Abstract
Heterogeneous prostatic carcinoma associated fibroblasts (CAF) contribute to tumor progression and resistance to androgen signaling deprivation therapy (ADT). CAF subjected to extended passaging, compared to low passage CAF, were found to lose tumor expansion potential and heterogeneity. Cell surface endoglin (CD105), known to be expressed on proliferative endothelia and mesenchymal stem cells, was diminished in high passage CAF. RNA-sequencing revealed SFRP1 to be distinctly expressed by tumor-inductive CAF, which was further demonstrated to occur in a CD105-dependent manner. Moreover, ADT resulted in further expansion of the CD105+ fibroblastic population and downstream SFRP1 in 3-dimensional cultures and patient derived xenograft tissues. In patients, CD105+ fibroblasts were found to circumscribe epithelia with neuroendocrine differentiation. CAF-derived SFRP1, driven by CD105 signaling, was necessary and sufficient to induce prostate cancer neuroendocrine differentiation in a paracrine manner. A partially humanized CD105 neutralizing antibody, TRC105, inhibited fibroblastic SFRP1 expression and epithelial neuroendocrine differentiation. In a novel synthetic lethality paradigm, we found that simultaneously targeting the epithelia and its microenvironment with ADT and TRC105, respectively, reduced castrate resistant tumor progression, in a model where either ADT or TRC105 alone had little effect.
Keywords: Prostate cancer, castrate resistant, synthetic lethality, CD105, stromal heterogeneity, cancer-associated fibroblasts, endoglin
Introduction
Prostate cancer (PCa) is a heterogeneous disease that results in the second highest cancer mortality in men. The standard of care for recurrent PCa is the disruption of androgen signaling. The eventual development of resistance to androgen signaling deprivation therapy (ADT) has no curative approach. We had previously identified transforming growth factor-beta (TGF-ß) signaling in fibroblasts as a determinant for prostate epithelial plasticity [1]. From the early steps of PCa initiation, the associated stromal fibroblastic cells begin to co-evolve with cancer progression and is predictive of recurrent disease and survival [2]. The tumor-inductive properties of cancer-associated fibroblasts (CAF) were originally coined as those that could convert non-tumorigenic epithelia to tumors and not simply based on proximity to cancer epithelia [3–5]. Additionally, prostatic CAF are attributed to tumor progression and therapeutic resistance to ADT [6, 7]. It is known that heterogeneous epithelial cell populations contribute to variable responses to treatments [8]. Whereas, the heterogeneity in the stromal fibroblastic cells is a concept less studied, especially in the context of resistance to therapy. Through a series of tissue recombination xenografts, we previously demonstrated the requirement of a heterogeneous mixture of mouse stromal fibroblasts with intact and deficient TGF-β signaling to induce the initial transformation of benign epithelia into malignant lesions [9]. However, better understanding of the heterogeneous human prostatic fibroblast populations that support the expansion of established tumors and contribute to recurrent disease is needed.
Histologically similar fibroblastic cells that circumscribe the glandular epithelia of the prostate vary biologically. At a simplistic level, cell surface proteins can be used to differentiate fibroblastic cells much like distinct populations of histologically similar immune cells were distinguished over thirty years ago. Furthermore, the function of these surface proteins can be cell type specific [10]. Mesenchymal stem cell proteins have emerged as markers for CAF in some tissues [11]. Among these markers, endoglin (CD105) was identified in both normal associated fibroblasts (NAF) and CAF. Interestingly, extended culture of tumor-inductive CAF, can result in the loss of their inductive capacity through a change in fibroblastic heterogeneity [12]. We exploited the fact that culturing CAF can diminish its tumorigenic potential as a tool to explore the consequence of stromal heterogeneity and paracrine determinants of tumor progression. Fibroblastic population drift resulting from culturing primary fibroblasts was revealed to diminish the CD105 population and ensuing effects of tumorigenesis. CD105 is a TGF-ß type III receptor that functions in promoting bone morphogenic protein (BMP) signaling and antagonizing TGF-ß signaling. CD105 is found on proliferating endothelial cells in development and tumorigenesis [13]. We hypothesized that the CD105+ CAF population critically mediates prostatic tumor epithelial differentiation and castrate resistance in a paracrine manner.
Therapeutics for late stage PCa target the androgen axis by blocking androgen synthesis or the androgen receptor. The phase III PREVAIL trial demonstrated that enzalutamide, a direct androgen receptor inhibitor, improved overall survival by 2 months in castration resistant prostate cancer (CRPC) patients who have not yet received chemotherapy [14]. Despite the initial efficacy of ADT many patients develop CRPC with the acquisition of neuroendocrine features by the cancer epithelia [15]. This paradox is a striking concept since de novo neuroendocrine PCa accounts for < 1% of patients, yet 10–15% of patients develop tumors having neuroendocrine features following ADT as a treatment-emergent adaptive response [16]. The role of CAF on neuroendocrine differentiation has not been reported. Interestingly, multiple paracrine factors have been demonstrated to support resistance to ADT, including IL-6, Wnt ligands, and IGF-1 [6, 17, 18]. Here, we identify a stromal CD105-expressing population in CAF that can mediate neuroendocrine differentiation and CRPC in a paracrine fashion. We further show that CD105 is highly druggable and can serve as a target complementing ADT to restore castrate sensitivity.
Results
Stromal heterogeneity is observed in human PCa specimens
Prostatic fibroblast populations directly from prostatectomy tissues were studied to determine heterogeneity of associated fibroblasts. Once we characterized these tissues as cancer or benign with H&E staining, we dissociated and depleted them for EpCAM+ epithelia and CD45+ lymphocytes by magnetic separation. FACS analysis was performed to distinguish for previously established mesenchymal stem cell surface markers (CD90, CD105, CD117, and Stro-1) within the remaining fibroblastic population of four patients. Fig 1A illustrates the distribution of the cell surface markers, based on the most abundant marker per population, color coded according to the termed dominant marker, with the co-expressed markers indicated, adding to the diversity of the individual populations. Similar to what has been reported in the past, we found CD90+ fibroblastic cells in the PCa tissues to be nearly double than that found in benign tissues [19, 20]. There was no statistical difference in benign and PCa in the CD105-dominant population. Expression of CD105 was further validated in 79 PCa and 16 benign tissues by immune-localization in a tissue array. In benign prostate tissues, CD105 immunohistochemical staining was primarily restricted to endothelial cells (Fig 1B). In PCa however, CD105 was primarily detected in the endothelia and heterogeneously expressed in stromal fibroblastic cells. We could not establish a correlation of Gleason grade to the expression of stromal fibroblastic CD105. Interestingly, staining for a neuroendocrine marker, chromogranin A, revealed its expression circumscribed by CD105+ fibroblasts (Figs 1C and Supplementary Figure S1). In the subset of 6 tissues that were positive for neuroendocrine differentiation, assessed by chromogranin A staining, stromal CD105 was also expressed in 83% of these same tissues; receiver operating characteristic (ROC) analysis provided an area under the curve (AUC) of 0.751 (p = 0.0026, Fig 1D). The treatment status of these patients was not known. Next, we used the R2 analysis platform to calculate the correlation coefficient between CD105 to a panel of nine neuroendocrine genes in a transcriptomic analysis of 545 PCa patient tissues (Figure 1E, R = 0.703 (p = 1.4×10−73) [21–23]. Taken together, the stromal fibroblastic CD105 population in tissues and primary cells was significantly associated with epithelial neuroendocrine differentiation (5).
Figure 1. Stromal CD105 expression is associated with neuroendocrine differentiation of the adjacent epithelia.
