Abstract
Background
This study sought to determine the 12-month effects of exercise increases on objective and subjective sleep quality in initially inactive older persons with mild to moderate sleep complaints.
Methods
A nonclinical sample of underactive adults 55 years old or older (n = 66) with mild to moderate chronic sleep complaints were randomly assigned to a 12-month program of primarily moderate-intensity endurance exercise (n = 36) or a health education control program (n = 30). The main outcome measure was polysomnographic sleep recordings, with additional measures of subjective sleep quality, physical activity, and physical fitness. Directional hypotheses were tested.
Results
Using intent-to-treat methods, at 12 months exercisers, relative to controls, spent significantly less time in polysomnographically measured Stage 1 sleep (between-arm difference = 2.3, 95% confidence interval [CI], 0.7–4.0; p = .003), spent more time in Stage 2 sleep (between-arm difference = 3.2, 95% CI, 0.6–5.7; p = .04), and had fewer awakenings during the first third of the sleep period (between-arm difference = 1.0, 95% CI, 0.39–1.55; p = .03). Exercisers also reported greater 12-month improvements relative to controls in Pittsburgh Sleep Quality Index (PSQI) sleep disturbance subscale score (p = .009), sleep diary–based minutes to fall asleep (p = .01), and feeling more rested in the morning (p = .02).
Conclusions
Compared with general health education, a 12-month moderate-intensity exercise program that met current physical activity recommendations for older adults improved some objective and subjective dimensions of sleep to a modest degree. The results suggest additional areas for investigation in this understudied area.
Keywords: Physical activity, Exercise, Sleep, Intervention, Older adult, Polysomnography, Subjective sleep
MUCH of the sleep literature has focused on clinical populations of poor sleepers, including individuals with clinical levels of insomnia (1). However, a sizeable proportion of the general midlife and older adult population reports chronic, albeit milder, sleep complaints that impair quality of life and result in health care visits (2). Short-term experimental studies have shown a positive impact of exercise on subjective sleep quality among mildly to moderately sleep-impaired adults (3–5). However, no study has examined experimentally the longer-term impacts of exercise increases on objectively measured sleep architecture as well as subjective sleep quality in older persons with mild to moderate sleep complaints (6). This study sought to answer the following primary question: Does increased community-based moderate-intensity exercise that meets current public health recommendations for older adults (7) improve objectively and subjectively measured sleep quality across 12 months among adults 55 years old or older with chronic mild to moderate sleep complaints?
METHODS
Study Design
Study eligibility consisted of: (a) age 55 years old or older; (b) not engaged in >60 minutes per week of moderate or more vigorous physical activity over the previous 6 months; (c) free of any medical condition that would limit participation in moderate-intensity exercise; (d) body mass index ≤ 35; (e) average alcohol intake ≤ 3 drinks per day and nonsmoker; (f) able to speak and understand English sufficiently to provide informed consent; (g) score ≥3 on at least two of three items of the Sleep Questionnaire and Assessment of Wakefulness (8) focused on getting to sleep, waking up during the night, and waking up in the morning; (h) free of sleep apnea or other clinically diagnosed sleep disorder, the former ruled out by a multivariate apnea prediction (MAP) score ≤ 0.8 (9) and an overnight pulse oximetry (Nellcor NPB-290) screening of <10% cumulative time of oxygen saturation (SaO2) < 90% and a desaturation index (>3% falls in SaO2 per sleep hour) <10/hour (10,11); (i) stable on all medications for at least 3 months prior to study enrollment; and (j) willing to be randomized to either of the two study arms. Major recruitment strategies featured a “Round the Clock” wellness promotion theme, which focused on enhancing overall health-related quality of life as opposed to sleep quality, in local media outlets. Individuals who met initial eligibility requirements underwent baseline assessment. Following stratification by gender, participants were randomly assigned (12) to one of two study arms: (i) moderate-intensity exercise delivered by trained study personnel in a community setting (local YMCA); or (ii) a health education control arm. Evaluation of enrollees in the two study arms occurred by staff blinded to participant arm assignment. Appropriate university institutional review boards approved the study protocol.
Following random assignment to study arm, all participants met with a staff health educator. Both arms received brief standard sleep hygiene recommendations and reading materials as recommended by national health organizations (13).
