaden the responding population. For example, ICIs have been combined with chemotherapy in lung, breast and gastric cancer, as well as multi-target TKIs in RCC and FGFR inhibitors in bladder cancer (Gandhi et al., 2018; Motzer et al., 2019a; Schmid et al., 2018; Tabernero et al., 2019). Further, there has been an extensive search for predictive biomarkers for initial ICI response. PD-L1 expression, tumor mutational burden, tumor infiltrating lymphocytes (TILs) or related gene expression signatures have been explored as potential predictors and various other markers are currently under investigation (Chen et al., 2016; Cristescu et al., 2018; Garon et al., 2015; Hugo et al., 2016; Johnson et al., 2016; Liu et al., 2019; McGranahan et al., 2016; Rizvi et al., 2018; Rizvi et al., 2015; Rodig et al., 2018; Topalian et al., 2012; Tumeh et al., 2014; Van Allen et al., 2015). Conversely, there have been no approved therapeutic breakthroughs yet for circumventing or reversing acquired resistance and there have been remarkably few published investigations on the characteristics or mechanisms of acquired resistance to ICIs.
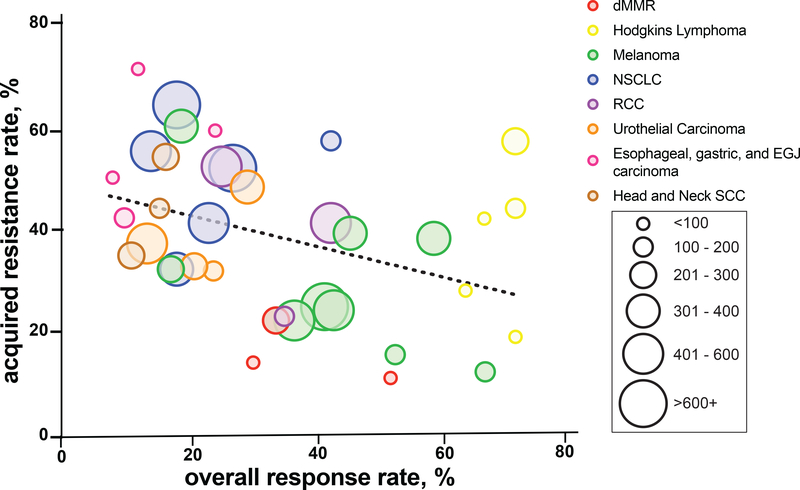
Unlike primary resistance, the rates of acquired resistance have not been routinely reported and thus are not clearly characterized across tumors types. We have attempted to infer the rates of acquired resistance based upon available duration of response data among different tumor types (Antonia et al., 2019; Armand et al., 2018; Balar et al., 2018; Burtness et al., 2019; Chen et al., 2019a; Cohen et al., 2019; Fehrenbacher et al., 2018; Fradet et al., 2019; Garon et al., 2019; Hamid et al., 2019; Herbst et al., 2016; Janjigian et al., 2018; Larkin et al., 2019; Le et al., 2017; Marabelle et al., 2020; Mok et al., 2019; Motzer et al., 2015; Motzer et al., 2019b; O’Donnell et al., 2019; Overman et al., 2017; Pires da Silva et al., 2020; Powles et al., 2018; Robert et al., 2019; Shah et al., 2019; Tykodi et al., 2019; Wang et al., 2017; Younes et al., 2016; Zandberg et al., 2019). From this data, it appears that rates of acquired resistance vary across multiple disease types (Table 1). For example, an updated report patients with melanoma treated with nivolumab demonstrated 39% of responders had progressed at five years follow up (Larkin et al., 2019). By contrast, in a recent pooled analysis of patients with NSCLC treated with nivolumab, up to 65% of responders had progressed at 4 years follow-up (Antonia et al., 2019). More generally across tumor types, there appears to be an inverse relationship between the overall response rate to PD-1 blockade and the frequency of acquired resistance among responders (Figure 1). This observation suggests disease-specific features of acquired resistance and contrast with the current typical understanding that, despite variable response rates across tumor types, if response occurs it is similarly durable across tumor types. With increasing experience with immunotherapy and longer-term follow-up, it is imperative that future studies report acquired resistance rates among responders to better quantify acquired resistance. Disease-specific or response-rate-related differences in the frequency of acquired resistance may reveal important biological insight into the distinct determinants of initial response, long-term durability, and acquired resistance.
Table 1.
Rate of acquired resistance and overall response by tumor type and study
| Disease Type | Study Name | Drug(s) | Line of therapy | ORR | Estimated acquired resistance rate | Endpoint used to estimate acquired resistance | Time landmark (months) |
|---|---|---|---|---|---|---|---|
| dMMR | Keynote 158 | Pembrolizumab | 2+ | 34 (80/233) | 22 | DOR KM curve | 24 |
| Le et al. | Pembrolizumab | 2+ | 53(46/86) | 11 | Rate | Last follow-up (med 13) | |
| Checkmate 142 | Nivolumab | 2+ | 31(23/74) | 14 | DOR KM curve | 12 | |
| Esophageal carcinoma | Keynote 180 | Pembrolizumab | 3+ | 10(12/121) | 42 | Rate | Last follow-up (med 6) |
| Esophageal, gastric, and EGJ carcinoma | Checkmate 032 | Nivolumab 1 + ipilimumab 3 | 2+ | 24 (12/49) | 59 | Rate | Last follow-up (med 24) |
| Checkmate 032 | Nivolumab 3 + ipilimumab 1 | 2+ | 8 (4/52) | 50 | Rate | Last follow-up (med 22) | |
| Checkmate 032 | Nivolumab | 2+ | 12 (7/59) | 71 | Rate | Last follow-up (med 28) | |
| Head and Neck SCC | Keynote 040 | Pembrolizumab | 2+ | 11 (26/247) | 35 | Rate | Last follow-up (med 8) |
| Keynote 048 | Pembrolizumab | 1 | 17 (51/301) | 54 | DOR KM Curve | 24 | |
| HAWK | Durvalumab | 2+ | 16(18/111) | 44 | Rate | Last follow-up (med 6) | |
| Hodgkin Lymphoma | Keynote 087 | Pembrolizumab | 2+* | 72 (151/210) | 57 | DOR KM curve | 24 |
| Checkmate 205 | Nivolumab | 2+* | 65 (41/63) | 28 | DOR KM curve | 12 | |
| Checkmate 205 | Nivolumab | 3+* | 68 (54/80) | 42 | DOR KM curve | 12 | |
| Checkmate 205 | Nivolumab | 3+* | 73 (73/100) | 44 | DOR KM curve | 12 | |
| Younes et al. | Nivolumab | 3+* | 73 (58/80) | 19 | Rate | Last follow-up (med 9) | |
| Melanoma | Pires da Silva et al | PD-(L)1 inhibitor + ipilimumab | 1 | 66 (93/140) | 12 | Rate | Last follow-up (med 16) |
| Checkmate 067 | Nivolumab + ipilimumab | 1 | 58 (183/314) | 38 | Rate | Last follow-up (med 54.6) | |
| Keynote 001 | Pembrolizumab | 1 | 52 (78/151) | 18 | Rate | Last follow-up (med 55) | |
| Checkmate 067 | Nivolumab | 1 | 45 (141/316) | 39 | Rate | Last follow-up (med 36.0) | |
| Keynote 001 | Pembrolizumab | 1+ | 41 (267/655) | 27 | Rate | Last follow-up (med 55) | |
| Wang et al. | PD-(L)1 inhibitor | 1+ | 34(166/488) | 22 | Rate | Last follow-up (med 33) | |
| Checkmate 067 | Ipilimumab | 1 | 19 (60/315) | 60 | Rate | Last follow-up (med 18.6) | |
| Keynote 006 | Pembrolizumab | 1+ | 42 (235/556) | 24 | DOR KM curve | 18 | |
| Keynote 006 | Ipilimumab | 1+ | 17 (46/278) | 32 | DOR KM curve | 18 | |
| Keynote 001 | Pembrolizumab | 1 | 42 (42/101) | 57 | Rate | Last follow-up (med 61) | |
| Keynote 042 | pembrolizumab | 1 | 27(174/637) | 52 | DOR KM curve | 24 | |
| NSCLC | Keynote 001 | Pembrolizumab | 2+ | 23(103/449) | 41 | Rate | Last follow-up (med 61) |
| CheckMate 017, 057, 063, and 003 | Nivolumab | 2+ | 18(122/664) | 64 | DOR KM curve | 48 | |
| Keynote 010 | Pembrolizumab2 | 2+ | 18 (62/344) | 32 | DOR KM curve | 12 | |
| OAK | Atezolizumab | 2+ | 14 (58/425) | 55 | Rate | Last follow-up (med 28) | |
| RCC | Checkmate 214 | Nivolumab + Ipilimumab | 1 | 42 (177/425) | 41 | Rate | Last follow-up (med 32) |
| Checkmate 025 | Nivolumab | 2+ | 25 (103/410) | 52 | Rate | Last follow-up (min 14) | |
| Keynote-427 | Pembrolizumab | 1 | 36 (40/110) | 23 | DOR KM curve | 6 | |
| Keynote-052 | Pembrolizumab | 1 | 29 (107/370) | 48 | DOR KM curve | 24 | |
