Abstract
Study Objectives
Mild-to-moderate obstructive sleep apnea (OSA) is highly prevalent in the general population; however, previous studies on its association with incident hypertension are mixed. We examined the association between mild and moderate OSA and incident hypertension in a large random general population sample.
Methods
From 1741 adults of the Penn State Cohort, 744 adults without hypertension or severe OSA (i.e. apnea/hypopnea index [AHI] ≥ 30 events/hour) were followed-up after 9.2 years. Mild OSA was defined as an AHI of 5 to 14.9 events/hour (n = 71), while moderate OSA as an AHI of 15 to 29.9 events/hour (n = 32). Incident hypertension was defined by a self-report of receiving antihypertensive medication and/or history of a diagnosis since their baseline study.
Results
After adjusting for multiple potential confounders, mild-to-moderate OSA was significantly associated with increased risk of incident hypertension (overall hazard ratio [HR] = 2.94, 95% confidence interval (CI) = 1.96–4.41; HR = 3.24, 95% CI = 2.08–5.03 for mild OSA and HR = 2.23, 95% CI = 1.10–4.50 for moderate OSA). Importantly, this association was modified by age (p-interaction < 0.05); while strong in young and middle-aged adults (HR = 3.62, 95% CI = 2.34–5.60), the association was lost in adults older than 60 years (HR = 1.36 95% CI = 0.50–3.72). Furthermore, the association of mild-to-moderate OSA with components of metabolic syndrome was strongest in young and middle-aged adults.
Conclusions
Mild-to-moderate OSA, even when asymptomatic, is associated with increased risk of incident hypertension, but the strength of association significantly decreases with age. Although older participants with asymptomatic mild-to-moderate OSA are not at significant risk of developing hypertension, early detection and intervention, including improving metabolic indices, is especially warranted in young and middle-aged adults.
Keywords: sleep apnea, sleep-disordered breathing, hypertension, cohort study, incidence
Statement of Significance.
Mild-to-moderate OSA is common and usually untreated. While the association of severe OSA with increased risk of incident cardiometabolic comorbidities is well-established, the association between mild-to-moderate OSA and incident hypertension has not been consistently demonstrated. In a large random general population sample of a wide age range, followed for 9.2 years, we observed that both mild and moderate OSA were significantly associated with the development of hypertension. Importantly, this association while most profound in young and middle-aged adults, it was lost in adults older than 60 years. Furthermore, preliminary analysis showed that the association of mild-to-moderate OSA and components of metabolic syndrome was stronger in young and middle-aged adults. Early detection and intervention for mild-to-moderate OSA is especially warranted in young and middle-aged adults.
Introduction
Obstructive sleep apnea (OSA) is highly prevalent in the general population, especially in its mild and moderate forms. In adults, the prevalence of mild (apnea/hypopnea index [AHI] ≥ 5 and < 15 events/hour) and moderate (AHI ≥ 15 and < 30 events/hour) OSA ranges from 9.4% to 45.7% [1–5] and from 5.4% to 15.7% [1–4], respectively; however, most of these participants are asymptomatic [2]. The lack of symptoms in this group of OSA patients, particularly in epidemiological samples, raise the question of when, and how best to treat, OSA in the mild-to-moderate range.
Although the association of severe OSA with increased risk of prevalent and incident cardiometabolic comorbidities, such as hypertension, is well-established [3–6], literature on mild and moderate OSA is inconsistent. Some, but not all, cross-sectional studies have reported that mild and/or moderate OSA is associated with prevalent hypertension or increased blood pressure (BP) [3–5] whereas the hazard ratio (HR) for mortality associated with mild-to-moderate OSA (AHI 15–29.9 events/hour) is negligible [7, 8].
To date, only three population-based longitudinal studies have examined the association between OSA and incident hypertension, and the findings are mixed. The Wisconsin Sleep Cohort Study reported that mild OSA (AHI 0.1–14.9 events/hour) was associated with increased presence (i.e. persistence and incidence) of hypertension after 4 years of follow-up compared to individuals without OSA (AHI = 0) [6]. In contrast, two other large studies, the Sleep Heart Health Study and the Vitoria Sleep Cohort Study, did not observe a significant association between mild and moderate OSA and incident hypertension [9, 10].
