Abstract
Background
Rotavirus disease rates dramatically declined among children <5 years of age since the rotavirus vaccine was introduced in 2006; population-level impacts remain to be fully elucidated.
Methods
Data from the Healthcare Cost and Utilization Project State Inpatient Databases were used to conduct a time-series analysis of monthly hospital discharges across age groups for acute gastroenteritis and rotavirus from 2000 to 2013. Rate ratios were calculated comparing prevaccine and postvaccine eras.
Results
Following vaccine introduction, a decrease in rotavirus hospitalizations occurred with a shift toward biennial patterns across all ages. The 0–4-year age group experienced the largest decrease in rotavirus hospitalizations (rate ratio, 0.14; 95% confidence interval, .09–.23). The 5–19-year and 20–59-year age groups experienced significant declines in rotavirus hospitalization rates overall; the even postvaccine calendar years were characterized by progressively lower rates, and the odd postvaccine years were associated with reductions in rates that diminished over time. Those aged ≥60 years experienced the smallest change in rotavirus hospitalization rates overall, with significant reductions in even postvaccine years compared with prevaccine years (rate ratio, 0.51; 95% confidence interval, .39–.66).
Conclusions
Indirect impacts of infant rotavirus vaccination are apparent in the emergence of biennial patterns in rotavirus hospitalizations that extend to all age groups ineligible for vaccination. These observations are consistent with the notion that young children are of primary importance in disease transmission and that the initial postvaccine period of dramatic population-wide impacts will be followed by more complex incidence patterns across the age range in the long term.
Keywords: rotavirus, vaccination, hospitalizations, gastrointestinal illness
Indirect impacts of infant rotavirus vaccination are apparent in the emergence of biennial patterns in rotavirus hospitalizations that extend to all ages. The initial postvaccine period of dramatic population-wide impacts is followed by more complex incidence patterns across age groups.
Before vaccine introduction in the United States, rotavirus was the leading cause of severe pediatric gastroenteritis, resulting in up to 70000 hospitalizations [1] and an estimated $319 million in healthcare costs annually [2]. Following pivotal clinical trial results [3, 4], 2 live, attenuated oral rotavirus vaccines, RotaTeq (Merck) and Rotarix (GlaxoSmithKline Biologicals) were included in the routine infant vaccination schedule in 2006 and 2008, respectively, according to recommendations from the Advisory Committee on Immunization Practices [5, 6]. By 2015, 73.2% of children aged 19–35 months had received a full course of rotavirus vaccine [7]. This is modest coverage compared with more established routine infant immunizations, such as the diphtheria and tetanus toxoids and acellular pertussis vaccine, with coverage of 95.0% for ≥3 doses in 2015 [7].
The direct effects of rotavirus vaccination have been clearly demonstrated, and evidence of potential indirect effects are emerging. Among children <5 years of age, rotavirus disease has declined dramatically in the decade following the introduction of the vaccines in the United States, with reductions in hospitalizations [8–10], emergency department visits [8, 11], and physician office visits [8, 10]. Introduction of rotavirus vaccination has also altered epidemiological patterns, with a switch from annual to biennial patterns in disease incidence and a delay in the seasonal peak in low-incidence years [12]. Moreover, there is evidence of potential indirect benefits of the vaccine program, with reductions in hospitalizations observed among unvaccinated children, probably because of reduced transmission from their vaccinated counterparts [9, 10, 13]. In addition, early data from the United States and select other early-introducing countries, suggest that these indirect benefits may extend to older children and adults [14, 15]. If true, these observations unmask a considerable severe disease burden outside the pediatric age range that may be preventable by infant immunization.
The longer-term impacts of infant rotavirus vaccination across all ages remain to be fully elucidated. Studies to date addressing these questions have been limited to relatively short-term postvaccine time periods [14, 15] or restricted to younger age groups [8–10, 13, 16]. Introduction of the rotavirus vaccine is changing epidemiologic patterns [12] and may lead to subtle shifts in circulating serotypes [17, 18]. Accordingly, evaluation of longer-term trends and analysis of older age groups is needed to identify the full public health impacts of infant rotavirus vaccination. We aimed to evaluate the population-wide impact, across all ages, of infant rotavirus vaccination on gastroenteritis and rotavirus hospitalizations by comparing the prevaccine and postvaccine periods in the United States. We examined the total effects among young children and the indirect effects to older children and adults using a large national discharge database.