| Urothelial Carcinoma | IMvigor210 | Atezolizumab | 1** | 24(28/119) | 32 | Rate | Last follow-up (med 29) |
| Keynote-045 | Pembrolizumab | 2+ | 21 (57/270) | 32 | DOR KM curve | 12 | |
| IMvigor211 | Atezolizumab | 2+ | 13 (62/467) | 37 | Rate | Last follow-up (med 17) | |
Abbreviations: ORR = objective response rate. dMMR = deficient mismatch repair tumors. EGJ = esophagogastric junction. SCC = squamous cell carcinoma. NSCLC = non-small cell lung cancer. RCC = renal cell carcinoma Med = median. Min = minimum. DOR = duration of response. KM = kaplan-meier. DOR KM curve refers to estimates inferred from DOR KM curve. Rate refers ratio of responders who progressed divided by all responders (CR/PR) detailed in report.
Prior therapies are autologous stem cell transplantation and/or brentuximab vedotin.
Acquired resistance data only available for first-line cohort.
Figure 1. Rate of acquired resistance and overall response by tumor type.
Different tumor types are represented by colored circle and number of patients is represented by size of the circle.
It is instructive to contrast the paucity of data on mechanisms of acquired resistance to immunotherapy with the growing knowledge about resistance to targeted therapy in oncogene driven cancers. For example, in EGFR-mutant lung cancer, an archetype for molecularly targeted therapy, knowledge of resistance to first-generation tyrosine kinase inhibitors (TKIs) identified a secondary EGFR point mutation which, in turn, led to the development of the third-generation EGFR-T790M targeted TKI osimertinib (Mok et al., 2017; Pao et al., 2005). Furthering the cycle, new investigations into the distinct mechanisms of acquired resistance to osimertinib have since yielded even further therapeutic successes (Piotrowska et al., 2018; Ramalingam et al., 2018; Schoenfeld et al., 2020).
Conversely in ICIs, despite the profound number of patients now treated with PD-(L)1 blockade, insight into the underpinnings of and rational therapeutic strategies to treat acquired resistance to ICI remain unrealized. Before reviewing what is known about acquired resistance to ICI, we sought to explore the barriers to progress thus far and propose potential solutions.
Barriers to investigation of acquired resistance mechanisms to immunotherapy
There are challenges to studying mechanisms of acquired resistance to immunotherapy that should be considered when reviewing the currently available data. Primary challenges include (1) Terminology: the lack of uniform terminology used to define, classify, and apply acquired resistance, (2) Tissue: difficulty acquiring optimal tumor samples for analyses, and (3) Tools: the absence of routine, effective tools to comprehensively interrogate and discovery mechanisms of immune resistance in the tumor, host, and/or microenvironment (Table 2). Deeper understanding of these issues is essential to interpreting prior reports and designing studies that can successfully and comprehensively identify mechanisms of acquired immune resistance in the future.
Table 2.
Barriers to insight in acquired resistance
| 1. Terminology: |
| • No consensus definition of acquired resistance |
| • Need to differentiate definitions and applications of patient-level and lesion-level acquired resistance |
| 2. Tissue: |
| • Infrequency of response and idiosyncratic acquired resistance stymies effective/efficient biospecimen collection |
| • Need to articulate and demonstrate clinical utility to tissue sampling at acquired resistance |
| 3. Tools: |
| • Analyses using bulk DNA/RNA next-generation sequencing have been helpful in molecularly targeted therapies, but more limited success for immune checkpoint inhibitors |
| • New tools are needed to interrogate the complexity of anti-tumor immunity |
Terminology
First, in order to analyze and interpret the results of multiple studies, a clear and consistent framework for defining acquired resistance should be established. For example, in EGFR-mutant lung cancer, relatively simple but specific guidelines were developed to define patients with acquired resistance and distinguish this specific disease state from other potentially confounding clinical scenarios. Among other considerations, the EGFR criteria stipulated patients should have 1) documented initial objective response (partial or complete response by RECIST or WHO) to therapy OR significant and durable (> 6 months) clinical benefit (stable disease defined by RECIST or WHO) and 2) systemic progression of disease while on continuous treatment with an EGFR-TKI within the last 30 days (Jackman et al., 2010). These criteria may seem so simple as to be self-evident, but have indeed facilitated consistency in evaluating new treatments for resistance and identifying mechanisms. And there are nuanced considerations when applying these criteria in the context of PD-(L)1 resistance.
No such uniform definition has been established for acquired resistance to immunotherapy. The Society for Immunotherapy of Cancer (SITC) has established an anti-PD-(L)1 taskforce and convened an anti-PD-(L)1 resistance workshop from which further discussion is forthcoming. We highlight two (of several) important features to be considered toward the goal of developing such a consensus framework:
1). Depth of initial response
Conceptually, a patient with acquired resistance would have first responded, before later developing resistance. What features constitute “a responder” still require consideration. It is likely uncontroversial to include patients with a RECIST-defined partial or complete response to ICIs, but it is debatable whether to include patients with stable disease of some duration in this definition. As a whole, the stable disease subgroup appears to derive improved survival with ICIs, although to a lesser degree than PR/CR (Antonia et al., 2019; Garon et al., 2019; Gettinger et al., 2018a; Larkin et al., 2019). More fundamentally, the stable disease category is likely a heterogenous mix of some patients with minor responses intermixed with indolent (but non-responsive) and progressive disease. Currently, there are not sufficiently reliable tools to distinguish among patients with stable disease who are responders (despite radiologically stable disease) versus who are non-responders to PD-1 treatment. Therefore, inclusion of stable disease may include this subset of responders whereas it also could also increase heterogeneity and obscure analysis of acquired resistance with patients who are non-responders. With utility far beyond the definition of acquired resistance, tools are needed to better resolve the heterogeneity of stable disease and adjudicate whom within this group should be considered a responder.
