Abstract
Introduction
Cancer cachexia is characterized by severe skeletal muscle mass loss, which is driven by decreased muscle protein synthesis and increased protein degradation. Daily physical activity and feeding behaviors exhibit diurnal fluctuations in mice that can impact the systemic environment and skeletal muscle signaling.
Purpose
We investigated the effect of cancer cachexia on the diurnal regulation of feeding, physical activity, and skeletal muscle mTORC1 signaling in tumor bearing mice. We also examined the impact of increased physical activity on diurnal behaviors and skeletal muscle mTROC1 signaling in the cancer environment.
Methods
Physical activity and feeding behaviors were measured for 4 consecutive days prior to sacrifice in male C57BL/6 (B6; N=24) and ApcMin/+ (MIN; N=22) mice at 7:00AM and 7:00PM under ad libitum condition. A subset of B6 (N=16) and MIN (N=19) mice were given wheel access for 2-weeks prior to diurnal behavior measurements. Gastrocnemius muscle protein expression was examined.
Results
MIN mice demonstrated altered diurnal fluctuations in feeding and activity, compared to the B6. Interestingly, cachexia did not alter MIN total food intake, but dramatically reduced cage physical activity. As a measurement of mTORC1 activity, 4E-BP1 phosphorylation increased following the dark cycle in B6 and pre-cachectic MIN mice, while rpS6 phosphorylation was only increased following the dark cycle in MIN mice. MIN 4E-BP1 phosphorylation at the end of the light cycle was significantly correlated with cachexia progression and reduced physical activity. Voluntary wheel running increased light cycle MIN 4E-BP1 phosphorylation and attenuated muscle mass loss.
Conclusion
The cancer environment can alter diurnal feeding and physical activity behaviors in tumor bearing mice, which are linked to the progression of cachexia and muscle wasting. Furthermore, suppressed physical activity during cachexia is associated with decreased skeletal muscle mTORC1 signaling.
Keywords: Cancer Survival, Circadian Rhythms, Wasting, muscle loss
Introduction
Cancer cachexia is a multifactorial wasting condition characterized by severe skeletal muscle mass loss with or without fat loss, gonadal dysfunction, elevated systemic inflammation (1), and skeletal muscle mass maintenance during cachexia’s progression has been linked to improved survival and quality of life (2). Cancer-induced activation of protein degradation and suppression of protein synthesis in skeletal muscle is a well characterized consequence of the disease and an established driver of cachexia (3). There are currently no approved treatments to prevent or attenuate cachexia’s progression, which is likely due to the complexity brought on by tumor heterogeneity and the cancer-induced systemic environment (4). However, there are discrepancies between preclinical cachexia models and cancer patients in the intracellular signaling that drive muscle wasting (5). Accounting for skeletal muscle sensitivity to feeding and physical activity, which fluctuate throughout the day, could underlie some of these inconsistencies (6). Most studies examining skeletal muscle protein turnover during cancer cachexia have either not controlled for feeding and cage activity behaviors or used a brief fast that has been variable in duration. The mechanistic understanding of cancer’s effect on skeletal muscle protein turnover could be greatly enhanced by accounting for daily fasting, food consumption, and physical activity.
Physical activity, feeding, and sleep are daily behaviors that can impact the systemic environment, individual tissues, and cells (7). These behaviors have diurnal fluctuations throughout the day; rodent physical activity and feeding are highest during the dark cycle. Tumor bearing mice and cachectic cancer patients consistently demonstrate a substantial reduction in physical activity (8–10). Additionally, recent findings suggest that feeding behaviors in cancer patients are minimally affected by appetite, but more dependent on chemotherapy symptoms and age, which suggests a multifactorial component to altered feeding behaviors (11). The effect of cachexia on food consumption in preclinical cancer models is equivocal, being either not changed, increased, or decreased depending on the severity of cachexia and the preclinical model examined (12, 13). However, the measurement of food consumption in preclinical cancer models is not standardized and has potential for wide variability. While altered feeding and physical activity patterns have established effects on normal systemic processes (14), circadian arrhythmicity has been suggested to be a factor in progressing tumorigenesis and impairing cancer treatment (15). Interestingly, exercise has been shown to re-establish mouse skeletal muscle molecular clock genes (CLOCK and Bmal1 genes) (16), thus providing a potential therapeutic effect of exercise on normalizing diurnal patterns. While behavior patterns and circadian rhythmicity have established roles in skeletal muscle anabolic signaling, we have an inadequate understanding of cancer’s impact on skeletal muscle diurnal regulation and if this could contribute to cachexia development.
Skeletal muscle protein turnover oscillates throughout the day in response to exercise and feeding (17). Interestingly, exercise and feeding prior to fasting and inactivity suppresses skeletal muscle protein degradation (18). Physical activity and feeding can stimulate skeletal muscle protein synthesis through mechanistic target of rapamycin complex 1 (mTORC1) signaling (19). Eukaryotic initiation factor-4E binding protein (4E-BP1) and ribosomal protein S6 (rpS6) are downstream effectors of mTORC1 that can initiate ribosome formation and translation initiation (20). Basal skeletal muscle anabolic signaling through mTORC1 is suppressed during the progression of cachexia in mice (3). While whole body protein turnover measurements in cachectic cancer patients have been shown to be elevated at baseline, the whole body and muscle anabolic response to feeding is suppressed in cachectic cancer patients (21, 22). Although basal muscle protein synthesis and mTORC1 activity in tumor bearing mice is suppressed during the progression of cachexia, there is an incomplete mechanistic understanding of physical activity and feeding interactions that impact cachectic skeletal muscle signaling.
The ApcMin/+ mouse is an established preclinical model of cancer cachexia that exhibits an overall reduction in cage activity and voluntary wheel activity (10, 23), without reduction in overall food consumption (12). Although great strides have been made in our understanding of aberrant muscle signaling in the basal cachectic condition, significant gaps remain in understanding the cancer environment’s impact on the integration of diurnal physiological inputs involving physical activity and feeding. Therefore, we investigated the effect of cancer cachexia on the diurnal regulation of feeding, physical activity, and skeletal muscle mTORC1 signaling in tumor bearing mice. The impact of increased physical activity on diurnal behaviors and skeletal muscle mTORC1 signaling in the cancer environment was also examined. We hypothesized that the cancer environment would suppress diurnal fluctuations in physical activity, which would be significantly associated with the progression of cachexia and attenuated skeletal muscle mTORC1 signaling. We also hypothesized that increased physical activity in ApcMin/+ mice would rescue the suppression of skeletal muscle mTORC1 signaling. Diurnal fluctuations that occur during the light and dark cycles involving feeding, cage activity, and skeletal muscle mTORC1 signaling were measured in wild-type and ApcMin/+ mice under normal free-living conditions. We increased physical activity level in a subset of wild-type and ApcMin/+ mice through free access to wheels in their cages for 2-weeks.
Methods
Animals
Male C57BL/6 (B6; N=41) and ApcMin/+ (MIN; N=41) mice were bred at the University of South Carolina Animal Resource Facility. MIN mice were initially purchased from Jackson Labs (Bar Harbor, ME, USA). Mice were kept on a 12:12h light/dark cycle beginning at 7:00AM and were given rodent chow ad libitum (Harlan Teklad Rodent Diet, #8604, Harlan, Indianapolis, IN, USA). Thus, 7:00AM and 7:00PM corresponds to the end of the dark and light cycle, respectively (Figure 1A). All experiments were approved by the University of South Carolina Institutional Animal Care and Use Committee.
