Abstract
Background
Vascular occlusion used during elective liver resection to reduce blood loss results in significant ischaemia reperfusion (IR) injury. This in turn leads to significant postoperative liver dysfunction and morbidity. Various pharmacological drugs have been used in experimental settings to ameliorate the ischaemia reperfusion injury in liver resections.
Objectives
To assess the relative benefits and harms of using one pharmacological intervention versus another pharmacological intervention to decrease ischaemia reperfusion injury during liver resections where vascular occlusion was performed during the surgery.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until January 2009.
Selection criteria
We included randomised clinical trials, irrespective of language or publication status, comparing one pharmacological agent versus another pharmacological agent during elective liver resections with vascular occlusion.
Data collection and analysis
Two authors independently identified trials for inclusion and independently extracted data. We analysed the data with both the fixed‐effect and the random‐effects models using RevMan Analysis. We planned to calculate the risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI) based on intention‐to‐treat analysis or available case analysis. However, all outcomes were only reported on by single trials, and meta‐analysis could not be performed. Therefore, we performed Fisher's exact test on dichotomous outcomes.
Main results
We identified a total of five randomised trials evaluating nine different pharmacological interventions (amrinone, prostaglandin E1, pentoxifylline, dopexamine, dopamine, ulinastatin, gantaile, sevoflurane, and propofol). All trials had high risk of bias. There was no significant difference between the groups in mortality, liver failure, or perioperative morbidity. The ulinastatin group had significantly lower postoperative enzyme markers of liver injury compared with the gantaile group. None of the other comparisons showed any difference in any of the other outcomes. However, there is a high risk of type I and type II errors because of the few trials included, the small sample size in each trial, and the risk of bias.
Authors' conclusions
Ulinastatin may have a protective effect against ischaemia reperfusion injury relative to gantaile in elective liver resections performed under vascular occlusion. The absolute benefit of this drug agent remains unknown. None of the drugs can be recommended for routine clinical practice. Considering that none of the drugs have proven to be useful to decrease ischaemia reperfusion injury, such trials should include a group of patients who do not receive any active intervention whenever possible to determine the pharmacological drug's absolute effects on ischaemia reperfusion injury in liver resections.
Plain language summary
No clear evidence that any pharmacological intervention is better than another in decreasing ischaemia reperfusion injury in liver resections
Elective liver surgery undertaken for a variety of reasons may require occlusion of the blood supply to the liver in order to reduce bleeding from the cut liver surface. This temporary interruption of blood supply causes liver damage for a variety of reasons. In experimental studies many drugs have shown some promise in decreasing liver damage caused by the occluded blood supply. The relative benefits of pharmacological agents compared with one another is unknown in the setting of liver damage caused by occlusion of the blood supply to the liver during surgery. We identified a total of five randomised trials evaluating nine different pharmacological interventions (amrinone, prostaglandin E1, pentoxifylline, dopexamine, dopamine, ulinastatin, gantaile, sevoflurane, and propofol). All trials had risk of bias ('systematic error') and risk of play of chance ('random errors'). There was no significant difference between the groups in mortality, liver failure, or postoperative complications. The ulinastatin group had significantly lower postoperative enzyme markers of liver injury compared with the gantaile group. None of the remaining pharmacological agents showed any significant difference in any of the remaining outcomes. However, there is a high risk of type I (erroneously concluding that an intervention is beneficial when it is actually not beneficial) and type II errors (erroneously concluding that an intervention is not beneficial when it is actually beneficial) because of the few trials included, the small sample size in each trial, and the risk of bias. Ulinastatin may have a protective effect relative to gantaile against liver injury sustained during elective liver surgery involving blood supply occlusion. The absolute benefit of ulinastatin in this setting remains unknown. None of the pharmacological agents can be recommended for routine clinical practice. Considering that none of the agents have been proven to be useful to decrease ischaemia reperfusion injury, such trials should include a group of patients who do not receive any active intervention whenever possible to determine their absolute effect on ischaemia reperfusion injury in liver resections.
Background
Elective liver resection is performed mainly for benign and malignant liver tumours (Belghiti 1993). The malignant tumours may arise primarily within the liver (hepatocellular carcinoma and cholangiocarcinoma) or be metastases from malignancies of other organs (Belghiti 1993; Chouker 2005). More than 1000 elective liver resections are performed annually in the United Kingdom alone (HES 2005).
