Abstract
Diffuse bilateral confluent air space opacities with air bronchograms may result from alveolar edema, pneumonia, or hemorrhage. Associated findings such as cardiac enlargement and pleural effusions help confirm the diagnosis of congestive heart failure; clinical findings of high fever, elevated white blood count, and productive cough favor pneumonia; and a history of hemoptysis may confirm pulmonary hemorrhage. This appearance is also seen in noncardiac edema from a variety of causes, including near-drowning, drug reactions, or acute respiratory distress syndrome. Pulmonary alveolar proteinosis is one of the few chronic diseases that causes this pattern.
Keywords: acute respiratory distress syndrome (ARDS), alveolar edema, congestive heart failure, diffuse pneumonia, pulmonary alveolar proteinosis, pulmonary hemorrhage, pulmonary vasculitis
Questions
-
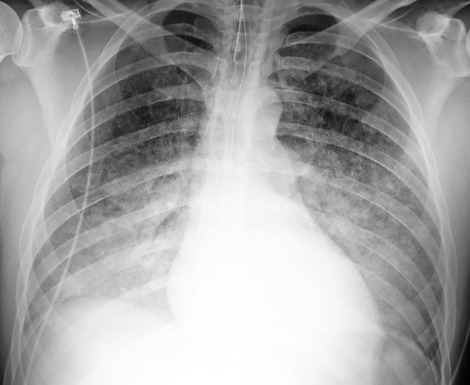
1.The images in Fig. 15.1, A and B , were of a patient who presented in the emergency department with a known diagnosis of granulomatosis with polyangiitis. Which one of the following complications is most likely?
-
a.Pneumonia.
-
b.Hemorrhage.
-
c.Diffuse alveolar damage (DAD).
-
d.Pulmonary edema.
-
e.Acute interstitial pneumonia (AIP).
-
a.
-
2.Which one of the following is not a sign of air space disease?
-
a.Diffuse coalescent opacities.
-
b.Air bronchograms.
-
c.Acinar nodules.
-
d.Fine reticular opacities.
-
e.Air alveolograms.
-
a.
-
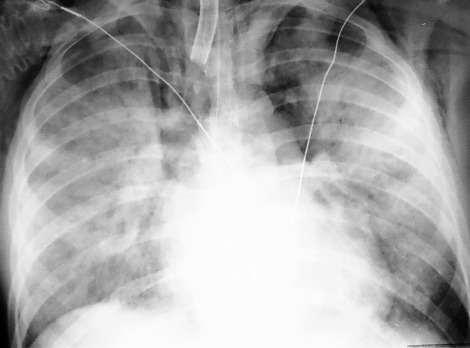
3.The asymmetric distribution of the diffuse coalescent opacities in Fig. 15.2 is suggestive of which diagnosis?
-
a.Pneumonia.
-
b.Goodpasture syndrome.
-
c.Chronic renal failure.
-
d.Alveolar proteinosis.
-
e.Congestive heart failure.
-
a.
-
4.A 24- to 48-hour delay in the development of pulmonary edema is commonly observed in which of the following conditions?
-
a.Congestive heart failure.
-
b.Pulmonary emboli.
-
c.Smoke inhalation.
-
d.Heroin reaction.
-
e.High-altitude pulmonary edema.
-
a.
Fig. 15.1.

Fig. 15.2.

Discussion
The diffuse air space consolidation22,60122601 shown in Fig. 15.1, A and B, is a classic appearance and consists of the following: coalescent or confluent opacities with ill-defined borders; butterfly-shaped perihilar distribution; ill-defined nodular opacities around the periphery of the process (“acinar pattern”)601,666601666; and interspersed small lucencies.457,460457460 Air-filled bronchi surrounded by the confluent opacities are seen as dark branching shadows. These were described by Fleischner162 as the “visible bronchial tree” and are commonly referred to as air bronchograms 150 (see Fig 15.2). The small, interspersed lucent spaces represent groups of air-filled alveoli surrounded by airless consolidated lung. The term air alveologram was applied to these lucent spaces by Felson150; they are the alveolar equivalent of the air bronchogram. The distribution of opacities caused by air space consolidation may be diffuse, lobar, or segmental (see Chapter 14). There is a tendency for air space opacities to be labile—that is, changing in severity over a short period of time on serial examinations.
Diffuse ground-glass opacity is occasionally used to describe less opaque, diffuse, confluent opacities seen on chest radiographs, but is more commonly used in reporting high-resolution computed tomography (HRCT). This differs from consolidation in degree of opacity and implies minimal disease. Ground-glass opacities appear on HRCT as gray areas of confluent attenuation that fail to obliterate normal vascular shadows. Ground-glass opacity demonstrated by HRCT results from minimal filling of the alveolar spaces or from thickening of the alveolar walls and septal interstitium.133 Reticular opacities, whether or not they are demonstrated on a chest radiograph or computed tomography (CT) scan, are not a finding of air space disease. (Answer to question 2 is d.)
Cardiac Pulmonary Edema
Pulmonary alveolar edema (Fig 15.3 ) is a classic example of a diffuse air space filling process (Chart 15.1 ). The presence of alveolar edema, however, does not imply the absence of interstitial edema. Cardiac pulmonary alveolar edema is always preceded by interstitial edema, but the extensive alveolar consolidation obscures the fine reticular opacities of the interstitial process. Radiologic documentation of the underlying interstitial process entails examination of areas not significantly involved by the alveolar filling process. When alveolar pulmonary edema is secondary to congestive heart failure, the alveolar edema often has a perihilar distribution, and Kerley B lines may be present in the costophrenic angles.