Interventions
Exercise
Exercise participants were instructed to attend exercise classes 2 days/week and exercise an additional 3 days/week on their own across the 12-month period (14). The 60-minute exercise classes, taught by trained staff in the morning and early afternoon hours, were composed primarily of moderate-intensity endurance exercises (e.g., aerobic movement using step platforms) (35–40 minutes/session), along with basic stretching, strengthening, and balance exercises (7). Participants wore Polar heart rate monitors to ensure that they achieved the target heart rate range (60%–85% of baseline treadmill-based peak heart rate). Participants were instructed to undertake their home-based exercise sessions at similar times of the day as the exercise class (i.e., before dinner). They were instructed to engage in at least 30 minutes of aerobic exercise in their training target heart rate range (using Polar monitors) at least 3 times/week as the major home-based activity.
Health education control
Individuals were offered 12 months of weekly 90-minute health education classes taught by trained study personnel that focused on a variety of nonphysical activity topics of interest to middle-aged and older adults such as nutrition, home safety, and foot care. Such classes are similar in format and frequency to those offered in many communities throughout the United States.
Measurement of Physical Activity
Stanford 7-day physical activity recall (PAR)
The PAR, a validated and extensively used structured interview (15), provided an estimate of energy expenditure across a 7-day period at baseline and 12 months.
Exercise attendance sheets and logs
Exercise instructors maintained attendance sheets recording individual participation in the exercise classes across the 12-month period (14). Exercisers also completed daily exercise logs that were turned into instructors weekly (3,14).
Measurement of Sleep Architecture
Nine-channel polysomnographic (PSG) recordings were made in participants’ homes using the Oxford Medilog MR95 digital recording system (Oxford Instruments, Oxford, U.K.). Electrode application followed standardized placements for electroencephalography (EEG) (C4-A1, C3-A2, Pz-A1), bipolar electrooculography (EOG), and surface mentalis electromyography (EMG) (16). An MR95 channel recorded ambient light, which was used to help determine the beginning of the sleep period (see below). Sleep was ad libitum.
Technologists arrived in participants’ homes between 7:00 and 8:00 PM for evening hook-up. Participants were instructed to press the event marker on the MR95 at the time they intended to go to bed. Participants removed electrodes in the morning, and technologists picked up the units shortly afterward. Participants maintained sleep diaries (see below) on nights of PSG recording. The beginning of the sleep period was defined using “lights out” as indicated on the MR95 illumination monitor. When this information was missing, the MR95 event marker as pressed by the participant was used to define “lights out.” Finally, when event marker information was missing, bedtime as indicated on the participant’s nightly sleep log was used. Retrospective examination indicated that the primary data sources for start of night were the luminescence monitor (46%) and event recorder (44%).
Upon morning collection of the MR95 equipment in participants’ homes, PSG data were de-identified and transferred to the Palo Alto Veterans Administration Medical Center (Woodward) for artifact removal and data compression. A total of 17 participant nights were eliminated from further analysis because of technical considerations (e.g., >25% of recording was unscoreable because of artifact, weak battery, etc.), resulting in a technical failure rate of 4.2%. Cleaned data were transferred to the Laboratory for Sleep, Aging and Chronobiology at Emory University Medical School (Bliwise) for stage scoring, which followed conventional scoring rules (16). Median inter-rater reliability (intra-class correlation coefficient) across the two trained and blinded PSG technologists was .90 for sleep stages. Scored data were subsequently transferred back to Stanford where data analysis occurred.
PSG data were collected for 3 nights during baseline and 2 nights at 6 and 12 months. Data were averaged from the 2nd and 3rd baseline nights (17) and for the 2 nights recorded at other time points when both nights were available. Only one participant had to be eliminated completely because of inadequate sleep recordings based on the above criteria. During the intervention, exercise participants were instructed to undertake their prescribed physical activity on at least one of the two PSG measurement days.
Measurement of Sleep: Self-Report Measures
The Pittsburgh Sleep Quality Index (PSQI)
The validated PSQI was used to evaluate rated sleep quality at baseline and 12 months (18). Seven “component” scores are generated (using a 0–3 scale) along with a summary global sleep quality score (range = 0–21).
Sleep diaries
Standard sleep diaries were used to provide an additional source of sleep data (19). Participants recorded sleep and lifestyle variables (e.g., caffeine intake) in a daily diary during a 2-week period at baseline and 12 months (3).
Measurement of Secondary Outcomes of Interest
Cardiorespiratory fitness and body mass index (BMI)
Participants underwent symptom-limited treadmill exercise testing at baseline and 12 months. Treadmill speed was increased gradually until the participant’s heart rate reached approximately 70% of age-predicted maximum heart rate. The participant walked at this speed for 4 minutes. Each subsequent stage was 2 minutes long, with grade increasing by 2%. The test continued until the participant requested to stop due to fatigue or until any one of the criteria listed under the American College of Sports Medicine (ACSM) standard stopping indications were met (20). Peak exercise oxygen consumption was measured during the exercise test (MedGraphics Corporation CPX/D; St. Paul, MN) and was defined as the average of the two highest oxygen consumption levels observed during the last minute of the exercise test (mL/kg/min). A physician supervised the test, and all staff administering the test were trained and certified. Body weight (kg) and height (meters) were assessed using standard procedures to obtain BMI (kg/m2) (21).
