Abstract
Diffuse malignant mesothelioma (DMM) is a tumor of serosal membranes with propensity for progressive local disease. Because current treatment options are largely ineffective, novel therapeutic strategies based on molecular mechanisms and the disease characteristics are needed to improve the outcomes of patients with this disease. Akt kinase interacting protein 1 (Aki1; Freud-1/CC2D1A) is a scaffold protein for the PI3K–PDK1–Akt signaling module that helps determine receptor signal selectivity for EGFR. Aki1 has been suggested as a therapeutic target, but its potential has yet to be evaluated in a tumor setting. Here, we report evidence supporting its definition as a therapeutic target in DMM. In cell-based assays, Aki1 silencing decreased cell viability and caused cell-cycle arrest of multiple DMM cell lines via effects on the PKA–CREB1 signaling pathway. Blocking CREB activity phenocopied Aki1 silencing. Clinically, Aki1 was expressed in most human DMM specimens where its expression correlated with phosphorylated CREB1. Notably, Aki1 siRNA potently blocked tumor growth in an orthotopic implantation model of DMMwhen administered directly into the pleural cavity of tumor-bearing mice. Our findings suggest an important role for the Aki1–CREB axis in DMM pathogenesis and provide a preclinical rationale to target Aki1 by intrathoracic therapy in locally advanced tumors.
Introduction
Diffuse malignant mesothelioma (DMM) is an aggressive tumor arising from the mesothelial cells of serosal membranes. Several etiologic factors, including exposure to asbestos (1, 2), have been reported to be involved in the development of DMM. With introduction of strict occupational protective measures, the incidence of DMM has already reached its peak in the United States (3); however, it is predicted that there will be increases in the number of DMM patients in Europe and Australia until 2020 (3) and in Japan until 2040 (2). DMM is most likely to advance locally and the extent of the disease is directly implicated in the prognosis of DMM patients (4). The prognosis of DMM patients is extremely poor because conventional treatment regimens, such as chemotherapy and radio-therapy are inefficient in controlling locally advanced disease and/or metastasis.
Clinical trials using EGFR tyrosine kinase inhibitors (EGFR-TKIs) for treatment of DMM have had disappointing results (5, 6). This mirrors non–small cell lung cancer (NSCLC) where efficacy of EGFR-TKIs does not correlate with EGFR expression levels and the activating mutations in EGFR found in NSCLC are extremely rare in DMM tumors (7, 8). In addition, previous reports have shown that unlike EGFR-mutated lung cancer cells, multiple receptor tyrosine kinases (RTK) are simultaneously activated in DMM cell lines (9). An increased understanding of the molecular mechanisms of DMM pathology will identify novel molecules and signaling pathways responsible for DMM local progression and provide new targets for therapy (4–6).
Akt kinase–interacting protein 1 (Aki1)/Freud-1/CC2D1A has been shown to be a scaffold protein in the PI3K–PDK1–Akt pathway (10). This protein selectively forms a complex with nonmutated EGFR and Akt in response to EGF stimulation, mediates Akt activation by PDK1, and contributes to cell survival and proliferation. Recently, we reported that Aki1 constitutively associates with mutated EGFR, including the T790M gatekeeper mutation, which is the most prevalent genetic alteration underlying acquired resistance to EGFR inhibitors. Aki1 has an important role as a determinant of receptor-selective signaling for mutant EGFR, and mediates the survival signal through activation of Akt (11). Aki1 is expressed in EGFR-mutant lung cancer (53/56 cases) and pancreatic cancer (25/60 cases; refs. 11, 12). Thus, Aki1 may have novel direct therapeutic potential for the treatment of many refractory malignancies.
Because we do not currently have drugs inhibiting this target, decreasing its activity through RNAi or targeting downstream pathways remain options. RNAi is a phenomenon of RNA-dependent sequence-specific gene silencing in mammalian cells. Recently, some drug delivery systems were reported for gene therapy using nonviral vectors for delivery of DNA, siRNA, and miRNA (13). In particular, in vivo delivery of siRNAs can be highly efficacious and of very low toxicity and several clinical trials using siRNA have shown promising results (13), although nonviral gene-based therapies have yet to be approved by the FDA (14).
In this study, we identify a crucial role for Aki1 in the survival of DMM cells through the CREB signaling pathway and show that targeting Aki1 or CREB with siRNA is effective in controlling DMM in vitro and Aki1 siRNA inhibited the growth of pleural disease in in vivo orthotopic models. To the best of our knowledge, this is the first experimental report to identify the pivotal roles of Aki1 in DMM that provides grounds for targeting Aki1 as novel therapeutic strategy.
Materials and Methods
Cell cultures and reagents
We used seven human DMM cell lines, one noncancerous mesothelial cell line (Met-5A), and the HEK293T cell line. Y-MESO-8A and Y-MESO-14 were established in Aichi Cancer Research Center Institute (15). NCI-H290 were provided by Dr. Adi F. Gazdar (University of Texas Southwestern Medical Center, Dallas, TX) and Dr. Yoshitaka Sekido (Aichi Cancer Research Center Institute, Aichi, Japan). Y-MESO-8A was authenticated by short tandem repeat analysis. Y-MESO-14 and NCI-H290 authentications were not performed. MSTO-211H, NCI-H2052, NCI-H2373, NCI-H2452, Met-5A, and HEK293T were obtained directly from the ATCC within 6 months of the experiments reported. DMM cell lines are divided into three histologic origins, an epithelioid type (NCI-H290 and NCI-H2452), a sarcomatoid type (NCI-H2052 and NCI-H2373), and a biphasic type (Y-MESO-8A, Y-MESO-14, and MSTO-211H; refs. 15–17). DMM cell lines were maintained in RPMI-1640 medium supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (50 g/mL), in a humidified CO2 incubator at 37°C. Met-5A cells were cultured according to the instructions of the ATCC.HEK293T cells were cultured in DMEM supplemented with 10% FBS, penicillin (100 U/mL), and streptomycin (50 g/mL), in a humidified CO2 incubator at 37°C. All cells were passaged for less than 3 months before renewal from frozen, early-passage stocks. Cells were regularly screened for Mycoplasma, using MycoAlert Mycoplasma Detection Kits (Lonza). PKA inhibitor KT5720 and CBP–CREB interaction inhibitor were obtained from Sigma-Aldlich and Calbiochem, respectively.