Fig. 15.3.

Pulmonary edema is one of the most common causes of diffuse bilateral confluent air space opacities. Associated pleural effusions and cardiac enlargement should confirm the diagnosis of pulmonary alveolar edema resulting from congestive heart failure.
Chart 15.1. Diffuse Air Space Opacities.
-
I.Edema
-
A.Cardiac failure
-
B.Noncardiac (see Chart 15.2)
-
A.
-
II.Exudate (pneumonias)
-
A.Bacteria50,11750117
-
B.Viruses95,235,38295235382
-
C.Mycoplasma159,250159250
-
D.Fungi93,372,450,53493372450534
-
E.Pneumocystis jiroveci pneumonia (also known as PCP)69,96,1146996114
-
F.Parasites (strongyloidiasis)653
-
G.Aspiration
-
H.Rickettsia (Rocky Mountain spotted fever)333,365333365
-
I.Tuberculosis405
-
J.Severe acute respiratory syndrome (SARS)44,70,408,4124470408412
-
A.
-
III.Hemorrhage
-
IV.Other
-
A.Pulmonary alveolar proteinosis176,249,457,487176249457487
-
B.Acute respiratory distress syndrome (ARDS)130,277,278,291,421,663130277278291421663
-
C.Acute interstitial pneumonia (AIP)12,25712257
-
D.Sarcoidosis (very unusual)386,457,467386457467
-
E.Mineral oil aspiration (exogenous cholesterol pneumonia)
-
F.Eosinophilic lung disease85,181,27485181274
-
G.Chemical pneumonitis
-
H.Drug reactions (see Chart 15.3)
-
A.
The latter sign confirms the underlying interstitial process. Other radiologic signs that may be associated with cardiopulmonary edema and can be helpful in suggesting the diagnosis include: (1) prominence of the upper lobe vessels454; (2) indistinctness of vessels291; (3) peribronchial cuffing390; (4) increased width of the vascular pedicle390; (5) pleural effusion, frequently with fluid in the fissures; and (6) cardiac enlargement with a left ventricular prominence. Correlation of the radiologic findings with clinical findings usually confirms the diagnosis. An electrocardiogram indicating cardiac enlargement or an old or acute myocardial infarction is also supportive evidence, whereas an S3 heart sound, neck vein distention, hepatomegaly, or peripheral edema usually confirm the diagnosis of congestive failure. Also, auscultation over the lungs usually reveals characteristic basilar rales.
Occasionally, alveolar edema is not distributed uniformly. As a result of gravity, when the patient is upright, the edema fluid has a predominantly lower lobe distribution, but when the patient is supine, the fluid tends to have a more posterior distribution. When the patient favors one side, the fluid tends to gravitate to the dependent side. The resolution of pulmonary edema is often not uniform, so that serial chest radiographs reveal a change in the distribution from diffuse perihilar opacities to a pattern of more uneven multifocal opacities. Other causes for atypical or nonuniform distribution of pulmonary edema are usually of pulmonary origin. The best known of these is severe emphysema, which results in a patchy distribution of the alveolar edema. Presumably, loss of vasculature in the emphysematous areas of the lung results in the development of edema in the more normal areas. Pulmonary embolism is a complication of pulmonary edema that may result in a nonuniform or patchy distribution of the alveolar edema. Two factors may determine the distribution of the air space edema following pulmonary embolism: (1) abrupt interruption of perfusion to an area of lung may prevent the development of typical pulmonary edema; and (2) severe ischemia of the lung may give rise to pulmonary hemorrhage. Clinical suspicion of pulmonary embolism in a patient with congestive heart failure will usually require computed tomography angiography (CTA).
Concomitant infection is another cause of uneven distribution of pulmonary edema. Like the diagnosis of pulmonary embolism, this requires correlation with the clinical history. An elevated temperature, leukocytosis, or purulent sputum should prompt a bacteriologic study to rule out superimposed pneumonia.
Cardiac enlargement in combination with diffuse alveolar opacities that are otherwise characteristic of pulmonary edema is not always a reliable indicator that the patient’s primary problem is a cardiac disorder. For instance, chronic renal failure with uremia can cause pulmonary edema (uremic pneumonitis) as well as hypertension and associated heart disease, with the result of cardiac enlargement. Not only does uremia cause true cardiomegaly, which is probably related to chronic hypertension, but it also may cause pericardial effusion. Thus, the pulmonary edema that results from chronic renal failure and uremia is typically associated with enlargement of the cardiac silhouette. Correlation with the clinical history should readily identify uremic pneumonitis.
In contrast to pulmonary alveolar edema and cardiac enlargement, the presence of a normal-sized heart might suggest a noncardiac form of pulmonary edema, but there are situations in which such patients may actually have cardiac pulmonary edema. These include acute cardiac arrhythmias and acute myocardial infarction, which result in pulmonary edema before dilation of the heart. Thus, there are at least two mechanisms for cardiac pulmonary edema with a normal-sized heart.