Since 2000, cross-sectional studies from the Penn State Adult Cohort and the Sleep Heart Health Study have shown that the association between OSA and hypertension is strongest in young and middle-aged adults [5, 11, 12]. Also since the early 2000s we have investigated and hypothesized that symptomatic sleep apnea is more frequent in patients with metabolic syndrome [13]. However, the modifying effect of age and metabolic syndrome on the association between OSA and hypertension has not been assessed in longitudinal studies.
The goal of this study was to examine the association between mild-to-moderate OSA and incident hypertension in a large random sample of the general population with a wide age range (20–82 years) over a long follow-up period (9.2 years). Secondary goals included the examination of the impact of age and components of the metabolic syndrome on the association of OSA and incident hypertension.
Methods
Study design and setting
Data were collected as part of a population-based study of sleep disorders (Penn State Adult Cohort), which used a two-phase protocol in order to recruit participants from various age groups [11, 14]. Participants included 741 men and 1000 women, selected randomly from the first phase, who completed a comprehensive evaluation including testing in the sleep laboratory.
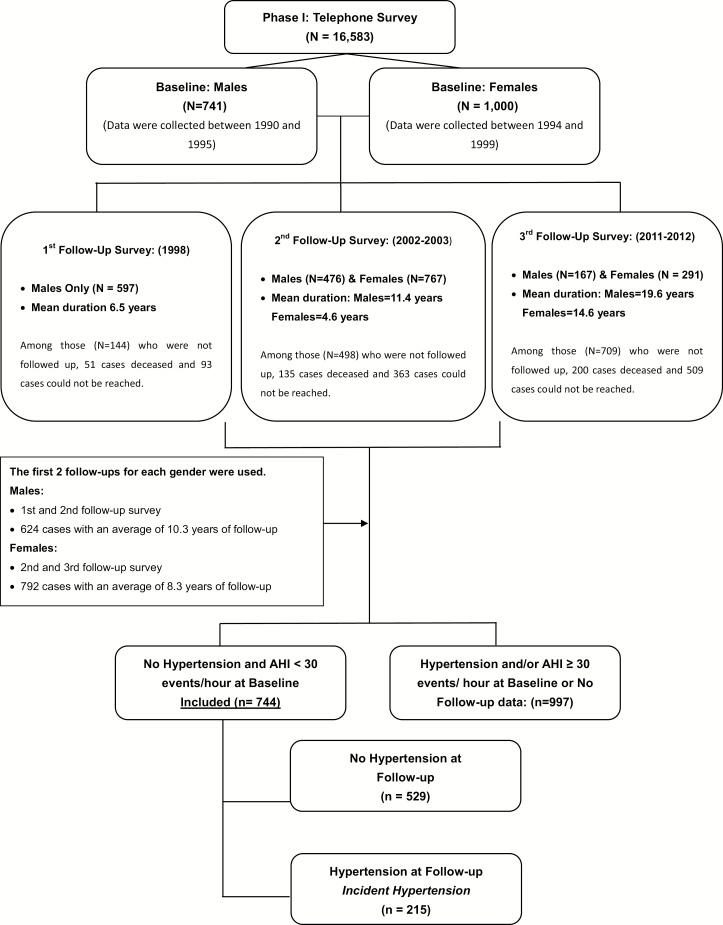
Of the 1741 participants, 1416 had at least one follow-up with an average duration of 9.2 years via telephone interview. The overall response rate was 81.3%. There were three follow-up surveys for males and two for females (Figure 1). In this study, we used data collected from the first two follow-ups for each gender (624 males with an average duration of 10.3 years and 792 females with an average duration of 8.3 years). After complete description of the follow-up study to the participants, verbal informed consent was obtained. The study procedure was approved by the Penn State University College of Medicine Institutional Review Board. Participants who participated in follow-up (n = 1461) compared to participants who did not participate in follow-up (n = 280), had significant lower percentages of minority (9% vs. 18.3%, p < 0.001) and female gender (55.8% vs. 64.4%, p = 0.005). No significant differences were observed in terms of baseline age, BMI, AHI and percentage of hypertension (all p-values > 0.05). Furthermore, there were no significant differences in terms of age, race, BMI, AHI, and percentage of hypertension at baseline among the 1741 who completed the baseline survey and the 1416 who completed the 9.2 years of follow-up, which indicates that characteristics of the 1461 follow-up participants and the 1741 baseline participants were similar.
Figure 1.
Participants’ flow in the study.