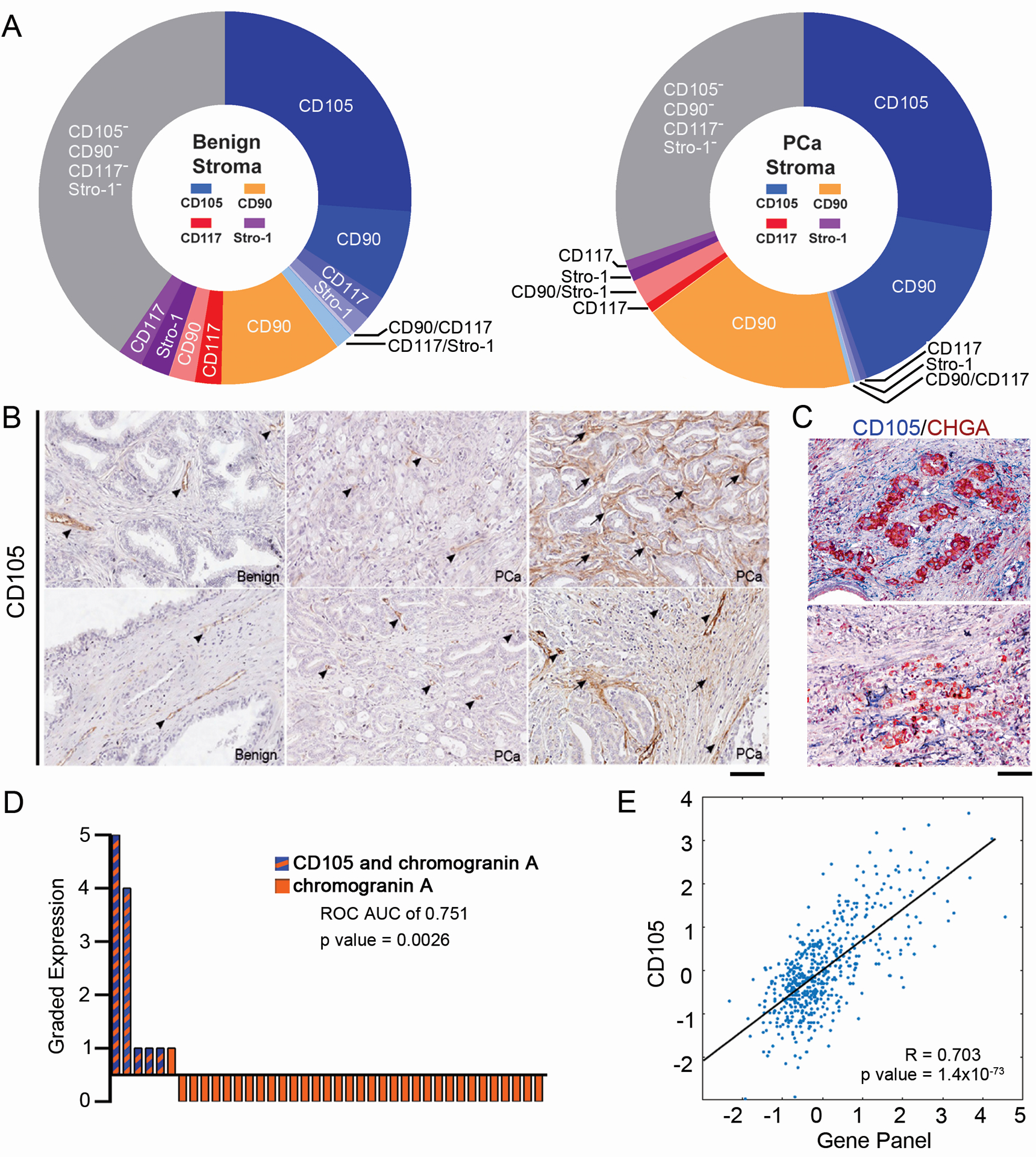
(A) Donut charts show a representative patient stromal makeup from benign or cancer prostatectomy tissue. The relative percent is indicated for the stromal populations based on FACS, n = 4. The dominant population, determined by the marker of greatest intensity per cell, are colored with shades of: blue (CD105), gold (CD90), red (CD117), purple (Stro-1). The double, triple, and quadruple stained cell populations are shown as lighter shades among their dominant population. Gray indicates negative staining for CD105, CD90, CD117, and Stro-1. (B) Immunohistochemical staining of CD105 (brown) from representative core sections of tissue arrays counterstained with hematoxylin. Arrow heads indicate CD105-positive blood vessels and arrows indicate CD105-positive stromal fibroblast staining, n=94. Scale bar represents 100 μm. (C) Representative serial sections from tissue cores stained for CD105 and chromogranin A, counterstained with hematoxylin, n = 39 paired tissues. A pseudo-colored overlay is shown to emphasize the localization of CD105 positive (blue) staining relative to chromogranin A positive staining (magenta). Scale bar represents 100 μm. (D) Waterfall plot indicates relative expression of epithelial chromogranin A (orange bars) and those that had co-expression of stromal CD105 (hatched orange and blue bars) on a graded scale of 0–5, where 5 was the greatest staining in paired cores, n = 39. (E) Scatter plot of the canonical correlation based on the R2 analysis platform between the neuroendocrine gene panel (AURKA, SCG3, MYCN, CGA, CGB, ENO2, NKX2.2, FOXA2) and CD105.
Cellular heterogeneity in carcinoma associated fibroblasts determine its tumor supportive property.
We next sought to confirm our finding from prostatectomy tissues with cultured CAFs and NAFs. Primary CAF cultures generated from prostatectomy tissues can promote the expansion of established tumor epithelia [5, 24]. However, routine culturing of primary prostate CAF can lead to its loss of tumor promoting potential. To characterize this observation, we compared primary cultured NAF, low passage CAF (3–7 passages) and high passage CAF (CAFHiP, > 8 passages) using FACS for CD90, CD105, CD117, and Stro-1. There was an overall statistical difference in the composition between NAF, CAF and CAFHiP populations (Fig 2A, p = 0.03). Interestingly, the most abundant fibroblastic population in NAF and CAF was the CD90+/CD105+ population compared to the CAFHiP (p = 0.03 and p = 0.06, respectively). In agreement with previous studies that prostatic fibroblasts can induce epithelial cell expansion [5], the xenografts with human CWR22Rv1 (22Rv1) PCa epithelia alone resulted in negligible tumors compared to tissue recombinant grafts with stromal fibroblastic cells (Fig 2B). Interestingly, there was no statistical difference between tissue recombinants using NAF and CAF in the progression of the established tumor epithelia, although the CAF provided a distinct growth advantage to non-tumorigenic BPH1 epithelia over NAF under similar conditions (data not shown). Regardless, tissue recombinants of 22Rv1 and CAFHiP resulted in tumor volumes larger than 22Rv1 epithelia alone (p < 0.05) but, significantly smaller than recombinants associated with NAF or CAF (p < 0.001 and p < 0.0001 respectively). Epithelial proliferation, as determined by Ki67 epithelial localization, was found to be greatest to least abundant in CAF > CAFHiP > NAF – associated recombinants (Fig 2C, D). However, CAFHiP-associated tumors had the lowest expression of survivin, compared to NAF or CAF recombinant tumors (Fig 2C, E). CAF-associated tumors were found to have significantly greater Ki67-expressing epithelia and reduced TUNEL staining, compared to NAF- or CAFHiP- associated tumors. This corroborated the concept of population drift, resulting from extended culturing of primary cells.
Figure 2. Stromal heterogeneity dictates tumor progression.
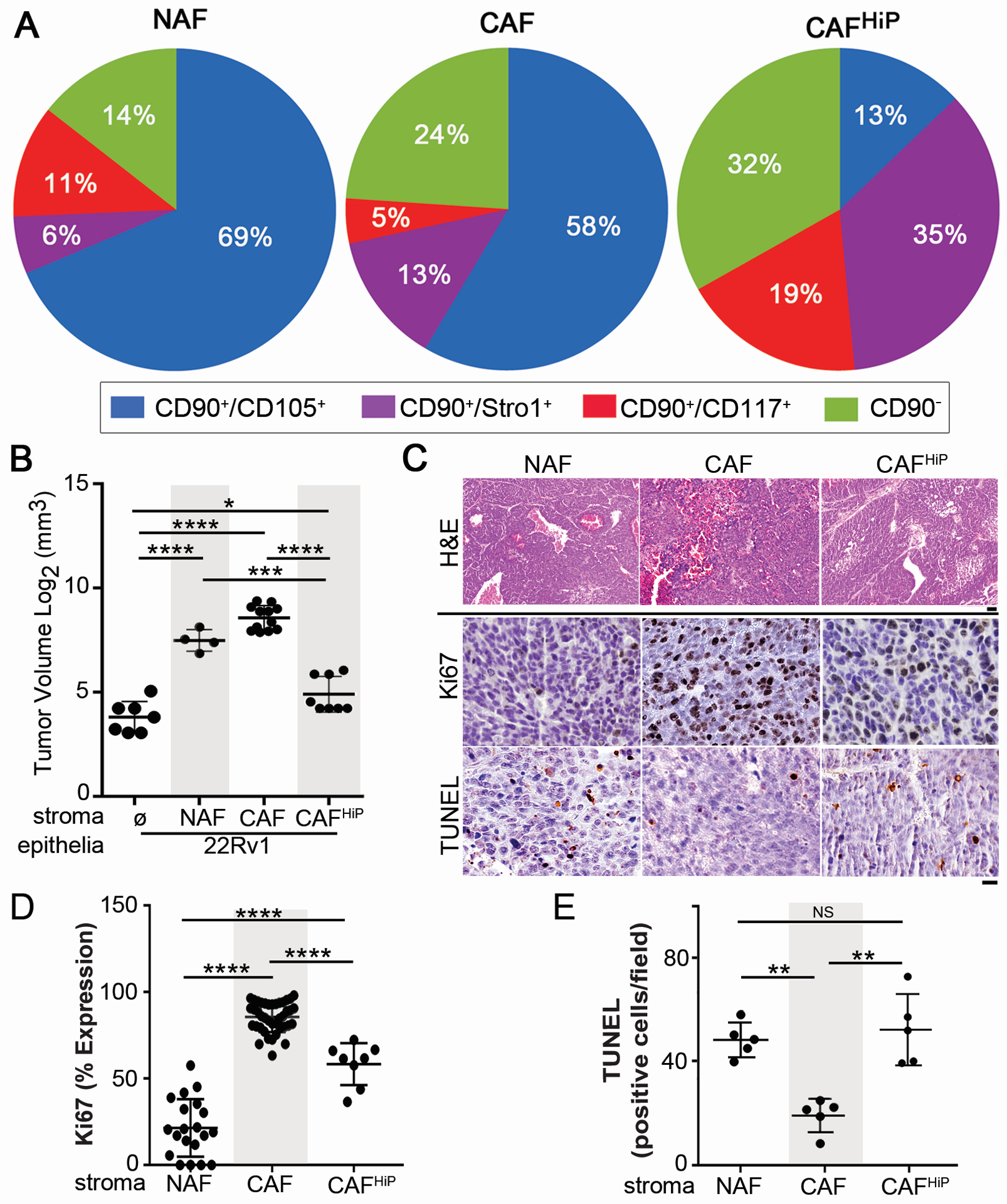
(A) Pie charts illustrate the relative ratio of the indicated stromal fibroblastic populations based on cell surface expression of the indicated markers: CD90+/CD105+ (blue), CD90+/Stro-1+ (purple), CD90+/CD117+ (red), and CD90− (green), n > 3. ANOVA analysis demonstrates NAF, CAF, and CAFHiP have distinct populations (p < 0.03). (B) Scatter plot indicates individual tumor volume (log transformed) for tissue recombinant tumors made up of 22Rv1 epithelia with the indicated fibroblastic populations. n > 4. (C) Histology for representative recombinant tumor sections of 22Rv1 with the indicated fibroblastic populations are shown. H&E staining shows tumor morphology (scale bar represents 64 μm). Ki67 and TUNEL immune-localization, with hematoxylin nuclear counterstain (scale bar represents 32 μm), is shown, n > 5. (D) The scatter plot shows quantitation of percent expression for Ki67 immunohistochemical staining, n > 8. (E) The scatter plot shows quantitation of the number of TUNEL positive nuclei per field by immunohistochemical staining, n > 5. For all, error bars are mean +/− SD, and p values of less than 0.05 were considered statistically significant (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Secreted frizzled related protein 1 expression is elevated in CAF stromal cell populations
To identify the differences in paracrine mediators among the three stromal cell types, we performed RNA-sequencing and segregated the genes based on their expression pattern. We first compared CAF to CAFHiP, as shown in the volcano plot comprised of the top 200 differentially regulated genes (Fig 3A). The top significantly upregulated genes were ependymin related 1 (EPDR1), lysyl oxidase (LOX), follistatin (FST), and SFRP1. Differential gene expression among CAF, CAFHiP, and NAF was analyzed based on the combined ranked ratios of CAF/CAFHiP and CAF/NAF. Candidate paracrine mediators (33 secreted genes, by gene ontology analysis from the top 200 differentially regulated genes) were plotted in a heatmap, revealing 9 genes expressed in both NAF and CAF, but not found in the CAFHiP (Fig 3B). Of the top significantly upregulated genes, only SFRP1 was co-expressed in both CAF and NAF populations. Presumably, paracrine mediators in the NAF and CAF, not expressed in CAFHiP, enabled the observed tumor expansion in Fig 2B. Therefore, the 9 genes common to NAF and CAF populations drew our attention as potential CD105-associated mediators of tumorigenicity.
