Abstract
Context
Glucagon increases energy expenditure; consequently, glucagon receptor agonists are in development for the treatment of obesity. Obesity negatively affects the reproductive axis, and hypogonadism itself can exacerbate weight gain. Therefore, knowledge of the effects of glucagon receptor agonism on reproductive hormones is important for developing therapeutics for obesity; but reports in the literature about the effects of glucagon receptor agonism on the reproductive axis are conflicting.
Objective
The objective of this work is to investigate the effect of glucagon administration on reproductive hormone secretion in healthy young men.
Design
A single-blinded, randomized, placebo-controlled crossover study was conducted.
Setting
The setting of this study was the Clinical Research Facility, Imperial College Healthcare NHS Trust.
Participants
Eighteen healthy eugonadal men (mean ± SEM: age 25.1 ± 1.0 years; body mass index 22.5 ± 0.4 kg/m2; testosterone 21.2 ± 1.2 nmol/L) participated in this study.
Intervention
An 8-hour intravenous infusion of 2 pmol/kg/min glucagon or rate-matched vehicle infusion was administered.
Main Outcome Measures
Luteinizing hormone (LH) pulsatility; LH, follicle-stimulating hormone (FSH), and testosterone levels were measured.
Results
Although glucagon administration induced metabolic effects (insulin area under the curve: vehicle 1065 ± 292 min.µU/mL vs glucagon 2098 ± 358 min.µU/mL, P < .001), it did not affect LH pulsatility (number of LH pulses/500 min: vehicle 4.7 ± 0.4, glucagon 4.2 ± 0.4, P = .22). Additionally, there were no significant differences in circulating LH, FSH, or testosterone levels during glucagon administration compared with vehicle administration.
Conclusions
Acute administration of a metabolically active dose of glucagon does not alter reproductive hormone secretion in healthy men. These data are important for the continued development of glucagon-based treatments for obesity.
Keywords: glucagon, luteinizing hormone, follicle-stimulating hormone, testosterone, reproduction
The global increase in the prevalence of obesity has fuelled the search for effective and safe treatments for obesity and related disorders. Glucagon is produced by pancreatic α-cells and increases energy expenditure, satiety, hepatic glucose output, and insulin secretion (1). Glucagon receptor agonists (together with other gut hormone receptor agonists) have been shown to reduce body weight and improve glucose tolerance in rodents and humans (2-6). Because the metabolic effects of glucagon receptor agonists are being used in the development of novel antiobesity treatments, it is important to determine whether there are additional direct effects of glucagon receptor agonism on reproductive hormone secretion aside from those due to changes in body weight. Furthermore, this information would improve our understanding of the dynamic metabolism-reproductive interface and help determine whether the impact of obesity on the endocrine reproductive axis could be in part mediated through glucagon.
Low reproductive hormone levels (known as male obesity secondary hypogonadism, MOSH) occurs in 25% to 40% of men with obesity (7). Consequently, if glucagon receptor agonism has a direct effect on the reproductive system (in addition to its metabolic effects), this would be highly clinically relevant because hypogonadism worsens insulin resistance, facilitates weight gain, impairs fertility, and worsens quality of life (7). Although some evidence (detailed as follows) suggests glucagon may influence reproductive hormone secretion, the nature of its effects on reproductive hormones has not been fully characterized. Additionally, no information about reproductive hormone levels were provided in studies exploring the metabolic effects of glucagon receptor (co-)agonists (2-6).
A study in ovariectomized estrogen-primed female rats demonstrated that 1 µg of glucagon injected into the medial preoptic area of the hypothalamus increased peak serum luteinizing hormone (LH) levels 4 hours postinjection (8). In humans, a 1 mg dose of glucagon given intravenously did not affect LH levels in the limited time frame studied (ie, 180 minutes) (9). In contrast, 0.03 mg/kg glucagon injected intramuscularly resulted in significant increases in serum LH in 11 of 13 people (peaking at 2 hours after glucagon administration), but again the effects of glucagon administration were measured for only 180 minutes (10).