Definition of incident hypertension
Hypertension at baseline and the two follow-up periods were defined by a self-report of receiving antihypertensive medication and/or history of a hypertension diagnosis, based on a standardized questionnaire completed by the participants in the evening of their sleep laboratory visit (baseline) or over the phone (follow-up). Of these 744 participants who did not have hypertension and their AHI < 30 at baseline, 215 developed hypertension at follow-up.
Sleep laboratory evaluation
All participants were evaluated for one night for 8-hours in the sleep laboratory [11, 14]. Apnea and hypopnea were defined as previously reported [5]. For the purpose of this study, mild OSA was defined as an AHI between 5 to 14.9 events/hour, and moderate OSA was defined as AHI between 15 to 29.9 events/hour. Normal control was defined as AHI < 5. BP was measured in the evening about 2 hours before the sleep recording using a pneumoelectric microprocessor-controlled instrument. Mean arterial pressure (MAP) was calculated as diastolic blood pressure (DBP)+1/3(systolic blood pressure, SBP-DBP).
Other measurements
As part of the baseline evaluations, body mass index (BMI) was calculated based on height and weight measured as part of the physical examination. The presence of diabetes or hyperlipidemia at baseline was defined as a self-report of receiving treatment for diabetes or having a fasting blood sugar >126 mg/dL or fasting triglyceride levels >150 mg/dl, respectively. The presence of excessive daytime sleepiness (EDS) was established based on two questions: “Do you feel drowsy or sleepy most of the day, but manage to stay awake?” and/or “Do you have any irresistible sleep attacks during the day?” Each question was answered on a Likert scale (0 = none, 1 = mild, 2 = moderate, 3 = severe), and the presence of EDS was defined as a moderate-to-severe report to either of the two questions. Participants’ daily consumption of tobacco was also assessed.
Follow-up measures taken through telephone interview included the standardized questionnaire that participants completed at baseline.
Statistical analyses
The design of this study included oversampling of those at higher risk for sleep disorder breathing and women with markedly higher BMI to increase the precision of the risk estimates. Because of this sampling strategy, numeric sampling weights were developed for the analysis so that the estimates could be inferred to the original target population [11, 14, 16]. We adjusted for the sampling weight in all analyses.
The effective sample size for the analysis was 744. The participants’ demographic characteristics and medical history were summarized and compared by using ANOVA, t-test, chi-square test, or Cochran–Mantel–Haenszel (CMH) tests as appropriate. To assess the association between OSA and time of developing incident hypertension, as defined above, multi-variable adjusted regression models were used. Since the hypertension status was evaluated at discrete time point, namely the time of follow-up interview, the time to develop hypertension is effectively interval censored. Therefore, we applied a discrete time-to-event hazard model [17] to examine the association between OSA and the associated hypertension risk. In effect, the discrete time-to-event hazard model reduced to a logistic regression model with a complementary log-log link function. The results are expressed as HRs and their corresponding 95% confidence interval (CI). In the regression models, age, race, sex, BMI, smoking status, baseline diabetes and MAP and treatment for OSA were included as covariables. To assess the potential effect modification of age in the association between OSA and incident hypertension, the significance of the interaction between age and mild-to-moderate OSA was tested. Furthermore, in order to appropriately interpret the results and choose a clinically meaningful age cut-off point, we estimated the age-specific HRs of developing hypertension based on the average demographic and clinical profiles of our sample. We further calculated the estimated HRs of incident hypertension for participants between 20 and 80 years of age, with a 10-year increment. Finally, to assess the association between mild-to-moderate OSA and metabolic syndrome components, and more importantly, whether the association differs by age group (i.e. <60 years vs. ≥60 years), multivariable adjusted logistic regression models were used. In these models, obesity (BMI > 30), diabetes (fasting glucose > 126 mg/dL or treatment for diabetes), and hypertriglyceridemia (TG > 150 mg/dL) were considered as dependent variables, while mild-to-moderate OSA was treated as the independent variable. We further introduced the interaction term between age group and OSA in the regression models to assess the potential effect modification of age in relationship between OSA and metabolic syndrome components. Major covariates, including race, sex, smoking status, and sampling weight, were adjusted in the models. The results were presented as multivariable adjusted odds ratio (OR) with the corresponding 95% CI.
A p < 0.05 was used to determine statistical significance in all analyses. All analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
Results
The overall incidence of hypertension was 21.5% (Table 1).