Figure 3. Differential gene expression in NAF, CAF, and CAFHiP stromal fibroblasts.
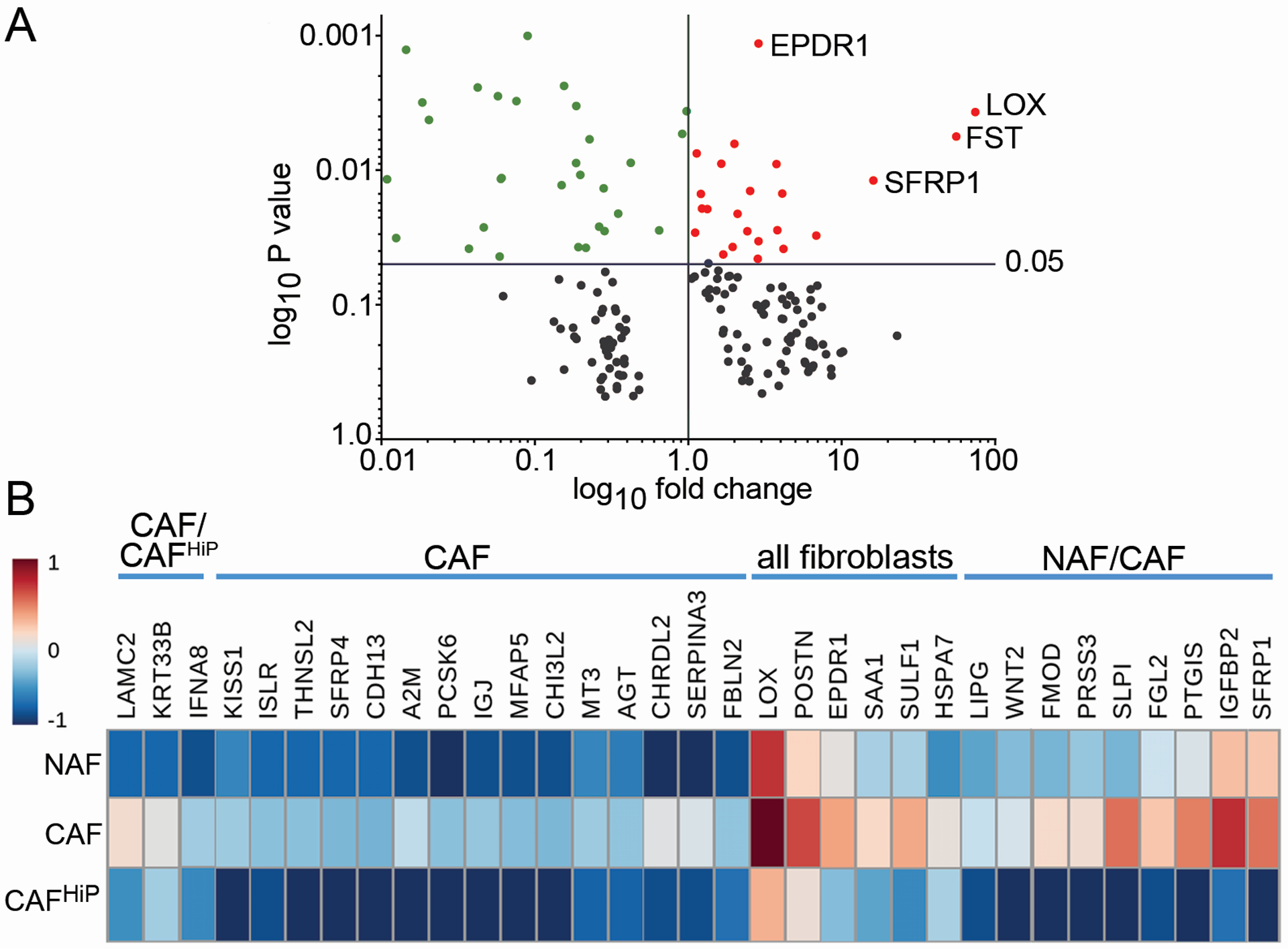
(A) The top 200 differentially expressed genes identified by RNA sequencing were plotted in a volcano chart to compare the ratio of CAFHiP to CAF. The effect size ratio was set to 1 indicating less effect below this threshold. Genes in the upper right quadrant (red) are considered significant with an effect greater than 1. Genes in the upper left quadrant (green) were considered significant with less effect. (B) Among the top 200 differentially expressed genes, 33 coded for secreted proteins, illustrated in the heat map following log transformation. The labels above the gene names highlight expression in NAF, CAF, and CAFHiP.
Stromal CD105 mediated SFRP1 induced PCa neuroendocrine differentiation
Next, a unique primary CAF line was identified to be spontaneously enriched in CD105 (CAFCD105en, 99% of the population) compared to primary CAF samples from other patients (56% ± 10), that maintained its high CD105 expression through passaging. A set of CAF-related genes based on previous studies and our RNA-sequencing data were assessed in NAF, CAF and the CAFCD105en cells. MMP-1, MMP-3, Cox-2, tenascin C (TNC), and secreted frizzled related protein 1 (SFRP1) were elevated in CAF more than 20-fold compared to NAF (Fig 4A). The expression of SFRP1 and TNC was further elevated in CAFCD105en compared to either NAF or CAF (p < 0.001). However, two-way ANOVA of the entire gene expression panel suggested that the CAF and CAFCD105en to be statistically similar. Notably, the traditional tumor-associated fibroblastic markers of alpha-smooth muscle actin, fibroblast activating protein (FAP), and IL-6 were not higher in the cultured CAF or CAFCD105en compared to NAF.
Figure 4. Androgen axis inhibition mediates paracrine SFRP1-mediated neuroendocrine differentiation.