Antibodies and Western blotting
Protein aliquots of 25 μg each were resolved by SDS polyacrylamide gel (Bio-Rad) electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad). After washing three times, the membranes were incubated with Blocking One (Nacalai Tesque, Inc.) for 1 hour at room temperature, and then incubated overnight at 4°C with primary antibodies to Akt, phospho-Akt (Thr308), p-CREB1, t-CREB1, p-PKAC, t-PKACα, HA, DYKDDDDK (FLAG), anti–beta-actin (13E5; 1:1,000 dilution; Cell Signaling Technology), Aki1 (1:1,000 dilution; Bethyl Laboratories), cyclin A, or EGFR antibodies (1:1,000 dilution; Santa Cruz Biotechnology). After washing three times, the membranes were incubated for 1 hour at room temperature with secondary Ab [horseradish peroxidase (HRP)–conjugated species-specific Ab]. Immunoreactive bands were visualized with SuperSignal West Dura Extended Duration Substrate Enhanced Chemiluminescent Substrate (Pierce Biotechnology). Each experiment was performed at least three times independently.
RNAi transfection
Stealth RNAi siRNA for Aki1 (HSS123402, HSS123400; Invitrogen), EGFR (HSS103114, HSS103116; Invitrogen), and Silencer Select siRNA for CREB1 (s3489, s3490; Invitrogen) were transfected with Lipofectamine RNAiMAX (Invitrogen) in accordance with the manufacturer’s instructions. Stealth RNAi siRNA Negative Control Low GC Duplex no.3 (Invitrogen) was used as scramble control throughout the experiment. Aki1, EGFR, and CREB1 knockdowns were confirmed by Western blotting analysis. Each experiment was performed at least in triplicate, and three times independently.
Proliferation assay
The cells were reseeded at 2 × 103 per well in 96-well plates, and incubated in antibiotic-containing RPMI-1640 with 10% FBS. After 24 hours of incubation, KT5720 (0.1 and 1 μmol/L), or CBP–CREB interaction inhibitor (0.1 and 0.3 μmol/L) was added to each well, and incubation was continued for a further 48 hours. These cells were then used for proliferation assay, which was measured using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium) dye reduction method. An aliquot of MTT solution (2 mg/mL; Sigma) was added to each well followed by incubation for 2 hours at 37°C as previously described (18). The media were removed and the dark blue crystals in each well were dissolved in 100 mL of DMSO. Absorbance was measured with an MTP-120 microplate reader (Corona Electric) at test and reference wavelengths of 550 and 630 nm, respectively. The percentage of growth is shown relative to untreated controls. Each sample was assayed in triplicate, with each experiment repeated at least three times independently.
Cell-cycle assay
Cells treated by Aki1 siRNA or scramble control siRNA were collected by centrifugation and resuspended at 1 × 106 cells/mL in propidium iodide (PI) staining buffer (0.1% sodium citrate, 0.1% Triton X-100, and 50 mg/mL PI) and were treated with 1 mg/mL RNase at room temperature for 30 minutes. Cell-cycle histograms were generated after analysis of PI-stained cells by FACS with a BD Biosciences FACScan. For each culture, at least 1 × 104 events were recorded. Histograms generated by FACS were analyzed by Mod-Fit cell-cycle analysis software (Verity) to determine the percentage of cells in each phase (G0–G1, S, and G2–M). Each sample was assayed in triplicate, with each experiment repeated at least two times independently.
Human phospho-kinase antibody array
We used the Human Phospho-Kinase Array Kit (R&D Systems) to measure the relative level of phosphorylation for 43 kinases and two related total proteins. The assay was performed according to the manufacturer’s instructions with modifications. Briefly, cells were cultured in RPMI-1640 containing 10% FBS, and then were lysed with array buffer before they reached confluence. The arrays were blocked with blocking buffer and incubated with 450 μg of cell lysate overnight at 4°C. Arrays were washed, incubated with an HRP-conjugated phospho-kinase antibody and treated with ECL solution (GE Healthcare), before being exposed to film. Each experiment was performed at least two times independently.
Luciferase reporter assay
We designed a dual-luciferase reporter driven by a 3x CRE consensus–binding sequence in the promoter region in addition to a TATA box, which was inserted into a lentiviral construct upstream of luciferase as previously described (19). Cells were stably transduced to express CRE wild-type reporters and ratios between the two were compared after RNAi transfection of Aki1 or scramble control. Each sample was assayed in triplicate, with each experiment repeated at least two times independently.
Plasmid construction
pCF plasmid–expressing empty Vector (Vector), FLAG-tagged human CREB (CREB-WT), or FLAG-tagged human CREB-S133A, which was CREB-kinase dead–mutated (CREB-KD) clones were purchased from Addgene, Inc. Phosphorylation of CREB on Serine133 is essential for the transcriptional function of CREB (20). The X-tremeGene HP (Roche) transfection reagent was used to transfect 211H cells with Vector, CREB-WT, or CREB-KD plasmid following the manufacturer’s instructions. 211H and HEK293T cells were stably transfected with pHM6 plasmid–expressing HA-tagged recombinant human WT-Aki1 proteins (Aki1-WT) or empty vector as a control protein by using X-tremeGene Transfection Reagent. The plasmid was constructed by Cancer Chemotherapy Center, Japanese Foundation for Cancer Research (Tokyo, Japan) as previously described (10). The successfully transduced clones were identified by G418 (Sigma-Aldrich) selection. Whole-cell lysates (25 μg) were subjected to Western blot analysis.
EGFP-Eluc gene transfection
The cDNA of EGFP from pIRES-EGFP Vector (Invitrogen) and Emerald Luc (Eluc) from Emerald Luc Vector (ELV-101, TOYOBO) were cloned into MaRXIVf Puro retrovirus vector as described previously (21). All cDNA were sequenced. Transfection was performed using Lipofectamine LTX and PLUS Reagent (Invitrogen), and retroviral infection into 211H cells was performed using the Retrovirus Packaging Kit (Ampho) according to the manufacturer’s instructions. Then, the cells were selected in Puromycin (Sigma-Aldrich).