The liver is subdivided into eight Couinaud segments (Couinaud 1999), which can be removed either individually or by right hemi‐hepatectomy (Couinaud segments 5 to 8), left hemi‐hepatectomy (segments 2 to 4), right trisectionectomy (segments 4 to 8), or left trisectionectomy (segments 2 to 5 and 8 ±1) (Strasberg 2000). Although every liver resection is considered major surgery, only resection of three or more segments is considered a major liver resection (Belghiti 1993).
Blood loss during liver resection is one of the important factors affecting the perioperative outcomes of patients (Shimada 1998; Yoshimura 2004; Ibrahim 2006). One of the methods that has been attempted to reduce blood loss during liver resection involves occluding the blood flow to the liver. Various methods of vascular occlusion have been attempted (Gurusamy 2009a). While the incidence of liver failure was not increased by most types of vascular occlusion, the enzymes indicative of liver parenchymal injury are elevated after vascular occlusion (Gurusamy 2009b) resulting in a variable degree of ischaemia reperfusion (IR) injury of the liver.
Ischaemia‐reperfusion injury of the liver is a complex multi‐path process. During the ischaemic phase mitochondrial oxidative phosphorylation is disrupted and adenosine tri‐phosphate (ATP) production is decreased leading to intracellular sodium accumulation and subsequent cellular damage. At the same time endothelial cells are primed to express surface adhesion molecules (Kupiec 2005). The reperfusion phase activates Kuppfer cells and T cells, leading to increased production of pro inflammatory cytokines and reactive oxygen species (Kupiec 2005). Neutrophils are recruited and activated resulting in propagation of the inflammatory cascade (Kupiec 2005). Many methods have been attempted to decrease the ischaemia reperfusion injury associated with prolonged duration of vascular occlusion including the use of ischaemic preconditioning (Azoulay 2006; Smyrniotis 2006; Gurusamy 2009c), in‐situ cooling (Kim 1996; Azoulay 2005), and the use of pharmacological agents. The various pharmacological agents that have been shown to ameliorate liver ischaemia reperfusion injury in experimental studies are discussed elsewhere (Koti 2003; Galaris 2006; Georgiev 2006). The relative efficacy of one pharmacological agent compared to a second pharmacological agent is important regardless of whether the absolute effect of the agent in reducing ischaemia reperfusion injury is known or not. This may determine which of the drugs that are of the same class or have similar mechanisms of action (eg, general anaesthetic agents) should be used in liver resection surgery performed under vascular control to reduce ischaemia reperfusion injury. For example, if either one of a drug 'A' or a drug 'B' is commonly used to anaesthetise patients undergoing liver resection surgery under vascular occlusion, then knowing which of drug 'A' or 'B' significantly reduces ischaemia reperfusion injury relative to one another, regardless of the absolute effect of each drug on ischaemia reperfusion injury, would guide clinicians in their choice of a drug.
We were not able to identify any systematic reviews or meta‐analyses comparing one pharmacological intervention versus another pharmacological intervention to decrease ischaemia reperfusion injury in liver resections performed under vascular control.
Objectives
To assess the relative benefits and harms of a pharmacological intervention versus another pharmacological intervention to decrease ischaemia reperfusion injury during liver resection where vascular occlusion was undertaken during the surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised clinical trials, irrespective of language, blinding, or publication status. We excluded quasi‐randomised studies (where the methods of allocating participants to a treatment are not strictly random, for example, date of birth, hospital record number, alternation) regarding benefits but planned to include them for adverse events resulting directly from the pharmacological intervention. However, we did not identify any such trials.
Types of participants
Patients who underwent elective liver resection surgery with vascular occlusion irrespective of the liver background (cirrhosis, steatosis, or normal liver); or the method and duration of the vascular occlusion; and whether ischaemic preconditioning was used or not.
Types of interventions
We included trials comparing one or more pharmacological interventions versus another pharmacological intervention, irrespective of the time, dose or pharmacological class of the administered drug.
Types of outcome measures
Primary outcomes
Peri‐operative mortality.
Liver decompensation/failure (however defined by authors).
Secondary outcomes
Peri‐operative morbidity (bile leak, intra‐abdominal collection, wound infection, renal impairment).
Intensive therapy unit (ITU) stay.
Hospital stay.
Blood transfusion requirements.
Blood loss.
Markers of liver function (bilirubin, prothrombin time).
Biochemical markers of liver parenchymal injury (aspartate aminotransferase (AST), alanine aminotransferase (ALT)).
Search methods for identification of studies
See: Cochrane Hepato‐Biliary Group methods used in reviews (Gluud 2009).