Noncardiac Pulmonary Edema
The preceding discussion suggests that the radiologic appearance of noncardiac pulmonary edema is similar to that of cardiac pulmonary edema.546 In general, the most helpful radiologic feature for distinguishing the two is the presence or absence of cardiac enlargement. Accurate assessment of heart size is often difficult. Technical factors—including supine and anteroposterior positioning, especially when done with portable units—may all contribute to cardiac magnification. Patient condition may also lead to inaccurate cardiac size estimation. Patients with emphysema often have cardiac enlargement, although the chest radiograph is suggestive of a normal or even small heart size. Aggressive intravenous (IV) fluid resuscitation may actually enlarge the heart and cause pulmonary edema. The evaluation of serial radiographs is especially useful for distinguishing a number of the causes of noncardiac edema because the evolution of the edema may be strikingly different. Many of the entities listed in Chart 15.2 may result in acute alveolar edema in the absence of the pulmonary vascular and interstitial changes that precede the edema because of either renal failure or cardiac failure. These entities tend to occur in very acute cases of pulmonary edema and are often best diagnosed by clinical correlation,5 as is shown in the following discussions of acute toxic inhalations, near-drowning, acute airway obstruction, drug reactions, and ARDS.
Chart 15.2. Noncardiac Pulmonary Edema.
-
I.
Chronic renal failure
- II.
-
III.
Anaphylaxis (e.g., penicillin, transfusion,62 radiologic contrast medium205)
-
IV.
Narcotics (e.g., morphine, methadone, cocaine, heroin)201,473,671201473671
-
V.
Drug reaction (e.g., interleukin-2 therapy,90,51890518 β-adrenergic drugs391)
-
VI.
Acute airway obstruction422 (e.g., foreign body)
-
VII.
Near-drowning448
-
VIII.
High altitude254
-
IX.
Fluid overload
-
X.
Cerebral (trauma, stroke, tumor)467
-
XI.
Hypoproteinemia
-
XII.
ARDS (early stages)277,291277291
-
XIII.
Pancreatitis494
-
XIV.
Amniotic fluid embolism537
-
XV.
Fat embolism
-
XVI.
Re-expansion following treatment of pneumothorax or large pleural effusion
-
XVII.
Organophosphate insecticide ingestion339
-
XVIII.
Hanta virus pulmonary syndrome291
Acute Toxic Inhalations
Nitrogen dioxide inhalation (silo filler’s disease) is an excellent model for acute toxic pulmonary edema. In the first few days after a grain storage silo is filled, nitrogen dioxide forms. The gas reacts with water in the respiratory tract to produce an irritation of the tracheobronchial tree and alveoli. In the acute phase, this disease has the radiologic appearance of bilateral diffuse alveolar edema. This phase is usually followed within a few days or weeks by complete resolution, although bronchiolitis obliterans may develop weeks to months later as a result of the small airway injury. Chest radiographs of patients with bronchiolitis obliterans often show a fine nodular or reticular pattern. The other chemicals listed in Chart 15.2 produce a similar reaction.
Smoke inhalation is the most common cause of death due to fires. Fire victims may have thermal injuries to the airways and are exposed to toxic gases, soot, and carbon monoxide. Inhalation of these substances may cause airway and alveolar injury with alveolar leak as the cause of pulmonary edema (Fig 15.4 ). Patients with smoke inhalation must be carefully monitored because the radiologic appearance of pulmonary edema may be delayed by as much as 24 to 48 hours (answer to question 3 is c). The risk of a delayed onset of edema is greatest in patients with low oxygen saturation and elevated carboxyhemoglobin, which reflects carbon monoxide poisoning with potential concomitant lung damage.445 Early onset of pulmonary edema indicates severe alveolar injury with increased risk of diffuse alveolar damage and ARDS.
Fig. 15.4.
Smoke inhalation produces diffuse bilateral air space opacities with a normal heart size. There is often delayed onset of edema following smoke inhalation. The presence of edema soon after the exposure indicates that the patient is at increased risk for diffuse alveolar damage.
Near-Drowning
Near-drowning448 is another important cause of noncardiac pulmonary edema. The history should confirm the diagnosis; however, aspiration of water provides only a partial explanation for the diffuse alveolar opacities that may develop in near-drowning victims. Again, there may be a delay of 24 to 48 hours before edema develops. Other mechanisms that may contribute to the development of this type of edema include prolonged hypoxia, respiratory obstruction, and fibrin degradation. Fibrin degradation raises the possibility of a subclinical consumptive coagulopathy with microembolization, which may lead to a diffuse pulmonary capillary leak and thus to pulmonary edema. Severe hypoxia may occur in a near-fatal–drowning victim, even when the initial chest radiograph is normal. Patients should therefore be followed for 24 to 48 hours to exclude a significant pulmonary injury.
Acute Airway Obstruction
The diagnosis of acute airway obstruction is usually made on the basis of the clinical history. The obstruction is frequently an aspirated object, such as a large bolus of food or a surgical sponge. The resultant pulmonary edema is usually related to severe hypoxia. This mechanism may be nearly identical to that described for near-drowning. The collection of alveolar fluid is most likely due to a diffuse alveolar leak caused by severe injury to the alveolar capillary membrane.422
Drug Reactions
Adverse reactions to a variety of drugs (Chart 15.3 ) may cause acute and chronic pulmonary responses.18,521852 These reactions have been described as chemotherapy lung, 551 but a large variety of drugs have pulmonary complications. These include antibiotics, narcotics, heart medications, arthritis drugs, radiographic contrast, and a number of chemotherapeutic agents. Acute drug reactions may cause the rapid development of diffuse, confluent air space opacities or patchy, multifocal, confluent opacities (as seen in Chapter 16). These acute reactions are the result of edema, hemorrhage, or DAD, which may resemble ARDS. Subacute and chronic reactions include eosinophilic pneumonia, COP, and NSIP. The more chronic reactions cause air space opacities in the early stages but later progress to cause reticular opacities, indicating a fibrotic reaction. Pleural effusions (see Chart 4.1) may also be associated with some of these reactions. This is especially true of drugs that are known to cause a lupus-like reaction.489
Chart 15.3. Pulmonary Drug Reactions.