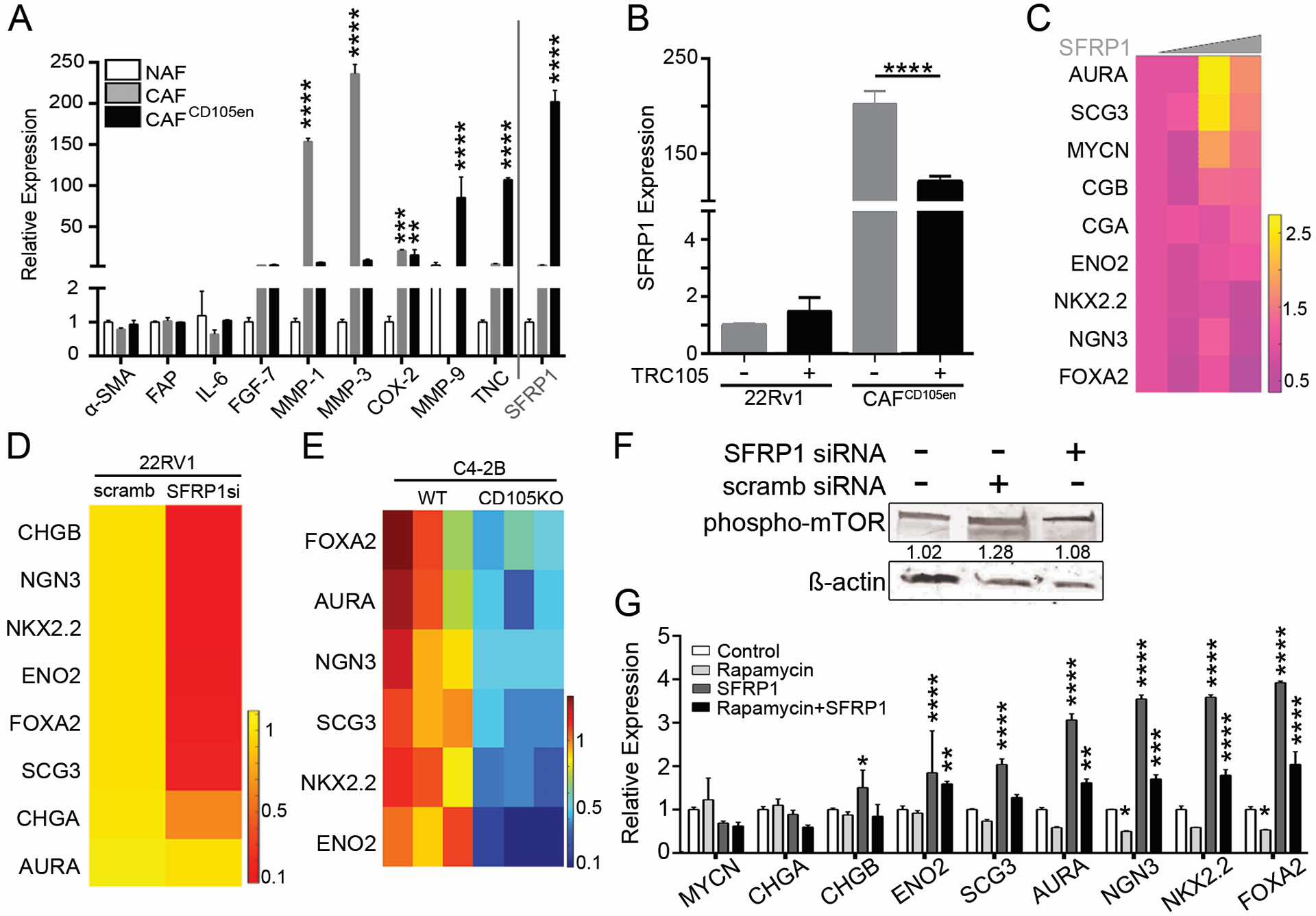
(A) Relative mRNA expression for the indicated genes is graphed for NAF (white), CAF (gray), and CAFCD105en (black) as mean +/− SD, n = 3. Primer sequences are listed in Supplementary Table S1. (B) Bar graph shows relative SFRP1 mRNA expression in human 22Rv1 and CAFCD105en regulated by TRC105 compared to IgG (control) treatment, n > 3. (C) Heat map shows the relative expression for the neuroendocrine gene panel in 22Rv1 cells, normalized to GAPDH, when treated with 0, 0.01, 0.1, and 1 μg/ml SFRP1, n = 3. Gradient scale from 2.5-fold increase (yellow) to 0.5-fold decrease (purple) indicates gene expression changes compared to control. (D) Heat map shows the relative expression for the neuroendocrine gene panel in 22Rv1 cells, normalized to β-actin, when treated with enzalutamide in combination with conditioned media from CAF nucleofected with scramble or siRNA against SFRP1, n = 3. Gradient scale from 1-fold increase (yellow) to 0.1-fold decrease (red) indicates gene expression changes compared to scramble control. (E) Heat map shows the relative expression for the neuroendocrine gene panel in C4–2B cells, normalized to β-actin, when treated with enzalutamide in combination with conditioned media from wild type fibroblasts or CD105 knockout fibroblasts, n=3. Gradient scale from 1-fold increase (red) to 0.1-fold decrease (blue) indicates gene expression changes compared to wild type conditioned media. (F) Immunoblot shows 22Rv1 cell protein expression for phosphorylated-mTOR and β-actin as the loading control, treated with control media or CAF-nucleofected with scramble or siRNA against SFRP1 as indicated for 72 hours. The ratio of phospho-mTOR/β-actin is shown. (G) Bar graph shows the relative gene expression for the indicated neuroendocrine differentiation genes in 22Rv1 cells treated with rapamycin (1 μM) and/or SFRP1 (0.1 ug/mL) for 72 hours, n = 3. * indicates significant gene expression compared to control. For all, error bars are mean +/− SD, and p values of less than 0.05 were considered statistically significant (**P<0.01, ***P<0.001, ****P<0.0001).
With the goal of identifying a neuroendocrine differentiation promoting paracrine factor, we directly tested if CD105 expression affected SFRP1 expression using a CD105 neutralizing antibody, TRC105. TRC105 blocked human CD105-mediated BMP2 activity without affecting TGF-ß signaling (Supplementary Figure S2) [25]. The low SFRP1 expression in 22RV1 was not affected by TRC105, however TRC105 significantly downregulated SFRP1 in CAFCD105en (p < 0.0001) (Fig 4B). Furthermore, the knockdown of CD105 in CAFCD105en by siRNA also resulted in decreased SFRP1 expression (Supplementary Figure S3). SFRP1 genes expression was found to be co-expressed with thrombospondin 1 (THBS1), platelet derived growth factor 1 (PDGFC), tectonic family member 1 (TCTN1), and zinc finger protein 449 (ZFN449), based on TCGA gene association query (Supplementary Figure S3)[26]. Of the four SFRP1 regulated genes, PDGFC, sonic hedgehog (target of TCTN1), and THBS1 are associated with tumor neuroendocrine differentiation. There was further evidence of the role of SFRP1 in neuroendocrine differentiation of PCa in the TCGA, where SFRP1 gene amplification was associated with neuroendocrine differentiation (Supplementary Figure S3). To test the role of SFRP1 on epithelial neuroendocrine differentiation more comprehensively, we treated cultured 22Rv1 with recombinant SFRP1 to find a significant induction of 9 PCa neuroendocrine differentiation genes [15] in a dose dependent manner (p < 0.001; Fig 4C). However, the same doses of SFRP1 had no effect on epithelial proliferation (Supplementary Figure S3). To further confirm the neuroendocrine response of stromal derived SFRP1 on epithelia, we knocked down SFRP1 by 10-fold using siRNA in CAFCD105en and used this conditioned medium to treat 22Rv1. The knockdown of SFRP1 significantly decreased the neuroendocrine differentiation gene panel without affecting proliferation, compared to control transfected CAFCD105en cells (p < 0.0001; Fig 4D and Supplementary Figure S3). To further confirm this mechanism in an alternative cell line, we treated C4–2B cells with enzalutamide in the presence of conditioned media from wild type mouse fibroblasts or CRISPR/Cas9 CD105-knockout mouse fibroblasts to measure the expression of the neuroendocrine differentiation gene panel (p < 0.0001, Fig 4E, Supplemental Figure S3). After confirming this mechanism at the transcriptional level, 22Rv1 were again treated with conditioned medium from CAF nucleofected with siRNA against SFRP1 compared to scramble control, which resulted in elevated mTOR phosphorylation (Ser 2448, associated with mTORC1 activation) (Fig 4F) [27, 28]. Furthermore, 22Rv1 were treated with combinations of recombinant SFRP1 and/or an mTOR inhibitor, rapamycin. The SFRP1-induced neuroendocrine gene panel was restored to near control levels with the addition of rapamycin, in support of the role of SFRP1 in neuroendocrine differentiation via mTOR signaling, in the absence of any effect on epithelial cell viability (p value < 0.0001, Fig 4G, Supplemental Figure S3). Fibroblast-derived SFRP1 was necessary and sufficient for epithelial neuroendocrine differentiation.
ADT induces neuroendocrine differentiation in a stromal CD105 dependent manner
To better understand the regulation of CD105 in both fibroblasts and epithelia, we generated 3D co-cultures with human 22Rv1 and mouse wild type prostatic fibroblasts. As patients can develop neuroendocrine prostate cancer when undergoing ADT, we used enzalutamide to reproduce this physiological state. Treatment with enzalutamide resulted in a three-fold increase in CD105 cell surface expression in epithelial and fibroblastic populations by FACS analysis, compared to vehicle (Fig 5A). To test the effect of ADT and CD105 inhibition directly on epithelial cells, we treated 22Rv1, C4–2B, or PC3 monolayer cultures with enzalutamide in the presence or absence of TRC105. Ki-67 expression was measured by FACS. We found that enzalutamide effectively downregulated the proliferation of AR expressing 22Rv1 and C4–2B cells (p < 0.01, Fig 5B). The administration of TRC105 had little proliferative effect in the presence or absence of enzalutamide treatment of 22Rv1 or C4–2B cells. Further determination of epithelial cell viability by MTT assay confirmed reduced viability of the cells with enzalutamide treatment (Supplementary Figure S4). TRC105 did not impact cell viability. The PC3 cells, with no androgen receptor expression, were predictably insensitive to either enzalutamide in the presence or absence of TRC105. In light of the observed epithelial proliferation with enzalutamide, we tested epithelial prostate specific androgen (PSA) expression by real-time PCR in co-cultured 22Rv1 or C4–2B with wild type mouse fibroblasts in the presence of enzalutamide. The co-cultured Rv1 demonstrated elevated PSA expression with enzalutamide in a dose dependent manner (p<0.05, Fig 5C). Whereas, PSA expression was significantly decreased in co-cultured C4–2B cells given enzalutamide (p < 0.05, Supplemental Figure S4). In line with elevated PSA expression by 22Rv1 when co-cultured with wild type fibroblasts, in 3D co-cultures of the same cells, enzalutamide caused a doubling of epithelial proliferation when normalized to vehicle control, (p < 0.01, Fig 5D). We utilized the species differences to target the epithelia with the human-specific CD105 neutralizing antibody, TRC105, or fibroblasts with the mouse-specific CD105 neutralizing antibody, M1043 [25]. At the dose used for this study (1 μg/ml), we found no cross-species reactivity of the two antibodies or alterations of TGF-ß signaling (Supplementary Figure S2). The co-cultures were treated with enzalutamide in the presence or absence of TRC105 and/or M1043. The resultant changes in epithelial proliferation were quantitated by FACS analysis for Ki67 expression of EpCAM+ cells. Treatment with either M1043 or TRC105 alone did not change epithelial proliferation compared to IgG control. However, combining enzalutamide with both M1043 and TRC105 reduced epithelial proliferation, compared to enzalutamide alone (p < 0.01). In support of fibroblastic expression of CD105 eliciting the dominant effect, M1043 (in the absence of TRC105) with enzalutamide resulted in a significant increase in epithelial cell death, measured by EpCAM+/annexin V staining, compared to control or enzalutamide (p < 0.01, Fig 5E). Thus, CD105+ fibroblastic population is consequential to castrate resistance due to cell-specific effects on epithelial proliferation and death in the context of ADT.