Because glucagon receptor agonists are being developed to treat obesity (2-6), which is frequently complicated by MOSH (7), it is important to conclusively determine whether glucagon receptor agonism can directly modulate the reproductive axis in men. Based on animal data, we hypothesized that glucagon could directly stimulate the reproductive axis; however, the evidence base in humans remains uncertain. Therefore, we conducted a single-blinded, placebo-controlled study to determine the direct effects of acute glucagon administration on the reproductive axis in men.
Methods
Participants
This study was performed in accordance with the Declaration of Helsinki and received ethical approval from the West London Research Ethics Committee (16/LO/0391) and was registered on the ISRCTN clinical trial registry (ISRCTN10114288). Following recruitment using online and print advertisements, screening, and informed consent, 18 healthy men (mean age 25.1 ± 1.0 years; mean body mass index [BMI] 22.5 ± 0.4 kg/m2; testosterone 21.2 ± 1.2 nmol/L) were entered into the study. Exclusion criteria included: BMI less than 18.5 or greater than 25 kg/m2, history of medical and psychological conditions, use of prescription, recreational or investigational drugs within the preceding 2 months, blood donation within 3 months of study participation, ingestion or inhalation of nicotine-containing substances within 3 months, alcoholism, or history of cancer.
Study visits
Each participant attended 2 study visits, 1 for glucagon administration and 1 for vehicle administration. The order of the infusions was randomized (using www.random.org) and participants were blinded as to the identity of the infusions. Glucagon infusions were prepared by dissolving 1 mg glucagon in 1 mL of sterile water and adding the glucagon solution to 49 mL Gelofusine (Braun). Glucagon was administered at a rate of 2 pmol/kg/min, a dose previously established to be metabolically active in humans (11). Rate-matched vehicle infusions comprised Gelofusine (Braun) only.
Participants fasted from 10 pm the night preceding each study visit. On the morning of each study visit, participants ate a standardized 200 kcal breakfast (OatSoSimple, Quaker Oats Ltd) at 6 am and arrived at the Clinical Research Facility at 8:15 am. After a period of acclimatization, 2 intravenous cannulae (1 in each arm) were inserted (1 cannula was used for blood sampling and the second for administering the infusion). Following baseline sampling, a glucagon/vehicle infusion was started at T = 0 min and continued until T = 500 min. A visual analog scale (0-10 cm), was used to measure participants’ self-reported nausea at T = –15 min, T = 240 min, and T = 470 min. Participants were given an ad libitum meal at T = 480 min. Blood samples were taken every 10 minutes throughout the study for assessment of serum insulin, LH, follicle-stimulating hormone (FSH), and testosterone (apart from during the meal).
Biochemical analyses
Serum insulin, LH, FSH, and testosterone were measured by North West London Pathology on the automated Abbott Architect platform. Chemiluminescent immunoassays were used to measure serum insulin (intra-assay and interassay coefficient of variation [CV]: ≤ 7%), serum LH (intra-assay and interassay CV: ≤ 5%), serum FSH (intra-assay and interassay CV: ≤ 10%), and serum testosterone (intra-assay and interassay CV: ≤ 8%). Blood glucose was measured using a FreeStyle Optimum Meter (Abbott Diabetes Care). Plasma glucagon was measured using a well-established in-house radioimmunoassay (intra-assay and interassay CV: ≤ 10%) (12).
Main outcome measures
The primary outcome measures were LH levels and LH pulsatility. Secondary outcomes included FSH and testosterone levels.