Table 1.
Baseline demographic, behavioral and sleep characteristics of the study groups
| Baseline characteristics | All | AHI (events/hour) | Incident hypertension | |||||
|---|---|---|---|---|---|---|---|---|
| N = 744 | <5N = 641 | 5–14.9N = 71 | 15–29.9N = 32 | P-value | NoN = 529 | YesN = 215 | P-value | |
| Mean (SD) | Mean (SD) | |||||||
| Age, years | 47.1 (12.5) | 46.6 (12.7) | 50.7 (11.5) | 57.1 (12.1) | <0.001 | 46.5 (12.9) | 49.2 (11.0) | 0.004 |
| ≤40 (%) | 31.0 | 32.3 | 20.3 | 7.1 | <0.001 | 33.2 | 22.6 | 0.006 |
| 41–59 (%) | 52.4 | 52.3 | 57.8 | 42.9 | 50.2 | 60.3 | ||
| ≥60 (%) | 16.6 | 15.4 | 21.9 | 50.0 | 16.5 | 17.1 | ||
| Gender (Male, %) | 48.0 | 44.9 | 84.4 | 77.8 | <0.001 | 49.1 | 44.0 | 0.166 |
| Race (Caucasian, %) | 94.6 | 94.7 | 93.8 | 88.9 | 0.419 | 94.7 | 93.6 | 0.501 |
| BMI, kg/m2 | 26.8 (4.7) | 26.5 (4.5) | 29.2 (6.5) | 29.3 (5.2) | <0.001 | 26.3 (4.3) | 28.3 (5.7) | <0.001 |
| Obesity (≥30, kg/m2) | 18.7 | 17.7 | 27.7 | 33.3 | 0.019 | 15.8 | 29.1 | <0.001 |
| Excessive daytime sleepiness (%) | 6.1 | 6.0 | 7.8 | 3.7 | 0.738 | 6.0 | 6.4 | 0.810 |
| SBP mmHg | 125.1 (14.6) | 124.9 (14.8) | 127.2(11.5) | 128.8(15.1) | 0.195 | 122.5(13.4) | 134.8 (14.9) | <0.001 |
| DBP mmHg | 78.5 (8.5) | 78.4 (8.4) | 79.5 (8.7) | 80.5 (12.1) | 0.273 | 77.0 (7.4) | 84.2 (9.7) | <0.001 |
| MAP mmHg | 94.1 (9.3) | 93.9 (9.3) | 95.4 (8.4) | 96.6 (12.2) | 0.156 | 92.1 (8.1) | 101.1 (10.2) | <0.001 |
| Glucose (mg/dL) | 100.0 (28.5) | 99.2 (27.2) | 110.8(39.1) | 102.9(39.4) | 0.009 | 99.6 (29.6) | 101.4 (24.3) | 0.423 |
| Diabetes mellitus (%) | 9.4 | 8.9 | 13.8 | 14.8 | 0.261 | 9.5 | 9.0 | 0.805 |
| Triglyceride (mg/dL) | 141.5(97.9) | 140.5(100.6) | 155.2(52.9) | 153.6(51.7) | 0.498 | 133.0(80.3) | 171.9(140.1) | <0.001 |
| Hyperlipidemia (%) | 42.2 | 40.6 | 54.7 | 67.9 | 0.002 | 40.4 | 48.3 | 0.031 |
| Depression (%) | 12.7 | 12.9 | 12.3 | 7.4 | 0.698 | 12.1 | 15.0 | 0.243 |
| Smoking (%) | 22.9 | 24.1 | 10.9 | 7.1 | 0.007 | 24.4 | 17.5 | 0.027 |
| Alcohol, drinks/day | 0.033 | 0.009 | ||||||
| None (%) | 72.8 | 73.7 | 63.1 | 60.7 | 72.3 | 74.8 | ||
| 1 (%) | 10.1 | 9.3 | 20.0 | 17.9 | 9.0 | 13.7 | ||
| ≥2 (%) | 17.2 | 17.0 | 16.9 | 21.4 | 18.7 | 11.5 | ||
| AHI, events/hour | 1.3 (3.6) | 0.4 (1.0) | 7.9 (2.1) | 19.6 (3.7) | <0.001 | 1.1 (3.3) | 2.1 (4.7) | <0.001 |
| OSA (%) | NA | <0.001 | ||||||
| <5, events/hour | 91.6 | 100 | 0 | 0 | 94.1 | 82.5 | ||
| 5–14.9, events/hour | 5.9 | 0 | 100 | 0 | 4.0 | 12.8 | ||
| 15–29.9, events/hour | 2.5 | 0 | 0 | 100 | 1.9 | 4.7 | ||
| Min SaO2 (%) | 91.4 (6.1) | 92.0 (5.8) | 85.1 (4.3) | 84.7 (5.2) | <0.001 | 91.5 (6.2) | 91.2 (5.5) | 0.505 |