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2009), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until January 2009 (Royle 2003). We have given the search strategies in Appendix 1 with the time span of the searches.
We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
Trial selection and extraction of data
We did not apply any language or publication status restrictions. KG and MA identified the trials for inclusion, independent of each other. We have also listed the excluded trials with the reasons for the exclusion.
KG and MA extracted the following data independently.
First author.
Year of publication of trial.
Country.
Inclusion and exclusion criteria.
Sample size.
Pharmacological drug name and dose.
Operation time.
Type of vascular exclusion.
Length of vascular exclusion (ischaemic time).
Proportion of major resections.
Proportion of patients with hepatosteatosis (fatty liver).
Proportion of cirrhotics.
Outcomes mentioned above.
Risk of bias (see below).
Any unclear or missing information was sought by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same patients ‐ completely or partially (by identifying common authors and centres) ‐ the authors of the trials were contacted to clarify whether the report had been duplicated. We resolved any differences in opinion through discussion.
Assessment of risk of bias
The authors followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2008) and the Cochrane Hepato‐Biliary Group Module (Gluud 2009; Gurusamy 2009d). The authors assessed the risk of bias in the trials independently, without masking of the trial names. Due to the risk of overestimation of beneficial intervention effects in randomised trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), we looked at the influence of methodological quality of the trials on the trial results by evaluating the reported randomisation and follow‐up procedures in each trial. If information was not available in the published trial, we contacted the authors in order to assess the trials correctly. We assessed the following:
Generation of the allocation sequence
Low risk of bias (if the allocation sequence was generated by a computer or random number table, drawing of lots, tossing of a coin, shuffling of cards, or throwing dice will be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure).
Uncertain risk of bias (if the trial was described as randomised, but the method used for the allocation sequence generation was not described).
High risk of bias (if a system involving dates, names, or admittance numbers were used for the allocation of patients. These studies are known as quasi‐randomised and were excluded from the review for benefits but were considered for inclusion for adverse effects).
Allocation concealment
Low risk of bias (if the allocation of patients involved a central independent unit, on‐site locked computer, or serially numbered, opaque, sealed envelopes).
Uncertain risk of bias (if the trial was described as randomised, but the method used to conceal the allocation was not described).
High risk of bias (if the allocation sequence was known to the investigators who assigned participants. Such quasi‐randomised studies were excluded from the review for benefits but were considered for inclusion for adverse effects, as mentioned before).
Blinding
Low risk of bias (if the trial was described as double blind).
Uncertain risk of bias (if the trial was described as double blind, but the method of blinding was not described).
High risk of bias (if the trial was not double blind).
Incomplete outcome data
Low risk of bias (the underlying reasons for missingness are unlikely to make treatment effects departure from plausible values, or proper methods have been employed to handle missing data).
Uncertain risk of bias (there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data is likely to induce bias on the estimate of effect).
High risk of bias (the crude estimate of effects (eg, complete case estimate) will clearly be biased due to the underlying reasons for missingness, and the methods used to handle missing data are unsatisfactory).
Selective outcome reporting
Low risk of bias (the trial protocol is available and all of the trial's pre‐specified outcomes that are of interest in the review have been reported or similar).
Uncertain risk of bias (there is insufficient information to assess whether the magnitude and direction of the observed effect is related to selective outcome reporting).
High risk of bias (perioperative mortality and liver decompensation have not been reported or not all of the trial's pre‐specified outcomes that are of interest in the review have been reported).
Other bias
Baseline imbalance
Low risk of bias (there was no baseline imbalance in important characteristics).
Uncertain risk of bias (the baseline characteristics were not reported).
High risk of bias (there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation).
Early stopping
Low risk of bias (sample size calculation was reported and the trial was not stopped or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was low).
Uncertain risk of bias (sample size calculations were not reported and it is not clear whether the trial was stopped early or not).
High risk of bias (the trial was stopped early due to an informal stopping rule or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high).
Academic bias
Low risk of bias (the author of the trial has not conducted previous trials addressing the same interventions).
Uncertain risk of bias (It is not clear if the author has conducted previous trials addressing the same interventions).
High risk of bias (the author of the trial has conducted previous trials addressing the same interventions).
Source of funding bias
Low risk of bias (the trial's source(s) of funding did not come from any parties that might have conflicting interest (eg, drug manufacturer).
Uncertain risk of bias (the source of funding was not clear).
High risk of bias (the trial was funded by a drug manufacturer).
We considered trials, which were classified as low risk of bias in sequence generation, allocation concealment, blinding, incomplete data, and selective outcome reporting as trials with low risk of bias.