Figure 5. Antagonizing the androgen axis increases CD105 with elevated neuroendocrine differentiation.
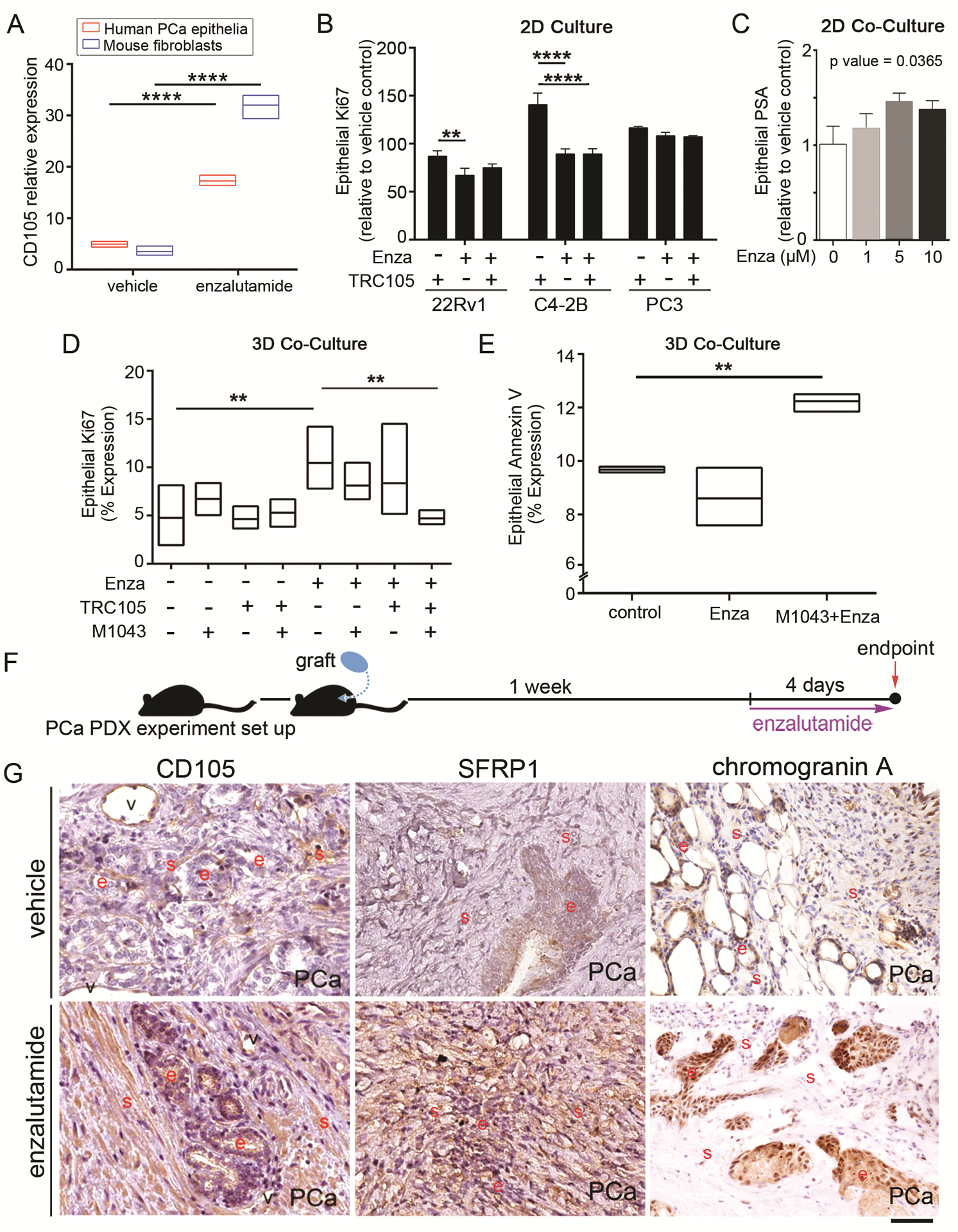
(A) CD105 expression in human epithelial (22Rv1) and wild-type mouse prostatic fibroblastic cells in a 3D co-culture model is regulated by enzalutamide treatment, as determined by FACS analysis, n = 3. (B) Epithelial proliferation of prostatic epithelia 22Rv1, C4–2B, and PC3 was determined by FACS analysis for Ki67+ cells in the presence and absence of TRC105 (1 ug/mL) and enzalutamide (5 μM), n = 3. (C) Epithelial PSA expression is shown from a 2D co-culture of 22Rv1 with wild-type mouse prostatic fibroblasts after a 16 hour treatment with indicated enzalutamide doses in hypoxia, n=3. (D) Epithelial proliferation of human 22Rv1, in a 3D co-culture model with mouse prostatic fibroblasts, were analyzed for double EpCAM+ and Ki67+ expression by FACS. The cultures were treated with TRC105, M1043, and/or enzalutamide for 72 hrs, n > 3. (E) Epithelial cell death of human 22Rv1, in a 3D co-culture model with mouse prostatic fibroblasts, were measured for double EpCAM+ and Annexin V+ expression by FACS. The cultures were treated with M1043 and/or enzalutamide for 72 hrs, n = 3. (F) In a PDX model, the mice were xenografted with untreated human prostatectomy tissue under the renal capsules. Tissues grew for one week and then the mice were treated with either vehicle or enzalutamide for 4 days. (G) Immunohistochemical localization of CD105, SFRP1, or Chromogranin A in benign or PCa tissues were counterstained with hematoxylin. Red letters were used to highlight positive staining in blood vessels (v), epithelia (e), and stroma (s), n > 5. Scale bar represents 100 μm. For all, error bars are mean +/− SD, and p values of less than 0.05 were considered statistically significant (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Next, we attempted to validate the associated increase in CD105 following ADT using PCa patient-derived xenograft (PDX) models. Treatment-naïve PCa tissues were grafted in the subrenal capsule and allowed to vascularize for one week before treatment with either vehicle or enzalutamide for 4 days (Fig 5F). The grafts were harvested and evaluated for the expression of CD105, SFRP1, and chromogranin A. As before, CD105 was predominantly immune-localized on the vascular endothelia in the absence of enzalutamide treatment (Fig 5G). However, enzalutamide treatment resulted in the upregulation of CD105 immuno-localization in both epithelial and CAF populations with SFRP1 upregulation in the PDX tissues. Double immunohistochemical staining indicated CD105+ fibroblasts proximal to chromogranin A+ epithelial cells in the enzalutamide treated mice, compared to vehicle treated mice (Supplementary Figure S5). Together, the data suggested that blocking the androgen axis was associated with elevated CD105 expression and PCa neuroendocrine differentiation.
Antagonizing CD105 can sensitize PCa to androgen targeted therapy
To determine if antagonizing CD105 sensitizes CRPC to ADT, we utilized an orthotopic castrate resistant tissue recombinant model comprised of human CAF and 22Rv1. The tumors were expanded for 3 weeks prior to castrating the mice. A subset of mice was also treated with enzalutamide, in the presence or absence of TRC105 for an additional 3 weeks to mimic secondary treatment after castration equivalent therapies have failed. Notably, TRC105, a human specific CD105 antagonist, does not impact host vascularization of the xenografts. Castrated mice had reduced tumor volumes compared to controls (p < 0.001; Fig 6A). Histologic measure of mitosis by phosphorylated-histone H3 was unchanged, however castration resulted in an expected increase in TUNEL staining (p < 0.0001; Fig 6B–C). In this CRPC xenograft model, the castrated mice given enzalutamide had tumor volumes and histologic measures of cell turnover statistically comparable to control intact mice. Mice treated with TRC105 alone had tumors smaller than vehicle (p < 0.05), with no notable changes in histology, proliferation, or cell death, compared to control. As patients on enzalutamide are normally at a castrate state, we combined castration with enzalutamide and compared the tumors to that generated following the addition of TRC105. We found substantially reduced tumor size with the addition of TRC105 compared to either control or castrated-enzalutamide treatment alone (p < 0.001 and < 0.01, respectively). Castrated mice given TRC105 resulted in the smallest tumor volume compared to control (p < 0.0001), with tumors too small to section for reliable histologic analysis. The marked chromogranin A staining associated with ADT (castrated mice given enzalutamide), was reduced by the added administration of TRC105 (p value <0.0001). Together, blocking the androgen axis was associated with increased CD105 contributing to neuroendocrine differentiation of PCa via fibroblasts derived paracrine SFRP1 (Fig 7).
Figure 6. Antagonizing the androgen axis and CD105 reduced tumor growth and neuroendocrine differentiation.