Mild-to-moderate OSA was independently and significantly associated with higher risk for incident hypertension compared to individuals without OSA at baseline (HR = 2.94, 95% CI 1.96–4.41), while adjusting for race, sex, baseline age, BMI, smoking, treatment for OSA, diabetes, and MAP (Table 2). In addition, after further stratifying participants based on severity of OSA, mild (HR = 3.24, 95% CI 2.08–5.03) and moderate (HR = 2.23, 95% CI 1.10–4.50), OSA were significantly associated with incident hypertension, respectively (Table 2). In sensitivity analyses, when we further adjusted for self-reported BMI changes across time points, the findings were similar and remained significant (mild-to-moderate OSA: HR = 3.06, 95% CI 2.04–4.59; mild OSA: HR = 3.30, 95% CI 2.12–5.13; moderate OSA: HR = 2.49, 95% CI 1.24–5.00, all p-value < 0.05).
Table 2.
Multivariable adjusted hazard ratio and 95% confidence intervals of incident hypertension in mild and moderate OSA
| Predictors | N | HR (95% CI) |
|---|---|---|
| Controls | 641 | Reference |
| Mild-to-moderate OSA | 103 | 2.94 (1.96–4.41) |
| Controls | 641 | Reference |
| Mild OSA | 71 | 3.24 (2.08–5.03) |
| Moderate OSA | 32 | 2.23 (1.10–4.50) |
All data present were adjusted for sampling weight, sex, race, baseline age, BMI, smoking, diabetes, mean arterial pressure and treatment for OSA. Controls were defined as AHI < 5 events/hour, Mild-to-moderate OSA was defined as AHI 5–29.9 events/hour, Mild OSA was defined as AHI 5–14.9 events/hour and Moderate OSA was defined as AHI 15–29.9 events/hour.
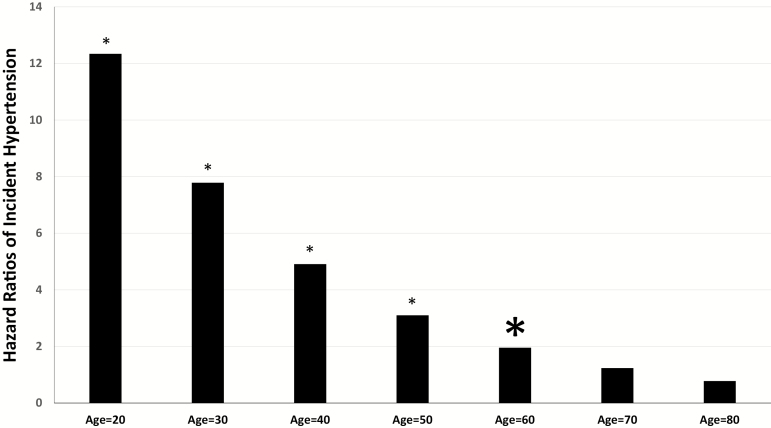
We found a significant interaction between mild-to-moderate OSA and age on incident hypertension (p < 0.05). The estimated HR of developing hypertension across each decade from 20 to 80 years of age was significantly higher in OSA patients compared to corresponding age participants who were free of OSA, in the young and middle-aged individuals 20–60 years old but became nonsignificant after the age of 60 (Figure 2). Similarly, incident hypertension was significantly associated with mild-to-moderate OSA among individuals whose age ≤60 years (HR = 3.62, 95% CI 2.34–5.60, p < 0.001), but not in those age > 60 years (HR = 1.36, 95% CI 0.50–3.72, p = 0.55). In sensitivity analyses, when we further adjusted for self-reported BMI changes across time point, the findings were similar. Interestingly, the majority of the moderate OSA cases (55.2%) were older than 60 years, while the majority of the mild OSA were younger than 60 years. Furthermore, there was no interaction of mild-to-moderate OSA and EDS on incident hypertension.