- I.
-
II.Hemorrhage489
-
A.Anticoagulants
-
B.Amphotericin B
-
C.Cytarabine
-
D.Cyclophosphamide
-
E.Penicillamine
-
A.
-
III.Diffuse alveolar damage (DAD)489
-
A.Bleomycin
-
B.Busulfan
-
C.Carmustine
-
D.Cyclophosphamide
-
E.Gold
-
F.Melphalan
-
G.Mitomycin
-
A.
-
IV.Eosinophilic pneumonia489
-
A.Nitrofurantoin
-
B.Nonsteroidal antiinflammatory drugs
-
C.Para-aminosalicylic acid
-
D.Penicillamine
-
E.Sulfasalazine
-
A.
- V.
-
VI.Nonspecific interstitial pneumonitis (NSIP)489
-
A.Amiodarone
-
B.Carmustine
-
C.Chlorambucil
-
D.Methotrexate
-
A.
Modified from Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: radiologic and pathologic manifestations. Radiographics. 2000;20:1245-59. Used with permission.
Anaphylaxis is an acute response to a variety of substances and is a cause of edema. Acute alveolar edema may occur following administration of IV radiologic contrast, morphine, heroin, and other opiates. Cocaine has been reported as a cause of both cardiac and noncardiac pulmonary edema.201,473201473 Although the mechanism for the noncardiac pulmonary edema is unknown, it is generally believed to represent an idiosyncratic reaction with an alveolar capillary injury. Since the opiates cause central nervous system depression, there may also be a relationship to neurogenic edema. Methadone, a slow-acting narcotic, may cause a slower onset of edema than heroin or morphine, and may also resolve more slowly.201,671201671 The typical radiographic pattern for narcotic-induced edema is diffuse, bilateral, confluent air space opacification without cardiomegaly and without pleural effusion. In contrast to narcotics, the allergic edema of interleukin-2 commonly causes interstitial edema (as seen in Chapter 18) with septal lines and peribronchial edema.90,51890518 This so-called allergic edema infrequently becomes more severe with the development of alveolar edema.291
Acute DAD causes permeability edema with the radiologic appearance of diffuse confluent opacities that may sometimes appear multifocal. Therefore, in its early stages, DAD may be indistinguishable from hydrostatic pulmonary edema. The acute edema is rapidly followed by cellular necrosis, inflammation, and later fibrosis. Bleomycin, busulfan, and cyclophosphamide are all possible causes of DAD, and the resulting ARDS is the most severe life-threatening drug reaction.
Eosinophilic pneumonia is a true allergic reaction. Diffuse confluent air space opacities with a peripheral distribution are typical. Histologic changes include infiltration of alveolar walls with eosinophils and other inflammatory cells. Peripheral eosinophilia is also a common finding. Eosinophilic pneumonia responds well to withdrawal of the medication but sometimes requires steroid therapy for complete resolution. Nitrofurantoin is a urinary antibiotic that may cause eosinophilic pneumonia.
COP, previously known as BOOP (bronchiolitis obliterans organizing pneumonia), is more likely to produce multiple areas of diffuse confluent opacity. Like eosinophilic pneumonia, the opacities tend to be in the periphery of the lung. CT often shows the areas of consolidation to be more nodular than expected from the radiograph. Even though there is histologic evidence of fibrosis, this reaction usually responds well to withdrawal of the drug and steroid therapy.489 Amiodarone, bleomycin, methotrexate, and nitrofurantoin are all possible causes of COP.
NSIP is more likely to present with minimal patchy or multifocal basilar opacities that may appear confluent on the chest radiograph. HRCT shows mainly ground-glass opacity with some reticular opacities. This reaction is more likely to progress to interstitial fibrosis with reticular opacities, honeycombing, and traction bronchiectasis.489 Amiodarone and methotrexate are also causes of NSIP.