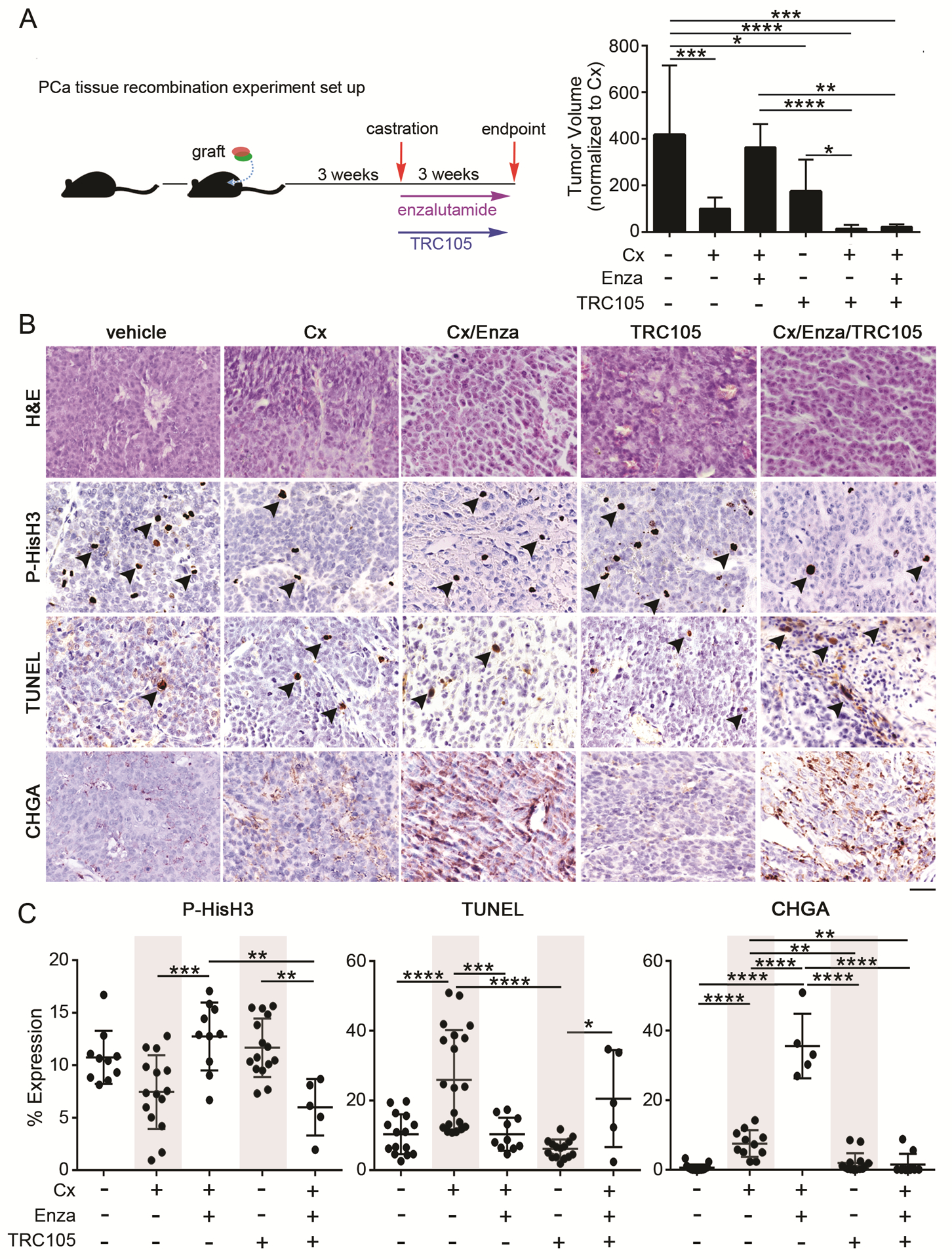
(A) Mice were orthotopically grafted with tissue recombinants of 22Rv1 and CAF. The mice were castrated, treated with TRC105, and/or enzalutamide. Bar graph shows tumor volumes normalized to castrated (Cx) mice. (B) H&E staining was followed by immune-localization for phosphorylated-histoneH3 (P-HisH3), TUNEL, and chromogranin A (CHGA). Scale bar represents 32 μm. (C) The scatter plots show the mitotic (PHis-H3), cell death (TUNEL), and chromogranin A positive staining indexes, mean +/− SD, n > 5. For all, error bars are mean +/− SD, and p values of less than 0.05 were considered statistically significant (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001).
Figure 7. Antagonizing the androgen axis increases CD105 and SFRP1 expression with elevated neuroendocrine differentiation.
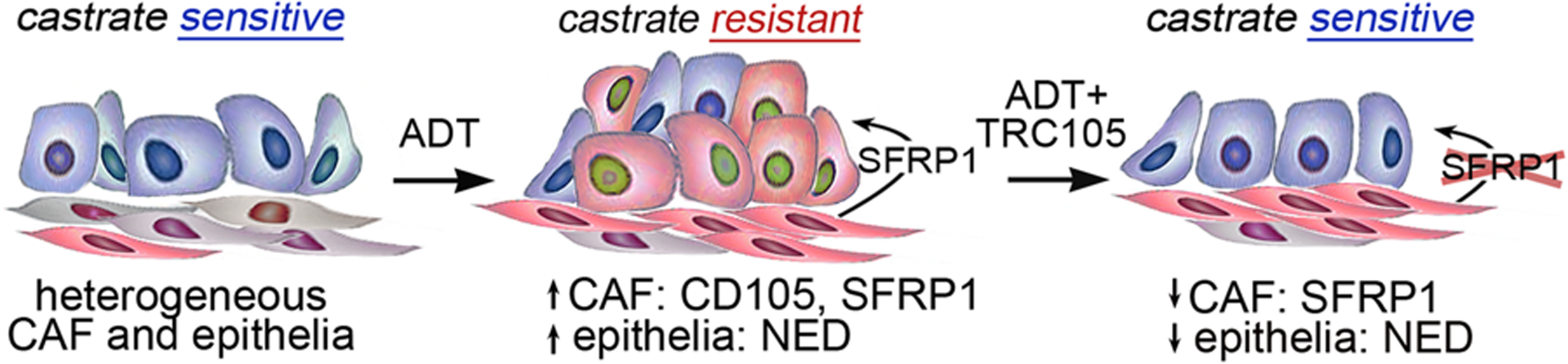
Diagram shows the evolution of prostate cancer stroma and epithelia. Castrate sensitive prostate cancer epithelia (blue top layer of cells) and stromal fibroblasts (bottom elongated layer of gray and CD105+ red cells) are both initially heterogeneous. After treatment with ADT, the epithelia and stroma express more CD105 (red). ADT induces SFRP1 secretion by fibroblasts that signal to the adjacent epithelia to induce neuroendocrine differentiation. The combined treatment with ADT and CD105 inhibition via TRC105 resulted in SFRP1 downregulation and reduced epithelial neuroendocrine differentiation in promoting castrate sensitivity.
Discussion
This study identifies the role of the CD105+ fibroblastic population in the heterogeneous stroma initiating a novel paracrine SFRP1 signaling axis critical to castrate resistant prostate cancer. We used a recognized change of the loss of tumor potentiation resulting from extended culturing of primary CAF to identify a mechanism for paracrine PCa differentiation. For the first time, we identified that CD105/BMP signaling is up regulated in response to ADT using mouse models. We found that stromal CD105 expression was associated with prostatic epithelial neuroendocrine differentiation in patient tissues and a mechanistic role for SFRP1 in the process (Fig 7). Ultimately, we discovered that combining androgen targeted therapy with a CD105 neutralizing antibody dramatically reduced neuroendocrine differentiation and resultant tumor size in a model of therapy resistant disease.
Stromal-epithelial interactions have defined organ development and cancer progression. We identified a sub-population of stromal fibroblastic cells that regulate androgen sensitivity of PCa epithelia. Previous studies have demonstrated the importance of CD105 in PCa progression [29]. However, the explicit decrease of CD105 in the CAFHiP cells initiated further interrogation of CD105 as it was similarly expressed in both NAF and CAF as well as fibroblasts from benign and PCa tissues. Validation of this culturing-associated population drift in CAFHiP also included reduction of the CD90+ population, reported to be a hallmark of tumor inductive CAF [19, 20]. FACS analysis enabled examination of the stromal population makeup on a per-cell basis, not just a total change in expression (Figs 1, 2). FACS analysis of benign and PCa tissues supported the previous reports regarding elevated CD90+ fibroblastic populations in CAF [19, 20]. There is precedence for tumor associated stromal cells to be derived from proliferative endothelia supporting breast cancer progression [30, 31]. The identification of the samples that exhibited neuroendocrine features and adjacent CD105+ fibroblasts directed us to further examine the role of CD105 in the context of ADT.