Sample Size and Statistical Analyses
No comparable exercise intervention data for sleep-impaired older adults were available for PSG-derived sample size determinations. A goal of the current study was the development of initial effect size estimates for those variables.
Prior to formal data analysis, principal components analysis with varimax rotation was applied to baseline data to reduce variable redundancies within each of the three sleep measurement domains (22). Components with eigenvalues ≥ 1.0 were used in identifying variables within each component with the greatest factor loadings for use in subsequent analysis (22).
Analysis of variance (ANOVA; general linear models procedure) (23) was used to evaluate baseline between-arm differences. Analysis of covariance (ANCOVA) (23), with baseline values as covariates, was used to assess 12-month change for all variables. Because Gender×Arm interactions were nonsignificant in all analyses, the results from the analyses without the interaction term are presented. Given that the central hypothesis was directional (i.e., whether increased exercise positively impacts sleep quality) and, to our knowledge, no published evidence has shown that participating in moderate physical activity during the first half of the day negatively impacts sleep quality, a one-sided α of .05 was applied as recommended by statistical experts in the field (24,25). This approach is appropriate and justified when the hypothesis being tested is aimed at one tail only, as was the case in the current investigation in which the experimental intervention was delivered to positively impact the outcome of interest (sleep) while the control intervention was developed to have no effect on sleep (i.e., a placebo) (26). This approach also helps to optimize power in early investigations of a question of interest when effect size estimates are lacking (24).
We incorporated intent-to-treat principles for all outcomes whereby, for participants with missing or incomplete data at 12 months, baseline values were used. The intent-to-treat analyses led to results that were reasonably comparable to those conducted using the available data only. We report the intent-to-treat results for all variables. For ANCOVAs that reached statistical significance, preplanned comparisons between arms were conducted using the SAS least squares means procedure (23). Because of the dearth of information examining exercise intervention effects on sleep variables in older adults with sleep complaints, we explored the relationships between the exercise intervention and the subgroup of PSG and subjective sleep variables found via principal components analysis to be reasonably distinct from one another within each sleep measurement domain. Given this, correction for multiple testing has been deemed less necessary, particularly in early investigations when identifying variables that may be sensitive to change with intervention is of major importance (27).
RESULTS
Participants
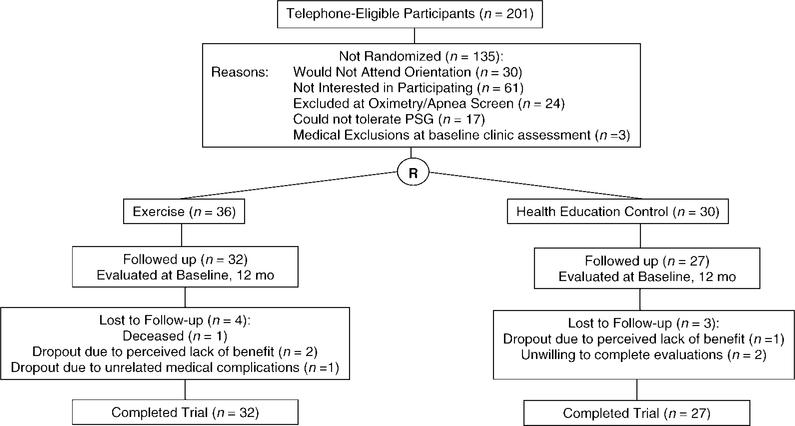
A summary of participant flow and retention is shown in Figure 1. Twelve-month retention rates were comparable across the two study arms (exercise = 32/36, or 89%; control = 27/30, or 90%). Those with 12-month PSG data were comparable to those without 12-month PSG data (n = 7; exercisers = 4; controls = 3) on the major baseline variables of interest, with the following exceptions: study completers had higher baseline VO2 peak (24.7 ± 4.3 vs 21.6 ± 4.0 for noncompleters, t[58] = 2.3, p = .03; differences between arms = not significant) and less impaired PSQI global sleep quality scores relative to noncompleters (7.7 ± 3.1 vs 11.0 ± 3.3, respectively, t[58] = 2.6, p=.01). Descriptive baseline data by arm are shown in Table 1 (no between-group baseline differences were found, p values >.05). Pretest and posttest descriptive data by arm for the intent-to-treat sleep analyses are summarized in Table 2.