Statistical methods
The study’s sample size (n = 18 per group) was chosen to provide 90% power to detect a difference in LH (between vehicle and glucagon administration) of 2 IU/L (SD 2.3 IU/L) at a significance level of .05 (13). LH pulsatility was assessed by J.V. (who was blinded to the identity and order of the intravenous infusions) using a validated deconvolution analysis (ie, a mathematical model of the underlying secretion and/or elimination rates of LH, derived from the LH concentration profile, which identifies and characterizes LH pulses and pulse orderliness [regularity]) (14, 15). Pulse orderliness refers to the regularity of pulsatile hormone secretion. In our study, LH pulse orderliness was determined by calculation of the approximate entropy (ApEn) of the profile of LH secretion as part of the deconvolution analysis. When LH pulsatile secretion is less orderly, it is more irregular and has a higher ApEn.
Longitudinal data were analyzed using generalized estimating equations with STATA 15.1 software (STATA Corp). Paired t tests were performed on parametric data and Wilcoxon matched-pairs signed rank tests were performed on nonparametric data using Prism 8.0.2 (GraphPad) software. P values of less than .05 were considered statistically significant. Data are presented as mean ± SEM.
Results
Confirmation of biological activity of glucagon administration
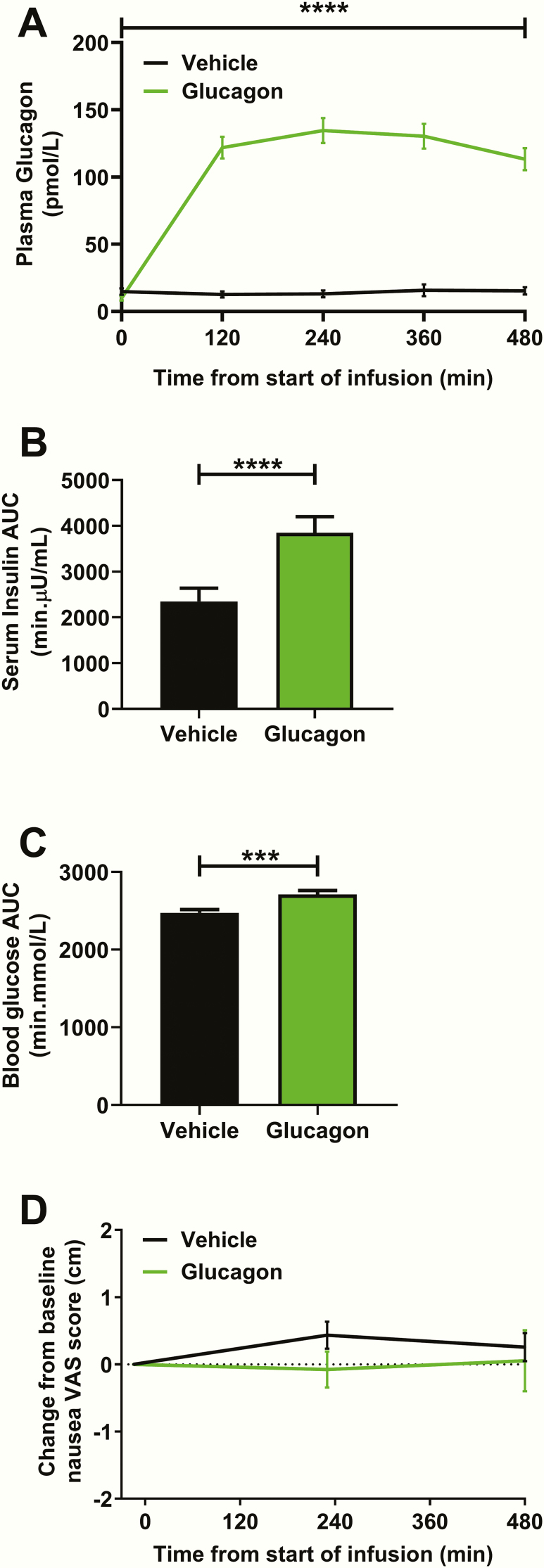
Intravenous infusion of glucagon at 2 pmol/kg/min was confirmed to significantly increase circulating plasma glucagon levels (Fig. 1A). Preinfusion insulin (vehicle 7.8 ± 1.0 µU/mL vs glucagon 10.4 ± 3.4 µU/mL, P > .99) and glucose (vehicle 5.1 ± 0.2 mmol/L vs glucagon 5.2 ± 0.2 mmol/L, P = .26) were similar in both groups. Glucagon administration resulted in a higher insulin area under the curve (AUC) (Fig. 1B) and glucose AUC compared to vehicle administration (Fig. 1C). Glucagon administration is recognized to cause nausea (9), which (as a form of stress) could have had a detrimental impact on reproductive hormone release (16). However, the dose of glucagon used in the present study although metabolically active (as shown earlier), did not cause nausea (Fig. 1D) and did not affect food intake (kcal eaten/kg body weight: vehicle 15.5 ± 0.3 vs glucagon 17.0 ± 1.3, P = .11).