METHODS
Data Sources
Hospitalization data from January 2000 through December 2013 were obtained from the Healthcare Cost and Utilization Project State Inpatient Databases, sponsored by the Agency for Healthcare Research and Quality, through an active collaboration. This database is a compilation of monthly discharge data from all community hospitals (all nonfederal, short-term general and specialty hospitals) within participating states [19]. Our analysis was restricted to 26 states for which data were available for the entire study period: Arizona, California, Colorado, Connecticut, Florida, Georgia, Hawaii, Iowa, Illinois, Kansas, Kentucky, Maryland, Massachusetts, Michigan, Missouri, North Carolina, New Jersey, New York, Oregon, South Carolina, Tennessee, Texas, Utah, Washington, Wisconsin, and West Virginia. According to the National Center for Health Statistics Bridged Race population data set (https://www.cdc.gov/nchs/nvss/bridged_race.htm), these 26 states represent approximately three-quarters (74.2%) of the total US population in 2013. This population data set was used to calculate rates in this analysis.
Two outcomes were separately assessed: rates of all-cause acute gastroenteritis (AGE) and rates of rotavirus gastroenteritis (RVGE) hospital discharges. Discharge data, coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), were extracted from the State Inpatient Databases to identify AGE and RVGE codes noted as the primary diagnosis or listed in 1 of 15 other possible diagnosis fields. Applicable ICD-9-CM codes for AGE are detailed elsewhere [20] and included bacterial, parasitic, and viral gastrointestinal illness of determined cause and presumed infectious or noninfectious gastrointestinal illness of undetermined cause. We defined a RVGE hospitalization as any discharge with ICD-9-CM diagnosis code 008.61. Rotavirus-coded discharge data were expected to provide the most specific indicator of rotavirus rates. However, restricting the analysis to only these episodes may underestimate the true rotavirus burden owing to limited testing for rotavirus in medical settings [21, 22]. Therefore, the broader classification of all-cause AGE was also analyzed to capture RVGE not identified as rotavirus related because of incomplete detection or miscoding.
Statistical Analysis
The impact of vaccination was estimated by comparing AGE and RVGE age-specific hospitalization rates per 10000 population before and after vaccine introduction using regression models. Monthly counts of AGE and RVGE hospitalizations were modeled using negative binomial regression, with a categorical variable representing postvaccine year and adjustment for changing population size using an offset of the log of population [23]. Analyses were performed using R software, version 3.5.0.
Three comparisons were made for each outcome to estimate rate ratios (RRs) and corresponding 95% confidence intervals (CIs). Monthly prevaccine rates overall (2000–2006) were compared with (1) monthly postvaccine rates overall (2008–2013), (2) monthly postvaccine rates for even (2008, 2010, and 2012) and odd (2009, 2011, 2013) postvaccine calendar years separately to assess biennial patterns, and (3) monthly postvaccine rates for each individual postvaccine calendar year to provide more detail on the potentially dynamic effects of vaccination over time. The year 2006 was included in the prevaccine period because the vaccine was not recommended until August of that year, and vaccine coverage was initially low [24]. The year 2007 was considered a transition period and was excluded from analyses. All analyses were performed separately for the age groups 0–4, 5–19, 20–59, and ≥60 years to assess the total and indirect effects of the vaccine across age groups. Age groups were selected by combining those with similar disease patterns and trends to increase power for the analyses. For AGE, we modeled data from the historic rotavirus season in the United States (January–June) to improve the model’s specificity for AGE cases that may be rotavirus related; rotavirus models included year-round data.
We aimed to adjust for potential exogenous secular trends (trends over time) with the inclusion of a sequential (continuous) time variable. The AGE model results were sensitive to the inclusion of time trends, so we further considered second- and third-order time variables. Based on the Akaike information criterion values, including higher-order time variables did not substantially improve model fit. RVGE hospitalization model results were not sensitive to the addition of a sequential time variable, so it was excluded. Rotavirus models controlled for the period 2000–2003 using an indicator variable to account for the increase in RVGE hospitalization rates that occurred just before 2004 among all age groups. The cause of this increase in RVGE hospitalization rates is uncertain but may be increased rotavirus testing in anticipation of vaccine introduction. Because we sought to identify the long-term impact of vaccine exposure on AGE and RVGE hospitalization rates, rather than short-term deviations from an “underlying pattern,” we chose to account for secular trends (where appropriate) and not consider autocorrelation.
Ethical Approval
This study was not subject to institutional review board approval at Emory University or the Centers for Disease Control and Prevention because it involved deidentified, aggregate data.
RESULTS
A total of 13527516 AGE hospitalizations from 2000 to 2013 were analyzed, including 224099 (1.7%) specified as RVGE.