Figure 1.
Study enrollment and retention.
Table 1.
Descriptive Statistics by Study Arm
| Variables | Exercise (N = 36) | Control (N = 30) |
|---|---|---|
| Gender | 12 Men, 24 Women | 10 Men, 20 Women |
| Mean age, y (SD) | 61.86 (6.33) | 60.90 (7.19) |
| Mean years of education (SD) | 16.22 (1.66) | 15.90 (2.06) |
| Percentage of white, non-Hispanic race | 88.9% | 80.0% |
| Percent married | 61.1% | 63.3% |
| Percent employed | 50.0% | 46.7% |
| OTC baseline sleep medication use, % | 19.4% | 10.0% |
| PAR, kcal/kg/d (SD) | ||
| Baseline (exercisers, n = 36; controls, n = 30) | 32.63 (0.86) | 32.74 (1.36) |
| Max VO2, mL/kg/min (SD) | ||
| Baseline (exercisers, n = 34; controls, n = 28) | 24.12 (4.72) | 24.19 (4.13) |
| Body mass index (SD) | ||
| Baseline (exercisers, n = 36; controls, n = 30) | 27.86 (4.06) | 27.18 (3.92) |
Notes: There were no statistically significant between-arm baseline differences (p values > .10).
SD = standard deviation; OTC = over-the-counter (nonprescription); PAR = Physical Activity Recall.
Table 2.
Selected Polysomnography and Self-Reported Sleep Variables (Intent-to-Treat Imputed Values) at Baseline and 12 Months, Means (SD), and Sample Sizes [N] by Study Arm
| Variables | Study Arm | Baseline (SD) | 12 Month (SD) |
|---|---|---|---|
| PSG data [N] | |||
| Sleep latency, min [36] | Exercise | 15.06 (17.12) | 14.22 (12.23) |
| [30] | Control | 17.74 (11.68) | 18.42 (13.55) |
| Number of awakenings: 1st third of sleep period [36] | Exercise | 4.65 (2.88) | 4.28 (2.72)* |
| [30] | Control | 3.26 (1.66) | 4.27 (2.46) |
| Number of awakenings: 2nd third of sleep period [36] | Exercise | 5.22 (2.89) | 5.73 (3.38) |
| [30] | Control | 4.36 (1.92) | 4.78 (1.87) |
| Number of awakenings: last third of sleep period [36] | Exercise | 8.78 (7.16) | 7.93 (3.92) |
| [30] | Control | 5.66 (2.72) | 7.62 (4.65) |
| % Sleep time in Stage 1 [36] | Exercise | 9.13 (3.96) | 7.88 (3.77)** |
| [30] | Control | 8.24 (3.51) | 9.37 (5.85) |
| % Sleep time in Stage 2 [36] | Exercise | 52.79 (8.17) | 53.68 (9.63)* |
| [30] | Control | 53.37 (8.67) | 50.96 (9.77) |
| % Sleep time in slow wave sleep [36] | Exercise | 1.31 (1.99) | 0.59 (1.68) |
| [30] | Control | 0.73 (1.32) | 0.23 (0.67) |
| Total sleep time, min [36] | Exercise | 361.58 (60.34) | 361.09 (65.48) |
| [30] | Control | 361.94 (63.58) | 373.95 (54.64) |
| Sleep efficiency, % [36] | Exercise | 80.95 (9.52) | 79.89 (10.53) |
| [30] | Control | 82.46 (9.37) | 81.09 (10.36) |
| Pittsburgh Sleep Quality Index (PSQI) | |||
| Sleep duration subscale [36] | Exercise | 0.89 (0.92) | 0.61 (0.84) |
| [30] | Control | 0.83 (0.87) | 0.57 (0.63) |
| ++Sleep disturbance subscale [36] | Exercise | 1.47 (0.51) | 1.31 (0.47)** |
| [30] | Control | 1.45 (0.51) | 1.57 (0.50) |
| Daytime dysfunction subscale [36] | Exercise | 1.19 (0.62) | 0.94 (0.71) |
| [30] | Control | 1.00 (0.53) | 1.13 (0.68) |
| +PSQI global score [36] | Exercise | 8.47 (3.61) | 6.36 (3.39)† |
| [30] | Control | 7.57 (2.78) | 6.57 (2.84) |
| Sleep diary | |||
| Sleep latency minutes [36] | Exercise | 38.44 (23.32) | 26.02 (18.5)** |
| [30] | Control | 23.08 (19.01) | 28.02 (21.04) |
| +++Feeling rested in morning [36] | Exercise | 3.58 (1.14) | 4.42 (1.52)* |
| [30] | Control | 3.83 (1.10) | 4.05 (1.24) |
| Number of awakenings [36] | Exercise | 2.59 (2.23) | 1.83 (1.40) |
| [30] | Control | 2.71 (2.01) | 2.28 (1.26) |
| Total sleep time [35] | Exercise | 7.53 (0.70) | 7.58 (0.78) |
| [29] | Control | 7.42 (0.87) | 7.84 (0.80) |
| Total nap time, h [23] | Exercise | 1.02 (0.52) | 0.92 (0.54) |
| [20] | Control | 0.64 (0.31) | 0.69 (0.38) |
Notes:
0–21 scale: lower score = better sleep quality
0–3 scale: lower score = less disturbance
0–8 scale: higher score = better rested.