Figure 1.
Confirmation of biological activity of administered glucagon. A, Glucagon levels were significantly higher during glucagon administration compared to during vehicle administration (vehicle vs glucagon from T = 0 min to T = 480 min, ****P < .001 using generalized estimating equation [GEE]). B, Insulin area under the curve (AUC) was significantly higher during glucagon administration compared to vehicle administration (****P < .001 using a Wilcoxon matched-pairs signed rank test). C, Glucose AUC was significantly higher during glucagon administration compared to vehicle administration (***P < .001 using a paired t test). D, There was no significant difference in self-reported (change from baseline) nausea during glucagon administration compared with vehicle administration (vehicle vs glucagon from T = –15 min to T = 470 min, P = .32 using GEE). Abbreviation: VAS, visual analog scale (0-10 cm).
Effects of glucagon administration on reproductive hormones
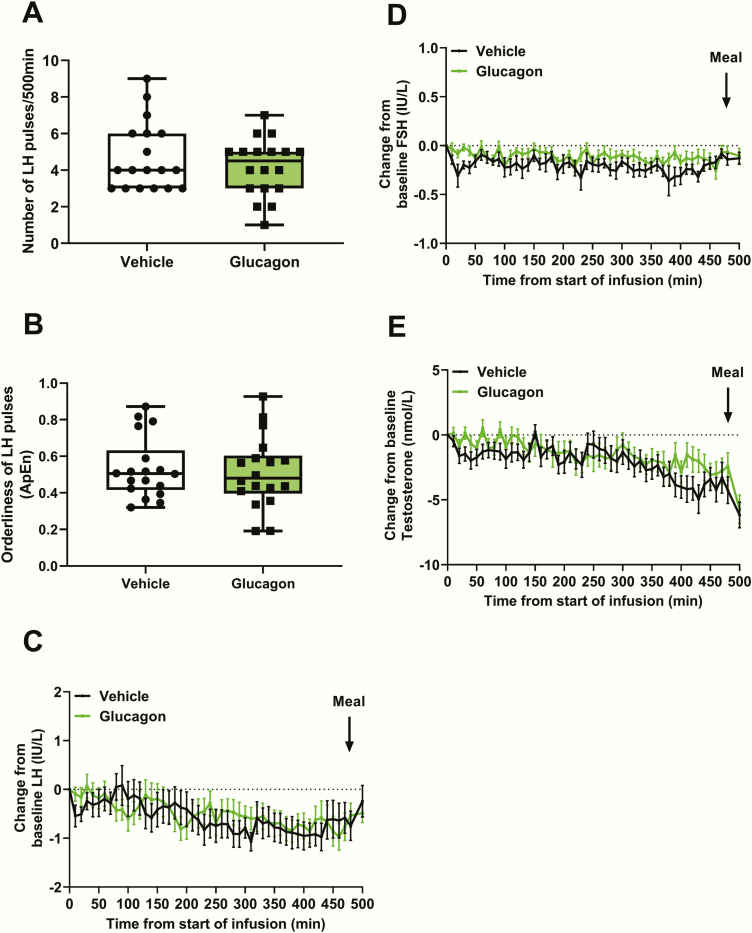
Glucagon administration did not affect the number of LH pulses (number of LH pulses/500 min: vehicle 4.7 ± 0.4 vs glucagon 4.2 ± 0.4, P = .22) (Fig. 2A), nor did it affect the orderliness (ie, regularity) of the LH pulses as measured by LH pulse ApEn (LH ApEn: vehicle 0.53 ± 0.04 vs glucagon 0.51 ± 0.05, P = .73) (Fig. 2B). Furthermore, starting from similar baseline levels (mean baseline LH: vehicle 3.6 ± 0.3 IU/L vs glucagon 3.6 ± 0.3 IU/L, P > .99), there was no significant change from baseline LH during glucagon administration compared to vehicle administration (Fig. 2C).