Additional nuance of how to interpret depth of response is also needed when considering patients treated with combinations with other active, orthogonal therapy such as chemotherapy. In such combinations, it is presently not feasible to differentiate whether initial response is attributable to either or both therapies, and therefore the nature of the associated resistance is uncertain.
One last consideration includes the importance of differentiating the application of the acquired resistance definition to patient-level response versus the lesion level response. The depth of response and occurrence of acquired resistance of a patient, using RECIST-defined best overall response or progression for example, may be most relevant for considering clinical trial eligibility and descriptive clinical reports. Meanwhile, the degree of response and acquired resistance at a specific metastatic site may be distinct and important for translational analysis of the underlying biology of acquired resistance at that specific site.
2). Drug exposure
The extent to which continued drug exposure should be required at the time of acquired resistance remains an open question. Unlike molecular therapy where patients will likely progress if therapy is discontinued, prior studies have demonstrated that patients can have ongoing responses long after cessation of ICI therapy. These include patients who have completed 1 – 2 year of therapy, along with patients who have stopped treatment early due to toxicity, even in some cases with minimal drug exposure (Garon et al., 2019; Gettinger et al., 2018a; Larkin et al., 2019; Schadendorf et al., 2017). Conversely, other recent analyses such as KEYNOTE-010 in NSCLC have also shown that 32% of responders had progressive disease after completion of 2 years of treatment (Herbst et al., 2020). And in a study (CheckMate-153) of patients with NSCLC who reached one year of treatment and then were randomized either to discontinue or continue treatment, progression-free survival thereafter was improved in those who continued therapy (Spigel et al., 2017). In general, prior reports on re-challenging patients with ICIs are relatively limited, show varying degrees of success with this strategy, and the patient-specific or tumor-specific features that predict re-response are not known (Garon et al., 2019; Gettinger et al., 2018a; Hamid et al., 2019; Herbst et al., 2020; Pires da Silva et al., 2020; Robert et al., 2019; Wang et al., 2017; Warner et al., 2019). Overall, it is unclear when the emergence of resistance to ICIs is attributable to cessation of therapy, but it appears to be a relative minority of patients and success of re-treatment is far from guaranteed.
Tissue
Importantly, in comparison to molecularly targeted therapy, response to ICI is less common and the occurrence of acquired resistance to ICIs is more idiosyncratic. In EGFR-mutant lung cancer, for example, ~80% patients treated with osimertinib will initially respond and nearly all responders will eventually develop acquired resistance (Soria et al., 2018). In this scenario, it is reasonably efficient to routinely collect pre-treatment tissue with the expectation that the vast majority of patients will both initially respond and later develop resistance, from which additional post-treatment tissue can then be examined. With ICIs, however, a much smaller proportion of patients are initially sensitive to treatment and, among those patients who do respond, the development of acquired resistance is variable. Therefore, in studies interested in acquired resistance specifically, it is inefficient to collect tissue from all patients at baseline knowing a relatively small fraction will respond and even smaller fraction will later develop resistance. Overall, the more unpredictable nature of acquired resistance to ICIs makes devising and executing successful correlative studies difficult.
The oligoprogressive pattern, which may be a common pattern of acquired resistance with ICIs (Gettinger et al., 2018b; Pires da Silva et al., 2020; Wang et al., 2017), also complicates the potentially ideal scenario of comparing site-matched tissue sample (as the acquired resistance site is unpredictable at baseline). This consideration also contrasts with molecular therapy, where resistance tends to (at least eventually) be systemic and therefore often feasible to interrogate the underlying biology of resistance from biopsy of any tumor site and/or blood (Barron et al., 2018; Ramalingam et al., 2018; Yang et al., 2013). Further, the role of tumor biopsies at the time of progression on immunotherapy may be questioned because of uncertain clinical actionability. In our experience, biopsies can be an essential clinical tool to guide management. Particularly in settings of indeterminate radiologic changes, biopsies can help distinguish inflammation or scar tissue or secondary malignancy or, indeed, acquired resistance. Thus, coordinated efforts to identify patients who have developed acquired resistance to ICIs will be critical. Multicenter and industry-academia cooperation is a potential remedy to small sample sizes and can help overcome the routine challenge of missing pre-treatment and/or post-treatment sampling.
Tools
Finally, the few number published reports on acquired resistance to ICIs may also reflect the few obvious discoveries using common molecular tools for examining the tumor and its microenvironment. By contrast, in targeted therapy analyses, the use of bulk next-generation sequencing of DNA and RNA has been successful to identify primary drivers of acquired resistance. Yet, studies of resistance to ICIs that similarly focused on bulk DNA and RNA sequencing of tumor samples have uncovered a limited number of acquired genomic alterations (discussed in more detail below, largely falling in antigen presentation and interferon-γ signaling pathways) (Anagnostou et al., 2017; Gettinger et al., 2017; Zaretsky et al., 2016). Instead, resistance to ICI may be vastly more nuanced, dynamic, and complex, involving at least three interactions (host, tumor, tumor microenvironment) and two compartments (intratumoral and systemic), necessitating a multifaceted approach and higher resolution investigation of the (epi)genetics, functional state, and geographic interconnections of both tumor and immune cells across broad space. Multiple factors including the evolution of neoantigens and neoantigen presentation, innate and adaptive immune response, epigenomic modifications, and tumoral heterogeneity which have all been associated with immune checkpoint inhibitor efficacy, are not well captured by traditional bulk sequencing from tumor samples and warrant further attention. Further, single cell imaging and/or sequencing approaches may be required to evaluate clonal evolution of tumors and the tumor immune microenvironment in response to ICIs.
With this context in mind, we here present a review of the clinical and pre-clinical studies on acquired resistance to ICIs to date.
Clinical data on acquired resistance to ICIs
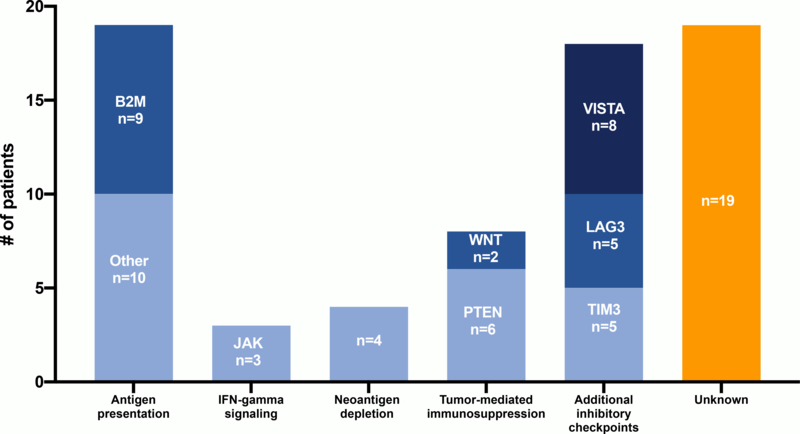
By any measure, the number of clinical cases of acquired resistance to ICI reported in the literature is remarkably small. We identified 13 clinical reports comprising 58 total patients with acquired resistance to ICIs across all tumor types (Abdallah et al., 2018; Anagnostou et al., 2017; Ascierto et al., 2017; Gettinger et al., 2017; Hu et al., 2018; Iams et al., 2019; Le et al., 2017; Sade-Feldman et al., 2017; Trujillo et al., 2019; Zaretsky et al., 2016) (Figure 2, Supplemental Table 1). Importantly, these reports use differing inclusion parameters for acquired resistance and represent a heterogenous population. While some reports require initial partial or complete response to ICI, others include cases of initial stable disease or “mixed responses” (Gettinger et al., 2017; Iams et al., 2019; Kakavand et al., 2017). Detailed analysis of the degree of response and acquired resistance of individual lesions from which tissue samples are derived may also be relevant when considering translational analyses.