Between-arm difference (analysis of covariance [ANCOVA]), p < .05, one-tailed.
Between-arm difference (ANCOVA), p ≤ .01, one-tailed.
Between-arm difference (ANCOVA), p = .07, one-tailed.
SD = standard deviation; PSG = polysomnography.
Adherence to the 12-Month Exercise Program
Participants demonstrated good overall adherence to the exercise program, with a mean of 3.7 ± 1.8 of 5 prescribed exercise sessions per week (74%) across the 12-month period (based on instructor-reported class attendance logs and participant logs). Across 12 months, mean number of home exercise sessions = 2.1 ± 0.9 and mean minutes of exercise per home session = 43.3 ± 19.4 (no significant differences between class and home sessions).
Participants randomized to the health education control arm had similarly good attendance (80%) at the weekly health education sessions across the 12-month period.
Change in 12-Month Physical Activity
At 12 months, using intent-to-treat analyses, exercisers (baseline-adjusted mean = 34.3 ± 2.2) showed significantly higher total energy expenditure levels compared to controls (baseline-adjusted mean = 32.9 ± 2.2) (F[1,65] = 5.6, p = .01, one-tailed).
Results from Principal Components Analyses of Sleep Measures
Home PSG
For the nine PSG variables, principal components analyses (PCA) yielded four components with eigenvalues ≥ 1.0, which together accounted for 76% of the total variance. Top-loading variables included percent time in Stage 2 sleep (Factor 1), percent time in Stage 1 sleep and awakenings during the first third of the sleep period (Factor 2), percent time in slow-wave sleep (SWS) (Factor 3), and sleep latency (Factor 4). Because Stage 1 sleep percentage and awakenings represent clinically distinct variables (28) and were only modestly correlated (Spearman r = 0.3), these five variables were included in subsequent analysis.
PSQI
For the seven PSQI subscales, PCA yielded three components with eigenvalues ≥ 1.0, which together accounted for 66% of the total variance. Top-loading variables included sleep duration (Factor 1), sleep disturbance (Factor 2), and daytime dysfunction (Factor 3). These three variables were included along with global PSQI score in subsequent analysis.
Two-week sleep diaries
PCA of the five sleep diary variables yielded four components with eigenvalues ≥ 1.0, which together accounted for 66% of the total variance. Top-loading variables included nighttime awakenings (Factor 1), sleep duration (Factor 2), sleep-onset latency and feeling rested in the morning (Factor 3), and amount of daytime napping (Factor 4). Because sleep-onset latency and feeling rested in the morning represent clinically distinct variables (19), all five variables were included in subsequent analysis.
Change in 12-Month Primary Sleep Outcomes: Home PSG
Using intent-to-treat methods, group differences were found on three of the five 12-month PSG sleep variables (Table 2). Exercisers showed significantly less percentages of time spent in Stage 1 sleep relative to controls (F[1,65] = 8.0, p = .003, one-tailed; group difference = 2.3, 95% confidence interval [CI], 0.7–4.0, effect size = 0.66). Exercisers also showed a significantly greater percentage of time spent in Stage 2 sleep relative to controls (F[1,65] = 3.2, p = .04, one-tailed; group difference = 3.2, 95% CI, 0.6–5.7, effect size = 0.41). Finally, exercisers had significantly fewer awakenings during the first third of the sleep period relative to controls (F[1,65] = 3.5, p = .03, one-tailed; group difference = 1.0, 95% CI, 0.39–1.55, effect size = 0.50). No statistically significant 12-month group differences were observed for other PSG variables. Of note, participants spent an average of 1% or less of their baseline sleep time in SWS sleep (Table 2). (Additional spectral analyses of delta band activity using the Fast Fourier Transform function also failed to yield significant results.)