Figure 2.
Effects of glucagon administration on reproductive hormone levels. A, The number of luteinizing hormone (LH) pulses was similar during glucagon and vehicle infusions (P = .22 using a Wilcoxon matched-pairs signed rank test). B, LH pulse orderliness (ie, approximate entropy [ApEn]—with zero representing perfect orderliness) was similar during glucagon and vehicle infusions (P = 0.73 using a Wilcoxon matched-pairs signed rank test). C, Change from baseline LH was similar during 2 pmol/kg/min intravenous glucagon infusion and rate-matched vehicle infusion (vehicle vs glucagon from T = 0 min to T = 500 min, P = .83 using generalized estimating equation [GEE]). Meal = ad libitum meal. D, Change from baseline follicle-stimulating hormone (FSH) was similar during 2 pmol/kg/min intravenous glucagon infusion and rate-matched vehicle infusion (vehicle vs glucagon from T = 0 min to T = 500 min, P = .11 using GEE). Meal = ad libitum meal. E, Change from baseline testosterone was similar during 2 pmol/kg/min intravenous glucagon infusion and rate-matched vehicle infusion (vehicle vs glucagon from T = 0 min to T = 500 min, P = .36 using GEE). Meal = ad libitum meal.
Starting from comparable baseline levels (mean baseline FSH: vehicle 2.7 ± 0.3 IU/L vs glucagon 2.8 ± 0.3 IU/L, P = .22), the change from baseline FSH was similar during glucagon administration compared to vehicle administration (Fig. 2D). Likewise, testosterone levels at baseline were similar (mean baseline testosterone: vehicle 23.3 ± 1.7 nmol/L vs glucagon 23.4 ± 1.7 nmol/L, P = .93), and there was no significant difference in the change in testosterone from baseline during glucagon and vehicle administration (Fig. 2E).
Discussion
We demonstrate that acute glucagon administration, at a dose that produces metabolic effects (as evidenced by higher insulin and glucose AUC compared to vehicle administration), does not affect the number of LH pulses nor LH pulse orderliness; nor does it affect LH, FSH, or testosterone levels. Previous human studies have suggested that glucagon administration either had no effects on reproductive hormone release (9) or had stimulatory effects on LH (10). However, these studies were performed on a small number of male volunteers with blood samples taken for a relatively short period of time, and testosterone levels were not reported in these studies (9, 10). Additionally, normal reproductive function is dependent on LH pulsatility as well as absolute LH levels (17), and LH pulsatility was not assessed in these previous studies (9, 10). Furthermore, the previous studies were performed more than 40 years ago, and differences in historical hormone measurement compared with modern techniques (including assay sensitivity and specificity) may limit interpretation of these previous studies’ results. Therefore, firm conclusions could not be drawn from the existing literature.
