Abstract
A high seroprevalence of hepatitis A virus (81%) among human immunodeficiency virus–negative high-risk men who have sex with men is likely why this community was largely spared from a recent hepatitis A virus outbreak in San Diego, California.
Keywords: hepatitis A, men who have sex with men, vaccination, immunity
Between November 2016 and October 2017, San Diego County, California, had an outbreak of hepatitis A virus (HAV), largely among persons who were homeless and/or used illicit drugs [1]. Historically, however, men who have sex with men (MSM) have higher risk of HAV infection than the general population and outbreaks have occurred among MSM communities [2]. This is why HAV vaccinations have been recommended on the national level for MSM since 1996 [3]. Given that MSM were not disproportionately affected in this outbreak, we hypothesized that the HAV seroprevalence was sufficient to offer herd immunity to the local MSM community. We measured the HAV immunoglobulin G (IgG) positivity rates among men who were testing for human immunodeficiency virus (HIV) at a local testing center. We chose this study population to evaluate MSM who were likely to be at high risk for HAV infection and perhaps not already engaged in healthcare, unlike MSM who have HIV and are receiving treatment.
A mathematical model of an HAV outbreak in Australia estimated the critical immunity threshold to be ≥70% for MSM populations in major urban centers [4]. However, data from a recent HAV outbreak in Lyon, France, demonstrated that >70% of MSM using preexposure prophylaxis for HIV had immunity to HAV, yet an HAV outbreak still affected this MSM population [5]. Because risk practices and demographics may differ among cultures, our study aimed to establish a benchmark level of HAV immunity for MSM in urban centers in the United States.
METHODS
The samples for this study came from men who tested for HIV at a local testing center between 1 February and 1 July 2017 (N = 921; 146 heterosexual men and 775 MSM). Immunity for HAV was defined as a positive HAV IgG result using the Aviva Systems Biology kit. Other data used in the analysis came from a 50-question demographic and health survey at the testing center. The difference in HAV seroprevalence between MSM and heterosexual men was analyzed using Fisher exact test. Logistic regression was used to model HAV seropositivity among MSM by age, race/ethnicity, sexual activity (number of partners, types of partners, and method of intercourse), homelessness, history of sexually transmitted infections, any reported drug use, risk for acquiring HIV infection, and zip code. Each predictor was modeled individually against HAV seropositivity. Predictors for multiple regression were initially selected if univariate P values were <.20, then iteratively tested with stepwise selection. Interactions between predictors were tested but were not found to significantly improve model fit. Model diagnostics were performed with a combination of likelihood ratio tests, Akaike information criterion, variance inflation factors, and residual plots. Participants with missing data among significant predictors in the final model were excluded from the analysis.
RESULTS
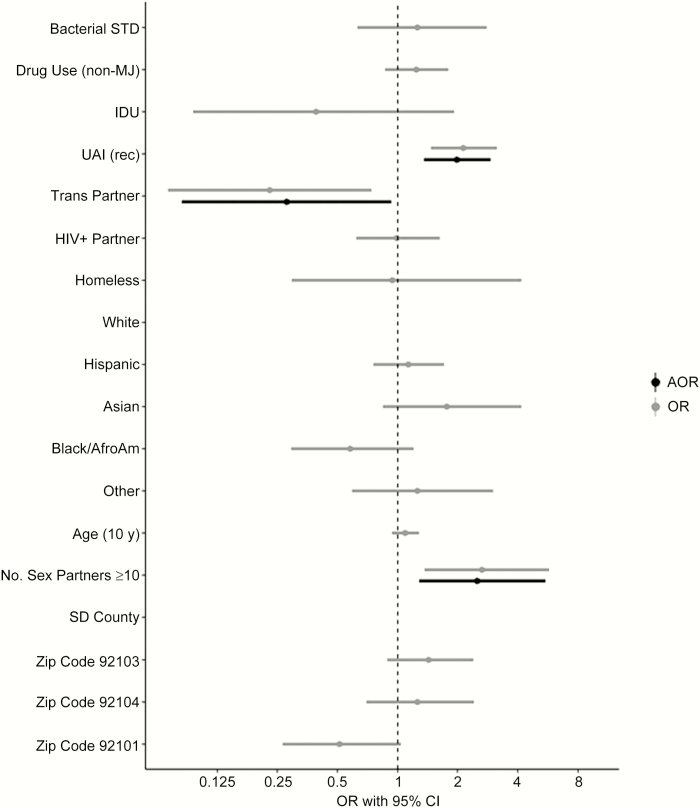
Study participants had a mean age of 34 years and 44.4% were non-Hispanic white. The seroprevalence of HAV was 58% (95% confidence interval [CI], 50.1%–65.9%) among heterosexual men and 81% (95% CI, 78.1%–83.6%) for MSM (odds ratio [OR], 3.09 [95% CI, 2.08–4.57]; P < .01). Among all male participants, there were no significant differences in HAV seropositivity based on age, race/ethnicity, reported drug use, injection drug use, homelessness, sex with HIV-infected partner, previous diagnosis of sexually transmitted infections, newly diagnosed acute HIV infection, or zip code of residence. There were independent, significantly higher proportions of HAV seropositivity among MSM who reported ≥10 sex partners in the previous 3 months (adjusted OR [AOR], 2.50 [95% CI, 1.28–5.49]; P = .01) and any receptive unprotected anal intercourse (AOR, 1.98 [95% CI, 1.35–2.92]; P < .01), and significantly lower proportions among MSM who reported sex with a transgender partner in the previous 3 months (AOR, 0.28 [95% CI, .08–.93]; P = .03) (Figure 1).