Figure 2. Summary of reported mechanisms of acquired resistance to immune checkpoint inhibitors.
The number of clinical cases by reported mechanism of acquired resistance mechanism are shown. Some cases report multiple acquired resistance mechanisms.
Although many cases have uncertain or unknown mechanisms, those with speculated mechanisms of resistance roughly fall into a few categories: 1) defects in antigen presentation, 2) interferon-γ (IFN-γ) signaling 3) neoantigen depletion 4) tumor-mediated immunosuppression/exclusion and 5) additional inhibitory checkpoints (Figure 3).
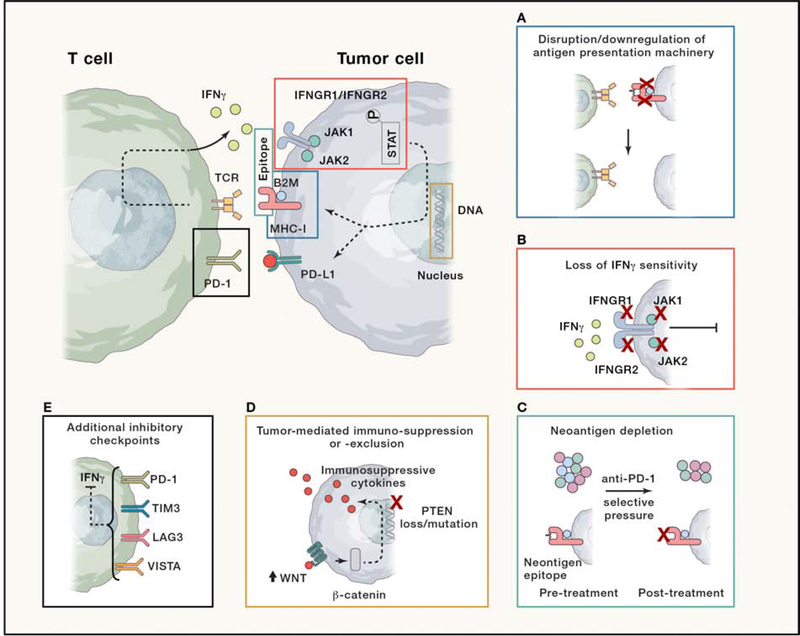
Figure 3. Schema of interaction between T cell and tumor cells, highlighting loci for proposed mechanisms of acquired resistance to immune checkpoint inhibitors.
Central cartoon depicts engagement between T cell (green) and tumor cell (blue). Transmembrane-bound T-cell receptor (TCR) and PD-1 receptor are depicted along T cell membrane, along with elaboration of IFN-γ cytokine granules into the extracellular space. Along the tumor cell membrane are the transmembrane-bound peptide-MHC class I receptor, stabilized by B2M, PD-L1 ligand, and IFNGR1/IFNGR2 heterodimer, bound by JAK1 and JAK2. Downstream of JAK1/JAK2 activates phospho-STAT, which engages with tumor cell DNA to regulate, among other targets, MHC-I and PD-L1 expression. Surrounding inset figures depict individual postulated mechanisms of acquired resistance that modulate or interfere with the normal engagement between a T cell and tumor cell. (A) Disruption/downregulation of antigen presentation machinery: Mutations or loss of MHC-I or B2M lead to loss of tumor antigen presentation and lack of TCR engagement. (B) Loss of IFN-γ sensitivity: mutations or loss of IFNGR1/IFNGR2 or JAK1/2 lead to insensitivity to IFN-γ in tumor microenvironment and resistance to anti-PD-1 mediated T cell response. (C) Neoantigen depletion: A clonally heterogenous tumor (blue, green, and purple circles) is affected by selective pressure from effective response to anti-PD-1 blockade, leading to loss of clones containing effective neoantigens (with blue circles lost post-treatment). Cartoon below depicts peptide-MHC-B2M complex with neoantigen epitope in pre-treatment setting, which is then lost post-treatment. (D) Tumor-mediated immunosuppression/exclusion: upregulated WNT signaling leads to stabilization of β-catenin or PTEN mutations/loss can ultimately yield immune-suppressive and -exclusionary cytokines that reduce infiltration and function of CD8+ T cells in the tumor microenvironment. (E) Additional inhibitory checkpoints: upregulation of additional immune checkpoints such as TIM3, LAG3, and VISTA can be found at the time of acquired resistance, and may reflect terminal exhaustion and fixed loss of effector function.
Defects in antigen presentation machinery
The activation of T-cell mediated immunity is dependent upon the recognition of antigens on major histocompatibility complexes (MHCs) of antigen presenting cells (Blum et al., 2013; Trombetta and Mellman, 2005). Tumor antigen presentation by MHC class I is mediated by the coordinated expression of multiple genes (Blum et al., 2013; Hulpke and Tampe, 2013; Trombetta and Mellman, 2005). One key gene, beta-2-microglobulin (B2M), is responsible for stabilization of MHC class 1 molecules at the cell surface and facilitation of loading with peptides (Hulpke and Tampe, 2013). Historically, following treatment with adoptive cell therapy or IL-2, loss of function mutations in B2M were shown to lead to MHC I loss and represent a molecular route of tumor escape from immune detection (Restifo et al., 1996). More recently, truncating alterations in B2M have been one of the few recurring findings in acquired resistance to ICIs. Zaretsky et al., for example, analyzed data from 4 melanoma patients with acquired resistance to immunotherapy and identified 1 patient with a homozygous acquired truncating B2M mutation (Zaretsky et al., 2016). Further studies in melanoma and other tumor types, have similarly found acquired defects in antigen presentation. In a study of 5 melanoma patients, Sade-Feldman et al. identified 1 patient with acquired B2M loss of heterozygosity (LOH) and 2 frameshift mutations and 1 patient that had 2 frameshift B2M alterations at the time of progression (Sade-Feldman et al., 2017). In a study of acquired resistance among 14 patients with lung cancer (10 with paired tumor tissue samples), 1 patient acquired homozygous loss of B2M (Gettinger et al., 2017). And in a report of 5 patients with mismatch repair deficient (MMR-d) tumors that received PD-1 blockade and developed progressive disease after initial response, 2 patients had acquired alterations of B2M (Le et al., 2017).
Importantly, in 5 of 6 cases of tumors harboring B2M genomic alterations described above, the patients’ tumors had bi-allelic, or homozygous, alterations of B2M. IHC also confirmed that many of these biallelic alterations were linked to expression loss of B2M and MHC class 1. Presumably tumors with a loss-of-function alteration in one allele and loss of heterozygosity (LOH) of the wild-type B2M allele have a similar biologic effect due to the lack of presence of functional B2M copy, supported by the findings of Sade-Feldman et al (Sade-Feldman et al., 2017). However, the biological and clinical impact of mono-allelic truncating B2M alterations with a retained wild-type B2M or missense mutations remains unclear. It is possible that a single copy of the wildtype B2M is sufficient to maintain proper function of the MHC complex and it should not be assumed that all B2M alterations will cause ICI resistance.