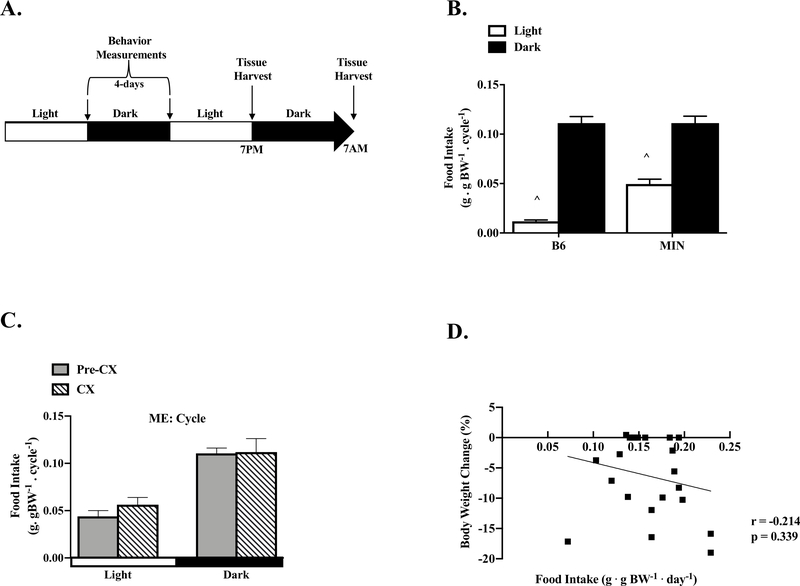
Figure 1: The Effect of Cancer Cachexia on Diurnal Regulation of Feeding.
Data is expressed as mean ± SEM or correlation coefficient and corresponding p value. A) Study Design. Four days prior to sacrifice, food intake, activity, and glucose were measured in B6 and MIN mice at the end of the light and dark cycle. Mice were sacrificed at either the end of the light cycle (7:00PM, Light) or dark cycle (7:00AM, Dark). B) B6 and MIN food intake per cycle. (B6: N=16, MIN: N=22). C) Pre-CX and CX MIN food intake per cycle. (Pre-CX; N=12, CX; N=10). D) Correlation of body weight change from peak to total food intake in the MIN. Abbreviations CX: Cachexia, ME: Main Effect. Two-way repeated measure ANOVAs were used to compare total food intake within genotypes across time (B, C). Spearman correlation was used to determine associations between total food intake and body weight change from peak in the MIN (D). ^ Different than all groups. Statistical significance was set a p<0.05.
Study 1 –Free-Living Experimental Design
Diurnal fluctuations were examined in B6 and MIN mice in the free-living condition. Male B6 (N=24) and MIN (N=22) mice between 12–18 weeks of age were acclimated to individual housing and animal handling 3 days prior to the start of their four-day monitoring. One week prior to sacrifice mice were single-housed in activity monitor cages (Opto-M3 Activity Meter, Columbus Instruments) for 4 consecutive days as previously described (12). Body weight, blood glucose, cage activity, and food intake were recorded at 7:00AM and 7:00PM (prior to the change of light/dark cycle) each day. At the completion of the 4th monitoring day B6 and MIN mice were sacrificed at either 7:00AM or 7:00PM under ad libitum condition.
Study 2 - Wheel Access Experimental Design
Following 2-weeks of unlimited access to wheels diurnal fluctuations were examined in B6 and MIN mice in the free-living condition. Male B6 (N=17) and MIN (N=19) mice between 12 and 15 weeks of age were acclimated to single housing and stainless steel activity wheels were placed in their cages (MiniMitter, Bend, OR) as previously described (23). Bicycle computers (Sigma, Germany) with a magnetic sensor measured speed, distance, and time; the data was recorded daily. Similar to experiment 1, body weight, blood glucose, wheel activity, and food intake were recorded at 7:00AM and 7:00PM (prior to the change of light/dark cycle) each day for 4 days following the 2-weeks of wheel access. At the completion of the 4th day of monitoring B6 and MIN mice were sacrificed at either 7:00AM or 7:00PM under ad libitum condition. The distance ran by MIN mice over the 2-week period varied widely, which allowed for the classification of mice into Low (N=10) or High (N=9) activity groups after the completion of the study. MIN mice that ran at least 1km per day were classified as high activity and MIN mice that achieved less than 1km per day were termed low activity.
Tissue Collection
Mice were euthanized with a subcutaneous injection of a ketamine-xylazine-acepromazine cocktail (1.4 ml/kg body weight) (23). Hindlimb muscles (soleus, plantaris, extensor digitorum longus, gastrocnemius, tibialis anterior and rectus femoris) and organs were rapidly excised, cleared of excess connective tissue, weighed, rinsed in PBS, and snap frozen in liquid nitrogen.
Intestinal Polyp Quantification
Intestinal segments were excised, cleaned with PBS, cut into equal segments, and stored in 10% neutral formalin until tumor count analysis. Intestinal polyps were analyzed after a deionized water rinse and 0.1% methylene blue staining. Total polyp counts were performed using dissecting micro-scope (model SMZ168, Motic, Xiamen, China) by an investigator blinded to the treatment.
Western Blotting
Briefly, frozen gastrocnemius muscle was homogenized in lysis buffer and protein concentration was determined by the Bradford method. Crude gastrocnemius muscle homogenates were fractionated on 10% SDS-polyacrylamide gels and transferred to PVDF membranes. Membranes were stained with Ponceau red to verify equal loading and transfer. Membranes were then blocked at room temperature for 1hr in 5% non-fat milk Tris-buffered saline with 0.1% Tween-20. Primary antibodies for phosphorylated 4E-BP1 (T37/44) (cat#2855, 1:1000), total 4E-BP1 (cat#9452, 1:2000), phosphorylated rpS6 (S240/244) (cat#2215, 1:2000), total rpS6 (cat#2708, 1:2000) were incubated overnight in 5% TBST milk. Membranes were then incubated in 5% milk-TBST containing anti-rabbit (cat#7074, 1:5000) IgG horseradish-peroxidase conjugated secondary antibodies for 1h at room temperature. All antibodies were from Cell Signaling Technology. Enhanced chemiluminescence (ECL) (GE Healthcare Life Sciences, Piscataway, NJ) was used to visualize the antibody-antigen interactions. Immunoblot images were collected using a digital imager (SynGene GBox, Frederick, MD) and quantified by densitometry using imaging software (Image J; NIH).
Plasma Interleukin-6 (IL-6)
Immediately prior to sacrifice blood was collected via retro-orbital sinus with heparinized capillary tubes, placed on ice, and centrifuged (10,000 × g for 10 min at 4°C). The supernatant was removed and plasma IL-6 concentrations were determined using an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (BioSource, Carlsbad, CA).