AGE Patterns
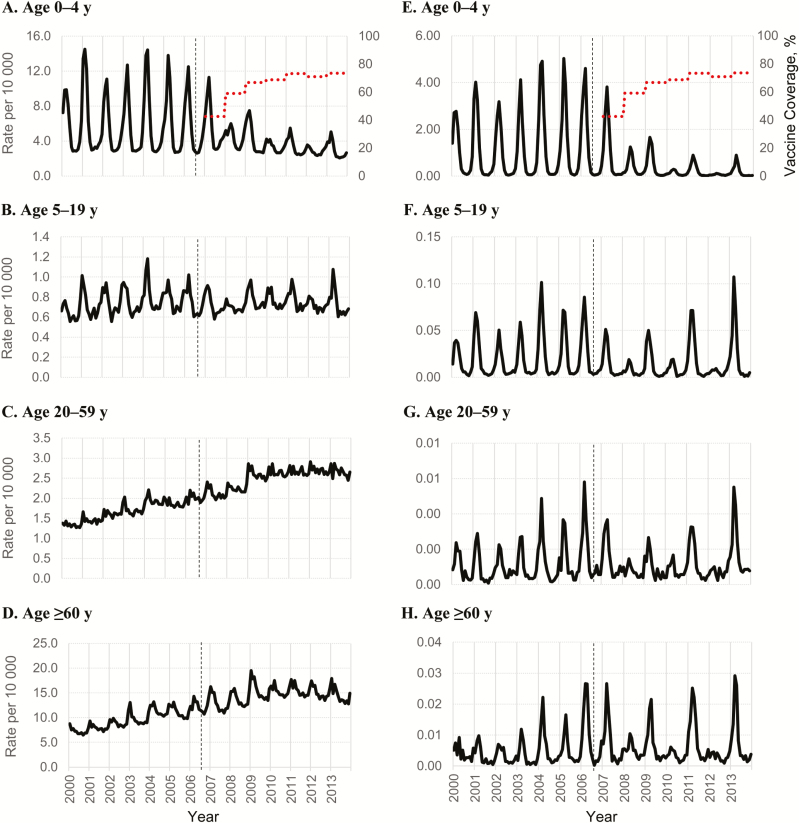
Using unrestricted (full-year) data, the highest rates of AGE hospitalization were among persons aged ≥60 years with an average monthly rate of 10.2 hospitalizations per 10000 age-specific population (unadjusted rate range, 3.3–24.3) during the prevaccine period, increasing to 15.7 per 10000 age-specific population (unadjusted rate range, 6.1–33.3) during the postvaccine period. A secular increasing trend began during the prevaccine period for this age group as well as among those aged 20–59 years (Figure 1C and 1D). In contrast, we observed no clear evidence of a secular trend in the 0–4- or 5–19-year age groups (Figure 1A and 1B).
Figure 1.
Monthly all-cause acute gastroenteritis (A–D) and rotavirus gastroenteritis (E–H) hospitalizations per 10000 age-specific population, 2000–2013. Data are from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. Data are full-year data (not restricted to January–June), for the purpose of visualizing the patterns in hospitalization rates. Dotted lines in parts (A–D) represent up-to-date vaccine coverage among children aged 19–35 months (2 or 3 doses, depending on vaccine manufacturer); the vertical black dashed lines denote introduction of rotavirus vaccine.
During the historic rotavirus season (January–June), we observed significant reductions in AGE hospitalizations for the 0–4-year age group in the postvaccine compared with the prevaccine period (Table 1; RR, 0.54; 95% CI, .34–.85), with slightly greater reductions in even than in odd calendar years (even-year RR, 0.50 [95% CI, .32–.78]; odd-year RR, 0.58 [.35–.95]) and generally decreasing RRs, punctuated by a subtle biennial pattern (Figure 2Aand Table 2). Similar patterns with smaller and nonsignificant reductions were observed among the 5–19-year age group (Figure 2B). No significant reductions in rates of AGE hospitalizations were observed for the 20–59- or ≥60-year age groups (Figure 2C and 2D). Similar patterns with more diluted impacts were observed in the unrestricted (full-year) comparative analyses (Supplementary Table 1).
Table 1.
Monthly All-cause Acute Gastroenteritis Hospitalization Rates and Adjusted Rate Ratios, Restricted to the Rotavirus Season: Prevaccine Period Compared With Postvaccine Period Overall and in Even and Odd Calendar Yearsa
| Age Group, y | Prevaccine Period | Postvaccine Period | Postvaccine Even Years | Postvaccine Odd Years | ||||
|---|---|---|---|---|---|---|---|---|
| Rateb | Range | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | |
| 0–4 | 8.04 | 2.89–14.49 | 4.19 | 0.54 (.34–.85)d | 3.92 | 0.50 (.32–.78)d | 4.47 | 0.58 (.35–.95)d |
| 5–19 | 0.79 | 0.43–1.84 | 0.79 | 0.84 (.68–1.06) | 0.75 | 0.81 (.65–1.01) | 0.83 | 0.88 (.69–1.12) |
| 20–59 | 1.72 | 0.78–3.43 | 2.62 | 0.93 (.74–1.18) | 2.55 | 0.94 (.75–1.18) | 2.69 | 0.93 (.72–1.20) |
| ≥60 | 10.24 | 3.34–24.33 | 15.74 | 0.82 (.58–1.17) | 15.33 | 0.83 (.59–1.18) | 16.16 | 0.81 (.55–1.19) |
Abbreviations: CI, confidence interval; RR, rate ratio.