Figure 2.
The estimated hazard ratio of incident hypertension across ages. The figure depicts the estimated hazard ratio of incident hypertension associated with mild-to-moderate OSA across ages with identical demographic (e.g. sex, race, and BMI) and clinical (e.g. prevalence of smoking, diabetes and treatment for OSA) characteristics. *p < 0.05.
Metabolic abnormalities in OSA at risk for incident hypertension: a preliminary analysis
Given the stronger association of mild/moderate OSA with incident hypertension in young and middle-aged adults as compared to older adults, we used multivariable adjusted logistic regression models to explore whether this association could be explained by a stronger association of OSA with components of the metabolic syndrome in young and middle-aged adults. Supplementary Table 1 presents the odds ratio 95% CI on the association between OSA and metabolic syndrome components among individuals < 60 and ≥60 years old. Individuals with OSA are at higher risk for obesity (BMI > 30 kg/m2), diabetes (fasting glucose levels ≥126 mg/dL and/or treatment for diabetes), and high TG (>150 mg/dL) in the younger group, but not in the older group. All interactions are significant, suggesting that the association between OSA and metabolic syndrome components is significantly different between young and old.
Discussion
The findings of this study indicate that mild-to-moderate OSA, even when asymptomatic, is a significant and independent risk factor for the development of hypertension, and that this effect is stronger in young and middle-aged adults. Furthermore, preliminary data suggest that the age-related association of OSA with incident hypertension may be explained by the stronger association of OSA with components of the metabolic syndrome in young and middle-aged adults. These findings have important implications in the clinical management of this highly prevalent disorder.
There have been three previous longitudinal studies that have evaluated whether mild-to-moderate OSA is an independent risk factor for incident hypertension. Two of them, the Sleep Heart Health Study [9] and Vitoria Sleep Cohort Study [10], did not find any significant association between mild-to-moderate OSA and hypertension. The negative findings of the Sleep Heart Health Study may be explained by that the population was primarily comprised of older adults with an average age of 62.9 ± 11.1 years (40–79 years) and the follow-up period was relatively short of only 5 years. In the Vitoria Sleep Cohort Study, the assessment of sleep apnea was performed via home polygraph, which is well-known not to correlate well with PSG findings, particularly in the milder forms of the disorder [18, 19]. The Wisconsin Sleep Cohort Study reported an increased presence of hypertension 4 years later, and mild sleep apnea was significantly associated with non-dipping of nocturnal SBP after an average of 7.2 years of follow-up [6, 20]. In this latter study the age range was relatively restricted (30–60 years) and the sample was comprised of State employees and not drawn from the general population. Our study is the first one to include a sample with a wide age range (20–82 years, 48.8 ± 13.6 years) randomly selected from the general population, which makes our findings more generalizable to the U.S. population.
Since 2000, data from the Penn State Adult Cohort and the Sleep Heart Health Study have shown that the association between OSA and hypertension is strongest in young and middle-aged adults [5, 11, 12]. Recently, a study from the Multiethnic Study of Atherosclerosis reported that OSA severity was associated with higher levels of glucose, white blood cell count, hsCRP, and triglyceride levels only in those age < 65 years [21]. However, due to the cross-sectional nature of these studies, the possibility that the lack of association between OSA and hypertension in elderly could have been due to a survival effect. The longitudinal design of our study demonstrates that OSA in older individuals (age > 60 years) is not as strongly associated with the development of hypertension. Relatedly, other studies have reported no association of severe OSA and mortality in men older than 70 years [8], whereas moderate OSA (RDI 20–39.9) was even associated with an “unexpected survival advantage” in the elderly [22]. In our study, the lack of a dose-response association between severity of OSA (mild vs. moderate) and incident hypertension may be related to the relatively small sample size of moderate OSA and that moderate OSA was more prevalent in older adults, who had the weakest association with incident hypertension.