Orthotopic model in SCID mice and in vivo RNAi
We used 5-week-old female SCID mice (Clea) for this study. All animal experiments were maintained under specific pathogen-free conditions throughout the study and complied with the Guidelines for the Institute for Experimental Animals, Kanazawa University Advanced Science Research Center (approval no. AP-081088). An orthotopic implantation model of human DMM was established as described previously (22). Briefly, 1 × 106 211H transfected the Eluc/EGFP gene cells (211H/Eluc) in100 μL of PBS were injected into the right pleural cavity of mice. On day 7 after cell inoculation, 100 μg of either scramble or Aki1 siRNA complexed with invivofectamine (Invitrogen) was injected into the right pleural cavity. siRNA and invivofectamine complex was prepared in accordance with the manufacturer’s instructions (Invitrogen). Aki1 knockdown in tumor tissue was confirmed by Western blotting analysis on day 7 after injection of siRNA complex. We evaluated tumor progression using in vivo imaging by the luminescence as described previously (21). The mice were sacrificed 19 days after tumor inoculation. Luminescence was evaluated twice per week from day 2 to day 19 after cell inoculation. All surgeries were performed under sodium pentobarbital anesthesia, and efforts were made to minimize the suffering of the animals.
Patients
A total of 68 tumor specimens were obtained from 67 DMM patients with written informed consent at the Ohio State University (Columbus, Ohio) and the Hyogo Prefectural Amagasaki Hospital (Amagasaki, Japan) in studies with Institutional Review Board approval. Of the 67 patients, 36 were epithelioid, 12 were biphasic, 10 were sarcomatoid, seven were desmoplastic, and two patients showed other types. Specimens from the 34 patients (35 tumors) were obtained in tissue microarray (TMA) at the Ohio State University, and the 33 patients (33 tumors) were obtained in the tissue at the Hyogo Prefectural Amagasaki Hospital.
Immunohistochemical studies for Aki1 and p-CREB
Paraffin-embedded tissue was cut at 4 μm and sections were placed on positively charged slides. Slides were then placed in a 60°C oven for 1 hour, cooled, deparaffinized, and rehydrated through xylene, and graded ethanol solutions to water. All slides were quenched for 5 minutes in a 3% hydrogen peroxide aqueous solution to block for endogenous peroxidase. Antigen retrieval was performed by Heat-Induced Epitope Retrieval, in which the slides were placed in a 1 x solution of Target Retrieval Solution, pH 6.0 (Dako, S1699) for 25 minutes at 96°C using a vegetable steamer (Black & Decker) and cooled for 15 minutes in solution. Slides were stained with the Intellipath Autostainer Immunostaining System. Detection system included Mach 3 Rabbit HRP (Biocare Medical; M3R531L) and Liquid DAB+ Chromogen (Dako; K346811). All incubations on the Autostainer were performed at room temperature. Slides were counterstained in Richard Allen hematoxylin, dehydrated through graded ethanol solutions, cleared with xylene, and coverslipped. Dilution experiments for immunohistochemical studies were performed in resection specimens according to the manufacturer’s instructions. On the basis of the observed expression patterns, the tumor cell staining in tissue and TMA was evaluated separately for p-CREB (1:700, clone 87G3; Cell Signaling Technology) and Aki1 (1:200; Aki1 aka CC2D1A, rabbit polyclonal; Sigma). Because p-CREB showed only nuclear staining that varied in intensity and distribution within a tumor, its expression was assessed in regard to the staining intensity as negative (0), low (1+), intermediate (2+), and high (3+). Because immunohistochemical studies for Aki1 showed only cytoplasmic staining evenly distributed within a tumor but varied in intensity, its expression was assessed as negative (0), low (1+), intermediate (2+), and high (3+).
Statistical analysis
Data from MTT assay, luciferase reporter assay, and tumor progression using in vivo imaging are expressed as means of ± SD. The statistical significance of differences was analyzed by one-way ANOVA and Spearman rank correlations performed with GraphPad Prism Ver. 6.0 (GraphPad Software, Inc.). For all analyses, a two-sided P value less than 0.05 was considered statistically significant.
Results
Specific downregulation of Aki1 inhibits cell viability and induces cell arrest in DMM cells
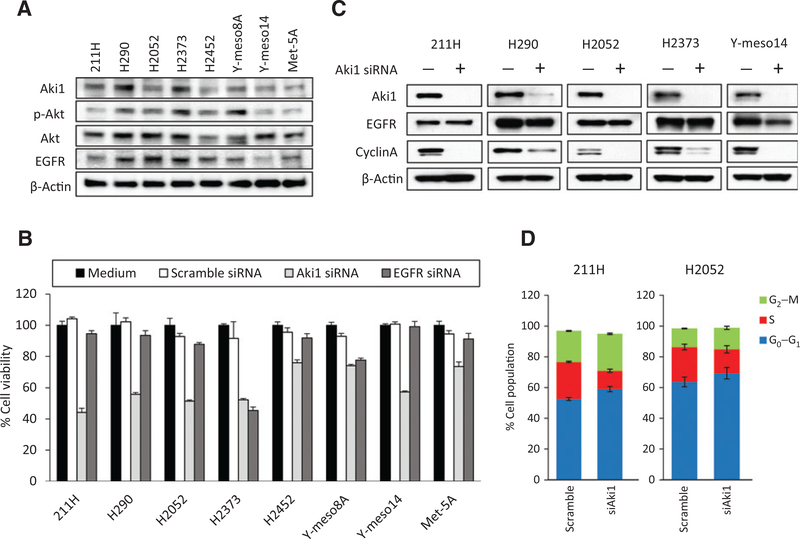
To assess the involvement of Aki1 in EGFR-mediated signaling of DMM cells, we first examined the expression of Aki1 protein and EGFR-related proteins (Akt and EGFR) in seven human DMM cell lines and compared them with an immortalized human mesothelial cell line, Met-5A, by Western blotting analysis (Fig. 1A). All of the cell lines examined expressed Aki1, Akt, and EGFR protein at various levels. However, the levels of Aki1 were generally higher in DMM cell lines than Met-5A. EGFR and phosphorylated Akt were also detected in all DMM and mesothelial cell lines at various levels. These results indicate that Aki1 expression is not correlated with EGFR-related proteins in DMM cells.
Figure 1.
Role of Aki1 on cell viability and cell cycle in malignant pleural mesothelioma cells. A, the expression of Aki1- and EGFR-related proteins in malignant pleural mesothelioma cell lines (211H, H290, H2052, H2373, H2452, Y-meso8A, and Y-meso14) and human mesothelial cell lines (Met-5A). They were lysed and the indicated proteins were detected by Western blotting analysis. B, cells were treated with Aki1, EGFR, or scramble control siRNA. After 72 hours incubation, cell viability was determined by MTT assay. C, cells were treated with scramble control or Aki1 siRNA. After 24 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting. D, cells were treated with scramble control or Aki1 siRNA. After 48 hours incubation, cell cycle was determined with an Annexin V–FITC Apoptosis Detection Kit I. The figure shows percentages of cell population (G2–M, S, and G0–G1).