aData from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. Adjusted RRs are from negative binomial regression comparing the prevaccine period (2000–2006) with the postvaccine period (2008–2013) overall and by even and odd calendar years, controlling for time and restricted to the rotavirus season, defined as January–June.
bUnadjusted rate per 10000 age-specific population.
cRR for comparison with prevaccine era.
dSignificant at the α = .05 level.
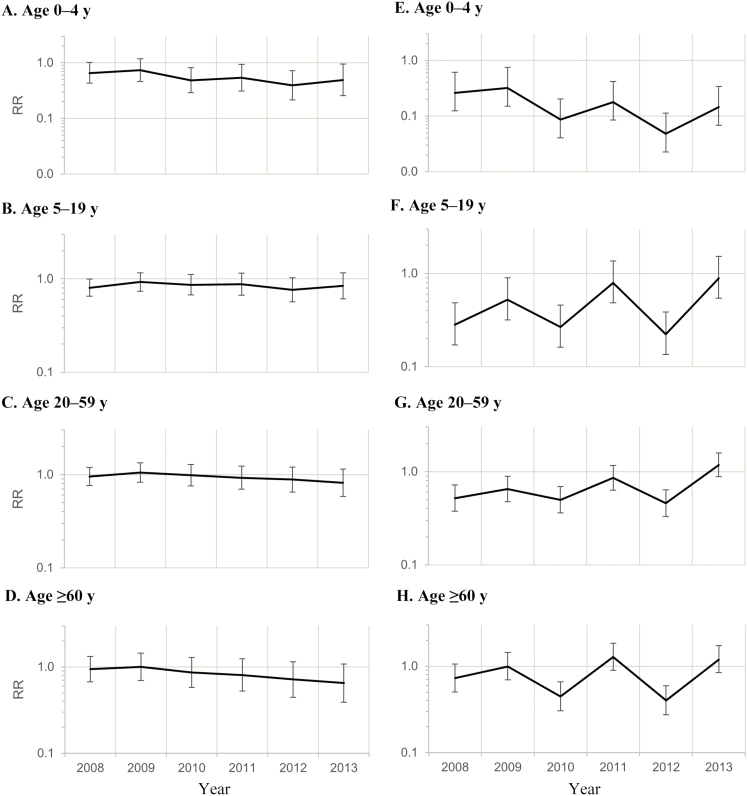
Figure 2.
Monthly all-cause acute gastroenteritis (AGE) (A–D) and rotavirus gastroenteritis (RVGE) (E–H) hospitalization rate ratios (RRs) comparing prevaccine (2000–2006) and postvaccine (2008–2013) periods by year. Data are from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. A–D, AGE data based on negative binomial regression using a sequential (continuous) time variable to control for potential exogenous secular trends, restricted to the rotavirus season (January–June). E–H, RVGE data based on negative binomial regression controlling for the pre-2004 period, using an indicator variable to account for the increase in RVGE hospitalization rates that occurred just before 2004, including all months.
Table 2.
Monthly All-cause Acute Gastroenteritis Hospitalization Rates and Adjusted RRs, Restricted to the Rotavirus Season: Prevaccine Period Compared With Postvaccine Period by Yeara
| Age Group, y | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | |
| 0–4 | 5.08 | 0.66 (.43–1.01) | 5.67 | 0.74 (.46–1.18) | 3.70 | 0.49 (.29–.81)d | 4.08 | 0.54 (.3–.94)d | 2.97 | 0.40 (.22–.72)d | 3.66 | 0.49 (.26–.94)d |
| 5–19 | 0.71 | 0.81 (.65–.99)d | 0.84 | 0.93 (.74–1.17) | 0.80 | 0.86 (.67–1.11) | 0.83 | 0.88 (.67–1.16) | 0.74 | 0.76 (.57–1.03) | 0.83 | 0.84 (.61–1.16) |
| 20–59 | 2.26 | 0.95 (.77–1.19) | 2.67 | 1.05 (.83–1.34) | 2.66 | 0.98 (.76–1.28) | 2.68 | 0.93 (.69–1.23) | 2.73 | 0.88 (.65–1.20) | 2.71 | 0.82 (.58–1.15) |
| ≥60 | 14.55 | 0.94 (.68–1.32) | 16.87 | 1.00 (.70–1.44) | 15.82 | 0.86 (.58–1.28) | 16.18 | 0.81 (.52–1.25) | 15.60 | 0.72 (.45–1.14) | 15.43 | 0.65 (.39–1.08) |
Abbreviations: CI, confidence interval; RR, rate ratio.
aData from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. Adjusted RRs are from negative binomial regression comparing the prevaccine period (2000–2006) with postvaccine period (2008–2013) by year, controlling for time and restricted to the rotavirus season (January–June).
bUnadjusted rate per 10000 age-specific population.
cRR for comparison with prevaccine era.
dSignificant at the α = .05 level.