From a methodological standpoint, the decreasing trend between mild-to-moderate OSA and hypertension with increased age could be accounted for by a “ceiling” effect. In other words, because hypertension becomes more common in older individuals, it may require a large sample to detect an effect of OSA on this disorder. However, even large studies have failed to show an association between BMI, a strong causative factor of OSA, and mortality in the elderly [23, 24]. From a mechanistic standpoint, a potential mechanism explaining this finding is delayed ischemic preconditioning, a state in which repeated sublethal ischemia confers protection from these problems [25]. In our study, the group with the highest probability of developing hypertension showed the strongest association with metabolic abnormalities, although, interestingly, most of these participants suffered from the milder form of the disorder. Based on these preliminary findings, we suggest that metabolic abnormalities are more frequent in young and middle-aged individuals with OSA at risk for hypertension.
Our study has several important clinical implications. Consistent with previous studies, it appears that mild-to-moderate OSA in the elderly is not associated with cardiovascular risks [5, 12]. Thus, older asymptomatic patients with an AHI in the range of 5–29.9 events/hour may not benefit from current gold standard treatment options, such as continuous positive airway pressure (CPAP), in terms of preventing hypertension. In those with concomitant symptoms, such as sleepiness and poor nighttime sleep, treatment may be beneficial, although hard evidence from randomized control trials is still largely missing [19, 26].
In young and middle-aged asymptomatic patients without comorbid cardiometabolic conditions, early detection and intervention to prevent the cardiometabolic sequelae associated with OSA is warranted. Given the stronger association of OSA with metabolic abnormalities in this age group, emphasis should be placed on yearly monitoring of indices of metabolic symptoms and lifestyle interventions, such as weight control, healthy diet, regular exercise, and stress management. Because AHI alone cannot satisfactorily predict the development of OSA-associated morbidities, future research should explore additional measures, such as inflammation biomarkers, to improve the prognostic value of AHI [27].
There are several strengths of this study: its prospective longitudinal study; a randomly selected sample from the general population; its wide age range (20–82 years); its long follow-up duration (9.2 years); and a detailed clinical history and physical examination that ensured careful control for multiple potential confounders.
Several limitations should be acknowledged and taken into account when interpreting our results. First, incident hypertension was defined by self-report and not by in-laboratory blood pressure measurements. However, mild-to-moderate OSA remained strongly and significantly associated with incident hypertension even after controlling for baseline MAP. Moreover, two sensitivity analyses were performed. When we entered baseline pre-hypertensive (≥120/80 mmHg) and hypertensive blood pressure (≥140/90 mmHg) status in the model, results did not differ significantly. Also, when we excluded individuals with baseline SBP ≥ 140 and/or DBP ≥ 90 mmHg, results remained similar and significant. In addition, self-reported hypertension has been used in other large epidemiological studies to ascertain incident hypertension [28, 29]. Second, we included 22 individuals who underwent CPAP treatment for OSA at some point in our main analyses because we could not be certain that their OSA was being treated optimally. However, when we further adjusted for OSA treatment in regression models, findings did not change and remained significant. Moreover, when we repeated the analyses after excluding participants with OSA treatment, findings remained very similar and significant (all p-values < 0.05). Third, it is possible that some participants with mild-to-moderate OSA may have shifted to the severe category over a 10-year period [30]. However, this does not negate our findings that young and middle-aged adults with mild-to-moderate OSA are at risk of hypertension either by worsening metabolic syndrome and/or severity of OSA. Furthermore, changes of BMI across time points may confound the association between OSA and the development of hypertension. However, we did not objectively measure BMI at follow-up surveys. When we further adjusted for changes of self-reported BMI (baseline minus follow-up) in regression models, the findings did not change and remained significant (all p-value < 0.05). Finally, different follow-up periods in men and women may affect the findings. The difference in the follow-up period between men and women was small (10.3 vs. 8.3 years). To minimize the potential bias induced by the difference in follow-up periods between men and women, we purposely used discrete time-to-event logistic regression hazard model, to take into account the time to follow up and this small difference. In addition, there was no significant interaction between OSA and gender (p = 0.68). The HRs (95% CI) of incident hypertension for mild-to-moderate OSA in males and females were 2.81 (1.78–4.45) and 3.43 (1.49–7.88), respectively. Finally, at the time of designing the Penn State cohort, the hypothesis that sleep apnea is more frequent in patients with metabolic syndrome was not primary, thus we have only some of the components of the metabolic syndrome and not the full profile of the disorder, that is, HDL and treatment for hyperlipidemia. Consequently, these results should be viewed only as preliminary and further studies should examine these associations.
In summary, mild and moderate OSA are significantly associated with increased risk for developing hypertension and this association is most profound in young and middle-aged adults. It appears that early detection and treatment of mild-to-moderate OSA through improving metabolic indices in young and middle-aged adults is warranted in order to prevent future cardiovascular disease.