To determine the role of Aki1 in DMM cell lines, we used specific siRNA (siRNA) to knockdown Aki1 or EGFR. Treatment with Aki1-specific siRNA decreased the viability of DMM cells by 40% or more in 5 of the 7 DMM cell lines examined (Fig. 1B and Supplementary Fig. S1). Treatment with an EGFR-specific siRNA decreased the cell viability in H2373 dramatically and Y-meso8A to a lesser degree; however, most DMM cell lines and the Met-5A line were left unaffected with EGFR knockdown. The effects of EGFR or Aki1 siRNAs in a noncancerous mesothelial cells, Met-5A, were only marginal. These results suggest that Aki1 knockdown inhibits viability of at least a subset of DMM cells and is independent of the EGFR signaling.
Western blotting analyses showed that Aki1 knockdown in the five most sensitive DMM cell lines, reduced cyclin A protein expression, consistent with the decrease in cell viability (Fig. 1C). Cyclin A is known to moderate the initiation and completion of DNA replication during S phase and reduction of cyclin A with Aki1 knockdown suggests that Aki1 contributes to the regulation of cell-cycle progression. Indeed, cell-cycle analysis confirmed that knockdown of Aki1 decreased the number of cells in S phase in 211H and H2052 (Fig. 1D).
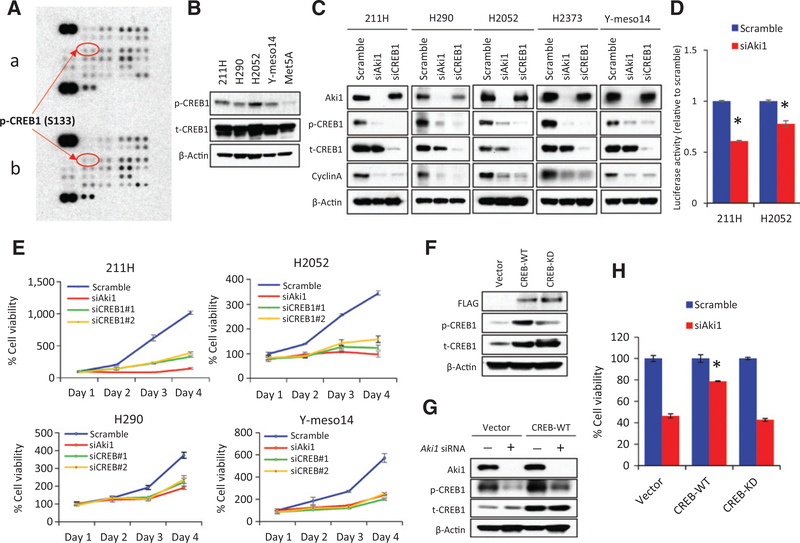
Aki1 maintains the viability of DMM cells through CREB1 activation
To elucidate the downstream activities regulated by Aki1 that are involved in cell viability of DMM cells, we performed a human phosphokinase array to compare Aki1 containing with Aki1 knockdown cells (Fig. 2A). Several kinase phosphorylation sites were changed with Aki1 knockdown. Interestingly, the CREB1 phosphorylation level at serine 133 (pS133) was decreased when cells were transfected with Aki1 siRNA. Examination of DMM cell lines by Western blot analysis for CREB1 pS133 indicates that all of the DMM cell lines appear to have higher levels of pS133 than the immortalized mesothelial cell line, Met-5A (Fig. 2B). Western blotting analyses confirmed the phosphokinase array result showing that Aki1 downregulation decreases phospho-CREB1 levels in multiple DMM cell lines (Fig. 2C). The Western blot analysis also showed that the specific downregulation of CREB1 by siRNA reduced cyclin A protein expression in five DMM cell lines, which is also seen with Aki1 knockdown. To evaluate how Aki1 loss affects CREB activity, we transfected DMM cell lines with a CRE reporter. The CRE-Luciferase reporter assay demonstrated that the treatment with Aki1 siRNA significantly inhibits CREB1 activity in 211H and H2052 cells compared with scramble control (P < 0.05 by one-way ANOVA; Fig. 2D).
Figure 2.
Aki1 maintains the viability of DMM cells through CREB1 activation. A, human kinase phosphorylation array analysis in 211H cells with scramble control siRNA (a) or Aki1 siRNA (b) after 48 hours incubation. B, total and phosphorylation of CREB expressions in four DMM (211H, H290, H2052, and Y-meso14) and mesothelial (Met-5A) cells. Cells were lysed and the indicated proteins were detected by Western blotting. C, luciferase activity assay against CREB1. Cells were stably transduced to express wild-type CRE reporters, which binds to CREB1 as a certain DNA sequences, and ratios between the two were compared after RNAi transfection of Aki1 or scramble control; *, P < 0.05 versus scramble control by one-way ANOVA. D, cells were treated with scramble control, Aki1, or CREB1 siRNA. After 24 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting analysis. E, cells were treated with Aki1, CREB1 (#1, #2), or scramble control siRNA. After 24, 48, 72, and 96 hours incubation, cell viability was determined by MTT assay. F, pCF plasmid–expressing empty vector (Vector), FLAG-tagged human CREB (CREB-WT), or FLAG-tagged human CREB-kinase dead–mutated (CREB-KD) clones were transfected into 211H cells. They were lysed and the indicated proteins were detected by Western blotting analysis. G, total and phosphorylation of CREB1 expressions in 211H cells with transfection of vector, CREB-WT, or CREB-KD clones were treated with Aki1 or scramble control siRNA. After 24 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting. H, 211H cells with transfection of vector, CREB-WT, or CREB-KD clones were treated with Aki1 or scramble control siRNA. After 72 hours incubation, cell viability was determined by MTT assay; *, P < 0.05 versus cells with vector or CREB-KD clones treated with Aki1 siRNA by one-way ANOVA.
We next evaluated the role of CREB1 activity in maintaining DMM cell viability. DMM cells treated with a CREB1 siRNA show remarkably reduced cell viability when compared with cells treated with the scrambled control (Fig. 2E and Supplementary Fig. S2). Notably, the reduction in cell viability is very similar to cells treated with Aki1 siRNA. The immortalized mesothelial cell line, Met-5A, does not appear to be as affected by CREB1 and Aki1 knockdown as the DMM cell lines (Supplementary Fig. S3). These findings suggest that the Aki1–CREB1 axis has an important role in maintaining the viability of DMM cells.