RVGE Patterns
Following vaccine introduction, a decrease in RVGE hospitalization rates occurred across all age groups (Table 3), with parallel trends and a shift toward biennial patterns (Figure 1E–1H) across all ages. The 0–4-year age group experienced the largest overall decrease in RVGE hospitalization rates comparing the pre- and postvaccine periods.
Table 3.
Monthly Rotavirus Gastroenteritis Hospitalization Rates and Adjusted Rate Ratios: Prevaccine Period Compared With Postvaccine Period Overall and in Even and Odd Calendar Yearsa
| Age Group, y | Prevaccine Period | Postvaccine Period | Postvaccine Even Years | Postvaccine Odd Years | ||||
|---|---|---|---|---|---|---|---|---|
| Rateb | Range | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | |
| 0–4 | 1.2051 | (0.045–5.023) | 0.2374 | 0.14 (.09–.23)d | 0.1810 | 0.10 (.06–.18)d | 0.2938 | 0.20 (.12–.35)d |
| 5–19 | 0.0226 | (0.000–0.257) | 0.0131 | 0.43 (.31–.59)d | 0.0068 | 0.26 (.18–.37)d | 0.0194 | 0.72 (.50–1.03) |
| 20–59 | 0.0012 | (0.000–0.009) | 0.0010 | 0.65 (.54–.79)d | 0.0007 | 0.49 (.39–.62)d | 0.0013 | 0.87 (.70–1.08) |
| ≥60 | 0.0049 | (0.000–0.042) | 0.0057 | 0.76 (.61–.96)d | 0.0036 | 0.51 (.39–.66)d | 0.0078 | 1.15 (.89–1.48) |
Abbreviations: CI, confidence interval; RR, rate ratio.
aData from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. Adjusted RRs are from negative binomial regression comparing the prevaccine period (2000–2006) with the postvaccine period (2008–2013), overall and in even and odd calendar years, controlling for the pre-2004 period.
bUnadjusted rate per 10000 age-specific population.
cRRs for comparison with prevaccine era.
dSignificant at the α = .05 level.
After introduction of the infant rotavirus vaccine, RVGE hospitalization rates among those aged 0–4 years declined by >85% (Table 3; RR, 0.14; 95% CI, .09–.23). A biennial pattern in RVGE hospitalization rates was apparent for this age group, with larger declines in the rate of RVGE hospitalization in even calendar years (RR, 0.10; 95% CI, .06–.18) than in odd calendar years (0.20; 95.12–.35). This pattern was further characterized by consistently decreasing rates over time for both even and odd postvaccine calendar years (Figure 2E).
The 5–19- and 20–59-year age groups both experienced a significant decline in RVGE hospitalization rates overall, primarily owing to the decline in RVGE hospitalizations that occurred in even calendar years after introduction of the vaccines (Table 3). No significant changes in RVGE hospitalization rates were observed for either age group when comparing prevaccine rates with rates for odd calendar years in the postvaccine period. Of note, analysis of RRs by individual postvaccine year revealed that even years were characterized by progressively lower rates, whereas odd years were typically associated with reductions in rates that diminished over time or modest, nonsignificant increases (Figure 2F and 2G; Table 4).
Table 4.
Monthly Rotavirus Gastroenteritis Hospitalization Rates and Adjusted Rate Ratios: Prevaccine Period Compared With Postvaccine Period by Yeara
| Age Group, y | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rateb | RRc (95% CI) |
Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | Rateb | RRc (95% CI) | |
| 0–4 | 0.3589 | 0.26 (.12–.61)d | 0.4383 | 0.32 (.15–.75)d | 0.1181 | 0.09 (.04–.20)d | 0.2450 | 0.18 (.08–.42)d | 0.0660 | 0.05 (.02–.11)d | 0.1981 | 0.14 (.07–.34)d |
| 5–19 | 0.0074 | 0.28 (.17–.48)d | 0.0138 | 0.52 (.32–.89)d | 0.0070 | 0.27 (.16–.46)d | 0.0209 | 0.79 (.48–1.36) | 0.0059 | 0.22 (.13–.38)d | 0.0234 | 0.89 (.54–1.52) |
| 20–59 | 0.0008 | 0.52 (.38–.72)d | 0.0010 | 0.65 (.48–.89)d | 0.0007 | 0.50 (.36–.69)d | 0.0013 | 0.86 (.63–1.16) | 0.0007 | 0.46 (.33–.64)d | 0.0017 | 1.18 (.88–1.59) |
| ≥60 | 0.0050 | 0.73 (.50–1.06) | 0.0067 | 0.99 (.70–1.44) | 0.0031 | 0.45 (.30–.66)d | 0.0087 | 1.28 (.90–1.85) | 0.0028 | 0.40 (.27–.59)d | 0.0081 | 1.20 (.84–1.73) |
Abbreviations: CI, confidence interval; RR, rate ratio.