Statistical Analysis
All results are reported as means ± standard error of measurement (SEM). Students unpaired t-test were used to compare tissue weights, total food consumption and total daily activity between B6 and MIN mice. Tissue weights, total food intake, and total daily activity were compared using students unpaired t-test in MIN mice stratified by severity pre-cachectic (Pre-CX) to cachectic (CX). Unpaired students t-test were used to compare tissue weights, food intake, and wheel activity in B6 and MIN mice given wheel access. Two-way repeated measures of ANOVAs were used to compare within genotype and between cycles for food intake and physical activity; comparing B6 to MIN, Pre-CX to CX, and MIN controls to MIN High Wheel Activity. Two-way ANOVAs were used to compare across genotypes and within cycles for blood glucose, stomach mass, liver mass, and skeletal muscle diurnal signaling. Unpaired student’s T-Test were used to compare mTORC1 signaling in MIN High Wheel Activity compared to MIN controls. To establish our model, pre-planned unpaired students t-test were performed in B6 mice to determine diurnal skeletal muscle signaling. Unpaired Students T-test were used to compare wheel speed between MIN Low Wheel Activity to MIN High Wheel Activity. When necessary, Tukey’s post hoc analysis was used. Pearson and Spearman correlations were used to determine associations. Statistical analysis was performed using GraphPad (Prism 8 for Mac OS X, La Jolla, Ca). Level of significance for all measures was set at p ≤ 0.05.
Study Approval
All experiments were approved by the University of South Carolina’s Institutional Animal Care and Use Committee.
Results
Cancer Cachexia Characteristics in Free Living MIN Mice - Study 1
Free-living MIN mice (N=22) in study 1, which were not given wheel access, were compared to free-living B6 mice (N=24). MIN had decreased final body weight −6.3%, hindlimb muscle mass −24%, testes mass −11%, and epididymal fat mass −72%, which are all consistent with the development of cachexia (Table 1). Despite the MIN mouse being older, peak body weight and tibia length at the end of the study were comparable to B6 mice. In contrast, MIN mice had increased plasma IL-6, tumor number, and spleen mass compared to B6 mice.
Table 1:
Free Living Male B6 and MIN Mice Characteristics
| Free Living Mice | Stratified Free Living Mice | Wheel Mice | ||||
|---|---|---|---|---|---|---|
| B6 | MIN | Pre-CX | CX | B6 | MIN | |
| Age (wks) | 12.5 ± 0.3 | 17.8 ±0.3 | 17.7± 0.5 | 17.8 ± 0.5 | 13.8 ± 0.5 | 15.8 ± 0.4 |
| N | 24 | 22 | 12 | 10 | 17 | 19 |
| Cachexia Indices | ||||||
| Max BW (g) | 24.1 ± 0.3 | 23.8 ± 0.4 | 23.7 ± 0.4 | 23.9 ± 0.7 | 23.4 ± 0.4 | 23.3 ± 0.4 |
| BW @ Sac (g) | 24.1 ± 0.3 | 22.2 ± 0.4* | 23.4 0± .4 | 20.8 ± 0.6# | 23.9 ± 0.4 | 22.5 ± 0.3** |
| BWΔ Peak (%) | 0.0 ± 0.3 | −6.3 ± 1.45* | −1.1 ± 0.6 | −12.7 ± 1.3# | 0 ± 0 | −3.9 ± 1.9** |
| Plasma IL-6 (pg·ml−1) | 0 ± 0 | 34 ± 6* | 21± 4 | 50 ± 10# | 0 ± 0 | 6.2 ± 2.4 |
| Tumor counts | 0 ± 0 | 60 ± 9* | 49 ± 27 | 81 ± 51 | 0 ± 0 | 32 ± 6** |
| Gastrocnemius (mg) | 117 ± 3 | 102 ± 4* | 113 ± 3 | 89 ± 4# | 122 ± 2 | 112 ± 3** |
| Hindlimb Muscle (mg) | 316 ± 7 | 241 ± 11* | 265 ± 9 | 212 ± 10# | 280 ± 10 | 264 ± 6** |
| Testes (mg) | 195 ± 3 | 175 ± 5* | 189 ± 4 | 158 ± 5# | 181 ± 3 | 178 ± 7 |
| Seminal Vesicle (mg) | 211 ± 12 | 106 ± 13* | 147 ± 15 | 57 ± 8# | 187 ± 7 | 147 ± 11** |
| Spleen (mg) | 140 ± 13 | 385 ± 31* | 341 ± 38 | 438 ± 47 | 125 ± 17 | 276 ± 29** |
| Epididymal Fat (mg) | 320 ± 15 | 91 ± 21* | 145 ± 28 | 30 ± 16# | 230 ± 12 | 158 ± 16 |
| Tibia Length (mm) | 16.8 ± 0.1 | 16.7 ± 0.1 | 16.8 ± 0.1 | 16.7 ± 0.1 | 16.6 ± 0.1 | 16.7 ± 0.1 |
| Animal Behaviors | ||||||
| Food Intake (g· BW−1· d−1) | 0.13 ± 0.01 | 0.16 ± 0.01* | 0.15 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.01 | 0.16 ± 0.02 |
| Activity (counts · d−1) | 27,301 ± 1,435 | 11,206 ± 1,607* | 14,384 ± 2,265 | 7,512 ± 2,055# | - | - |
| Rearing Up (counts · d−1) | 826 ± 160 | 607 ± 160 | 982 ± 245 | 157 ± 42# | - | - |
| Wheel Distance (km) | - | - | - | - | 88.5 ± 19.0 | 33.5 ± 9.4** |
| Time Active (min) | - | - | - | - | 1,705 ± 247 | 979 ± 28.3** |
Data is expressed as mean ±SEM. First, we determined the effect of cancer on cachexia indices and animal behaviors (Left Column) (B6; N=24, MIN; N=22). We then compared the severity of all of the MIN mice by body weight loss (Middle Column). Pre-cachectic mice (Pre-CX) had < 5% body weight loss and cachectic mice (CX) > 5% body weight loss (Pre-CX; N=12, CX; N=10). Lastly, we conducted a 2-week wheel study where mice were given free access to wheels for two weeks (Right Column). Hindlimb muscle mass is the sum weight of the soleus, plantaris, gastrocnemius, tibialis anterior, extensor digitorum longus, and rectus femoris. A subset of mice was used to monitor animal behaviors (B6; N=16, MIN; N=22). Abbreviations: wks: weeks, BW: Body Weight, BWC: Body Weight Change from Peak, g: Grams, IL-6: Interleukin-6, pg · ml−1: picograms per milliliter, mg: Milligrams, g · gBW−1 · d−1: grams per gram of body weight per day, mm: millimeter. Unpaired Students t-test were used to compare cachexia indices and animal behaviors of B6 to MIN, then to compare severity within the MIN, and to compare B6 and MIN wheel groups. Statistical significance was set a p<0.05.
Different than B6
Different than Pre-CX.
Different than B6 Wheel.
The MIN mouse is a genetic model of intestinal and colon tumorigenesis that causes inherent variability in tumor number, which allows for differential progressions of cachexia based and variability in overall tumor burden. MIN mice were classified as either pre-cachectic (<5% body weight loss; Pre-CX) or cachectic (>5% body weight loss; CX). Pre-CX and CX mice were of similar age, peak body weight, and tibia length; therefore, body size was relatively similar between severities (Table 1). As expected, CX mice had greater loss of body weight −12.7%, hindlimb muscle mass −20%, testes mass −16%, and epididymal fat mass −79% compared to Pre-CX mice at sacrifice. Plasma IL-6 was increased 140% in the CX mice, while there was only a trend (p=0.087) for greater tumor number. Spleen mass was unaffected by cachexia severity (p=0.124).