By comparison, the present study is the first to extensively characterize the effect of acute glucagon administration on reproductive hormone release in men using validated methodology (blinded deconvolution analysis of LH pulsatility) for an extended period of time (ie, 500 minutes) using a protocol designed to detect clinically relevant differences while avoiding potential confounding factors. The study duration was chosen to ensure sufficient numbers of LH pulses occurred for adequate pulse analysis because the time between LH pulses in healthy men has been shown to range from 15 minutes to more than 135 minutes, with a mean interpulse interval of 111minutes when samples are taken every 10 minutes (18). Therefore, it would be expected that on average 4 LH pulses would occur during the 500-minute period during which glucagon was administered in our study; and our protocol (ie, blood samples taken every 10 minutes) ensured the LH pulses that occurred would be detected. Fasting and eating both can affect reproductive hormone levels, thus we designed the study to avoid both extremes of prandial status. Fasting suppresses LH release and pulsatility (13), and conversely testosterone levels are suppressed in the immediate postprandial period (19). Therefore, study participants consumed a meal at 6 am before the start of the glucagon infusion to minimize the confounding effects of prandial status on reproductive hormone secretion.
It would be interesting to examine the effects of chronic glucagon receptor agonist administration on reproductive hormones in the future because there may be differences between acute effects (as reported in this study) and chronic effects of glucagon receptor agonism, and currently there are no reports of the effects of recently developed agonists on reproductive hormone secretion (2-6). However, interpretation of the direct effects of glucagon agonism on reproductive hormones using these agents will be challenging because the weight loss associated with these agents would be likely to improve hypogonadism if present.
A possible explanation for the absence of an effect of glucagon administration on reproductive hormone levels is nausea, which can be caused by glucagon receptor agonism (9). Nausea (as a form of stress) may have a detrimental impact on reproductive hormone release (16). However, the dose of glucagon used in this study produced some of the expected metabolic effects (ie, an increase in glucose and insulin concentrations) but did not increase nausea (compared to vehicle administration). In contrast to some of the literature, the dose of glucagon administered in our study did not reduce food intake. This may be due to differences in study methodology because, in our study, food intake was assessed more than 8 hours after previous nutrient ingestion, whereas in other studies reporting a reduction in food intake with glucagon administration, food intake was assessed 20 to 210 minutes after previous nutrient ingestion (20, 21).
Another plausible explanation for our findings is that peripherally administered glucagon was unable to act centrally and therefore could not influence hypothalamic function. However, there is evidence to suggest that intravenously administered glucagon crosses the blood-brain barrier (22). Therefore, it is reasonable to conclude that central glucagon receptor agonism is also not likely to result in acute changes in reproductive hormone secretion.
Our finding that glucagon administration does not affect reproductive hormones in healthy men provides important data on potential off-target effects in the ongoing development of glucagon receptor agonists for the treatment of obesity. Based on our data, therapeutic glucagon receptor agonists are likely to produce metabolic effects without having adverse direct effects on the reproductive system. Furthermore, chronic exposure to glucagon receptor agonists may improve the reproductive hormone status of men with obesity-related hypogonadism because weight loss itself can ameliorate hypothalamic hypogonadism in obese men (7).
Novel glucagon/glucagon-like peptide-1 (GLP-1) receptor dual agonists and glucagon/GLP-1/glucose-dependent insulinotropic peptide (GIP) receptor triagonists are currently being developed to treat obesity and associated comorbidities (2-6). However, the effects of these agents on reproductive hormone secretion has not been reported (2-6, 23), but they are unlikely to have direct effects on reproductive hormones based on studies using individual constituent hormone receptor agonists. Acute administration of GLP-1 does not alter reproductive hormone levels in healthy men (24). Chronic treatment with GLP-1 receptor agonists has been reported to improve reproductive hormone levels in hypogonadal men with obesity and/or type 2 diabetes, but this may be a consequence of the weight loss associated with GLP-1 receptor agonists (25, 26). In rodents, incubation of pituitary cells with GIP resulted in LH and FSH release, whereas central administration of GIP to female rats lowered circulating FSH levels but had no effect on circulating LH levels (27).