Funding
This study was supported by National Heart Lung and Blood Institute (NHLBI) R01 HL40916 and HL5193.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgments
The work was performed at the Sleep Research and Treatment Center at the Penn State University Milton Hershey Hospital, and the staff (C. Criley, P. Cain, S. George, and T. Miksiewicz) is especially commended for their efforts.
References
- 1. Heinzer R, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arnardottir ES, et al. Obstructive sleep apnea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47(1):194–202. [DOI] [PubMed] [Google Scholar]
- 3. Young T, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 4. Nieto FJ, et al. Pickering TG: association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. [DOI] [PubMed] [Google Scholar]
- 5. Bixler EO, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160(15):2289–2295. [DOI] [PubMed] [Google Scholar]
- 6. Peppard PE, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. [DOI] [PubMed] [Google Scholar]
- 7. Young T, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8. Punjabi NM, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Connor GT, et al. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009;179(12):1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cano-Pumarega I, et al. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med. 2011;184(11):1299–1304. [DOI] [PubMed] [Google Scholar]
- 11. Bixler EO, et al. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. [DOI] [PubMed] [Google Scholar]
- 12. Haas DC, et al. Age-dependent associations between sleep-disordered breathing and hypertension: importance of discriminating between systolic/diastolic hypertension and isolated systolic hypertension in the Sleep Heart Health Study. Circulation. 2005;111(5):614–621. [DOI] [PubMed] [Google Scholar]
- 13. Vgontzas AN, et al. Sleep apnea is a manifestation of the metabolic syndrome. Sleep Med Rev. 2005;9(3):211–224. [DOI] [PubMed] [Google Scholar]
- 14. Bixler EO, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services (DHHS), National Center for Health Statistics.Third National Health and Nutrition Examination Survey, 1988–1994. NHANES III Laboratory Data File. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. https://wwwn.cdc.gov/nchs/nhanes/nhanes3/DataFiles.aspxAccessed January 12, 1998. [Google Scholar]
- 16. Kish L. Survey Sampling. New York, NY: John Wiley & Sons, Inc; 1965. [Google Scholar]
- 17. Prentice RL, et al. Regression analysis of grouped survival data with applications to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 18. Kuna ST, et al. ; ATS/AASM/ACCP/ERS Committee on Ambulatory Management of Adults with OSA. An official ATS/AASM/ACCP/ERS workshop report: research priorities in ambulatory management of adults with obstructive sleep apnea. Proc Am Thorac Soc. 2011;8(1):1–16. [DOI] [PubMed] [Google Scholar]
- 19. McNicholas WT, et al. Mild obstructive sleep apnea: clinical relevance and approaches to management. Lancet Respir Med. 2016;4(10):826–834. [DOI] [PubMed] [Google Scholar]
- 20. Hla KM, et al. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31(6):795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geovanini GR, et al. Age and sex modify the association between OSA and traditional and novel cardiovascular risk factors: the multi-ethnic study of atherosclerosis (MESA) [Abstract]. Sleep. 2017;40 (Suppl);A166. [Google Scholar]
- 22. Lavie P, et al. Unexpected survival advantage in elderly people with moderate sleep apnea. J Sleep Res. 2009;18(4):397–403. [DOI] [PubMed] [Google Scholar]
- 23. Stevens J, et al. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. [DOI] [PubMed] [Google Scholar]
- 24. Bender R, et al. Effect of age on excess mortality in obesity. JAMA. 1999;281(16):1498–1504. [DOI] [PubMed] [Google Scholar]
- 25. Lavie L, et al. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses. 2006;66(6):1069–1073. [DOI] [PubMed] [Google Scholar]
- 26. Chowdhuri S, et al. ; ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea. An official American Thoracic Society research statement: impact of mild obstructive sleep apnea in adults. Am J Respir Crit Care Med. 2016;193(9):e37–e54. [DOI] [PubMed] [Google Scholar]
- 27. Gaines J, Kong L, Li M, et al. C-reactive protein improves the ability to detect cardiometabolic risk in mild-to-moderate obstructive sleep apnea[J]. Physiol Rep. 2017;5(18). doi:10.14814/phy2.13454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandez-Mendoza J, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gangwisch JE, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23(1):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.