Despite being one of the more common recurring findings in acquired resistance, biallelic B2M alterations appear to be uncommon at baseline and are not a clearly established mechanism of primary resistance to ICIs. For example, in one report examining pre-treatment tissue of patients with NSCLC treated with PD-1 blockade, only 1 of 240 (0.4%) patients had biallelic alterations in B2M (Rizvi et al., 2018). Notably, this patient has loss of B2M expression confirmed by immunohistochemistry and nevertheless experienced a partial response to PD-1 blockade. Sade-Feldman found B2M alterations to be enriched in non-responders to anti-CTLA4 therapy compared to responders, but did not find enrichment in a dataset of ant-PD-(L)1 therapy (Sade-Feldman et al., 2017). Further, a recent analysis in MSI-H colorectal cancer has shown that most patients with B2M mutations and protein expression loss were still sensitive to ICIs (Middha et al., 2019), illustrating that mechanisms of acquired resistance may be fundamentally distinct from primary resistance.
Additionally, it is important to distinguish genomic etiologies of MHC loss via B2M alterations versus other causes of expression loss of the MHC. In Gettinger et al., significantly reduced levels of MHC class 1 protein and B2M protein were also identified in 4 out of 9 and in 3 out 9 patients, respectively, with no corresponding molecular alterations of B2M (Gettinger et al., 2017). In these cases, additional unknown genomic or nongenomic factors may modulate MHC class 1 expression and influence resistance to ICIs.
Defects in IFN-γ signaling
Release of IFN-γ from effector T cells triggers a signaling cascade in tumor cells via the JAK-STAT pathway that mediates both MHC class I and PDL1 expression and can induce tumor cell death in multiple ways (Bach et al., 1997). One of the critical first steps within this pathway is activation of receptor associated kinases JAK1 and JAK2 via IFN-γ binding to the heterodimeric IFNGR1/IFNGR2. Recent clinical reports have revealed cases of inactivating mutations of JAK1 or JAK2, which may contribute to progression on ICIs (Gao et al., 2016; Shin et al., 2017; Sucker et al., 2017; Zaretsky et al., 2016).
In Zaretsky et al., 2 patients with who progressed on ICIs after 1 and 2 years had acquired loss of function mutations in genes encoding JAK1 (JAK1 Q503*) or JAK2 (JAK2 F547 splice-site mutation) (Zaretsky et al., 2016). The mutations were upstream of the kinase domains and presumably caused truncation or nonsense-mediated decay of the JAK protein. Similar to the scenario with B2M alterations described above, no wild-type JAK1 or JAK2 allele was retained as the mutations occurred concurrently with the LOH of the wild-type allele at the time of progression. Cell lines established from the patient with the acquired JAK2 mutation and CRISPR-Cas9 engineered cell lines with analogous JAK1 or JAK2 mutations also confirmed a total loss of JAK protein and a loss of tumor cell sensitivity to IFN-γ, with a corresponding lack of MHC class 1 and PDL1 expression (Zaretsky et al., 2016). An additional analysis of cell lines from 6 melanoma patients with alterations of the JAK-STAT pathway (JAK1 n=4, JAK2 n=1, STAT1 n=1) validated that only patients with homozygous deficiency of JAK (n = 2) either through LOH or biallelic genomic alterations appeared insensitive to the effects IFN-γ (Sucker et al., 2017). Patients with heterozygous mutations still demonstrated strong IFN-γ pathway activation with elevated surface expression of HLA class 1 and PD-L1 surface expression (Sucker et al., 2017).
JAK1, JAK2, and IFGNGR alterations have been reported in the setting of primary resistance to immunotherapy (Gao et al., 2016; Shin et al., 2017). In an analysis of 16 patients with melanoma who received CTLA4 inhibitors, genomic loss of other key IFN-γ genes such as IRF1 and amplification of critical IFN-γ pathway inhibitors such as SOCS1 and PIAS4 were also strongly enriched in tumors of non-responder patients (Gao et al., 2016). However, clinical reports of acquisition of these alterations are lacking and the extent to which additional IFN-γ pathway genomic alterations beyond JAK1 and JAK2 contribute to acquired resistance to ICIs is unclear.
Neoantigen depletion
Neoantigen-specific T cells may be key drivers of role response to ICIs (McGranahan et al., 2016; Rizvi et al., 2015; van Rooij et al., 2013). Consequently, loss of the somatic mutations encoding putative tumor-specific neoantigens either through clonal selection, epigenetic repression, or copy number loss may lead to subsequent immune evasion and clinical progression (Rosenthal et al., 2019).
In a clinical report of 4 patients with NSCLC who developed acquired resistance to ICIs, no loss-of-function mutations in HLA genes such as B2M or JAK1 or JAK2 were identified (Anagnostou et al., 2017). Rather, exome data of pre vs post-treatment tissue identified loss, at the time of acquired resistance, of several mutations computationally predicted to generate neoantigens. In vitro, there was clonal T-cell expansion when stimulated with many of the lost neoantigen peptides, suggesting these predicted neoantigens were immunologically relevant and may have driven the selective pressure to eliminate these clones and permit the outgrowth of acquired resistance. Relatedly, immunoselection via loss of T-cell-recognized antigens after adoptive T-cell transfer has similarly been described (Verdegaal et al., 2016).
Tumor-mediated immunosuppression/exclusion
In preclinical models, loss of the tumor suppressor PTEN, which is critical in the regulation of PI3K activity, increases expression of immunosuppressive cytokines and decrease T cell effector IFN-γ leading to inhibition of T-cell mediated infiltration and immunity (Peng et al., 2016). PTEN loss has also been observed in cases of acquired resistance. For example, in a recent report of a patient with metastatic uterine leiomyosarcoma who initially had a near complete response to pembrolizumab for greater than 2 years, biallelic PTEN loss was acquired at the time of resistance (George et al., 2017). Similarly, in a report of two patients with melanoma who developed acquired resistance to immune therapies, one patient also developed biallelic PTEN loss (Trujillo et al., 2019).
Of note, in Trujillo et al, the second patient initially showed a durable partial response to a melanoma-peptide/interleukin-12 vaccine and subsequently developed new treatment-resistant metastases had showed new robust tumor expression of β-catenin (Trujillo et al., 2019). Similar to PTEN loss, WNT-β-catenin activity has been linked to the production of immunosuppressive cytokines, alterations in priming of dendritic cells, promotion of regulatory T cells, and lack of significant T cell infiltration in melanoma, supporting B-catenin’s role in ICI resistance (Spranger et al., 2015; Spranger et al., 2017; Yaguchi et al., 2012; Zhao et al., 2018).
Additional inhibitory checkpoints
Some reports have described upregulated expression of other T cell checkpoint at the time of acquired resistance, including TIM3 (Gettinger et al., 2017; Koyama et al., 2016), LAG3 (Gettinger et al., 2017) and V-domain Ig suppressor of T cell activation (VISTA) (Kakavand et al., 2017), although whether these changes are causally associated with resistance is unclear. Although such checkpoints may in some contexts be functionally associated with T cell exhaustion and terminal dysfunction, they may also be associated with T cell activation (Blank et al., 2019). Additional data may be needed to understand the functional T cell state in these examples and, ultimately, the contribution to acquired resistance.
Unclear mechanisms of resistance
We caution that even in many of the reports discussed above, it is challenging to verify or identify a specific mechanism of resistance in many cases. In some reports, no mechanism is proposed and in others, overlapping mechanisms are presented, or the mechanism of resistance is inferred from circumstantial data. Thus, the true landscape of acquired resistance to ICIs remains largely uncertain.