Changes in 12-Month Secondary Sleep Outcomes: Self-Reported Sleep Quality
PSQI
Using intent-to-treat methods, exercisers reported lower 12-month sleep disturbance subscale scores compared to controls (F[1,63] = 5.0, p = .014, one-tailed, effect size = 0.55).
Two-week sleep diaries
Using intent-to-treat methods, exercisers, compared to controls, reported reduced sleep-onset latency (F[1,62] = 6.1, p = .008, one-tailed, effect size = 0.63) and feeling more rested in the morning (F[1,62] = 3.6; p = .03, one-tailed, effect size = 0.47). There were no between-group differences reported for bedtime, daytime napping, or behavioral variables such as alcohol or caffeine intake, the timing or number of warm baths or showers, or use of sleep medications (all p values > .05, one-tailed).
Relationships Between Objective and Self-Reported 12-Month Sleep Measures
For those PSG variables achieving statistical significance, Pearson-product moment correlation analyses were conducted with the available self-report measures. A larger Stage 2 sleep percentage was associated with more positive PSQI global sleep scores (r = −0.29, p < .03, two-tailed), as well as more positive sleep diary ratings of nighttime awakenings and feeling rested in the morning (r = −0.28 and 0.31, respectively, p ≤ .03, two-tailed). A lower number of awakenings during the first third of the sleep period was associated with more positive PSQI sleep duration subscale ratings (r = −0.27, p = .04, two-tailed). No significant correlations were found for Stage 1 sleep percentage.
Acute Effects of Exercise on PSG-Based Sleep Quality
To explore whether exercise during the day acutely impacted PSG-measured sleep that evening, exercisers with two consecutive PSG measurements that included one exercise and one non-exercise day were evaluated. At 12 months, 12 individuals fit this pattern. Paired-comparison t tests conducted on all nine PSG variables indicated no significant differences between exercise and non-exercise days (p values ≥ .35). Nor were significant differences observed when this group was combined with 11 additional exercisers who had consecutive exercise–no exercise PSG nights at the interim 6-month visit (total n = 23, p values ≥ .19).
Change in Other Secondary Outcomes: Cardiorespiratory Fitness and Body Weight
Using intent-to-treat analyses, exercisers showed significantly greater 12-month cardiorespiratory fitness (baseline-adjusted mean = 24.7 ± 2.2) compared to controls (baseline-adjusted mean = 23.1 ± 2.3) (F[1,61] = 7.6, p = .004, one-tailed). Exercisers also had significantly lower BMI (baseline-adjusted mean = 27.1 ± 1.2) compared to controls (baseline-adjusted mean = 28.0 ± 1.3) (F[1,65] = 8.5, p = .002, one-tailed). These between-arm differences are comparable to or greater than changes in these variables obtained in other community-based exercise intervention studies that typically targeted younger individuals (21,29).
DISCUSSION
To our knowledge, this study is the first to evaluate the sustained effects of a moderate-intensity exercise program on home-based PSG in older adults with sleep complaints. In a recent meta-analysis reviewing 23 behavioral intervention trials for insomnia, just three used PSG (30). Use of repeated nights of home-based PSG recordings increases the contextual validity of the PSG assessment over that occurring within the more typical context of a sleep laboratory (31). The results suggest that increases in moderate-intensity primarily endurance exercise may have modest positive effects on several dimensions of objectively measured sleep architecture as well as on subjective aspects of sleep. The significant 12-month shift observed from Stage 1 to Stage 2 sleep and the reduced number of awakenings observed during this early phase of sleep represents a small to moderate effect size (32), similar to those reported in a meta-analysis of the associations of exercise with increased Stage 2 and SWS in individuals without insomnia (33). The low levels of baseline SWS seen in our older participants—similar to other populations of older adults (28)—may have mitigated the detection of increases in those sleep stages. Notably, there are no well-accepted pharmacologic or nonpharmacologic interventions to increase SWS. Our data suggest that increases in regular moderate-intensity exercise levels commensurate with those identified in current population-wide recommendations, including those for older adults (7), can yield measurable changes in sleep architecture typically associated with better sleep. The significant associations found between 12-month Stage 2 sleep percentages and several self-reported sleep outcomes provide additional evidence that the increase in Stage 2 sleep percentage may be associated with more positive sleep perceptions. The potential public health impacts of these changes deserve further investigation. Of note, the intervention resulted in physical activity and fitness changes of similar magnitude to other community-based studies targeting younger populations (21,29).