Figure 1.
. Abbreviations: AfroAm, African American; AOR, adjusted odds ratio; CI, confidence interval; HIV+, human immunodeficiency virus infected; IDU, injection drug user; MJ, marijuana; OR, odds ratio; rec, receptive; SD, San Diego; STD, sexually transmitted disease; UAI, unprotected anal intercourse.
DISCUSSION
These data suggest that herd immunity among the MSM community in San Diego was achieved with 81% HAV seropositivity, and that participants with more sex partners and condomless anal receptive sex also had the highest HAV seropositivity. This could provide a good vaccination benchmark for at-risk communities during HAV outbreaks.
In the San Diego outbreak, 590 known infections occurred in San Diego, California, between November 2016 and June 2018, largely in people who were homeless and use illicit drugs (34%), those who were only homeless (15%), and those who only use illicit drugs (13%). Among 402 male cases, 14 (3.5%) were MSM and ranged in age from 24 to 68 years (median, 36 years). Two (14%) were neither homeless nor used illicit drugs. Six cases (42%) received 1 dose of the HAV vaccine, but only 1 case received it far enough in advance of exposure to be considered effective [6]. Among all cases, mortality was high with 20 deaths (3.3%), and none were MSM [1]. This demographic pattern is more similar to outbreaks occurring currently in Kentucky, Utah, and West Virginia than those occurring in Michigan and Los Angeles, where MSM represent 14% and 58% of the infected male populations, respectively [7, 8]. Of note, the Los Angeles outbreak developed much later (September 2017) and remains much smaller than the San Diego outbreak (43 vs 590 total cases). Because the proportion of infected persons was large for MSM in Michigan and Los Angeles, we postulate that HAV seroprevalence sufficient for herd immunity was not present in those communities. Our study was limited because we do not know the baseline HAV seroprevalence among people who are homeless or use illicit drugs during the San Diego outbreak that may have predisposed them to infection. It is also important to consider that there may not have been sufficient overlap between MSM and people experiencing homelessness in San Diego or those using illicit drugs; however, 11 of the 14 MSM who did become infected in the San Diego outbreak were either homeless or reported use of illicit drugs. Additionally, we do not know whether the high HAV seroprevalence among MSM study participants was through vaccination or previous exposure, although large HAV and hepatitis B virus vaccination awareness campaigns were directed to the local MSM community in 2005–2008 and 2011. Further seroprevalence and vaccination data in various risk groups across outbreaks are needed to corroborate our findings.
Notes
Acknowledgments. The authors appreciate the technical assistance from Dr Eric McDonald and Dr Corey Peak.
Financial support. This work was supported by James Pendleton Foundation and National Institutes of Health (NIH) (grant numbers AI036214 and AI106039).
Potential conflicts of interest. M. P., A. F., S. L., C. A., D. S., and B. S. have received grants from the NIH. R. S. has received grants from the NIH, Gilead Sciences, and CytoDyn; personal fees from VIR and Pfizer; and other from CytoDyn and Antiva Biosciences. A. S. has received personal fees from the County of San Diego, Public Health Services. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. San Diego County Health and Human Services Agency. Hepatitis A outbreak 2018. Available at: https://www.sandiegocounty.gov/content/sdc/hhsa/programs/phs/community_epidemiology/dc/Hepatitis_A/outbreak.html. Accessed 21 June 2018. [Google Scholar]
- 2. Gorgos L. Sexual transmission of viral hepatitis. Infect Dis Clin North Am 2013; 27:811–36. [DOI] [PubMed] [Google Scholar]
- 3. Advisory Committee on Immunization Practices; Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1–23. [PubMed] [Google Scholar]
- 4. Regan DG, Wood JG, Benevent C, et al. Estimating the critical immunity threshold for preventing hepatitis A outbreaks in men who have sex with men. Epidemiol Infect 2016; 144:1528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charre C, Ramiere C, Roque-Afonso AM, et al. Hepatitis A outbreak in HIV-infected MSM and in PrEP-using MSM despite a high level of immunity, Lyon, France, January to June 2017. Euro Surveill 2017; 22. doi: 10.2807/1560-7917.ES.2017.22.48.17-00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases 2018. Available at: https://www.cdc.gov/vaccines/pubs/pinkbook/hepa.html. Accessed 21 June 2018. [Google Scholar]
- 7. Michigan Health Department. Michigan hepatitis A 2016–2018 outbreak summary 2018 Available at: https://www.michigan.gov/documents/mdhhs/HepA_Summ_County_SEMI2016_updated91517_601552_7.pdf. Accessed 21 June 2018.
- 8. Los Angeles County Department of Public Health. Hepatitis A outbreak 2018. Available at: http://publichealth.lacounty.gov/acd/Diseases/HepA/OB.htm. Accessed 21 June 2018.