The biologic heterogeneity of advanced PCa is attributed to its resistance to ADT [32]. Although not mutually exclusive, therapeutic resistance can be a result of a population of cells that are selected by the attrition of sensitive ones or through an active acquisition of adaptive features [33].These models are based on cell-autonomous mechanisms of resistance. Although NAF and CAF were originally coined for their respective lack of or capacity to initiate tumorigenesis [3–5], when combined with established cancer epithelia, we found their shared ability to promote expansion of 22Rv1 cells (Fig 2). We used a recognized decrease in tumorigenicity by extended culturing of CAF to identify the nature of the drift in fibroblastic populations. Specific up regulation of the CD105+ fibroblastic population in response to ADT mediated neuroendocrine differentiation of PCa epithelia in a paracrine manner downstream of SFRP1 (Fig 3, 4). SFRP1, originally recognized to antagonize Wnt ligands, has previously been associated with epithelial branching morphogenesis in the prostate [34, 35]. SFRPs, have more recently been shown to play a role in promoting Wnt diffusion and correlated with tumor progression [36–38]. Importantly, SFRP1 and SFRP2 are also reported to have Wnt-independent effects [39], and the capacity to inhibit androgen receptor transcriptional activity [40, 41]. The ability for recombinant SFRP1 to induce neuroendocrine features in PCa cells, inclusive of aurora kinase, n-myc, and secretogranin-3, supported its Wnt-promoting activity (Fig 4). Stromal CD105 expression was associated with prostatic epithelial neuroendocrine differentiation, in patient tissues as well as the PDXs when administered ADT (Figs 1, 5). Consequently, we found that combining ADT with TRC105 dramatically reduced tumor size in a model of ADT resistant disease (Fig 6). The acute changes in the PDX model correlated with the longer-term tissue recombinant xenograft model (6 weeks). These findings go beyond the known association of elevated vascular CD105 with poor PCa prognosis, in providing a mechanism for the paracrine mediated fibroblast CD105-SFRP1 response to ADT [42]. Although, we focus on stromal SFRP1 eliciting neuroendocrine differentiation on prostatic epithelia, other differentially expressed genes found in our sequencing analysis in CAF vs NAF or CAFHiP may also play roles as has been demonstrated by IGFBP2 [43]. The genes in our circus plot (PDGF, TCTN1, ZNF449 and THBS) are reported to be co-expressed with SFRP1 and known to be involved in neuroendocrine differentiation (Supplemental Figure S2) [44, 45]. However, there is no claim that these genes are necessary or sufficient to induce neuroendocrine differentiation like SFRP1. The relation of the other genes in the circus plot to CD105 is yet to be determined. Our current work provides the foundation for subsequent studies to provide a well-rounded understanding of prostatic neuroendocrine differentiation.
The strategy to combine ADT and CD105 antagonism is an example of synthetic lethality. We identified a mechanism for paracrine-mediated CRPC and addressed a means for its therapeutic resolution. Since ADT promoted CD105 expression in both fibroblastic and epithelial cells (Fig 5), the cells are more susceptible to its antagonism. The roll of CD105 in fibroblasts and epithelia are likely quite different, as the epithelia do not respond to ADT by expressing SFRP1 nor was the epithelia able to develop resistance on its own. We do acknowledge the potential for CD105 effects outside of the scope of this study. Notably, elevated PSA expression in 2D co-cultures of 22Rv1 with wild type fibroblasts treated with enzalutamide corresponded with elevated proliferation in 3D co-cultures of 22Rv1 and wild type fibroblasts treated with enzalutamide. A similar phenomenon has been seen in patients, where PSA flares result from enzalutamide treatment [46]. As demonstrated in our 3D co-cultures, inhibition of both stromal and epithelial CD105 was necessary to suppress the proliferative effects of ADT on prostate cancer epithelia. However, TRC105 had little direct effect on the proliferation or viability of mono-cultured PCa epithelia in the presence or absence of enzalutamide (Figs 5 and Supplemental Figure S2), much like the lack of the progression-free survival change in CRPC patients given single agent TRC105 [47]. Overall these findings support the idea that simultaneous targeting of both stromal and epithelial cell compartments with TRC105 and ADT may provide a better therapeutic option for CRPC patients, however its clinical validation is required.
Materials and Methods
Cell culture and reagents
Primary fibroblasts were grown out of prostatectomy specimens from Cedars-Sinai Medical Center or the Greater Los Angeles Veterans Affairs under respective Institutional Review Boards [9, 48]. The designation of NAF and CAF were determined by tissue recombination with BPH1 non-tumorigenic prostatic epithelia [3–5]. Mouse primary fibroblasts were grown out of prostate specimens using the same method as human fibroblasts. Conditioned media was generated by plating NAF or CAF at a density to reach confluence at the end of 72 hours, at which time the cultured media, centrifuged, and supernatant used fresh or stored at −80°C [7, 48]. Target cells were treated with 50% conditioned media in combination with 50% control media. Viability assays were done using MTT reagent (M6494, Life Technologies) was used as directed.
Generation of CD105 knockout mouse fibroblasts
293T cells were transfected at a confluence of 70% using BioT (Bioland, CA), Cas9 vector (52962, Addgene), pCMV-dR8.91 (8455, Addgene), pCMV-VSVG (8454, Addgene) following the BioT protocol per manufacturer’s protocol with a 9:1 ratio of Delta 8.9 to VSVg. 24h after transfection the medium was replaced with only 50% of the volume and virus was collected after 48h and 72h and filtered with a sterile 0.45uM sterile syringe filter. Target wild-type mouse fibroblasts were seeded in 6 well plates at 200,000 cells/well. Virus was added with Polybrene (5 ug/mL, Sigma, MA) to the cell suspension at different ratios for a total of 2mL 1:1, 1:5, 1:10, 1:100, 1:1,000 and an uninfected control. 48h after infection, virus was replaced with 2ml of fresh medium. Selection was performed using blasticidin (5ug/mL). The well which had a 10% infection ratio was selected to ensure one infection per cell.
Once the mouse fibroblasts stably expressed CAS9, an IDT gblock was designed with the sequence: AAGGTCGGGCAGGAAGAGGGCCTATTTCCCATGATTCCTTCATATTTGCATATACGATACAAGGCTGTTAGAGAGATAATTAGAATTAATTTGACTGTAAACACAAAGATATTAGTACAAAATACGTGACGTAGAAAGTAATAATTTCTTGGGTAGTTTGCAGTTTTAAAATTATGTTTTAAAATGGACTATCATATGCTTACCGTAACTTGAAAGTATTTCGATTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCGxxxxxxxxxxxxxxxxxxxxGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTT, where the xxx were replaced by the sgRNA sequence: GGTTCGCGCGGGGATCCGAA. Fibroblasts were transfected with the gblock using the IDT reverse transfection protocol (IDT, Illinois). Single cell clones were selected, grown out, and verified with FACS.
3D organotypic co-culture
A modified version of the 3D organotypic co-culture system was generated in collagen matrix gels were prepared by mixing five volumes of rat tail collagen I with two volumes of matrigel (NCI), one volume of 10x DMEM medium (GE Healthcare Life Sciences), and one volume of FBS (Atlanta Biologicals), 22Rv1 and primary mouse prostatic fibroblasts were combined in a 1:3 ratio [49]. Nylon squares were coated with collagen and placed on metal grids in a 6-well plate. Gel plugs (150 μl) were transferred onto the nylon squares, media was added to the level of the nylon mesh and incubated at 37°C with 2% O2. The cells were expanded in the matrix for 72 hours, to be subsequently dissociated with collagenase and dispase for FACS analysis.
FACS analysis
FACS experiments were performed with eBiosciences antibodies: anti-human Stro-1-FITC (340105), anti-human CD90-PE (12-0909-42), anti-human CD105-APC (17-1057-41), anti-mouse CD105-APC (17-1051-82), anti-human CD117- PE-Cy5 (15-1178-41), anti-human Ki67-PECy7 (25-5699-41), anti-human EpCAM-FITC (53-8326-41), and anti-human EpCAM-PE (12-9326-41); and BD antibody: anti-human Annexin V (BD 556422). EpCAM (130-061-101, Miltenyi Biotec) and CD45 (130-045-801, Miltenyi Biotec) beads were used to negatively select for epithelial and immune cells, respectively, prior to FACS. BD LSRII was used to collect the data for analysis using FlowJo software v10.3. EpCAM+ cells were gated for measuring epithelial CD105, AnnexinV, or Ki67 expression in 3D co-cultures.
Animal studies
Male beige/SCID mice (Envigo), 6–8 or 10–12 weeks old, were used for sub-renal capsule or prostatic orthotopic grafting, respectively, as previously described [3, 18]. In accordance with institutional animal care and use committee approval, 2×105 22Rv1 cells and 6×105 stromal cells were suspended in 20 μL type I collagen to be grafted into the subrenal capsule of mice, randomized with equal numbers to each treatment group without blinding, castrated after seven days and sacrificed 21 days after castration. For orthotopic xenografts, mice were castrated after three weeks, they were treated 3 times weekly with enzalutamide (1 mg/mouse oral gavage) and/or TRC105 (50 μg/mouse i.v.) and sacrificed 21 days after castration. Tumor volume was calculated using the modified ellipsoid formula volume3 = π/6 × (width)2 × length. For PDX models, prostatectomy tissues were xenografted into the sub-renal capsules and mice were treated after one week with enzalutamide.
Statistical analysis
FACS comparisons were normalized with arcsine square root transformation and then followed with ANOVA analysis. After log transformation, a student’s t test was used to compare fibroblastic populations. Relative expression within each group of FACS data was plotted with Prism (GraphPad software) v6.07 using the pie or donut chart features.