Diurnal Feeding Behavior and Cancer Cachexia – Study 1
Neither B6 or MIN mice demonstrated diurnal fluctuation in muscle mass, gonadal tissue, and spleen mass (data now shown). Total daily food intake normalized to body mass was increased 231% in MIN mice when compared to B6 mice (Table 1). B6 mice exhibited a strong diurnal fluctuation in food intake, being increased 10-fold in the dark cycle (Figure 1B). MIN mice also exhibited a significant but attenuated diurnal fluctuation in food intake normalized to body mass; food intake was increased 2.2-fold during the dark cycle. In a subset of 12wk male B6 (N=10) and MIN (N=10) mice, there was no difference in relative food intake (B6: 0.15 ± 0.004g/g BW; MIN: 0.16 ± 0.009, p=0.333). The normal diurnal fluctuation in food intake are altered in MIN mice. Interestingly, MIN consumed 400% more food during the light cycle than B6 mice.
We then examined the effect of cachexia on diurnal regulation of food intake (Figure 1C). Daily food intake was not different between Pre-CX and CX MIN mice (Table 1), and the diurnal fluctuation to increase food intake 100% during the dark cycle was not altered by cachexia severity. There was no relationship between total daily food intake and body weight loss in MIN mice (Figure 1D). Additionally, no other cachexia variables were significantly associated with total food intake (Table 2). While these results suggest that total food consumption is not related to cachexia progression in MIN mice, the disruption of the diurnal regulation of feeding behavior occurs prior to the development of cachexia.
Table 2:
Total Food and Activity Relationship to Cachexia Indices and Skeletal Muscle Signaling the MIN for Study 1
| Factor 1 | Factor 2 | r | p |
|---|---|---|---|
| Cachexia Indices | |||
| Food Intake (g·gBW−1·day−1) | Gastrocnemius (mg) | −0.237 | 0.289 |
| Epididymal Fat (mg) | −0.007 | 0.975 | |
| Testes (mg) | −0.181 | 0.412 | |
| IL-6 (pg·ml−1) | 0.066 | 0.768 | |
| Activity Counts (g·gBW−1·day−1) | Gastrocnemius (mg) | 0.486 | 0.022 |
| Epididymal Fat (mg) | 0.324 | 0.152 | |
| Testes (mg) | 0.482 | 0.023 | |
| IL-6 (pg·ml−1) | −0.310 | 0.161 | |
| Rearing Up Counts (g·gBW−1·day−1) | Gastrocnemius (mg) | 0.305 | 0.167 |
| Epididymal Fat (mg) | 0.359 | 0.110 | |
| Testes (mg) | 0.432 | 0.045 | |
| IL-6 (pg·ml−1) | −0.270 | 0.225 | |
| Light Cycle Muscle Signaling | |||
| BWL from Peak (%) | 4E-BP1 T37/44 | −0.715 | 0.013 |
| rpS6 S240/244 | 0.250 | 0.458 | |
| Food Intake (g·gBW−1·cycle−1) | 4E-BP1 T37/44 | 0.194 | 0.567 |
| rpS6 S240/244 | −0.068 | 0.842 | |
| Activity Counts (g·gBW−1cycle−1) | 4E-BP1 T37/44 | −0.632 | 0.037 |
| rpS6 S240/244 | 0.583 | 0.060 | |
| Rearing Up Counts (g·gBW−1·cycle−1) | 4E-BP1 T37/44 | −0.278 | 0.408 |
| rpS6 S240/244 | 0.622 | 0.041 | |
Data is expressed as correlation coefficient and corresponding p value. Comparisons are made between total food and activity (factor 1) and either cachexia indices or muscle signaling (factor 2). First, we determined the relationship of total food and activity to cachexia indices (Upper Rows) (MIN; N=22). We then determined the relationship of total food and activity to skeletal muscle signaling following the Light Cycle (Lower Rows) (MIN; N=11). Sections are separated by double black lines. Abbreviations: g · gBW−1 · d−1: grams per gram of body weight per day, counts· d−1: activity counts per day, mg: milligrams, g: grams, pg· ml−1: picogram per milliliter. Spearman correlations were used to determine associations between total food and activity to cachexia indices and total food and activity to skeletal muscle signaling following the light cycle. Statistical significance was set a p<0.05. Italicized and bolded indicates a significant association.
Diurnal Cage Activity and Cancer Cachexia – Study 1
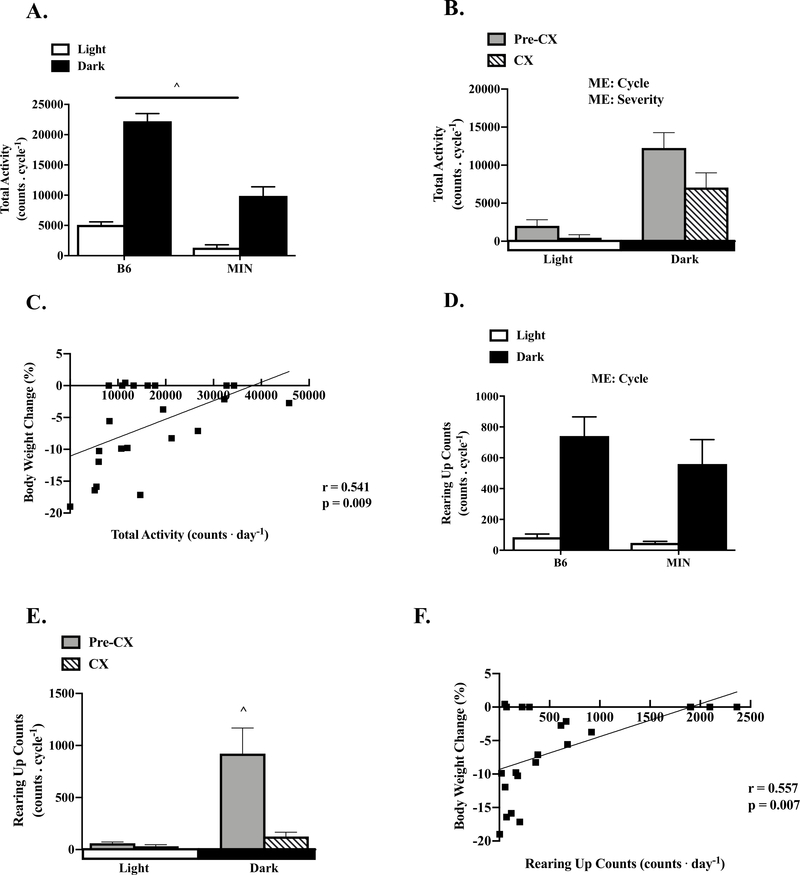
As expected, MIN mice had reduced 58% total daily cage activity when compared to the B6 (Table 1). B6 mice exhibited a strong diurnal fluctuation in cage activity, being increased 336% in the dark cycle (Figure 2A). MIN mice also exhibited a significant diurnal fluctuation in cage activity; being increased 651% in the dark cycle. Interestingly, MIN had decreased cage activity during the light and dark cycle compared to the B6. In a subset of 12wk male B6 (N=10) and MIN (N=10) mice, there was no difference in cage activity (B6: 19,453 ± 1,319 counts/day; MIN: 21,999 ± 2,747 counts/day, p=0.796). Total cage activity was decreased 48% in CX mice compared to Pre-CX mice (Table 1). There was a main effect to increase cage activity during the dark cycle and a main effect for cachexia severity to decrease cage activity regardless of cycle. There was a significant relationship between total daily cage activity and body weight loss in MIN mice (Figure 2C). Additionally, total cage activity was associated to gastrocnemius muscle mass and testes mass (Table 2). These data suggest that decreased cage activity is related to cachexia progression in the MIN mouse.