Insights from new tools
The sparse clinical data illustrates that the reliance on common molecular tools may not fully reveal the underlying mechanisms of acquired resistance. Instead, broader and deeper exploration of the relationship between the tumor microenvironment, host immunity, and tumor may be required to understand the functional and geographic nature of immune response and immune checkpoint inhibitor efficacy. There are new emerging techniques may further facilitate this pursuit.
Systematic, genome-wide scale CRISPR-Cas9 mutagenesis screens have identified candidate genes and pathways critical for modulating anti-tumor immunity and, therefore, potentially relevant to understanding acquired resistance (Manguso et al., 2017; Pan et al., 2018; Patel et al., 2017). These studies converge on the importance of the IFN-γ receptor signaling pathway and reveal other potential mediators of ICI sensitivity and resistance. Patel et al. highlighted the role of APLNR, a G-protein-coupled receptor that is mutated in multiple cancer types, in immunotherapy resistance and showed that APLNR regulates T cell response via modulation of JAK1 and IFN-γ signaling (Patel et al., 2017). Manguso et al. demonstrated activating mutations in the tyrosine-protein phosphatase nonreceptor type 2 (Ptpn2), which negatively regulates JAK1 and JAK2, contributes to resistance to ICIs through IFN-γ resistance (Manguso et al., 2017). Finally, Pan et al., which also found Ptpn2 and IFNγ signaling was enriched in ICI resistance, reveals critical components of the SWI/SNF chromatin remodeling complex can suppress IFNγ signaling and thereby mediate T cell antitumor immunity (Pan et al., 2018).
Vredevoogd et al. also performed a genome wide CRISPR-Cas9 screen of IFN-γ receptor-deficient melanoma to identify IFN-γ independent pathways involved in anti-tumor immunity. In doing so, they uncovered that tumor necrosis factor (TNF) receptor associated factor 2 (TRAF2) inactivation within the TNF signaling pathway contributes to T-cell elimination (Vredevoogd et al., 2019).
Single cell sequencing has also further refine understanding of key regulators of distinct T cell states, which may define the balance between effective anti-tumor immunity or resistance. In particular, better understanding of the characteristic features and mechanisms underlying T cell exhaustion with chronic antigen stimulation may be critical to predicting patterns of response and reinvigorating T cell response after acquired resistance. (Blank et al., 2019).
Sade-Feldman et al. profiled the transcriptomes of individual immune cells (48 samples from 32 patients with melanoma) receiving immunotherapy, enabling the detection of individual T cell populations and associated markers associated with ICI responders and non-responders (Sade-Feldman et al., 2018). In this report, TCF1, a protein within the Wnt/β-catenin signaling pathway, emerged as a top marker of response and linked to a cluster of T cells with expression of genes of memory, activation, and cell survival. TCF1 has previously been described a critical factor for differentiation, self-renewal, and persistence of memory CD8+ T cells and shown to reinvigorate and sustain effective immunity of CD8+ T cells against chronic lymphocytic choriomeningitis mouse virus (LCMV) infection upon anti-PD-1 treatment (Blank et al., 2019; Chen et al., 2019b; Hudson et al., 2019; Im et al., 2016; Utzschneider et al., 2016; Zhou et al., 2010).
In another recent report, Miller et al. compared single-cell expression profiles from TIL CD8+ T cells in murine models of LCMV chronic infections versus tumors to further characterize T cell exhaustion (Miller et al., 2019). They identify similar, overarching traits in both models and highlight two critical subpopulations of T cells in the tumor microenvironment; progenitor exhausted CD8+ T cells and terminally exhausted CD8+ T cells. The report demonstrates that the frequency of these subsets may account for differential responses and resistance to immunotherapy. Further, progenitor T cells, which may proliferate and differentiate into exhausted CD8+ T cells upon PD-1 blockade, may be necessary for sustained anti-PD-1-driven responses. Future clinical studies are required to determine if and when depletion of progenitor T cells may play a role in acquired resistance to ICIs.
Interrogation of peripheral blood samples has also facilitated better understanding of the relationship of systemic and intratumoral T-cell responses to PD-1 blockade. In metastatic melanoma, sequential immune profiling of the peripheral blood has shown increased Ki67 expression among exhausted T cells after PD-1 blockade and evidence that immunologic reinvigoration and response may be detected in the periphery (Huang et al., 2017). Two recent studies have further identified early changes in a subset of peripheral CD8+ memory effector cytotoxic T cells was associated with subsequent response to PD-1 blockade in melanoma (Fairfax et al., 2020; Valpione et al., 2020). Additionally, paired single-cell RNA and T cell receptor sequencing demonstrated that expanded TCRs on-treatment and T cell response to PD-1 blockade may actually be derived from peripheral circulation rather than baseline TILs (Yost et al., 2019). Likewise, serial peripheral sampling and single-cell analyses may be a valuable, complementary process to paired tumor tissue for investigating and monitoring acquired resistance to immunotherapy and require further exploration in future correlative studies.
Conclusions
Acquired resistance to ICI is a common clinical phenotype about which relatively little mechanistic insight is known. The clinical development of next generation immunotherapies for patients with primary and acquired resistance is robust, but success has been limited. More effective therapeutic strategies may be achieved though deeper understanding of the underlying biology, permitting precision deployment of immunotherapies beyond immune checkpoint inhibitors. Collaborative efforts are needed to overcome the barriers toward a deeper understanding of the underlying biology of acquired resistance to ICIs. This progress will ultimately enable rational future drug and cellular therapy development to prevent, circumvent, or reverse resistance to ICIs.
Supplementary Material
Supplemental Table 1. Patient characteristics and mechanisms of acquired resistance to immune checkpoint inhibitors. The ICI treatment, cancer type, initial response, time on therapy, site of resistance biopsy and reported mechanism of resistance are detailed in each patient case.
Acknowledgement of research support
Supported by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748) and the Druckenmiller Center for Lung Cancer Research at MSK; MDH is a Damon Runyon Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (CI-98-18) and is a member of the Parker Institute for Cancer Immunotherapy.