To further prove that CREB1 activity is important in DMM cell viability and is downstream of Aki1, we surmised that enforced expression of CREB1 might overcome Aki1 knockdown and maintain cell viability. To do this, we transfected 211H cells with empty Vector, CREB-WT, and a mutated CREB that lacks transcriptional activity, CREB-S133A. Western blot analysis of transfected 211H cells clearly shows an increase in both the total CREB1 and phosphorylated CREB1 (Fig. 2F). Treatment of the transfected cells with Aki1 siRNA reduced the phosphorylation of CREB1 in the vector control cells as expected. Although phosphorylated CREB1 in CREB-WT transfected cells was reduced in the Aki1 knockdown cells, it was still expressed at a relatively high level compared with vector control (Fig. 2G). More importantly, cells expressing wild-type CREB had a significantly higher viability than vector control cells or cells expressing the mutated form of CREB (P < 0.05 by one-way ANOVA; Fig. 2H). These findings confirm that there is indeed an Aki1–CREB axis and that increased CREB1 activity downstream of Aki1 is important in maintaining cell viability of DMM cells.
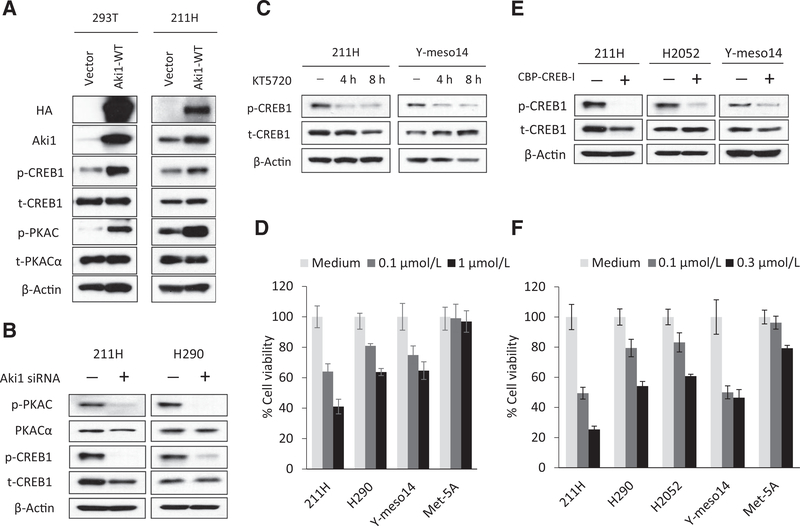
Aki1 regulates CREB by modulating protein kinase A phosphorylation and activity
To gain a better understanding of how Aki1 regulates CREB1 activity, Aki1 was overexpressed and PKA, a major regulator of CREB phosphorylation at S133, was examined. To do this, we made an Aki1 expression construct that was transfected into both HEK293T and 211H cells. The exogenously expressed Aki1 protein induced the phosphorylation of CREB1 and the catalytic subunit of Protein Kinase A (PKAC) in both cell lines (Fig. 3A). Alternatively, treatment of two DMM cell lines with Aki1 siRNA reduced PKAC phosphorylation (Fig. 3B). We also determined the effect of the PKA inhibitor KT5720 on the cell viability of DMM and Met-5A cells. Treatment of DMM cells with KT5720 reduces CREB1 phosphorylation after 4 and 8 hours incubations (Fig. 3C). KT5720 also inhibits the growth of DMM cells in a dose-dependent manner with little effect on the Met-5A cells (Fig. 3D). In addition, we investigated the efficacy of the CBP–CREB interaction inhibitor (CBP–CREB-I) on the cell growth of DMM and Met-5A cells. CBP–CREB-I inhibits the CREB1 phosphorylation in DMM cells (Fig. 3E) and strongly suppresses the viability of DMM cells, with marginal effects on the Met-5A cells (Fig. 3F). Thus, our findings clearly show that Aki1 mediates the activation of the PKA–CREB axis in DMM cells and may provide an attractive therapeutic option in DMM (Supplementary Fig. S4).
Figure 3.
Aki1 regulates CREB by modulating protein kinase A phosphorylation and activity. A, HEK293T and 211H cells were stably transfected with pHM6 plasmid–expressing HA-tagged recombinant human WT-Aki1 proteins (Aki1-WT) or empty vector (Vector). They were lysed and the indicated proteins were detected by Western blotting analysis. B, cells were treated with scramble control or Aki1 siRNA. After 24 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting. C, cells were treated with KT5720 (0.1 μmol/L). After 4 and 8 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting. D, cells were treated with KT5720 (0.1 and 1.0 μmol/L). After 72 hours incubation, cell viability was determined by MTT assay. E, cells were treated with CBP–CREB interaction inhibitor (CBP–CREB-I; 0.1 μmol/L). After 4 hours incubation, cells were lysed and the indicated proteins were detected by Western blotting. F, cells were treated with CBP-CREB-I (0.1 and 0.3 μmol/L). After 72 hours incubation, cell viability was determined by MTT assay.
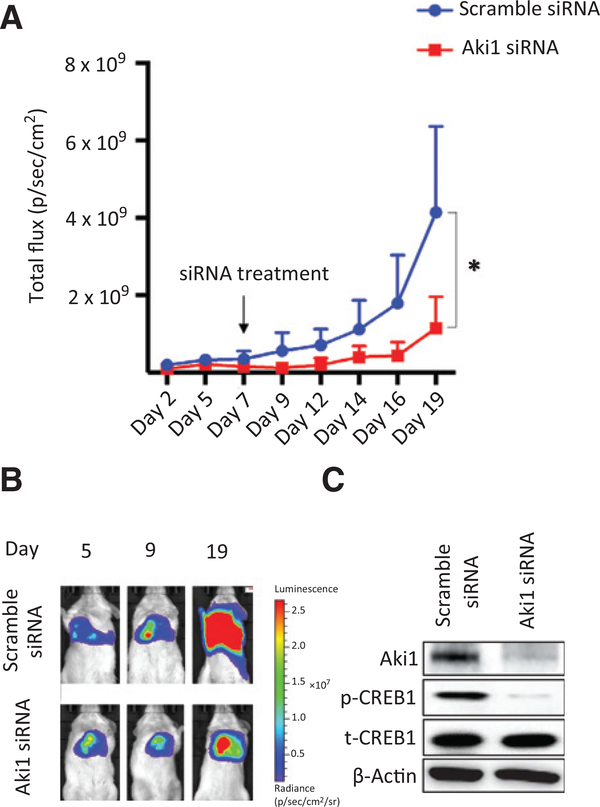
Direct injection of Aki1 siRNA into the pleural cavity of a DMM orthotopic mouse model dramatically decreases tumor formation
We next examined the antitumor potential of Aki1 siRNA by directly injecting the siRNA into pleural cavity of a DMM orthotopic mouse model. The DMM 211H cell line expressing luciferase was implanted into the pleural cavity of immunocompromised mice. Seven days after implantation of the DMM cells Aki1 siRNA or a control siRNA complexed with invivofectamine was injected as a single dose. Bioimaging was used to monitor disease progression. The single injection of Aki1 siRNA into the pleural cavity significantly inhibited tumor growth in comparison with that of scramble control 12 days after Aki1 treatment (P < 0.05 by one-way ANOVA; Fig. 4A and B). We confirmed the knockdown of Aki1 and the inhibition of CREB1 phosphorylation in tumors by Western blotting analysis (Fig. 4C). These results clearly indicate the therapeutic potential of Aki1 siRNA against DMM localized to the pleural cavity.