Subjectively, 12-month improvements were observed in nighttime sleep disturbance (PSQI), sleep latency (diaries), and feeling rested in the morning (diaries). Given that it is the perceptual aspects of sleep that motivate physician visits, use of pharmacologic sleep aids, and other forms of medical intervention, subjective aspects of sleep remain an important domain for investigation (34). Similar to the behavioral treatment literature for insomnia, the exercise intervention did not have a significant impact on total sleep time in this older adult sample (30).
Prevalence studies suggest that more than half of the older population report some degree of sleep problems (2). Our participants probably represent this broader range of individuals experiencing “suboptimal” sleep for their age, as opposed to individuals with more severe sleep complaints that are represented in clinic- or laboratory-based insomnia studies.
It is conceivable that the increased light exposure accompanying home-based exercise sessions occurring outdoors could have had a positive impact on sleep quality (6). We do not have data on what percentage of home-based exercise sessions occurred outdoors, and thus were not able to differentiate these potential light effects from more general exercise effects.
Although the current investigation represents a sample size two- to threefold larger than trials typically undertaken in the sleep-exercise field, it is quite possible that we still lacked statistical power for some objectively measured sleep variables (e.g., sleep latency, SWS), as well as discerning acute effects of exercise on sleep architecture. While PCA was used to reduce redundancies among the sleep variables, a reasonably large number of tests were conducted to better understand which sleep variables might deserve further investigation. Given this, we urge caution in interpreting the study results.
The applicability of our findings to very old adults remains uncertain. An observational study including many individuals older than 80 years showed that regular physical activity was protective for insomnia (35). Although one trial implementing a limited physical activity regimen in nursing home patients (mean age = 84 years) was unsuccessful in impacting actigraphically measured sleep (36), a second study of nursing home residents (mean age = 87), which evaluated a multicomponent intervention that included daily low-level exercise, reported improved actigraphically measured rest/activity rhythms (37). Additional research with adults older than 80 years is clearly warranted.
Finally, the average changes in self-reported sleep quality found were smaller than those often reported in cognitive-behavioral or pharmacological intervention studies in the sleep area (1), although those studies typically have targeted a different population (clinically impaired, such as those presenting at a sleep clinic). Although statistically significant, our PSG results suggest effects that were modest and less dramatic than we originally expected. Increases in visually scored SWS following exercise in younger participants, for example, have been reported and were expected here. Perhaps owing to the relatively low amounts of SWS seen at baseline in our older participants, such changes were not detected. In contrast, several sleep architecture variables did show intervention-related changes that were entirely consistent with better sleep. Stage 1 sleep, typically considered a transition state from waking to sleep, was decreased with exercise and was converted to a sleep EEG pattern consistent with more stable sleep (i.e., increased Stage 2). The fact that sleep continuity in the first third of the night also showed such trends for improvement is compatible with studies of pharmacologic (38) and nonpharmacologic (39) interventions that suggest that effects during early portions of the night, rather than the duration of the night, are most sensitive to intervention-based sleep improvements.
In light of the demonstrated positive impacts of regular moderate-intensity exercise on a range of health outcomes in addition to sleep in middle-aged and older adults (40), exercise interventions merit continued investigation among sleep-impaired adult populations.
ACKNOWLEDGMENTS
This study was supported by U.S. Public Health Service grant RO1MH58853 (Dr. King).
We are indebted to Jill Thomson and Shannon Morris for assistance in intervention delivery; Karen Bolen, Jessica Morton, and Julia Wu for data collection; Kathy Lee for measurement input; and Clete Kushida for clinical input. Thanks to Ann Kollrack, Wendy Stegman, Elaine Bailey, and Lorraine Stewart for assistance with sleep scoring and data processing.