Figure 2: The Effect of Cancer Cachexia on Diurnal Regulation of Total Cage and Rearing Up Activity.
Data is expressed as mean ± SEM or correlation coefficient and corresponding p value. A) B6 and MIN total cage activity per cycle. (B6: N=16, MIN: N=22). B) Pre-CX and CX MIN total activity per cycle. (Pre-CX; N=12, CX; N=10). C) Correlation of body weight change from peak to total cage activity in the MIN. D) B6 and MIN cage rearing activity per cycle. (B6: N=16, MIN: N=22). E) Pre-CX and CX cage rearing activity per cycle. (Pre-CX; N=12, CX; N=10). F) Correlation of body weight change from peak to cage rearing activity in the MIN. Abbreviations CX: Cachexia, ME: Main Effect. Two-way repeated measures ANOVA were used to compare cage activity within genotypes across time (A, B, D, E). Spearman correlation was used to determine associations between cage activity counts and body weight change from peak in the MIN (C, F). ^ All groups are different. Statistical significance was set a p<0.05.
Cancer Cachexia and Diurnal Cage Rearing Activity – Study 1
Rearing up activity in mice has been linked to the willingness to explore and respond to stimuli (24). Total cage rearing activity was not different between B6 and MIN mice (Table 1). There was a strong diurnal fluctuation in cage activity. There was a main effect of rearing activity to increase 700% during the dark cycle independent of genotype. We then examined the effect of cachexia on diurnal regulation of cage rearing activity (Figure 2E). Total daily rearing activity was reduced 84% in cachectic MIN mice compared to Pre-CX MIN mice (Table 1). The strong diurnal fluctuation in rearing activity present in Pre-CX MIN mice was absent in cachectic MIN mice. Furthermore, total rearing up activity demonstrated a strong relationship with body weight loss in MIN mice (Figure 2F). MIN mice rearing up activity was also significantly correlated with testes mass (Table 2). These results suggest that rearing up activity is reduced during the progression of cachexia in MIN mice.
Cancer Cachexia and Diurnal Blood Glucose, Stomach and Liver Mass – Study 1
The effect of cancer on the diurnal regulation of blood glucose, stomach and liver mass were examined. There was a main effect to decrease 10% blood glucose after the dark cycle (Light: 124 ± 3 mg•dl−1; Dark: 115 ± 2 mg•dl−1). There was also a main effect for MIN mice to have lower blood glucose 14% than B6 regardless of cycle (B6: 129 ± 3 mg•dl−1; MIN: 111 ± 3 mg•dl−1). Interestingly, light cycle blood glucose levels were significantly correlated with gastrocnemius muscle mass, total cage and rearing activity (data not shown). There was a main effect for increased liver mass 16% during the dark cycle (Light: 1.2 ±.05g; Dark: 1.5 ± 0.05g) and a main effect for the MIN to have larger liver 19% than B6 mice (B6: 1.2 ± 0.04g; MIN: 1.5 ± 0.06g). However, no cachexia variables were significantly associated with liver mass (data not shown). There was a main effect to increase stomach mass 31% during the dark cycle (Light: 359 ± 25mg; Dark: 533 ± 34mg) and a main effect for the MIN to have heavier 33% stomach than the B6 (B6: 360 ± 22mg; MIN: 532 ± 37mg). Light cycle stomach mass was significantly associated with body weight loss, epididymal fat mass, plasma IL-6, and rearing activity (data not shown). These data suggest blood glucose, stomach and liver mass exhibit diurnal fluctuations in B6 and MIN mice; however, the MIN mouse exhibits suppressed blood glucose and increased stomach mass.
Cancer Cachexia and Skeletal Muscle mTORC1 Signaling – Study 1
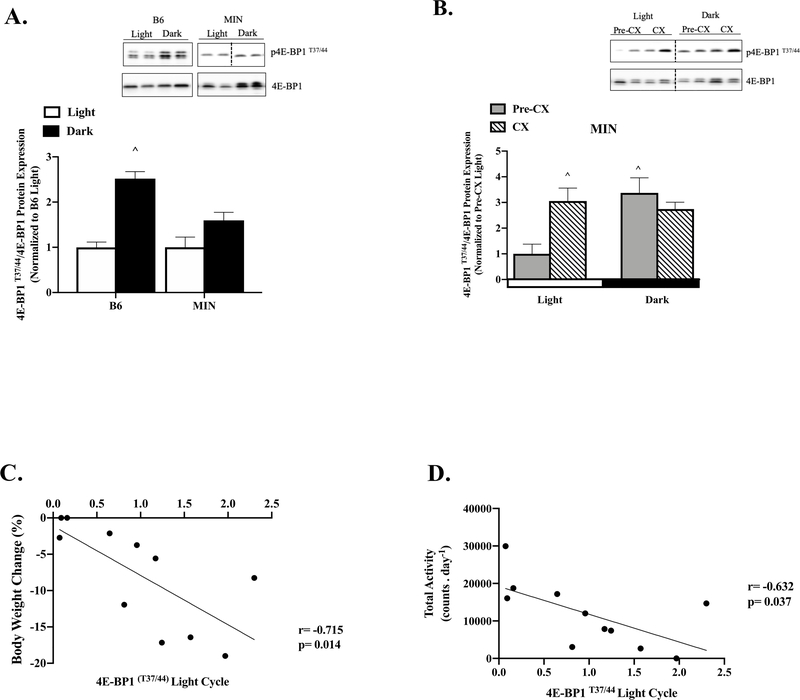
4E-BP1 phosphorylation exhibited a strong diurnal fluctuation in B6 mice, being increased 150% during the dark cycle, which was no present in MIN mouse muscle (Figure 3A). While pre-cachectic MIN mice exhibited a strong diurnal fluctuation in 4E-BP1 phosphorylation, being increased 240% during the dark cycle, this was not present in cachectic MIN mice (Figure 3B). Interestingly, cachectic mouse skeletal muscle demonstrated increased 4E-BP1 phosphorylation 200% in the light cycle and 170% in the dark cycle compared to pre-cachectic mice. The phosphorylation of 4E-BP1 during the light cycle was significantly associated with body weight change from peak (Figure 3C), however this relationship was lost in the dark cycle (r=0.248; p=0.462). 4E-BP1 phosphorylation in the light cycle also demonstrated a significant association with total cage activity (Figure 3D) and gastrocnemius muscle mass (r=−0.787; p=0.004). Interestingly, there was not a diurnal fluctuation in B6 muscle rpS6 phosphorylation, which is also a downstream target of mTORC1 (Figure 3E). However, MIN mice exhibited a strong diurnal fluctuation in rpS6 phosphorylation, which was increased 70% during the dark cycle. There was a main effect to increase rpS6 phosphorylation 70% during the dark cycle independent of cachexia severity (Figure 3F). There was no light or dark cycle relationship between rpS6 and body weight change from, nor any other cachexia variables (data not shown). However, rpS6 phosphorylation after the light cycle had a significant relationship with total rearing up activity (Figure 3G). These data suggest that cachexia differentially alters the diurnal regulation of mTORC1 signaling through downstream mediators 4E-BP1 and rpS6, and disruptions occurring after the light cycle are closely associate with bodyweight loss and cage activity level.