The diagnosis of drug reaction is best suggested by a history of medication with any of the drugs known to produce pulmonary reactions. In patients who are undergoing chemotherapy for cancer, the differential includes the following: (1) opportunistic infection; (2) diffuse hemorrhage; (3) drug reaction; and (4) spread of the primary tumor.94
Acute Respiratory Distress Syndrome
ARDS is a complex clinical syndrome that may occur after a variety of severe pulmonary injuries130 including trauma, shock, sepsis, severe pulmonary infection, transfusion reaction, or cardiopulmonary bypass. These conditions cause an alveolar capillary injury with leakage of edematous fluid into the alveolar spaces. This is so severe that increasing concentrations of inspired oxygen are required to maintain adequate arterial oxygen saturation, while at the same time high ventilator pressures are needed to combat the decreasing lung compliance. The radiologic appearance is that of diffuse coalescent opacities similar to those described for alveolar edema, alveolar hemorrhage, or diffuse air space pneumonia. The sequence of events in patients with ARDS, however, is different from that in patients with typical pulmonary edema. Unlike cardiac pulmonary edema, which clears in response to therapy, the edema in ARDS may persist for days to weeks. As the diffuse coalescent opacities begin to clear, an underlying reticular pattern emerges. Patients who succumb to the illness usually have a complex pulmonary reaction that includes the formation of hyaline membranes, extensive fibrosis, and development of areas of organizing pneumonia. Grossly, the lungs are stiff and firm. The mechanisms for this catastrophic course are not completely understood. It has been suggested that diffuse intravascular clotting and platelet aggregation within the capillary bed (disseminated intravascular coagulation) probably lead to interstitial edema, altered capillary permeability, atelectasis, and hyaline membrane formation. This is pathologically described as DAD and includes injury to the capillary endothelium and alveolar epithelium.291 Oxygen toxicity may also be a factor in the pathogenesis of many cases of ARDS. In addition, bacterial superinfection is very common. This entity should be suspected on the basis of the clinical presentation and the presence of persistent, diffuse, coalescent opacities (Fig 15.5 ).
Fig. 15.5.
Diffuse pulmonary consolidations in this case are the result of acute respiratory distress syndrome. This appearance is radiologically indistinguishable from diffuse pneumonia and other causes of pulmonary alveolar edema. In the early stages of diffuse alveolar damage, the alveoli are filled by edema resulting from alveolar capillary leak. The pneumomediastinum is the result of barotrauma caused by positive pressure ventilation.
Acute Interstitial Pneumonia
AIP is a fulminant form of interstitial pneumonia that was originally described by Hamman and Rich.217 It is an idiopathic interstitial pneumonia that is often grouped with usual interstitial pneumonia, desquamative interstitial pneumonia, and NSIP. It has a different clinical course in that it occurs in previously healthy individuals and is rapidly progressive with a poor prognosis. The histologic findings are described as DAD and are similar to findings of ARDS. It has even been described as an idiopathic form of ARDS.
The first phase is an exudative reaction that is followed by a proliferative reaction. The chest radiograph typically shows diffuse air space opacities. HRCT may show additional findings of ground-glass opacities and reticular or linear opacities. The proliferative phase may begin after the first week. Additional CT findings that result from the retracting fibrosis during the proliferative phase include architectural distortion, traction bronchiectasis, and cystic spaces.12,25712257
Re-expansion Pulmonary Edema
Re-expansion pulmonary edema is an infrequent complication that occurs after treatment of pneumothorax or a large pleural effusion. Rapid reinflation of the lung probably causes alveolar capillary injury initiated by ischemia. Re-expansion edema is most likely when the lung has been collapsed for a prolonged period of time. In cases of pneumothorax the history often suggests a delay in treatment of more than 24 hours (Fig 15.6, A and B ).
Fig. 15.6.

A, This large right pneumothorax has caused near-complete collapse of the right lung. B, Following treatment of the pneumothorax with a thoracostomy tube, the patient developed diffuse confluent opacity throughout the right lung. This has been described as re-expansion pulmonary edema.
Other Causes
Other causes of pulmonary edema that must be diagnosed on the basis of the clinical history include high-altitude pulmonary edema,254 amniotic fluid embolism,537 and fat embolism. As indicated in the previous discussion of smoke inhalation and near-drowning, there may be a delay in the development of the diffuse coalescing opacities with fat embolism, but it should not exceed the time of the fracture by more than 24 to 48 hours. Repositioning or orthopedic manipulation of a fracture occasionally accounts for fat emboli occurring days to weeks after the initial fracture. In the absence of a history of manipulation, however, the development of pulmonary opacities from days to weeks after a fracture is more suggestive of other diagnoses, such as venous thromboembolism or pneumonia.
Pulmonary Hemorrhage
Hemorrhage is an important cause of diffuse coalescent opacities because it may lead to extensive air space consolidation (see Fig 15.1, A and B). Some of the causes of pulmonary hemorrhage, such as anticoagulant therapy and pulmonary contusion, are easily identified from the clinical history. Hemoptysis is a common clinical finding because a large amount of blood fills the lungs.
Bleeding disorders that may lead to pulmonary hemorrhage include hemophilia, anticoagulation therapy, and hematologic malignancy. The differential of diffuse alveolar consolidation in the leukemic patient is that of: (1) opportunistic infection; (2) drug reaction; (3) diffuse hemorrhage; (4) leukemic infiltration; and (5) pulmonary edema. Severe hemoptysis confirms the diagnosis of diffuse pulmonary hemorrhage in such a clinical setting, but the absence of hemoptysis does not exclude the diagnosis.
Drug reactions that result in pulmonary hemorrhage produce radiologic appearances identical to those of alveolar edema. The chest radiograph may show either diffuse, symmetric, confluent opacities with a perihilar or basal distribution, or the pattern of multifocal opacities. Sometimes, HRCT may show that the distribution is more patchy or multifocal than suspected from the radiograph. Additional HRCT findings include ground-glass opacities that indicate less severe alveolar hemorrhage. Anticoagulants, amphotericin B, and some of the cytotoxic drugs are all possible causes of acute pulmonary hemorrhage.489
Trauma is not a common cause of diffuse pulmonary hemorrhage, but in the setting of severe trauma, diffuse air space opacities may result from pulmonary contusion (Fig 15.7, A and B ). More frequently, trauma results in asymmetric, localized, or multifocal areas of contusion. The radiologic presentations range from minimal ground-glass opacities on CT to multiple areas of air space opacity or even diffuse air space consolidation. Associated fractures, extrapleural hematoma, and pleural effusion are common associated findings but are not always present. A history of rapid deceleration injury, blunt trauma, or penetrating trauma can confirm the diagnosis of contusion with pulmonary hemorrhage.