aData from 26 US states from the Healthcare Cost and Utilization Project’s State Inpatient Databases; see Data Sources, in Methods, for the full list of states. Adjusted RRs are from negative binomial regression comparing the prevaccine period (2000–2006) with the postvaccine period (2008–2013) by year, controlling for the pre-2004 period.
bUnadjusted rate per 10000 age-specific population.
cRRs for comparison with prevaccine era.
dSignificant at the α = .05 level.
Among persons aged ≥60 years, patterns similar to those observed for the 5–19- and 20–59-year age groups were seen, including overall declines in hospitalization rates in the postvaccine period compared with prevaccine years (Figure 2H and Table 3; RR, 0.76; 95% CI, .61–.96) and significant declines in the even postvaccine years (0.51; .39–.66). In the odd postvaccine years, we observed no change in RVGE rates in 2009 (Table 4; RR, 0.99; 95% CI, .70–1.44) and nonsignificant rate increases in 2011 and 2013 (1.28 [.90–1.85] and 1.20 [.84–1.73], respectively), compared with the prevaccine period.
DISCUSSION
Our analysis of 6 years of postvaccine data offers several insights into the complex longer-term population-level effects of infant rotavirus vaccination. Rotavirus vaccination had a substantial impact on RVGE hospitalizations across age groups during the 6-year period, highlighting the role of infants as drivers of infection transmission. These indirect effects are evidenced by parallel trends in the incidence of RVGE hospitalizations whereby biennial temporal patterns emerged among all age groups. Overall reductions in the incidence of RVGE hospitalizations were observed for children, adolescents, and adults during the postvaccine period, with diminished indirect impacts as age increased. Remarkably, by 2013, there was a suggestion in the data that RVGE hospitalization rates among the 20–59- and ≥60-year age groups may have slightly exceeded prevaccine levels. Infant vaccination programs increase the average age of infection; further years’ data will be need to determine whether there is an absolute increase in disease rates in older age groups.
The United States was one of the first countries to introduce a rotavirus vaccine nationally [5, 25], and we were therefore uniquely positioned to assess the longer-term temporal trends in RVGE hospitalization rates. A central strength of this study is the use of national AGE and RVGE hospital discharge data spanning 6 years after the introduction of the rotavirus vaccine and including data on older children, adolescents, and adults. This is the longest age stratified time-series analysis of the rotavirus vaccine era trends to date. The 26 states included in the analysis represent 74.2% of the national population and include all cases occurring in community hospitals (all nonfederal, short-term general and specialty hospitals) in these states. The time-series method enabled us to assess the dynamic effects of the vaccine while accounting for background secular trends. These results extend findings from previous studies that suggested possible indirect impacts of infant vaccination among older children and adults using data from the first few years after vaccine introduction [14, 15, 26–28]. This study also provides a more detailed look at temporal patterns by analyzing both pre- and postvaccine rates overall but also for individual years, enabling the identification of biennial patterns previously noted [12, 14].
Potentially confounding our analysis were secular changes in RVGE and AGE rates, particularly among older adults, that may have been unrelated to vaccine introduction. We aimed to control for these trends by including time variables in our models, a technique used in some [14, 15] but not all [9, 29] previous studies. Depending on the direction of the secular trend, not accounting for time confounding may lead to an overestimation or underestimation of vaccine impact. Without genotype data, we are unable to determine whether the new patterns of RVGE hospitalizations during the vaccination era are associated with previously circulating rotavirus strains or the emergence of less commonly observed strains—a question raised by recent evaluations [17, 18, 30]. The role of vaccine-induced pressures on circulating strains should be considered cautiously, because vaccination has been shown to induce cross-protective immunity [31] and there is evidence of strain diversity by geography and over time, unrelated to vaccination [32].
Important biases may arise from rotavirus testing in the clinical setting. It is well recognized that not all patients hospitalized for AGE are tested for rotavirus, even among young children, in whom the burden is most appreciated [21, 22]. The ICD-9-CM coding for rotavirus has demonstrated high specificity (97%) but low sensitivity (estimated between 25% and 47%) for children <5 years of age during the prevaccine era [33], and there is limited information about the sensitivity and specificity of rotavirus coding among older age groups [34, 35], with trends in testing practices and coding validity largely unknown. We are also unable to assess how seasonality of rotavirus (ie, changes in rotavirus prevalence) may affect the positive and negative predictive value of rotavirus tests.