Figure 3: Effect of Cancer Cachexia on Diurnal Regulation of Skeletal Muscle mTORC1 Signaling.
Data is expressed as mean ± SEM. A) 4E-BP1 T37/44 to total 4E-BP1 protein expression in B6 and MIN mice following the Light or Dark Cycle. Data is expressed as fold change from B6 Light. B) 4E-BP1 T37/44 to total 4E-BP1protein expression in Pre-CX and CX MIN mice following the Light or Dark Cycle. Data is expressed as fold change from Pre-CX Light. C) Correlation of body weight change from peak to p-4E-BP1 signaling following the light cycle in the MIN. D) Correlation of total activity to p-4E-BP1 signaling following the light cycle in the MIN. E) rpS6 S240/244 to total rpS6 protein expression in B6 and MIN mice following the Light or Dark Cycle. Data is expressed as fold change from B6 Light. F) rpS6 S240/244 to total rpS6 protein expression in Pre-CX and CX MIN mice following the Light or Dark Cycle. Data is expressed as fold change from Pre-CX Light. G) Correlation of total rearing up counts to p-rpS6 signaling following the light cycle in the MIN. Abbreviations: 4E-BP1:4E-binding protein-1, rpS6: ribosomal protein S6, ME: Main Effect. All protein analysis was completed in the gastrocnemius muscle. Two-way ANOVAs were used to compare 4E-BP1 and rpS6 signaling within genotypes across time (A, B, E, F). Spearman correlation was used to determine associations (C, D). Dotted line signifies same gel. ^ Different than all groups. B6; N=12 per time point, MIN; N=11 per time point, Pre-CX; N=6 per time point, CX; N=5 per time point. Statistical significance was set a p<0.05.
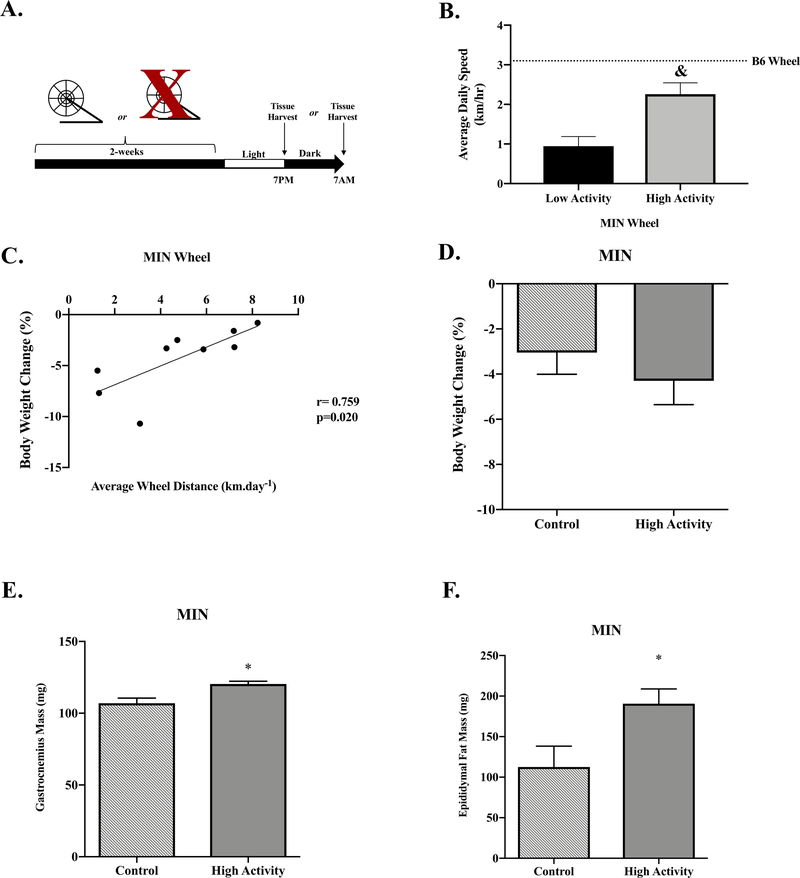
Wheel Access and Cancer Cachexia Progression - Study 2
As expected, high activity mice had increased daily wheel speed (Figure 4B), total running distance (Low: 3.1 ± 1.6 km; High: 67.2 ± 11.9 km) and total time active (Low: 233 ± 135 min; High: 2133 ± 383 min) compared to low activity MIN mice. High wheel activity MIN mice had reduced body weight loss compared to low activity MIN mice (Low: −9.3 ± 1.5%; High: −4.3 ± 1.1%). Interestingly, higher average daily wheel distance was associated with less body weight loss in the high activity MIN mice (Figure 4C). Low activity MIN mice did not demonstrate an association between running distance and body weight loss, which suggests a potential activity threshold for this relationship (r=0.158; p=0.662). To determine the effect of wheel activity on cachexia progression, we compared free-living MIN mice from study 1 matched for body weight loss (control) compared to High wheel activity MIN mice. Tumor number in MIN mice was not significantly affected by wheel access (Control: 52 ± 11; High: 33 ± 8). However, plasma IL-6 was significantly reduced in wheel access MIN mice when compared to control MIN mice (Control: 24.9 ± 4.2 pg/ml−1; High: 5.1 ± 3.4 pg/ml−1). High activity MIN mice had increased gastrocnemius muscle mass and epididymal fat mass compared to control MIN mice (Figure 4E & 4F). These data provide evidence that increased wheel activity attenuates fat mass and muscle mass loss during the initiation of cachexia.
Figure 4: Effect of Wheel Activity on Cancer Cachexia Progression.
Data is expressed as mean ± SEM. A) Study Design. Between 12–15 weeks of age B6 and MIN mice were given access to wheels for two weeks. Mice were either sacrificed at either the end of the light cycle (7:00PM, Light) or dark cycle (7:00AM, Dark) under ad libitum condition. B) Average daily wheel speed in Low Activity and High Activity MIN mice. Dashed line represents B6 Wheel. C) Body weight change relationship to average wheel distance in High activity MIN mice. D) Body weight loss matched controls were compared to High Activity MIN Wheel mice. Body weight change in MIN free-living controls compared to MIN High Activity. E) Gastrocnemius muscle mass in MIN Control compared to MIN High activity E) Epididymal Fat Mass in MIN controls compared to MIN High activity. G) MIN Control and High Activity relative food intake per cycle. (Control: N=16, High Activity: N=9). H) MIN Control and High Activity blood glucose. I) 4E-BP1 T37/44 to total 4E-BP1 protein expression in Control and High Activity MIN mice following the Light Cycle. Data is expressed as fold change from Control MIN. J) rpS6 S240/244 to total rpS6 protein expression in Control and High Activity MIN mice following the Light Cycle. Dotted line signifies same gel. Data is expressed as fold change from Control MIN. Abbreviations: mg: milligrams, 4E-BP1:4E-binding protein-1, rpS6: ribosomal protein S6, BWL: Body weight loss, mg: milligrams, g.gBW−1.cycle−1: gram of food intake per gram of body weight per cycle. All protein analysis was completed in the gastrocnemius muscle (Control: N=8, High Activity: N=5). Unpaired Students T-Test were used to compare average daily speed between low activity to high activity MIN mice (B), and body weight change, gastrocnemius muscle mass, and epididymal fat mass in weight match control MINs to High Activity MINs (D, E, F). Spearman correlations were used to determine body weight change relationship to average wheel distance (C). Two-way repeated measure ANOVAs were used to compare relative food intake and blood glucose (G, H). Two-way ANOVAs were used to compare skeletal muscle mTORC1 signaling in control MINs to High Activity MINs (I, J). &Different than Low Activity, *Different than Control. Statistical significance was set a p<0.05.