In conclusion, this comprehensive study demonstrates that in healthy men, acute glucagon administration (at a dose that produces metabolic effects) does not affect LH pulse number, or LH pulse orderliness, nor does it affect circulating LH, FSH, and testosterone levels. This provides important mechanistic insight into the direct effects of glucagon receptor agonism on the male reproductive axis. Furthermore, it remains possible that a stimulatory effect of glucagon agonism could be observed in obese men with MOSH with low baseline LH levels and/or pulsatility (28). Consequently, it would be important to now perform similar studies in patient groups such as obese men with MOSH.
Acknowledgments
Financial Support: The Section of Endocrinology and Investigative Medicine is supported by grants from the Medical Research Council (MRC), Biotechnology and Biological Sciences Research Council, the National Institute for Health Research (NIHR), an Integrative Mammalian Biology Capacity Building Award, an FP7-HEALTH-2009-241592 EuroCHIP grant, the NIHR Imperial Biomedical Research Centre Funding Scheme, an MRC Clinical Research Training Fellowship (Grant number MR/M004171/1 to C.I.), the NIHR Clinical Research Networks (to D.P.), an MRC Clinical Research Training Fellowship (Grant number MR/N020472/1 to R.R), an MRC Clinical Research Training Fellowship (Grant number MR/R000484/1 to L.Y.), grants from the MRC and Wellcome Trust (to T.T.), an NIHR Clinician Scientist Award (Grant number CS-2018-18-ST2-002 to A.A.), the National Health Service (NHS) (to A.N.C.), and an NIHR Professorship (Grant number RP-2014-05-001 to W.S.D.). This article presents independent research funded by the NIHR and supported by the NIHR Imperial Clinical Research Facility and NIHR Imperial Biomedical Research Centre at Imperial College Healthcare NHS Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Glossary
Abbreviations
- ApEn
approximate entropy
- BMI
body mass index
- GIP
glucose-dependent insulinotropic peptide
- GLP-1
glucagon-like peptide-1
- LH
luteinizing hormone
- MOSH
male obesity secondary hypogonadism
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Campbell JE, Drucker DJ. Islet α cells and glucagon—critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329-338. [DOI] [PubMed] [Google Scholar]
- 2. Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finan B, Yang B, Ottaway N, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21(1):27-36. [DOI] [PubMed] [Google Scholar]
- 4. Jall S, Sachs S, Clemmensen C, et al. Monomeric GLP-1/GIP/glucagon triagonism corrects obesity, hepatosteatosis, and dyslipidemia in female mice. Mol Metab. 2017;6(5):440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ambery P, Parker VE, Stumvoll M, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607-2618. [DOI] [PubMed] [Google Scholar]
- 6. Tillner J, Posch MG, Wagner F, et al. A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21(1):120-128. [DOI] [PubMed] [Google Scholar]
- 7. Dhindsa S, Ghanim H, Batra M, Dandona P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care. 2018;41(7):1516-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitsugi N, Hashimoto R, Yoshida K, Arita J, Kimura F. Effects of preoptic injection of glucagon on luteinizing hormone secretion in ovariectomized rats with or without estrogen priming. Exp Clin Endocrinol. 1988;91(2):135-142. [DOI] [PubMed] [Google Scholar]
- 9. Eddy RL, Jones AL, Hirsch RM. Effect of exogenous glucagon on pituitary polypeptide hormone release. Metabolism. 1970;19(10):904-912. [DOI] [PubMed] [Google Scholar]
- 10. Gilboa Y, Hertzeanu I, Rosenberg T. Effect of glucagon on serum LH. Horm Metab Res. 1975;7(4):357-358. [DOI] [PubMed] [Google Scholar]