However, only confounders that vary over time, such as a change in testing practices, would bias our results; we have no evidence to suggest that such a change did or did not occur. The percentage of AGE case patients who tested positive for rotavirus has declined sharply among children since introduction of the rotavirus vaccine [12, 13], so the decline in rotavirus cases after vaccine introduction cannot be attributed to reduced testing alone. Nonetheless, any potential changes in testing practices are unlikely to account for the biennial pattern observed. Differences in the patterns between AGE and RVGE hospitalization rates are probably due to the nonspecific nature of the AGE outcome, which captures gastroenteritis cases from diverse causes.
The current study reveals the important role that infants play as drivers of rotavirus infection among all age groups, the indirect effects of infant vaccinations among older children, adolescents, and adults, and the emergent biennial patterns of RVGE hospitalization across age ranges. The biennial pattern in RVGE hospitalization rates could be a result of changing rates of susceptibles within the population. Vaccine coverage combined with acquired immunity could be high enough to transiently raise herd immunity such that transmission is low and allow for a short-term accumulation of susceptibles, particularly among young children, spurring biennial epidemics that spread across age groups [13, 36].
The indirect effects of infant vaccination are apparent in the reduced incidence of RVGE among older age groups observed immediately after introduction of the vaccine. These findings are probably a result of higher transmissibility from children and social contact patterns that result in children being the primary drivers of infection [37]. A related pattern observed in the data is the reduced relative effect of infant RVGE vaccination on unvaccinated individuals over time and as age group among the unvaccinated increases and over time. Seasonal patterns among older age groups are likely to be closely linked to incidence among young children, but population immunity and transmission within the age group may play an increasing role [38].
Further understanding of the long-term impacts of rotavirus vaccination across the age range could be gained with additional data from the postvaccine period and from comparable early-introducing countries. Data from 2014 and beyond would enable us to determine whether the relationships identified in this study continue in future years. Indeed, infant vaccination can create immunity gaps in older ages, as has been observed for varicella [39] and rubella [40]. Although our analysis shows the substantial influence of infant immunization across the age range, the source of infection among adults and their role in rotavirus transmission is not clear and should be the subject of future studies. Because nearly all adults in our study would have acquired rotavirus antibodies as children in the prevaccine era [5], our findings support the notion that immunity is not lifelong [34]. Data on vaccine effectiveness by year could provide further insight into these trends.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors are grateful to Juan Leon of the Emory University Rollins School of Public Health for his review of this manuscript. We also sincerely thank and acknowledge the Healthcare Cost and Utilization Project state partners for their active support of this evaluation (https://www.hcup-us.ahrq.gov/sidoverview.jsp).
Disclaimer. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Healthcare Research and Quality.
Potential conflicts of interest. B. A. L. has received personal fees from Takeda Pharmaceuticals for service on their Norovirus Advisory Board, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Charles MD, Holman RC, Curns AT, Parashar UD, Glass RI, Bresee JS. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J 2006; 25:489–93. [DOI] [PubMed] [Google Scholar]
- 2. Widdowson MA, Meltzer MI, Zhang X, Bresee JS, Parashar UD, Glass RI. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics 2007; 119:684–97. [DOI] [PubMed] [Google Scholar]
- 3. De Vos B, Vesikari T, Linhares AC, et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatr Infect Dis J 2004; 23:S179–82. [DOI] [PubMed] [Google Scholar]
- 4. Salinas B, Pérez Schael I, Linhares AC, et al. Evaluation of safety, immunogenicity and efficacy of an attenuated rotavirus vaccine, RIX4414: a randomized, placebo-controlled trial in Latin American infants. Pediatr Infect Dis J 2005; 24:807–16. [DOI] [PubMed] [Google Scholar]
- 5. Parashar UD, Alexander JP, Glass RI; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2006; 55:1–13. [PubMed] [Google Scholar]
- 6. Cortese MM, Parashar UD; Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep 2009; 58:1–25. [PubMed] [Google Scholar]
- 7. Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Dietz V. Vaccination coverage among children aged 19–35 months—United States, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:1065–71. [DOI] [PubMed] [Google Scholar]
- 8. Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125:e208–13. [DOI] [PubMed] [Google Scholar]
- 9. Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617–24. [DOI] [PubMed] [Google Scholar]