Fig. 15.7.

A, Pulmonary contusion should be suspected as the cause of diffuse air space opacities in patients who have sustained major thoracic trauma. In this case, the extensive air space opacities are bilateral but more severe on the right. The patient was in a motor vehicle collision and experienced major rapid deceleration and blunt force trauma. B, Coronal computed tomography shows confluent opacities with air bronchograms on the right and lobular ground-glass opacities on the left.
Goodpasture syndrome,522 although not a common entity, must be seriously considered in a patient with hemoptysis, hematuria, and diffuse air space consolidations. The diagnosis is usually confirmed by renal biopsy with specific immunofluorescent stains. Because these patients have antibodies to their glomerular basement membrane, the condition has been renamed antiglomerular basement membrane disease. 441
Idiopathic pulmonary hemosiderosis is another rare pulmonary disease that causes diffuse pulmonary hemorrhage. The radiologic pattern depends on the stage of the disease. In the acute phases, there are bilateral, diffuse coalescent opacities with air bronchograms. As the disease resolves, the clearing may be patchy, leaving the multifocal ill-defined opacities described in Chapter 16. This entity tends to be recurrent with the development of an interstitial pattern that is frequently of a fine reticular nature and occurs as a late complication of the disease. This generally follows many recurrences of the acute alveolar hemorrhage. During the phases of the hemorrhage, the fine reticular pattern is obscured by extensive alveolar consolidation.
Granulomatosis with polyangiitis is a diffuse pulmonary vasculitis that may produce either localized or diffuse pulmonary hemorrhage (see Fig 15.1, A and B; answer to question 1 is b). This diagnosis is easily confirmed when the classic triad of pulmonary, nasopharyngeal, and renal involvement is present. The limited form of granulomatosis with polyangiitis requires lung biopsy for confirmation. Other patterns associated with this entity include multiple ill-defined opacities and multiple lesions that may cavitate (see Chapters 16 and 24Chapter 16Chapter 24). Systemic lupus erythematosus is another collagen vascular disease that sometimes causes diffuse pulmonary hemorrhage.441
Inflammatory Diseases
Acute pulmonary infections are a major consideration in the differential of diffuse coalescent opacities. The entity is commonly distinguished from pulmonary edema on clinical grounds. Patients with diffusely coalescent bilateral pneumonias are usually profoundly ill, with an elevated temperature, elevated white blood cell count, severe dyspnea, and productive sputum.
Pneumonia
Bronchopneumonias are the most common infections producing diffuse coalescent opacities. Gram-negative organisms are particularly notorious for producing such fulminant pneumonias.235 This pattern is frequently preceded by radiographs showing multifocal ill-defined opacities like those described in Chapter 16. There is a tendency for the patient to have some volume loss because of the bronchial inflammation. The loss of volume may cause one lobe to appear predominantly involved during the course of the illness. In some cases, the radiologic patterns of bronchopneumonia and pulmonary edema are identical, but an asymmetric, patchy, or even unilateral presentation (see Fig 15.2) is more consistent with the diagnosis of bronchopneumonia. (Answer to question 2 is a.) It must be remembered that pulmonary edema may also produce a patchy or asymmetric distribution when there is underlying disease such as emphysema or pulmonary embolism. Clinical and laboratory data may be useful in distinguishing bronchopneumonia from pulmonary edema. Bronchopneumonia should result in a febrile response with productive purulent sputum and leukocytosis. Culture of sputum and blood usually confirms the diagnosis and identifies the organisms.67
Viral Pneumonia
Viral pneumonias are best known for producing a diffuse interstitial pattern—usually a fine reticular or fine nodular pattern—but fulminant cases may also lead to diffuse air space consolidations. The appearance varies depending on the severity, ranging from ground-glass opacities with areas of consolidation to near-complete alveolar consolidation. This has been reported in detail with descriptions of SARS.70,322,42770322427 Viral pneumonia may be especially severe in immunologically compromised patients, in particular those with hematologic malignancies, acquired immunodeficiency syndrome (AIDS), or an organ transplant, and especially those who are receiving immunosuppressive therapy. Severe viral pneumonia with air space consolidation is infrequently encountered in the otherwise normal patient.
ARDS is a well-documented complication of a number of viral and mycoplasma infections,159 and it is a likely explanation for the pattern of diffuse air space opacities in patients with viral pneumonia. Chickenpox pneumonia carries a very high risk for this complication, especially in pregnant patients. ARDS is also a concern with emerging infections including SARS.44,42044420 This complication could also be the cause for a high mortality rate in an influenza pandemic.
Aspiration Pneumonia
Aspiration pneumonia is another cause of diffuse coalescent opacities that should be diagnosed by correlating the radiologic appearance with the clinical setting. Aspiration pneumonia may produce diffuse bilaterally coalescent opacities, although these tend to be more localized than in pulmonary edema. Because the aspirated material usually goes to the dependent portions of the lung, the distribution of the radiologic abnormality is directly related to the position of the patient at the time of aspiration. Material aspirated while the patient is in the upright position tends to go to the medial basal segments of the lung and to the right middle lobe, whereas in the supine patient aspirated material tends to collect in the superior segments of the lower lobes and the posterior segments of the upper lobes. Knowledge of the material aspirated and of the patient’s position at aspiration often confirms the diagnosis. The clinical setting is very important. For example, the postoperative or comatose patient is very susceptible to aspiration, and alcoholic patients are especially prone to aspiration pneumonia.