Figure 4.
Direct injection of Aki1 siRNA into the pleural cavity of a DMM orthotopic mouse model dramatically decreases tumor formation. 211H transfected the Eluc/EGFP gene cells (211H/Eluc; 1 × 106 cells per 100 mL of PBS) and were injected into the right pleural cavity of 5-week-old male SCID mice. On day 7 after cell inoculation, 100 μg of either scramble or Aki1 siRNA complexed with invivofectamine was injected into the right pleural cavity. A, luminescence was evaluated twice per week from day 2 to day 19 after cell inoculation. Bars, SE is shown for groups of 8 mice; *, P < 0.05 by one-way ANOVA. B, the appearance of representative mice is shown. C, the harvested tumors, which are on day 7 after injection of siRNA complex, were examined for Aki1 and phosphorylated CREB1 by Western blotting analysis.
Aki1 and phosphorylated CREB are frequently expressed in human DMM tumors
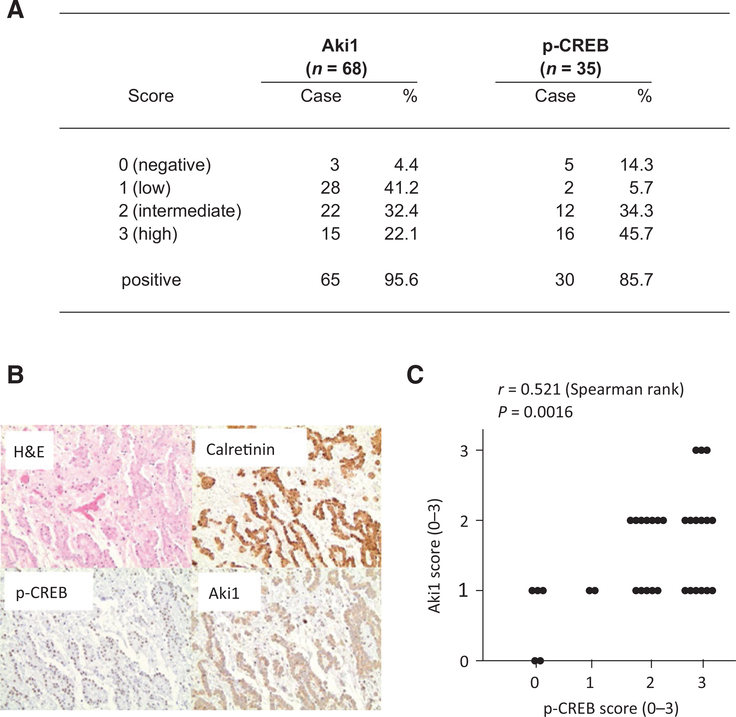
We examined Aki1 expression in 68 clinical specimens obtained from 67 DMM patients (Fig. 5A and B and Supplementary Fig. S5). Of 68 DMM tumors, Aki1 was expressed at some level in 65 (95.6%). High cytoplasmic Aki1 protein expression was detected in 15 (22.1%), intermediate in 22 (32.4%), low in 28 (41.2%), and negative expression in 3 (4.4%) of 68 DMM tumors. Aki1 protein expression was detected in 37 of 37 (100%) of epithelioid DMM, 8 of 10 (80%) sarcomatoid DMM, and 12 of 12 (100%) biphasic DMM (Supplementary Fig. S6A). We next examined phosphorylated CREB expression in 35 clinical specimens obtained from 34 DMM patients (Fig. 5A and B and Supplementary Fig. S5). Of 35 DMM tumors, phosphorylated CREB was expressed at some level in 30 tumors (85.7%). High nuclear expression of phosphorylated CREB protein was detected in 16 (45.7%), intermediate in 12 (34.3%), low in 2 (5.7%), and negative expression in 5 (14.3%) of 35 DMM tumors. Phosphorylated CREB was detected in 20 of 21 (95%) epithelioid, in 1 of 5 (20%) sarcomatoid, and in 6 of 6 (100%) biphasic DMM (Supplementary Fig. S6A). There was a statistically significant correlation between expression of Aki1 and phosphorylated CREB (Spearman rank correlations = 0.521; P = 0.002, N = 34; Fig. 5C). The epithelioid DMM demonstrated the strongest correlation between expression levels of Aki1 and phosphorylated CREB (Spearman rank correlations = 0.561; P = 0.009, N = 21; Supplementary Fig. S6B). These findings suggested involvement of phosphorylated CREB in Aki1-mediating signaling in DMM tumors, especially epithelioid types.
Figure 5.
Aki1 and phosphorylated CREB are frequently expressed in DMM. Clinical specimens from DMM patients evaluated for Aki1 and phosphorylated CREB by immunohistochemistry. A, high cytoplasmic Aki1 protein expression was detected in 15 (22.1%), intermediate in 22 (32.4%), low in 28 (41.2%), and negative expression in 3 (4.4%) of 68 DMM tumors. High nuclear expression of phosphorylated CREB protein was detected in 16 (45.7%), intermediate in 12 (34.3%), low in 2 (5.7%), and negative expression in 5 (14.3%) of 35 DMM tumors. B, representative images of epithelioid DMM, hematoxylin and eosin stain (H&E), showing diffuse expression of calretinin, p-CREB, and Aki1. C, the correlation of expression scores between Aki1 and phosphorylated CREB in tumors of DMM patients (Spearman rank correlations = 0.521; P = 0.002, N = 34).
Discussion
Prior observations where the knockdown of EGFR by siRNA hardly affected cell viability of DMM (23), suggested that targeting molecules downstream of the multiple RTKs is a more effective therapeutic option.