- 10. Cortese MM, Tate JE, Simonsen L, Edelman L, Parashar UD. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J 2010; 29:489–94. [DOI] [PubMed] [Google Scholar]
- 11. Shah MP, Tate JE, Steiner CA, Parashar UD. Decline in emergency department visits for acute gastroenteritis among children in 10 US states after implementation of rotavirus vaccination, 2003 to 2013. Pediatr Infect Dis J 2016; 35:782–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aliabadi N, Tate JE, Haynes AK, Parashar UD, Centers for Disease Control and Prevention (CDC). Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2015; 64:337–42. [PMC free article] [PubMed] [Google Scholar]
- 13. Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US counties, 2006–2009. Clin Infect Dis 2011; 53:245–53. [DOI] [PubMed] [Google Scholar]
- 14. Gastañaduy PA, Curns AT, Parashar UD, Lopman BA. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA 2013; 310:851–3. [DOI] [PubMed] [Google Scholar]
- 15. Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204:980–6. [DOI] [PubMed] [Google Scholar]
- 16. Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med 2011; 365:1108–17. [DOI] [PubMed] [Google Scholar]
- 17. Gurgel RQ, Correia JB, Cuevas LE. Effect of rotavirus vaccination on circulating virus strains. Lancet 2008; 371:301–2. [DOI] [PubMed] [Google Scholar]
- 18. Pitzer VE, Patel MM, Lopman BA, Viboud C, Parashar UD, Grenfell BT. Modeling rotavirus strain dynamics in developed countries to understand the potential impact of vaccination on genotype distributions. Proc Natl Acad Sci U S A 2011; 108:19353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heathcare Cost and Utilization Project (HCUP). SID database documentation. 2016. Available at: www.hcup-us.ahrq.gov/db/state/siddbdocumentation.jsp. Accessed 6 March 2017. [Google Scholar]
- 20. Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics 2014; 134:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel MM, Tate JE, Selvarangan R, et al. Routine laboratory testing data for surveillance of rotavirus hospitalizations to evaluate the impact of vaccination. Pediatr Infect Dis J 2007; 26:914–9. [DOI] [PubMed] [Google Scholar]
- 22. Chang HG, Glass RI, Smith PF, Cicirello HG, Holman RC, Morse DL. Disease burden and risk factors for hospitalizations associated with rotavirus infection among children in New York State, 1989 through 2000. Pediatr Infect Dis J 2003; 22:808–14. [DOI] [PubMed] [Google Scholar]
- 23. Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol 2013; 42:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention, Advisory Committee on Immunization Practices. Rotavirus vaccination coverage and adherence to the advisory committee on immunization practices (ACIP)-recommended vaccination schedule—United States, February 2006–May 2007. MMWR Morb Mortal Wkly Rep 2008; 57:398–01. [PubMed] [Google Scholar]
- 25.International Vaccine Access Center. VIEW-hub report: global vaccine introduction and implementation. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health, 2016. [Google Scholar]
- 26. Atchison C, Collins S, Brown D, Ramsay ME, Ladhani S. Reduction in rotavirus disease due to the infant immunisation programme in England; evidence from national surveillance. J Infect 2015; 71:128–31. [DOI] [PubMed] [Google Scholar]
- 27. Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:S25–9. [DOI] [PubMed] [Google Scholar]
- 28. Anderson EJ, Shippee DB, Weinrobe MH, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis 2013; 56:755–60. [DOI] [PubMed] [Google Scholar]
- 29. Leshem E, Tate JE, Steiner CA, Curns AT, Lopman BA, Parashar UD. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. JAMA 2015; 313:2282–4. [DOI] [PubMed] [Google Scholar]
- 30. Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:847–56. [DOI] [PubMed] [Google Scholar]
- 31. Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis 2011; 203:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Payne DC, Szilagyi PG, Staat MA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J 2009; 28:948–53. [DOI] [PubMed] [Google Scholar]
- 33. Hsu VP, Staat MA, Roberts N, et al. Use of active surveillance to validate International Classification of Diseases code estimates of rotavirus hospitalizations in children. Pediatrics 2005; 115:78–82. [DOI] [PubMed] [Google Scholar]
- 34. Anderson EJ, Katz BZ, Polin JA, Reddy S, Weinrobe MH, Noskin GA. Rotavirus in adults requiring hospitalization. J Infect 2012; 64:89–95. [DOI] [PubMed] [Google Scholar]
- 35. Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis 2004; 4:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 2009; 325:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 2008; 5:e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cardemil CV. Two rotavirus outbreaks caused by genotype G2P[4] at large retirement communities: cohort studies. Ann Intern Med 2012; 157:621. [DOI] [PubMed] [Google Scholar]
- 39. Papaloukas O, Giannouli G, Papaevangelou V. Successes and challenges in varicella vaccine. Ther Adv Vaccines 2014; 2:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Trentini F, Poletti P, Merler S, Melegaro A. Measles immunity gaps and the progress towards elimination: a multi-country modelling analysis. Lancet Infect Dis 2017; 17:1089–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.