Chronic aspiration is often more difficult to confirm and requires careful evaluation of the patient’s history. For example, air space consolidation in the right middle lobe in an older patient who is otherwise not particularly ill should prompt suspicion of an exogenous lipoid pneumonia—mineral oil aspiration. This type of aspiration pneumonia should not result in diffuse confluent opacities. Patients with disturbances of esophageal motility, obstructive lesions of the esophagus, and head or neck tumors are all candidates for chronic aspiration. Aspiration may also be the underlying factor in the tendency for these patients to have gram-negative pneumonias. Gram-negative pneumonias are a much more likely cause of diffuse confluent opacities than is uncomplicated chronic aspiration.
Opportunistic Pneumonia
Immunologically compromised patients are susceptible not only to common pyogenic, viral, and fungal pneumonias but also to a more virulent infection. Viral infections that may cause minimal abnormality in a patient with a competent immune system may cause a fatal hemorrhagic pneumonia in a patient with severe immunosuppression. Fungi that are nonpathogenic in patients with normal immunity may cause diffuse coalescent opacities in the patient who is immunologically compromised.423 These uncommon pathogenic fungi include Aspergillus, Candida, Cryptococcus, and Phycomycetes.372 Infection by Phycomycetes is commonly called mucormycosis. Both Phycomycetes and Aspergillus invade the pulmonary vessels, leading to a diffuse hemorrhagic pneumonia and even pulmonary necrosis or gangrene.
PCP is an AIDS-defining disease and one of the most common infections in patients with AIDS.196,605196605 It is not expected to occur until the CD4 cell count has dropped to less than 200 cells/μl.223,559223559 The organisms spread through the airways and interstitium with minimal or no visible abnormality on the initial radiograph. During the earliest stages, gallium scans and HRCT are more sensitive for early diagnosis. The earliest chest radiograph appearance is a subtle, fine, reticular pattern, but many cases follow a more fulminant course, with rapid development of diffuse coalescent opacities (Fig 15.8, A-C ). This results from alveolar wall injury followed by filling of the air spaces with plasma proteins, inflammatory cells, and organisms. The chest radiographic appearance is often that of diffuse symmetric coalescent opacities and resembles noncardiac edema. CT often confirms a mixed pattern of reticular interstitial opacities with thickening of the interlobular septa, ground-glass opacities, and alveolar consolidation. The combination of interlobular septal thickening and ground-glass opacities is common and has been described as the “crazy-paving pattern.”221,490221490 An atypical upper lobe distribution may be seen in patients who are receiving prophylactic treatment with aerosolized pentamidine.69,966996 Associated pleural effusions are rare in patients with pneumocystis, occurring as infrequently as in 2% of patients, and should be considered evidence of another diagnosis.
Fig. 15.8.
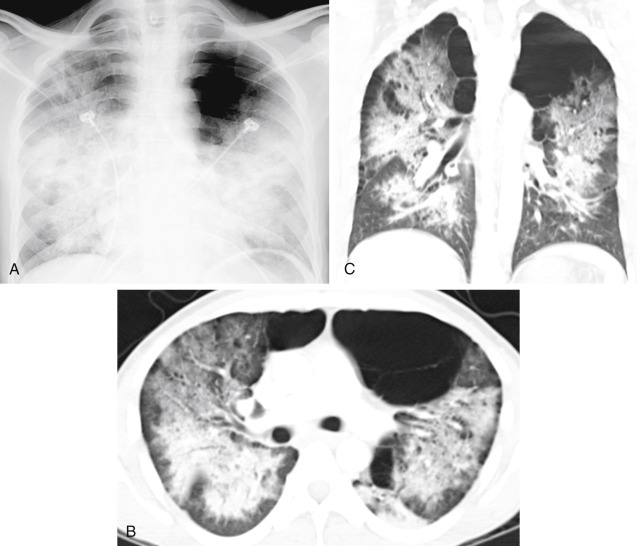
A, Diffuse bilateral confluent opacities in a patient with AIDS are strongly suggestive of pneumocystis pneumonia. B, Axial computed tomography (CT) confirms the presence of extensive bilateral alveolar consolidations and ground-glass opacities. The upper lobes are spared because of severe bullous disease. C, Coronal CT shows the opacities to have air bronchograms and a distribution resembling pulmonary edema.
Pyogenic pneumonias typically occur in the earlier stages of HIV infection—that is, before the first AIDS-defining disease with a CD4 count between 200 and 500 cells/μl. Bacterial pneumonias may be severe and cause a pattern of diffuse consolidations in approximately 20% of cases, but lobar consolidations are more common with a frequency of 50%. The most common organisms are Haemophilus influenzae and Streptococcus pneumoniae.106
Chronic Diffuse Consolidations
Diffuse coalescent opacities usually indicate either an acute process, such as pulmonary edema, or the acute phase of a chronic relapsing disease, such as idiopathic pulmonary hemosiderosis. However, although rare, there are a few conditions that cause persistent chronic diffuse pulmonary consolidations. This somewhat rare radiologic presentation may be seen with chronic granulomatous diseases and pulmonary alveolar proteinosis.