- 11. Chakravarthy M, Parsons S, Lassman ME, et al. Effects of 13-hour hyperglucagonemia on energy expenditure and hepatic glucose production in humans. Diabetes. 2017;66(1):36-44. [DOI] [PubMed] [Google Scholar]
- 12. Izzi-Engbeaya C, Comninos AN, Clarke SA, et al. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab. 2018;20(12):2800-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergendahl M, Aloi JA, Iranmanesh A, Mulligan TM, Veldhuis JD. Fasting suppresses pulsatile luteinizing hormone (LH) secretion and enhances orderliness of LH release in young but not older men. J Clin Endocrinol Metab. 1998;83(6):1967-1975. [DOI] [PubMed] [Google Scholar]
- 14. Jayasena CN, Abbara A, Veldhuis JD, et al. Increasing LH pulsatility in women with hypothalamic amenorrhoea using intravenous infusion of kisspeptin-54. J Clin Endocrinol Metab. 2014;99(6):E953-E961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29(7):823-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science. 1986;231(4738):607-609. [DOI] [PubMed] [Google Scholar]
- 17. Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207(4437):1371-1373. [DOI] [PubMed] [Google Scholar]
- 18. Veldhuis JD, Evans WS, Urban RJ, Rogol AD, Johnson ML. Physiologic attributes of the luteinizing hormone pulse signal in the human. Cross-validation studies in men. J Androl. 1988;9(2):69-77. [DOI] [PubMed] [Google Scholar]
- 19. Iranmanesh A, Lawson D, Veldhuis JD. Glucose ingestion acutely lowers pulsatile LH and basal testosterone secretion in men. Am J Physiol Endocrinol Metab. 2012;302(6):E724-E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geary N, Kissileff HR, Pi-Sunyer FX, Hinton V. Individual, but not simultaneous, glucagon and cholecystokinin infusions inhibit feeding in men. Am J Physiol. 1992;262(6 Pt 2):R975-R980. [DOI] [PubMed] [Google Scholar]
- 21. Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T. Effect of oxyntomodulin, glucagon, GLP-1, and combined glucagon +GLP-1 infusion on food intake, appetite, and resting energy expenditure. J Clin Endocrinol Metab. 2015;100(12):4541-4552. [DOI] [PubMed] [Google Scholar]
- 22. Banks WA, Kastin AJ. Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res Bull. 1985;15(3):287-292. [DOI] [PubMed] [Google Scholar]
- 23. Ambery PD, Klammt S, Posch MG, et al. MEDI0382, a GLP-1/glucagon receptor dual agonist, meets safety and tolerability endpoints in a single-dose, healthy-subject, randomized, phase 1 study. Br J Clin Pharmacol. 2018;84(10):2325-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeibmann A, Zahedi S, Simoni M, Nieschlag E, Byrne MM. Glucagon-like peptide-1 reduces the pulsatile component of testosterone secretion in healthy males. Eur J Clin Invest. 2005;35(9):565–572. [DOI] [PubMed] [Google Scholar]
- 25. Jensterle M, Podbregar A, Goricar K, Gregoric N, Janez A. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr Connect. 2019;8(3):195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giagulli VA, Carbone MD, Ramunni MI, et al. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology. 2015;3(6):1094-1103. [DOI] [PubMed] [Google Scholar]
- 27. Ottlecz A, Samson WK, McCann SM. The effects of gastric inhibitory polypeptide (GIP) on the release of anterior pituitary hormones. Peptides. 1985;6(1):115-119. [DOI] [PubMed] [Google Scholar]
- 28. George JT, Veldhuis JD, Tena-Sempere M, Millar RP, Anderson RA. Exploring the pathophysiology of hypogonadism in men with type 2 diabetes: kisspeptin-10 stimulates serum testosterone and LH secretion in men with type 2 diabetes and mild biochemical hypogonadism. Clin Endocrinol (Oxf). 2013;79(1):100-104. [DOI] [PubMed] [Google Scholar]