Previously, we identified Aki1 as a scaffold protein critical for transmitting the signal through the EGFR–PI3K–PDK1–Akt pathway. Downregulation of Aki1 dramatically decreased cell viability in EGFR-mutant lung cancer cell lines (11). These data suggested that Aki1 had a role in aberrant signaling through the EGFR–PI3K–Akt pathway. Our screening approach through monitoring altered kinase phosphorylation in Aki1 knockdown DMM cells led to the discovery that Aki1 was signaling through a different pathway. On the basis of in vitro studies, we showed for the first time, that Aki1 is critical in proliferation and cell viability of DMM. This process is independent of EGFR signaling and involves CREB1 activation by modulating PKA phosphorylation.
The observation that phosphorylation of CREB1 was decreased by knockdown of Aki1 agrees with previous studies in nonsyndromic mental retardation where Aki1 is a regulator of the cAMP–PKA pathway in neuronal cell differentiation (24, 25). Mutant mice with a truncated Aki1 showed defective PKA activation and CREB phosphorylation in the neuronal system (25). Hence, Aki1 has important roles in regulating the activity of the PKA–CREB axis in neuronal differentiation and in brain development as well as in malignant tumors.
CREB1 is a 43 kDa basic/leucine zipper transcription factor that mediates signaling from extracellular stimulation and can be phosphorylated and activated by many kinases, including PKA, protein kinase C, Ca2+/calmodulin-dependent kinase, extracellular signal–regulated kinases, Akt, and p90 ribosomal S6 kinase (26). Phosphorylation of CREB1 on Serine 133 has an important role in the transcriptional activity of CREB1 by promoting the interaction with the coactivator CREB-binding protein (CBP)/p300. CREB promotes cell proliferation, cell-cycle progression, and survival of myeloid cells through induction of specific target genes (27). In addition, the overexpression and activation of CREB1 has been associated with poor prognosis in nonsmokers with NSCLC (28), melanoma metastasis (29), and acute myelogenous leukemia (30). With regard to the roles of CREB1 in DMM, CREB-induced inflammation is associated with cell growth and asbestosis-related DMM (31). Recent studies in DMM cells, both in vitro and in vivo, as well as our study, demonstrate that CREB inhibition by RNAi or small molecules, results in significant attenuation of proliferation, migration, and drug resistance to cytotoxic chemotherapy, such as doxorubicin (31, 32). In addition, our study demonstrates that treatment with small-molecule inhibitors targeting PKA or CREB reduces proliferation of DMM cells in vitro
Our data would suggest that inhibition of Aki1, or a downstream pathway component such as PKA, would be an effective strategy to treat DMM. However, Aki1 is a scaffold protein without a known small-molecule inhibitor. An inhibitor of PKA would be an option, and finding a PKA inhibitor is currently an area of active research (33, 34). Phase I trials of an antisense RNA against the PKA regulatory subunit, RIα, were performed in multiple solid tumors (35). This was a systemic approach with i.v. infusion of the antisense RNA, and although it appeared that PKA activity decreased as measured by serum extracellular PKA (ECPKA) activity, there were no objective responses to treatment. A previous study showed that tumor growth of several cancer types was blocked by multiple i.p. injections of siRNAs for Zcchc11, Lin28A, or Lin28B using a xenograft model (36). A recent article reports the pleural and peritoneal injection of a recombinant human p53-containing adenovirus to control malignant effusions (37). This treatment provided excellent local control of the effusion, was safe, and could be an option for patients unable to undergo standard chemotherapeutic treatments. As for a local therapy using pleural injection, we have demonstrated the efficacy of a single direct injection into the pleural cavity of a siRNA to Aki1 in an orthotopic mouse model of DMM. Given the unique aspects of mesothelioma biology, and particularly its localization to the pleural cavity in most cases, direct injection of an antisense RNA could be practical, and provide local control and reduce side effects associated with standard systemic cytotoxic chemotherapy. Phosphorylated CREB1 has been shown to be increased in mesothelioma compared with reactive mesothelial hyperplasia and normal lung tissue (31). The results of human tissue analysis in our study are in keeping with that observation, but also for the first time document that Aki1 is also frequently overexpressed and correlates with expression of phosphorylated CREB in DMM. Among DMM subtypes, the strongest correlation between the expression levels of these molecules was seen in epithelioid DMM, although the cell-based analysis showed the high expression of both proteins even in sarcomatoid type. We cannot explain why these correlations were not apparent in our TMA samples, but is likely related to the small number of sarcomatoid samples in our TMA. Further experiments are needed to confirm these histologic differentiations in DMM.
In summary, we have demonstrated that Aki1 has roles in the cell growth, cell cycle, and transcription though the activation of PKA–CREB1 signaling for DMM cells. Direct application of a single dose of Aki1 siRNA into the pleural cavity of mice significantly inhibited the growth of 211Hcells within the pleural cavity in an orthotropic implantation model. Furthermore, Aki1 is frequently expressed in DMM tumors and correlated with phosphorylation of CREB1, especially epithelioid types. Our data provide a rationale for targeting Aki1 by intrathoracic therapy in patients with local invasive DMM tumors.
Supplementary Material
Acknowledgments
The authors appreciate that NCI-H290 was provided by Dr. Adi F. Gazdar (University of Texas Southwestern Medical Center, Dallas, TX) and Dr. Yoshitaka Sekido (Aichi Cancer Research Center Institute, Aichi, Japan). The authors thank Dr. Takayuki Nakagawa and Mr. Kenji Kita (Cancer Research Institute, Kanazawa University, Kanazawa, Japan) for technical advice and for technical support.