REFERENCES
- 1.Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 1999;22:1134–1156. [DOI] [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. [DOI] [PubMed] [Google Scholar]
- 3.King AC, Oman RF, Brassington G, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults: a randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 4.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20:95–101. [DOI] [PubMed] [Google Scholar]
- 5.Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:768–776. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for problems in later life. Sleep Med Rev. 2004;8:47–62. [DOI] [PubMed] [Google Scholar]
- 7.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Miles LE. A sleep questionnaire In: Guilleminault C, ed. Sleeping and Waking Disorders: Indications and Techniques. Menlo Park, CA: Addison-Wesley; 1982:383–413. [Google Scholar]
- 9.Maislin G, Dinges DF, Pack FM, Pack AI. Validity study of the Multivariable Apnea Index (MAP) in an elderly population. Sleep Res. 1996;25:288. [Google Scholar]
- 10.George CF, Millar TW, Kryger MH. Identification and quantification of apneas by computer-based analysis of oxygen saturation. Am Rev Respir Dis. 1988;137:1238–1240. [DOI] [PubMed] [Google Scholar]
- 11.Series F, Marc I, Cormier Y, LaForge J. Utility of nocturnal home oximetry for case finding in patients with suspected sleep apnea hypopnea syndrome. Ann Intern Med. 1993;119:449–453. [DOI] [PubMed] [Google Scholar]
- 12.Efron B Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 13.Vitiello MV. Effective treatments for age-related sleep disturbances. Geriatrics. 1999;54:47–52. [PubMed] [Google Scholar]
- 14.King AC, Pruitt LA, Phillips W, Oka R, Rodenburg A, Haskell WL. Comparative effects of two physical activity programs on measured and perceived physical functioning and other health-related quality of life outcomes in older adults. J Gerontol Med Sci. 2000;55A:M74–M83. [DOI] [PubMed] [Google Scholar]
- 15.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804. [DOI] [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A, eds. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research; 1968. [Google Scholar]
- 17.Woodward SH, Bliwise DL, Friedman MJ, Gusman F. First night effects in PTSD inpatients. Sleep. 1996;19:312–317. [DOI] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14:331–338. [PubMed] [Google Scholar]
- 19.Lichstein KL, Durrence HH, Riedel H, Taylor DJ, Bush AJ. Epidemiology of Sleep: Age, Gender, and Ethnicity. Mahwah, NJ: Lawrence Erlbaum; 2004. [Google Scholar]
- 20.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 7th Ed. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 21.King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group-versus home-based exercise training in healthy older men and women: a community-based clinical trial. JAMA. 1991;266:1535–1542. [PubMed] [Google Scholar]
- 22.Morrison DF. Multivariate Statistical Methods, 4th Ed. Belmont, CA: Thomson Brooks/Cole; 2004. [Google Scholar]
- 23.Spector PC, Goodnight JH, Sall JP, Sarle WS. The GLM procedure. In: SAS User’s Guide: Statistics. Version 5. Cary, NC: SAS Institute Inc.; 1985:433–506. [Google Scholar]
- 24.Kraemer HC, Thiemann S. How Many Subjects? Statistical Power Analysis in Research. Newbury Park: Sage; 1987. [Google Scholar]
- 25.Hays WL. Statistics, 5th Ed. Fort Worth, TX: Harcourt Brace; 1994. [Google Scholar]
- 26.Jaccard J, Becker MA. Directional versus Nondirectional Tests. Belmont, CA: Wadsworth Publishing Company; 1990. [Google Scholar]
- 27.Maxwell SE, Delaney HD. Two-way between subjects factorial designs In: Designing Experiments and Analyzing Data: A Model Comparison Perspective. Belmont, CA: Wadsworth Publishing Company; 1990:259–260. [Google Scholar]
- 28.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. [DOI] [PubMed] [Google Scholar]
- 29.Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW 3rd, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: a randomized trial. JAMA. 1999;281:327–334. [DOI] [PubMed] [Google Scholar]
- 30.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25:3–14. [DOI] [PubMed] [Google Scholar]
- 31.Edinger JD, Fins AI, Sullivan RJ Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–1126. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 33.Youngstedt SD, O’Connor PJ, Dishman RK. The effects of acute exercise on sleep: a quantitative synthesis. Sleep. 1997;20:203–214. [DOI] [PubMed] [Google Scholar]
- 34.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004). Sleep. 2006;29:1398–1414. [DOI] [PubMed] [Google Scholar]
- 35.Morgan K Daytime activity and risk factors for late-life insomnia. J Sleep Res. 2003;12:231–238. [DOI] [PubMed] [Google Scholar]
- 36.Alessi CA, Schnelle JF, MacRae PG, et al. Does physical activity improve sleep in impaired nursing home residents? J Am Geriatr Soc. 1995;43:1098–1102. [DOI] [PubMed] [Google Scholar]
- 37.Martin JL, Marler MR, Harker JO, Josephson KR, Alessi CA. A multicomponent nonpharmacological intervention improves activity rhythms among nursing home residents with disrupted sleep/wake patterns. J Gerontol A Biol Sci Med Sci. 2007;62:67–72. [DOI] [PubMed] [Google Scholar]
- 38.Monti JM. Effect of zolpidem on sleep in insomniac patients. Eur J Clin Pharmacol. 1989;36:461–466. [DOI] [PubMed] [Google Scholar]
- 39.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavior therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–1864. [DOI] [PubMed] [Google Scholar]
- 40.U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]