Chronic Granulomatous Diseases
Chronic granulomatous diseases rarely cause diffuse coalescent opacities. Fungal infections may produce this pattern in immunologically compromised patients but rarely in the uncompromised host. Although rarely seen in tuberculosis, this pattern may occur when there is extensive pulmonary hemorrhage in conjunction with aspiration of blood to other portions of the lung from a cavitary lesion, or when patients with miliary tuberculosis develop secondary pulmonary edema or ARDS.405 Once this complication develops, the underlying miliary nodules are obscured by pulmonary edema.
Sarcoidosis is a rare cause of diffuse bilaterally symmetric consolidations that may resemble pulmonary edema. The mechanism for this pattern is considered in detail in Chapter 16. Sarcoidosis more often causes multifocal ill-defined opacities, which may have air bronchograms, than a pattern of diffuse confluent opacities. In contrast to the other entities considered in this chapter, there may be a striking disparity between the severity of the radiologic appearance of sarcoidosis and the clinical well-being of the patient. Although the abnormalities may appear to be very extensive, patients with sarcoidosis are often only mildly dyspneic and may otherwise be asymptomatic.
Alveolar Proteinosis
Alveolar proteinosis176,487176487 is an unusual disease that results in diffuse bilateral confluent opacities that often have air bronchograms (Fig 15.9, A and B ). Occasionally, a fine nodular pattern with ill-defined borders may be seen around the periphery of the confluent opacities, but these are not interstitial nodules like the nodules discussed in Chapter 17. They represent acinar nodules and are the smallest unit of air space filling that can be detected radiologically. CT usually confirms extensive air space consolidation, but also reveals ground-glass opacities and thickened interlobular septa,249 or the crazy-paving pattern.490 These consolidations may appear acutely and resolve spontaneously or may persist, requiring pulmonary lavage. The time required for their spontaneous resolution is highly variable. Alveolar proteinosis is also observed to be a chronic relapsing disease and one of the few diseases that may produce diffuse air space consolidation while the patient remains relatively asymptomatic. In fact, the radiologic presentation of diffuse bilateral air space consolidations that are either recurrent or chronic in a patient who complains only of mild dyspnea strongly suggests this diagnosis.
Fig. 15.9.

A, Diffuse bibasilar confluent opacities fade into the more normal-appearing aerated lung with an intermediate, poorly defined, ground-glass appearance. This radiologic appearance is similar to pulmonary edema, hemorrhage, and diffuse pneumonia, but this patient’s opacities were chronic and relapsing. B, Axial computed tomography (CT) through the upper lungs confirms extensive ground-glass opacities with associated thickening of the interlobular septa. This combination is described as the crazy-paving pattern. These chest radiographic and CT patterns in combination with the history of a chronic and relapsing clinical course are the expected presentation of pulmonary alveolar proteinosis.
Top 5 Diagnoses: Diffuse Air Space Opacities
-
1.
Edema
-
2.
Pneumonia
-
3.
ARDS
-
4.
Pneumocystis
-
5.
Hemorrhage
Summary
Diffuse coalescent opacities with air bronchograms, air alveolograms, and acinar nodules constitute the classic radiologic appearance of alveolar disease.
Pulmonary edema is the most common cause of this pattern. Pulmonary edema may be divided into cardiac and noncardiac categories on the basis of cause. Clinical correlation is extremely important in identifying the cause in pulmonary edema of noncardiac origin.
ARDS is a complex clinical syndrome that results in diffuse coalescing opacities. The diagnosis should be suspected when the patient has acute pulmonary edema following severe injury or shock, particularly after a drug reaction, gram-negative sepsis, transfusion reactions snake bite, or the use of a pump oxygenator in cardiopulmonary bypass surgery.
Diffuse pneumonias are another extremely important cause of diffuse coalescent opacities. They should be easily distinguished from pulmonary edema when seen in an acutely ill and toxic febrile patient. The radiologic appearance is of little value in determining the specific organism. Bacteria, viruses, and fungi may also produce this appearance. Viruses and fungi are more likely in the immunologically compromised host, and a prompt specific diagnosis is essential because failure to initiate immediate therapy may result in death.
Diffuse pulmonary hemorrhage with extensive bilateral confluent opacities is frequently but not invariably associated with hemoptysis. Clinical correlation is essential for determining the cause of diffuse pulmonary hemorrhage (e.g., anticoagulation therapy, hemophilia, leukemia, or trauma). Biopsy is required for a diagnosis of the idiopathic sources of such hemorrhage, including Goodpasture syndrome, pulmonary hemosiderosis, and granulomatosis with polyangiitis.
Answer Guide
Legends for introductory figures
-
Fig. 15.1
A, Pulmonary hemorrhage fills the alveoli with blood and often causes bilateral symmetric, coalescent opacities. This patient presented with massive hemoptysis. The bleeding was the result of pulmonary vasculitis from granulomatosis with polyangiitis. B, Coronal reconstruction emphasizes the diffuse alveolar consolidation with air bronchograms.
-
Fig. 15.2
Bronchopneumonia has caused consolidation of the entire right lung. Note the air-filled branching bronchial tree. Air bronchograms are a reliable sign of pulmonary consolidation.
Answers
-
1.
b
-
2.
d
-
3.
a
-
4.
c