Grant Support
This study was supported by Grants-in-Aid for Cancer Research (25860638 to T. Yamada), Scientific Research on Innovative Areas “Integrative Research on Cancer Microenvironment Network” (22112010A01 to S. Yano) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and the Dellapezze Family Foundation (D.P. Carbone).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Broaddus VC. Asbestos, the mesothelial cell and malignancy: a matter of life or death. Am J Respir Cell Mol Biol 1997;17:657–9. [DOI] [PubMed] [Google Scholar]
- 2.Morinaga K, Kishimoto T, Sakatani M, Akira M, Yokoyama K, Sera Y. Asbestos-related lung cancer and mesothelioma in Japan. Ind Health 2001;39:65–74. [DOI] [PubMed] [Google Scholar]
- 3.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591–603. [DOI] [PubMed] [Google Scholar]
- 4.Pasello G, Favaretto A. Molecular targets in malignant pleural mesothelioma treatment. Curr Drug Targets 2009;10:1235–44. [DOI] [PubMed] [Google Scholar]
- 5.Garland LL, Rankin C, Gandara DR, Rivkin SE, Scott KM, Nagle RB, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 2007;25:2406–13. [DOI] [PubMed] [Google Scholar]
- 6.Govindan R, Kratzke RA, Herndon JE II, Niehans GA, Vollmer R, Watson D, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res 2005;11:2300–4. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch FR, Varella-Garcia M, Bunn PA Jr, Franklin WA, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non–small cell lung cancer. J Clin Oncol 2006;24:5034–42. [DOI] [PubMed] [Google Scholar]
- 8.Mezzapelle R, Miglio U, Rena O, Paganotti A, Allegrini S, Antona J, et al. Mutation analysis of the EGFR gene and downstream signalling pathway in histologic samples of malignant pleural mesothelioma. Br J Cancer 2013;108:1743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawaguchi K, Murakami H, Taniguchi T, Fujii M, Kawata S, Fukui T, et al. Combined inhibition of MET and EGFR suppresses proliferation of malignant mesothelioma cells. Carcinogenesis 2009;30:1097–105. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura A, Naito M, Tsuruo T, Fujita N. Freud-1/Aki1, a novel PDK1-interacting protein, functions as a scaffold to activate the PDK1/Akt pathway in epidermal growth factor signaling. Mol Cell Biol 2008;28:5996–6009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada T, Takeuchi S, Fujita N, Nakamura A, Wang W, Li Q, et al. Akt kinase-interacting protein1, a novel therapeutic target for lung cancer with EGFR-activating and gatekeeper mutations. Oncogene 2013;32:4427–35. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsubo K, Yamada T, Zhao L, Jin TF, Takeuchi S, Mouri H, et al. Expression of Akt kinase-interacting protein 1, a scaffold protein of the PI3K/PDK1/Akt pathway, in pancreatic cancer. Pancreas 2014;43:1093–100. [DOI] [PubMed] [Google Scholar]
- 13.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Nonviral vectors for gene-based therapy. Nat Rev Genet 2014;15:541–55. [DOI] [PubMed] [Google Scholar]
- 14.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater 2013;12:967–77. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi T, Karnan S, Fukui T, Yokoyama T, Tagawa H, Yokoi K, et al. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci 2007;98:438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannetti E, Zucali PA, Assaraf YG, Leon LG, Smid K, Alecci C, et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin.Br J Cancer 2011;105: 1542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Wang W, Yamada T, Matsumoto K, Sakai K, Bando Y, et al. Pleural mesothelioma instigates tumor-associated fibroblasts to promote progression via a malignant cytokine network. Am J Pathol 2011;179: 1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yano S, Wang W, Li Q, Matsumoto K, Sakurama H, Nakamura T, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479–87. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman JM, Amann JM, Park K, Arasada RR, Li H, Shyr Y, et al. LKB1 Loss induces characteristic patterns of gene expression in human tumors associated with NRF2 activation and attenuation of PI3K-AKT. J Thorac Oncol 2014;9:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2001;2:599–609. [DOI] [PubMed] [Google Scholar]
- 21.Nanjo S, Nakagawa T, Takeuchi S, Kita K, Fukuda K, Nakada M, et al. In vivo imaging models of bone and brain metastases and pleural carcinomatosis with a novel human EML4-ALK lung cancer cell line. Cancer Sci 2015;106: 244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakataki E, Yano S, Matsumori Y, Goto H, Kakiuchi S, Muguruma H, et al. Novel orthotopic implantation model of human malignant pleural mesothelioma (EHMES-10 cells) highly expressing vascular endothelial growth factor and its receptor. Cancer Sci 2006;97:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jänne PA, Taffaro ML, Salgia R, Johnson BE. Inhibition of epidermal growth factor receptor signaling in malignant pleural mesothelioma. Cancer Res 2002;62:5242–7. [PubMed] [Google Scholar]
- 24.Al-Tawashi A, Gehring C. Phosphodiesterase activity is regulated by CC2D1A that is implicated in non-syndromic intellectual disability. Cell Commun Signal 2013;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Tawashi A, Jung SY, Liu D, Su B, Qin J. Protein implicated in nonsyndromic mental retardation regulates protein kinase A (PKA) activity. J Biol Chem 2012;287:14644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res 2009;15:2583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankar DB, Cheng JC, Kinjo K, Federman N, Moore TB, Gill A, et al. The role of CREB as a proto-oncogene in hematopoiesis and in acute myeloid leukemia. Cancer Cell 2005;7:351–62. [DOI] [PubMed] [Google Scholar]
- 28.Seo HS, Liu DD, Bekele BN, Kim MK, Pisters K, Lippman SM, et al. Cyclic AMP response element-binding protein overexpression: a feature associated with negative prognosis in never smokers with non–small cell lung cancer. Cancer Res 2008;68:6065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braeuer RR, Zigler M, Villares GJ, Dobroff AS, Bar-Eli M. Transcriptional control of melanoma metastasis: the importance of the tumor microenvironment. Semin Cancer Biol 2011;21:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho EC, Mitton B, Sakamoto KM. CREB and leukemogenesis. Crit Rev Oncog 2011;16:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, et al. Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am J Pathol 2009;175: 2197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westbom CM, Shukla A, MacPherson MB, Yasewicz EC, Miller JM, Beuschel SL, et al. CREB-induced inflammation is important for malignant mesothelioma growth. Am J Pathol 2014;184:2816–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enns LC, Ladiges W. Protein kinase A signaling as an anti-aging target. Ageing Res Rev 2010;9:269–72. [DOI] [PubMed] [Google Scholar]
- 34.Tortora G, Ciardiello F. Protein kinase A as target for novel integrated strategies of cancer therapy. Ann N Y Acad Sci 2002;968:139–47. [DOI] [PubMed] [Google Scholar]
- 35.Mani S, Goel S, Nesterova M, Martin RM, Grindel JM, Rothenberg ML, et al. Clinical studies in patients with solid tumors using a second-generation antisense oligonucleotide (GEM 231) targeted against protein kinase A type I. Ann N Y Acad Sci 2003;1002:252–62. [DOI] [PubMed] [Google Scholar]
- 36.Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, et al. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 2011;147:1066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Hu Y, Wang J, Zhang S, Tao H, Jing S, et al. Efficacy of recombinant adenoviral human p53 gene in treatment of malignant pleural or peritoneal effusions. Zhongguo Fei Ai Za Zhi 2013;16:153–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.