Wheel Access and Diurnal Signaling with– Study 2
Food intake was not associated with wheel distance regardless of activity level. However, high wheel running MIN mice consumed significantly more food per gram of body weight in the dark cycle when compared to control MIN mice (Figure 4G). Despite an increase in relative food intake, there was no effect of high wheel activity on the diurnal fluctuation in blood glucose, which decreased 8% following the dark cycle (Figure 4H). The diurnal fluctuation in stomach and liver mass was also not altered by high wheel activity (data not shown).
In the free-living condition, we report that reduced physical activity alters mTORC1 activation. Therefore, we examined the effect of wheel running activity on skeletal muscle mTORC1 signaling in MIN mice. High wheel activity increased MIN mouse skeletal muscle 4E-BP1 phosphorylation 119% after the light cycle when compared to control mice (Figure 4I). However, light cycle rpS6 was not altered by wheel activity (Figure 4J). These data suggest that 2-weeks of high wheel activity in MIN mice can increase muscle 4E-BP1 phosphorylation during the light cycle when compared to control MIN mice matched for body weight loss.
Discussion
While cancer’s ability to disrupt the normal regulation of patient diurnal behaviors has been established for many years (25), we have only recently began to understand the potential negative implications of these behavior changes on systemic and cellular processes that can promote cachexia. Physical activity and feeding patterns have clear potential to regulate wasting through the control of muscle protein turnover and metabolism (26). A reduction in overall cage activity has been identified in several preclinical cancer cachexia models (10). However, the diurnal variation in physical activity and its relationship to the progression of cachexia has not been established. Changes in food consumption in preclinical cancer models are equivocal, and these disparities appear dependent on the tumor model, severity of cachexia, in addition to the methodology and duration of food consumption monitoring. While the MIN mouse has not demonstrated reduced food intake before the initiation of severe cachexia (12), the effect of cachexia on the diurnal feeding patterns in pre-clinical cachexia models has not been previously reported. Diurnal fluctuations in feeding and cage activity are well documented in wildtype mice, and illustrate that food consumption and cage activity are significantly higher during the dark cycle (27). However, cancer cachexia’s impact on these diurnal behaviors is not well understood. Therefore, we investigated the effect of cancer cachexia progression on the diurnal regulation of feeding, physical activity and skeletal muscle mTORC1 signaling in an established preclinical model of cancer cachexia. We report the novel finding that diurnal feeding and physical activity behaviors are altered by the cancer environment and linked to the progression of cancer cachexia. Moreover, diurnal regulation of skeletal muscle mTORC1 activity, which can integrate feeding and physical activity stimuli to regulate muscle protein synthesis, was also impaired by cachexia. Interestingly, low physical activity levels were closely associated with cancer cachexia progression and skeletal muscle mTORC1 signaling. Taken together, our findings of different diurnal fluctuations in feeding and activity behaviors in the cancer environment suggest a potential therapeutic target for the treatment of cachexia that warrants further investigation.
Tumor bearing mice and cachectic cancer patients consistently demonstrate a substantial reduction in physical activity (8–10). Reducing inactivity in cancer patient can improve physical function and reduce fatigue (28). Additionally, cancer patient health is improved by exercise (29). Our findings demonstrate low physical activity levels in tumor bearing mice are significantly associated with severe body weight loss and lower gastrocnemius muscle mass. However, there was a dose effect of wheel activity; increased wheel running distance per day was associated with less body weight change. Disuse-induced muscle atrophy has been extensively examined and can cause muscle mass loss, low grade inflammation, and circadian arrhythmicity of clock genes (30–32). Furthermore, brief periods of inactivity can alter muscle signaling that regulates metabolism and protein turnover (33), and similar pathways have been implicated for driving the progression of cachexia. It has been suggested that disuse atrophy is a hidden component of muscle wasting that accompanies disease (34). Thus, there is a need to understand the role of both the cancer environment and inactivity-associated disuse if effective therapeutic treatments for cachexia are to be achieved. During the initiation of cachexia we have previously identified that MIN mice exhibit reduced voluntary wheel speed (23), reduced overall daily activity, and increased muscle fatigue (35). We report that increasing MIN activity level by 2-weeks of wheel running access was able to maintain gastrocnemius muscle mass and fat pad mass independent of overall weight loss. Interestingly, early studies with the MIN mouse strain reported inherent behavior differences compared to wild-type mice that involved a circling behavior (36). While these circling behaviors could impact cage physical activity measurements, they have been reported to be bred out of the MIN strain that is carried by Jackson Laboratories (36), and we have never observed the circling behavior in MIN mice from our breeding colony. Additionally, when comparing 12wk B6 and MIN mice, we found no differences in relative food intake or cage activity. Therefore, we propose that identified differences that occur between the B6 and MIN mouse can be attributed to the cancer environment. Taken together, we postulate that reduced physical activity contributes to decreased skeletal muscle function and serves to exacerbate cachexia progression, which could be prevented through increased activity.
In addition to the activation of protein degradation, basal muscle protein synthesis control by mTORC1 is suppressed during the progression of cachexia in MIN mice (37). mTORC1 signaling cascade’s sensitivity to feeding and activity are well documented. Independent of disease, muscle unloading can suppress muscle protein synthesis through mTORC1; muscle unloading also decreases mTORC1’s sensitivity to feeding (38). We extend these observations through the examination of diurnal mTORC1 activity in free living, ad libitum fed MIN mice during the progression of cachexia. While 4E-BP1 and rpS6 are downstream phosphorylation targets of mTORC1, only 4E-BP1 demonstrated diurnal fluctuations in wild-type gastrocnemius muscle by increasing following the dark cycle. Recent investigations examining rapamycin sensitive and resistant mTORC1 phosphorylation targets suggest differential sensitivity of 4E-BP1 and S6 phosphorylation to rapamycin and nutrients (39), and might explain our reported diurnal differences in wild-type 4E-BP1 and rpS6 phosphorylation when in the ad libitum condition. We report that cachexia suppressed or enhanced muscle mTORC1 signaling. Cachexia progression suppressed the diurnal fluctuation in 4E-BP1, which involved cachectic mice having increased 4E-BP1 phosphorylation in the light cycle. This increase in light cycle phosphorylation was significantly associated with increased body weight loss and decreased cage activity. While further investigation is needed to understand the ramifications of altered light cycle 4E-BP1 activity, it is interesting to consider the consequences of the altered diurnal feeding behavior in MIN mice, which consumed more food in the light cycle when compared to wild-type mice. Interestingly, rpS6 only exhibited diurnal fluctuation in MIN mice, being induced after the dark cycle. High rpS6 phosphorylation was significantly associated with more cage rearing up activity. An interesting outcome of our current study is that cachectic muscle from free living, ad libitum fed mice did not exhibit a decrease in either 4E-BP1 or rpS6 phosphorylation, which contrasts with the well documented suppression of basal mTORC1 activity in cachectic muscle measured after a brief (3–5h) fast at a non-uniform time of day (3). Taken together, the altered feeding behavior and skeletal muscle light cycle signaling changes suggest that cancer cachexia may cause increased sensitivity to the fasting state. Further study is warranted to determine if the cancer environment alters and accelerates muscle physiological responses to the fasting condition, which may explain discrepancies and equivocal results in the examination of cachectic muscle found in the scientific literature, as varied fasting times combined with mouse sacrifices performed at different times of the day have clear potential to alter signaling associated with protein turnover in skeletal muscle.