For the RNA-seq data, raw sequencer data was processed using Illumina’s RTA and CASAVA pipeline software, which includes image analysis, base calling, and sequence quality scoring. Moreover, we analyzed the RNA-seq data with our transcriptome analysis pipeline, which uses the Tophat software package for performing gapped alignments against the reference genome, DESeq for detecting differential gene expression. The log-fold change and a noise filter of minimum number (2) of FPKM for a gene were used to identify differentiated genes. The complete data set was uploaded in the GEO repository, accession # GSE99744. For the volcano plot, the top 200 genes among the RNA-sequencing data comparing CAFHiP and CAF groups were analyzed using the grouped volcano plot feature with Prism. For the heat map, we pulled out the top 33 secreted genes among RNA-sequencing data, ratio values were generated for CAF/CAFHiP and CAF/NAF gene values. Next, the ratio values were ranked for each ratio value among all the genes analyzed, with the highest value having a rank of 1. The ranks of CAF/CAFHiP and CAF/NAF ratio values were summed. The sum values of the two ranks were then ranked. The lowest sum value had the lowest rank, which inversely correlated with the most significant gene expression. MATLAB was used for heatmap creation with gene-wise hierarchical clustering. Average linkage and Euclidean distances were calculated un-supervised. Two-way ANOVA analysis over all genes indicated statistically significant variations in expression patterns.
cBioPortal was used to check SFRP1 mutation, deletion, and amplification frequency and correlations across publicly available data sets generated by the TCGA Research Network: http://cancergenome.nih.gov/ as described previously (Cerami et al., 2012; Gao et al., 2013). Multiple comparisons for in vitro data were evaluated with one-way or two-way analysis of variance (ANOVA). The tumor data was analyzed using one-way ANOVA for multiple comparisons. Results were expressed as individual data points or as the mean ± S.D. p values of less than 0.05 were considered statistically significant. Relative expression within each group of FACS data was plotted with Prism software using the pie or donut chart features. The concordance of stromal CD105 population and epithelial chromogranin A expression was measured with receiver operating characteristic (ROC) curve and the area under the ROC curve (AUC). The p value for AUC (c-statistic) was determined with Mann–Whitney U test. All calculations were performed with ROC package in R. Multiple comparisons for in vitro data were evaluated with one-way or two-way analysis of variance (ANOVA). The tumor data was analyzed using one-way ANOVA for multiple comparisons.
Additional Materials and Methods can be found in the electronic supplementary material.
Supplementary Material
Acknowledgements
We also thank Charles Theurer at Tracon Pharmaceuticals, Inc. for the TRC105 and M1043 neutralizing antibodies. The RNA Sequencing and histology slide scanning was performed at the Cedars-Sinai Genomics Core and Microscopy Core, respectively. This work was supported by grants from the National Cancer Institute (CA108646) and Veterans Affairs (BX001040) to NAB.
References
- 1.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303: 848–851. [DOI] [PubMed] [Google Scholar]
- 2.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res 2003; 9: 4792–4801. [PubMed] [Google Scholar]
- 3.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD et al. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res 2001; 61: 8135–8142. [PubMed] [Google Scholar]
- 4.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59: 5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res 1991; 51: 3753–3761. [PubMed] [Google Scholar]
- 6.Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res 2008; 68: 4709–4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qi J, Tripathi M, Mishra R, Sahgal N, Fazli L, Ettinger S et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell 2013; 23: 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Sousa EMF, Vermeulen L, Fessler E, Medema JP. Cancer heterogeneity--a multifaceted view. EMBO Rep 2013; 14: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiskowski MA, Jackson RS 2nd, Banerjee J, Li X, Kang M, Iturregui JM et al. Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res 2011; 71: 3459–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhowmick NA, Ghiassi M, Aakre M, Brown K, Singh V, Moses HL. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc Natl Acad Sci U S A 2003; 100: 15548–15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennen WN, Chen S, Denmeade SR, Isaacs JT. Quantification of Mesenchymal Stem Cells (MSCs) at sites of human prostate cancer. Oncotarget 2013; 4: 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Doi Y et al. Upregulation of cancer-associated myofibroblasts by TGF-beta from scirrhous gastric carcinoma cells. Br J Cancer 2011; 105: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 2003; 22: 6557–6563. [DOI] [PubMed] [Google Scholar]
- 14.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014; 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltran H, Tagawa ST, Park K, MacDonald T, Milowsky MI, Mosquera JM et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol 2012; 30: e386–389. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal RR, Feng FY, Small EJ. Emerging Categories of Disease in Advanced Prostate Cancer and Their Therapeutic Implications. Oncology (Williston Park) 2017; 31: 467–474. [PubMed] [Google Scholar]
- 17.Dayyani F, Varkaris A, Araujo JC, Song JH, Chatterji T, Trudel GC et al. Increased serum insulin-like growth factor-1 levels are associated with prolonged response to dasatinib-based regimens in metastatic prostate cancer. Prostate 2013; 73: 979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee J, Mishra R, Li X, Jackson RS, 2nd, Sharma A, Bhowmick NA. A reciprocal role of prostate cancer on stromal DNA damage. Oncogene 2014; 33: 4924–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.True LD, Zhang H, Ye M, Huang CY, Nelson PS, von Haller PD et al. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Mod Pathol 2010; 23: 1346–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate 2009; 69: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl).
- 22.Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS One 2013; 8: e66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011; 1: 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T et al. Prostate tumor progression is mediated by a paracrine TGF-beta/Wnt3a signaling axis. Oncogene 2008; 27: 7118–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolan-Stevaux O, Zhong W, Culp S, Shaffer K, Hoover J, Wickramasinghe D et al. Endoglin requirement for BMP9 signaling in endothelial cells reveals new mechanism of action for selective anti-endoglin antibodies. PLoS One 2012; 7: e50920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Xu Y, Helseth DL Jr., Gulukota K, Yang S, Pesce LL et al. Zodiac: A Comprehensive Depiction of Genetic Interactions in Cancer by Integrating TCGA Data. J Natl Cancer Inst 2015; 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanayama M, Hayano T, Koebis M, Maeda T, Tabe Y, Horie S et al. Hyperactive mTOR induces neuroendocrine differentiation in prostate cancer cell with concurrent up-regulation of IRF1. Prostate 2017. [DOI] [PubMed] [Google Scholar]
- 28.Sun F, Zhang ZW, Tan EM, Lim ZLR, Li Y, Wang XC et al. Icaritin suppresses development of neuroendocrine differentiation of prostate cancer through inhibition of IL-6/STAT3 and Aurora kinase A pathways in TRAMP mice. Carcinogenesis 2016; 37: 701–711. [DOI] [PubMed] [Google Scholar]
- 29.Romero D, O’Neill C, Terzic A, Contois L, Young K, Conley BA et al. Endoglin regulates cancer-stromal cell interactions in prostate tumors. Cancer Res 2011; 71: 3482–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013; 15: 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 2016; 18: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017; 355: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joesting MS, Cheever TR, Volzing KG, Yamaguchi TP, Wolf V, Naf D et al. Secreted frizzled related protein 1 is a paracrine modulator of epithelial branching morphogenesis, proliferation, and secretory gene expression in the prostate. Dev Biol 2008; 317: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng L, Sun D, Fan W, Zhang Z, Li Q, Jiang T. Diagnostic value of SFRP1 as a favorable predictive and prognostic biomarker in patients with prostate cancer. PLoS One 2015; 10: e0118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteve P, Sandonis A, Ibanez C, Shimono A, Guerrero I, Bovolenta P. Secreted frizzled-related proteins are required for Wnt/beta-catenin signalling activation in the vertebrate optic cup. Development 2011; 138: 4179–4184. [DOI] [PubMed] [Google Scholar]
- 37.Siamakpour-Reihani S, Caster J, Bandhu Nepal D, Courtwright A, Hilliard E, Usary J et al. The role of calcineurin/NFAT in SFRP2 induced angiogenesis--a rationale for breast cancer treatment with the calcineurin inhibitor tacrolimus. PLoS One 2011; 6: e20412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Zhu D, Chen F, Qian M, Wei H, Chen W et al. SFRP2 augments WNT16B signaling to promote therapeutic resistance in the damaged tumor microenvironment. Oncogene 2016; 35: 4321–4334. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci 2008; 121: 737–746. [DOI] [PubMed] [Google Scholar]
- 40.Kawano Y, Diez S, Uysal-Onganer P, Darrington RS, Waxman J, Kypta RM. Secreted Frizzled-related protein-1 is a negative regulator of androgen receptor activity in prostate cancer. Br J Cancer 2009; 100: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joesting MS, Perrin S, Elenbaas B, Fawell SE, Rubin JS, Franco OE et al. Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res 2005; 65: 10423–10430. [DOI] [PubMed] [Google Scholar]
- 42.Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate 2015; 75: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Degraff DJ, Aguiar AA, Sikes RA. Disease evidence for IGFBP-2 as a key player in prostate cancer progression and development of osteosclerotic lesions. Am J Transl Res 2009; 1: 115–130. [PMC free article] [PubMed] [Google Scholar]
- 44.Laskaratos FM, Rombouts K, Caplin M, Toumpanakis C, Thirlwell C, Mandair D. Neuroendocrine tumors and fibrosis: An unsolved mystery? Cancer 2017; 123: 4770–4790. [DOI] [PubMed] [Google Scholar]
- 45.Zikusoka MN, Kidd M, Eick G, Latich I, Modlin IM. The molecular genetics of gastroenteropancreatic neuroendocrine tumors. Cancer 2005; 104: 2292–2309. [DOI] [PubMed] [Google Scholar]
- 46.Igarashi A, Fukagai T, Morita M, Hayashi K, Koshikiya A, Ogawa Y et al. Initial Experience of the Enzalutamide Treatment for Castration-Resistant Prostate Cancer. Nihon Hinyokika Gakkai Zasshi 2016; 107: 155–161. [DOI] [PubMed] [Google Scholar]
- 47.Karzai FH, Apolo AB, Cao L, Madan RA, Adelberg DE, Parnes H et al. A phase I study of TRC105 anti-endoglin (CD105) antibody in metastatic castration-resistant prostate cancer. BJU Int 2015; 116: 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS 2nd et al. Altered TGF-beta signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res 2011; 71: 1272–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol 1999; 112: 681–691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