Disclosures: MDH receives research funding from Bristol-Myers Squibb; is paid consultant to Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Blueprint, Achilles Therapeutics, PACT Pharma, Immunai, and Shattuck Labs; receives travel support/honoraria from AstraZeneca, Eli Lilly, Merck, and BMS; and a patent has been filed by MSK related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah N, Nagasaka M, Abdulfatah E, Shi D, Wozniak AJ, and Sukari A (2018). Non-small cell to small cell lung cancer on PD-1 inhibitors: two cases on potential histologic transformation. Lung Cancer (Auckl) 9, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, et al. (2017). Evolution of Neoantigen Landscape During Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. CD-16–0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro Carpeno J, Pluzanski A, Burgio MA, Garassino M, Chow LQM, Gettinger S, et al. (2019). Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. The Lancet Oncology 20, 1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, et al. (2018). Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. Journal of Clinical Oncology 36, 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascierto ML, Makohon-Moore A, Lipson EJ, Taube JM, McMiller TL, Berger AE, Fan J, Kaunitz GJ, Cottrell TR, Kohutek ZA, et al. (2017). Transcriptional Mechanisms of Resistance to Anti-PD-1 Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 3168–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach EA, Aguet M, and Schreiber RD (1997). The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annual review of immunology 15, 563–591. [DOI] [PubMed] [Google Scholar]
- Balar AV, Dreicer R, Loriot Y, Perez-Gracia JL, Hoffman-Censits JH, Petrylak DP, Heijden MSVD, Ding B, Shen X, and Rosenberg JE (2018). Atezolizumab (atezo) in first-line cisplatin-ineligible or platinum-treated locally advanced or metastatic urothelial cancer (mUC): Long-term efficacy from phase 2 study IMvigor210. Journal of Clinical Oncology 36, 4523–4523. [Google Scholar]
- Barron F, Cardona AF, Corrales L, Ramirez-Tirado LA, Caballe-Perez E, Sanchez G, Flores-Estrada D, Zatarain-Barron ZL, and Arrieta O (2018). Characteristics of progression to tyrosine kinase inhibitors predict overall survival in patients with advanced non-small cell lung cancer harboring an EGFR mutation. Journal of thoracic disease 10, 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Tessier A, Baldini C, Martin P, Champiat S, Hollebecque A, Postel-Vinay S, Varga A, Bahleda R, Gazzah A, Michot JM, et al. (2018). Outcomes of long-term responders to anti-programmed death 1 and anti-programmed death ligand 1 when being rechallenged with the same anti-programmed death 1 and anti-programmed death ligand 1 at progression. Eur J Cancer 101, 160–164. [DOI] [PubMed] [Google Scholar]
- Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. (2019). Defining ‘T cell exhaustion’. Nat Rev Immunol 19, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, and Cresswell P (2013). Pathways of antigen processing. Annual review of immunology 31, 443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. (2010). Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., Psyrri A, Basté N, Neupane P, Bratland Å, et al. (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (London, England) 394, 1915–1928. [DOI] [PubMed] [Google Scholar]
- Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K, et al. (2016). Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer discovery 6, 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, et al. (2017). Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. Journal of Clinical Oncology 35, 2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, et al. (2019a). Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 134, 1144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, et al. (2019b). TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855.e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al. (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England) 393, 156–167. [DOI] [PubMed] [Google Scholar]
- Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, Sher X, Liu XQ, Lu H, Nebozhyn M, et al. (2018). Pan-tumor genomic biomarkers for PD-1 checkpoint blockade–based immunotherapy. Science (New York, NY) 362, eaar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H, Mahé EA, Cooper R, Woodcock V, Traill Z, et al. (2020). Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nature Medicine 26, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher L, von Pawel J, Park K, Rittmeyer A, Gandara DR, Ponce Aix S, Han JY, Gadgeel SM, Hida T, Cortinovis DL, et al. (2018). Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 13, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petryla DP, Choueir TK, Necch A, et al. (2019). Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of > 2 years of follow-up. Ann Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. (2018). Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. The New England journal of medicine 378, 2078–2092. [DOI] [PubMed] [Google Scholar]
- Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, et al. (2016). Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 167, 397–404.e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn M-J, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, et al. (2019). Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. Journal of Clinical Oncology 37, 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. (2015). Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. 372, 2018–2028. [DOI] [PubMed] [Google Scholar]
- George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, Lipschitz M, Amin-Mansour A, Raut CP, Carter SL, et al. (2017). Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity 46, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al. (2017). Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. CD-17–0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, et al. (2018a). Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non–Small-Cell Lung Cancer: Results From the CA209–003 Study. 36, 1675–1684. [DOI] [PubMed] [Google Scholar]
- Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, Kavathas P, Chiang A, Lilenbaum R, Zelterman D, et al. (2018b). Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 13, 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al. (2019). Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Annals of Oncology 30, 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England) 387, 1540–1550. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, Arvis CD, Majem M, Forster MD, Monnet I, et al. (2020). Long-Term Outcomes and Retreatment Among Patients With Previously Treated, Programmed Death-Ligand 1Positive, Advanced NonSmall-Cell Lung Cancer in the KEYNOTE-010 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, Jco1902446. [DOI] [PubMed] [Google Scholar]
- Hu ZI, Hellmann MD, Wolchok JD, Vyas M, Shia J, Stadler ZK, Diaz LA Jr., and O’Reilly EM (2018). Acquired resistance to immunotherapy in MMR-D pancreatic cancer. Journal for immunotherapy of cancer 6, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. (2017). T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, et al. (2019). Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1(+) Stem-like CD8(+) T Cells during Chronic Infection. Immunity 51, 1043–1058.e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. (2016). Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 165, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulpke S, and Tampe R (2013). The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends in biochemical sciences 38, 412–420. [DOI] [PubMed] [Google Scholar]
- Iams WT, Beckermann KE, Almodovar K, Hernandez J, Vnencak-Jones C, Lim LP, Raymond CK, Horn L, and Lovly CM (2019). Small Cell Lung Cancer Transformation as a Mechanism of Resistance to PD-1 Therapy in KRAS-Mutant Lung Adenocarcinoma: A Report of Two Cases. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 14, e45–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, Lynch T, Johnson BE, and Miller VA (2010). Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 28, 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al. (2018). CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36, 2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DB, Estrada MV, Salgado R, Sanchez V, Doxie DB, Opalenik SR, Vilgelm AE, Feld E, Johnson AS, Greenplate AR, et al. (2016). Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 7, 10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakavand H, Jackett LA, Menzies AM, Gide TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV, and Scolyer RA (2017). Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Modern Pathology 30, 1666–1676. [DOI] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, et al. (2016). Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature Communications 7, 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. (2019). Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. 381, 1535–1546. [DOI] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, NY) 357, 409–-413.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Amon L, Zimmer L, Gutzmer R, Satzger I, Loquai C, et al. (2019). Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 25, 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manguso RT, Pope HW, Zimmer MD, Brown FD, Yates KB, Miller BC, Collins NB, Bi K, LaFleur MW, Juneja VR, et al. (2017). In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 547, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Le DT, Ascierto PA, Giacomo AMD, Jesus-Acosta AD, Delord J-P, Geva R, Gottfried M, Penel N, Hansen AR, et al. (2020). Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of Clinical Oncology 38, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, et al. (2016). Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, NY) 351, 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middha S, Yaeger R, Shia J, Stadler ZK, King S, Guercio S, Paroder V, Bates DDB, Rana S, Diaz LA Jr., et al. (2019). Majority of B2M-Mutant and -Deficient Colorectal Carcinomas Achieve Clinical Benefit From Immune Checkpoint Inhibitor Therapy and Are Microsatellite Instability-High. JCO precision oncology 3, 10.1200/PO.1218.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, et al. (2019). Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nature Immunology 20, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. (2017). Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. The New England journal of medicine 376, 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England) 393, 1819–1830. [DOI] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. (2015). Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. 373, 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. (2019a). Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. 380, 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, et al. (2019b). Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. The Lancet Oncology 20, 1370–-1385.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Bhatia S, Lipson EJ, Sharfman WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM, Reddy SA, et al. (2019). Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 37, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell PH, Balar AV, Vuky J, Castellano DE, Bellmunt J, Powles T, Bajorin DF, Grivas P, Hahn NM, Plimack ER, et al. (2019). KEYNOTE-052: Phase 2 study evaluating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC)— Updated response and survival results. Journal of Clinical Oncology 37, 4546–4546. [Google Scholar]