Overall, our results provide novel insight into diurnal fluctuations that involve feeding and physical activity during the progression of cancer cachexia. We report that the cancer environment induces significant alterations in diurnal feeding and physical activity behaviors, which can be linked to disrupted skeletal muscle mTORC1 signaling and the progression of cancer cachexia. Cachexia progression dramatically reduced physical activity which was linked to altered skeletal muscle mTORC1 signaling through 4E-BP1 phosphorylation. Furthermore, mTORC1 activity was significantly associated with MIN mouse cage activity level and rearing activity. Increased physical activity in MIN mice provided by 2-weeks of wheel access increased muscle mass and fat mass. Furthermore, increased running distance was associated with less body weight loss in mice that ran at least 1 kilometer a day. Wheel activity also increased dark cycle feeding in MIN mice and increased light cycle 4E-BP1 phosphorylation. Taken together, the impact of disrupted diurnal behaviors induced by cancer need to be considered when examining potential drivers of muscle wasting since these changes would normally occur under free-living conditions. Further investigations are warranted to establish how exercise and physical activity could counteract cancer-induced disruptions to diurnal behaviors that can regulate skeletal muscle wasting.
Acknowledgements
This work was supported by National Institute of Health Grants RO1 CA-121249 (NCI) to J.A. Carson; SPARC Graduate Research Grant from the Office of the Vice President for Research at the University of South Carolina to B.R. Counts. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
References
- 1.Fearon K, Strasser F, Anker SD et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 2.Bye A, Sjoblom B, Wentzel-Larsen T et al. Muscle mass and association to quality of life in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle. 2017;8(5):759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White JP, Baynes JW, Welle SL et al. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS One. 2011;6(9):e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson LJ, Albrecht ED, Garcia JM. Update on Management of Cancer-Related Cachexia. Curr Oncol Rep. 2017;19(1):3. [DOI] [PubMed] [Google Scholar]
- 5.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–66. [DOI] [PubMed] [Google Scholar]
- 6.Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms. 2015;30(2):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 Spec No 2:R271–7. [DOI] [PubMed] [Google Scholar]
- 8.Nissinen TA, Hentila J, Penna F et al. Treating cachexia using soluble ACVR2B improves survival, alters mTOR localization, and attenuates liver and spleen responses. J Cachexia Sarcopenia Muscle. 2018;9(3):514–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer. 2004;90(5):996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol (1985). 2014;116(8):1078–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solheim TS, Blum D, Fayers PM et al. Weight loss, appetite loss and food intake in cancer patients with cancer cachexia: three peas in a pod? - analysis from a multicenter cross sectional study. Acta Oncol. 2014;53(4):539–46. [DOI] [PubMed] [Google Scholar]
- 12.Narsale AA, Puppa MJ, Hardee JP et al. Short-term pyrrolidine dithiocarbamate administration attenuates cachexia-induced alterations to muscle and liver in ApcMin/+ mice. Oncotarget. 2016;7(37):59482–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwarkasing JT, Boekschoten MV, Argiles JM et al. Differences in food intake of tumour-bearing cachectic mice are associated with hypothalamic serotonin signalling. J Cachexia Sarcopenia Muscle. 2015;6(1):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SW, Yoshihara T, Machida S, Naito H. Circadian rhythm of intracellular protein synthesis signaling in rat cardiac and skeletal muscles. Biochem Biophys Rep. 2017;9:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder EA, Esser KA. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc Sport Sci Rev. 2013;41(4):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol (1985). 2009;106(5):1692–701. [DOI] [PubMed] [Google Scholar]
- 18.Hodson N, McGlory C, Oikawa SY et al. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol. 2017;313(6):C604–C11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1 Pt 1):C120–7. [DOI] [PubMed] [Google Scholar]
- 20.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. [DOI] [PubMed] [Google Scholar]
- 21.van Dijk DP, van de Poll MC, Moses AG et al. Effects of oral meal feeding on whole body protein breakdown and protein synthesis in cachectic pancreatic cancer patients. J Cachexia Sarcopenia Muscle. 2015;6(3):212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JP, Phillips BE, Smith K et al. Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am J Clin Nutr. 2012;96(5):1064–70. [DOI] [PubMed] [Google Scholar]
- 23.Baltgalvis KA, Berger FG, Pena MM, Mark Davis J, White JP, Carson JA. Activity level, apoptosis, and development of cachexia in Apc(Min/+) mice. J Appl Physiol (1985). 2010;109(4):1155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mormont MC, Levi F. Circadian-system alterations during cancer processes: a review. Int J Cancer. 1997;70(2):241–7. [DOI] [PubMed] [Google Scholar]
- 26.Hardee JP, Counts BR, Carson JA. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am J Lifestyle Med. 2019;13(1):46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harfmann BD, Schroder EA, Kachman MT, Hodge BA, Zhang X, Esser KA. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet Muscle. 2016;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer. 2015;137(2):471–80. [DOI] [PubMed] [Google Scholar]
- 29.Segal R, Zwaal C, Green E et al. Exercise for people with cancer: a systematic review. Curr Oncol. 2017;24(4):e290–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudrappa SS, Wilkinson DJ, Greenhaff PL, Smith K, Idris I, Atherton PJ. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front Physiol. 2016;7:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol. 2013;45(10):2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadek K, Macklon N, Bruce K, Cagampang F, Cheong Y. Hypothesis: Role for the circadian Clock system and sleep in the pathogenesis of adhesions and chronic pelvic pain? Med Hypotheses. 2011;76(3):453–6. [DOI] [PubMed] [Google Scholar]
- 33.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. American journal of physiology. Cell physiology. 2004;287(4):C834–43. [DOI] [PubMed] [Google Scholar]
- 34.Malavaki CJ, Sakkas GK, Mitrou GI et al. Skeletal muscle atrophy: disease-induced mechanisms may mask disuse atrophy. J Muscle Res Cell Motil. 2015;36(6):405–21. [DOI] [PubMed] [Google Scholar]
- 35.VanderVeen BN, Hardee JP, Fix DK, Carson JA. Skeletal muscle function during the progression of cancer cachexia in the male Apc(Min/+) mouse. Journal of applied physiology. 2018;124(3):684–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–4. [DOI] [PubMed] [Google Scholar]
- 37.White JP, Puppa MJ, Gao S, Sato S, Welle SL, Carson JA. Muscle mTORC1 suppression by IL-6 during cancer cachexia: a role for AMPK. Am J Physiol Endocrinol Metab. 2013;304(10):E1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimkus KL, Jefferson LS, Gordon BS, Kimball SR. Repressors of mTORC1 act to blunt the anabolic response to feeding in the soleus muscle of a cast-immobilized mouse hindlimb. Physiol Rep. 2018;6(20):e13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang SA, Pacold ME, Cervantes CL et al. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science. 2013;341(6144):1236566. [DOI] [PMC free article] [PubMed] [Google Scholar]