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al. (2017). Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. The Lancet Oncology 18, 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D, Kobayashi A, Jiang P, Ferrari de Andrade L, Tay RE, Luoma AM, Tsoucas D, Qiu X, Lim K, Rao P, et al. (2018). A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science (New York, NY) 359, 770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, and Varmus H (2005). Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2, e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, et al. (2017). Identification of essential genes for cancer immunotherapy. Nature 548, 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X, et al. (2016). Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery 6, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, Marcoux N, Banwait MK, Digumarthy SR, Su W, et al. (2018). Landscape of acquired resistance to osimertinib in EGFR-mutant NSCLC and clinical validation of combined EGFR and RET inhibition with osimertinib and BLU-667 for acquired RET fusion. Cancer discovery 8, 1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires da Silva I, Lo S, Quek C, Gonzalez M, Carlino MS, Long GV, and Menzies AM (2020). Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer 126, 86–97. [DOI] [PubMed] [Google Scholar]
- Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (London, England) 391, 748–757. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Cheng Y, Zhou C, Ohe Y, Imamura F, Cho BC, Lin M-C, Majem M, Shah R, Rukazenkov Y, et al. (2018). LBA50Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Annals of Oncology 29. [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. The New England journal of medicine 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, and Rosenberg SA (1996). Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. Journal of the National Cancer Institute 88, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N, et al. (2018). Molecular Determinants of Response to Anti-Programmed Cell Death (PD)-1 and Anti-Programmed Death-Ligand 1 (PD-L1) Blockade in Patients With Non-Small-Cell Lung Cancer Profiled With Targeted Next-Generation Sequencing. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36, 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, NY) 348, 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al. (2019). Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. The Lancet Oncology 20, 1239–1251. [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, Chang H, Lovitch SB, Horak C, Weber JS, et al. (2018). MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Science translational medicine 10. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, Lund T, Tanić M, Reading JL, Joshi K, et al. (2019). Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane J-P, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, et al. (2017). Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nature Communications 8, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, et al. (2018). Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013.e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, Lao CD, Chesney J, et al. (2017). Efficacy and Safety Outcomes in Patients With Advanced Melanoma Who Discontinued Treatment With Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. Journal of Clinical Oncology 35, 3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, et al. (2018). Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. The New England journal of medicine 379, 2108–2121. [DOI] [PubMed] [Google Scholar]
- Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, Chang JC, Paik PK, Offin M, Arcila ME, et al. (2020). Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT, et al. (2019). Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA oncology 5, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W, Sandoval S, Torrejon DY, et al. (2017). Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer discovery 7, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, et al. (2018). Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. The New England journal of medicine 378, 113–125. [DOI] [PubMed] [Google Scholar]
- Spigel DR, McCleod M, Hussein MA, Waterhouse DM, Einhorn L, Horn L, Creelan B, Babu S, Leighl NB, Couture F, et al. (2017). Randomized results of fixed-duration (1-yr) vs continuous nivolumab in patietns (pts) with advance non-small cel lung cancer (NSCLC). Ann Oncol 28, v460–v496. [Google Scholar]
- Spranger S, Bao R, and Gajewski TF (2015). Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235. [DOI] [PubMed] [Google Scholar]
- Spranger S, Dai D, Horton B, and Gajewski TF (2017). Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer cell 31, 711–723.e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucker A, Zhao F, Pieper N, Heeke C, Maltaner R, Stadtler N, Real B, Bielefeld N, Howe S, Weide B, et al. (2017). Acquired IFNgamma resistance impairs anti-tumor immunity and gives rise to T-cell-resistant melanoma lesions. Nat Commun 8, 15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero J, Cutsem EV, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Salguero HRC, et al. (2019). Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. 37, LBA4007–LBA4007. [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 366, 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA, Atkins MB, Leming PD, et al. (2019). Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non–Small Cell Lung Cancer Treated With Nivolumab. JAMA oncology 5, 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta ES, and Mellman I (2005). Cell biology of antigen processing in vitro and in vivo. Annual review of immunology 23, 975–1028. [DOI] [PubMed] [Google Scholar]
- Trujillo JA, Luke JJ, Zha Y, Segal JP, Ritterhouse LL, Spranger S, Matijevich K, and Gajewski TF (2019). Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. Journal for immunotherapy of cancer 7, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tykodi SS, Donskov F, Lee J-L, Szczylik C, Malik J, Alekseev BY, Larkin JMG, Matveev VB, Gafanov R, Tomczak P, et al. (2019). First-line pembrolizumab (pembro) monotherapy in advanced clear cell renal cell carcinoma (ccRCC): Updated results for KEYNOTE-427 cohort A. Journal of Clinical Oncology 37, 4570–4570. [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, et al. (2016). T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45, 415–427. [DOI] [PubMed] [Google Scholar]
- Valpione S, Galvani E, Tweedy J, Mundra PA, Banyard A, Middlehurst P, Barry J, Mills S, Salih Z, Weightman J, et al. (2020). Immune awakening revealed by peripheral T cell dynamics after one cycle of immunotherapy. Nature Cancer 1, 210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al. (2015). Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science (New York, NY) 350, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. (2013). Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 31, e439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, et al. (2016). Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 536, 91–95. [DOI] [PubMed] [Google Scholar]
- Vredevoogd DW, Kuilman T, Ligtenberg MA, Boshuizen J, Stecker KE, de Bruijn B, Krijgsman O, Huang X, Kenski JCN, Lacroix R, et al. (2019). Augmenting Immunotherapy Impact by Lowering Tumor TNF Cytotoxicity Threshold. Cell 178, 585–599.e515. [DOI] [PubMed] [Google Scholar]
- Wang DY, Eroglu Z, Ozgun A, Leger PD, Zhao S, Ye F, Luke JJ, Joseph RW, Haq R, Ott PA, et al. (2017). Clinical Features of Acquired Resistance to Anti-PD-1 Therapy in Advanced Melanoma. Cancer immunology research 5, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner AB, Palmer JS, Shoushtari AN, Goldman DA, Panageas K, Callahan MK, Wolchok JD, Postow MA, and Chapman PB (2019). Responders to anti-PD1 therapy: Long-term outcomes and responses to retreatment in melanoma (mel). Journal of Clinical Oncology 37, 9513–9513. [Google Scholar]
- Yaguchi T, Goto Y, Kido K, Mochimaru H, Sakurai T, Tsukamoto N, Kudo-Saito C, Fujita T, Sumimoto H, and Kawakami Y (2012). Immune suppression and resistance mediated by constitutive activation of Wnt/beta-catenin signaling in human melanoma cells. Journal of immunology (Baltimore, Md : 1950) 189, 2110–2117. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, Wang Z, Xu CR, Huang YS, Wang BC, et al. (2013). Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung cancer (Amsterdam, Netherlands) 79, 33–39. [DOI] [PubMed] [Google Scholar]
- Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, McNamara KL, Granja JM, Sarin KY, Brown RA, et al. (2019). Clonal replacement of tumor-specific T cells following PD-1 blockade. Nature Medicine 25, 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, et al. (2016). Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. The Lancet Oncology 17, 1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, Ready NE, Clement PM, Even C, Jang RW, et al. (2019). Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 107, 142–152. [DOI] [PubMed] [Google Scholar]
- Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, et al. (2016). Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. 375, 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Xiao C, Evans KS, Theivanthiran T, DeVito N, Holtzhausen A, Liu J, Liu X, Boczkowski D, Nair S, et al. (2018). Paracrine Wnt5a-beta-Catenin Signaling Triggers a Metabolic Program that Drives Dendritic Cell Tolerization. Immunity 48, 147–160.e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, and Xue H-H (2010). Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity 33, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Patient characteristics and mechanisms of acquired resistance to immune checkpoint inhibitors. The ICI treatment, cancer type, initial response, time on therapy, site of resistance biopsy and reported mechanism of resistance are detailed in each patient case.