Statistical methods
We performed the analyses according to the recommendations of The Cochrane Collaboration (Higgins 2008) and the Cochrane Hepato‐Biliary Group Module (Gluud 2009) using the software package RevMan 5 (RevMan 2008). For dichotomous outcomes reported on by two or more trials, we planned to calculate the risk ratio with 95% confidence interval. However, all dichotomous outcomes were only reported on by single trials, and, therefore, we performed Fisher's exact test on such outcomes. For continuous variables, we calculated the mean difference with 95% confidence interval. We planned to use the random‐effects model (DerSimonian 1986) and the fixed‐effect model (DeMets 1987). In case of discrepancy between the two models we planned to report both results; otherwise we planned to report only the results from the fixed‐effect model. However, because of the inclusion of only one trial under each comparison, we did not perform a meta‐analysis. We planned to explore heterogeneity by chi‐squared test with significance set at P value 0.10, and the quantity of heterogeneity was measured by I2 (Higgins 2002). However, because of the inclusion of only one trial under each comparison, measurement of heterogeneity was not applicable.
We performed the analysis on an intention‐to‐treat basis (Newell 1992) whenever possible. Otherwise, we adopted the available case analysis. In case we found zero‐event trials in statistically significant outcomes, we planned to perform a sensitivity analysis with and without empirical continuity correction factors as suggested by Sweeting et al (Sweeting 2004). However, we found no such outcomes.
Subgroup analysis
We planned to perform the following subgroup analyses.
Trials with low risk of bias compared to trials with high risk of bias.
Major resection compared to minor resections.
Cirrhosis compared to no cirrhosis.
Steatosis compared to no steatosis
Different methods of vascular occlusion compared to each other.
Employment of ischaemic preconditioning before vascular occlusion compared to no employment.
None of the trials in this review were of low risk of bias. The remaining subgroup analyses were not performed because of the lack of the necessary information in the trial reports and because of the few trials included under each comparison.
Bias exploration
We planned to use a funnel plot to explore bias (Egger 1997; Macaskill 2001). We planned to use asymmetry in funnel plot of trial size against treatment effect to assess bias. We also planned to perform linear regression approach described by Egger et al to determine the funnel plot asymmetry (Egger 1997). However, we could not explore bias using funnel plots or linear regression because of the small number of trials included under each comparison.
Results
Description of studies
We identified a total of 636 references through the electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 75), MEDLINE (n = 283), EMBASE (n = 171), and Science Citation Index Expanded (n = 107). We excluded 161 duplicates and 427 clearly irrelevant references through reading abstracts. Fourty eight references were retrieved for further assessment. No references were identified through scanning reference lists of the identified randomised trials. Of the 48 references, we excluded 42 because of the reasons listed under the table Characteristics of excluded studies. In total, six publications describing five randomised trials fulfilled the inclusion criteria. All the five were completed trials and could provide data for the analyses (Marx 2000; Orii 2000; Stadheim 2000; Li 2004; Beck‐Shimmer 2008). Details of the participants, interventions, and outcomes for each of the included trials are shown in the table Characteristics of included studies. Details of the proportion of major liver resections, type of vascular occlusion, parenchymal transection method used, and the mean ischaemic times are shown in Table 1. All the trials assessed the different pharmacological agents in open liver resections. The five trials included in this review compared the following interventions:
1. Operative details.
| Trial | Major resections (%) | Vascular occlusion* | Parenchymal transection | Mean ischaemic time (min) ‐ intervention 1** | Mean ischaemic time (min) ‐ intervention 2** |
| Beck‐Shimmer 2008 | 28 (43.8%) | CPTC | Kelly clamp | 36 | 35 |
| Li 2004 | not stated | CPTC | not stated | 18 | 17 |
| Marx 2000 | 19 (100%) | CPTC | not stated | 26 | 27 |
| Orii 2000 | 0 (0%) | IPTC | not stated | 66 | 71 |
| Stadheim 2000 | 23 (100%) | Afferent and efferent vessels | not stated | not stated | not stated |
IPTC: intermittent portal triad clamping. CPTC: continuous portal triad clamping. ** No significant difference between intervention and control group mean ischaemic times (P > 0.05).
Dopexamine versus dopamine (Marx 2000).
Prostaglandin E1 versus amrinone (Orii 2000).
Prostaglandin E1 versus pentoxifylline (Stadheim 2000).
Ulinastatin (protease inhibitor) versus gantaile (Li 2004).
Sevoflurane versus propofol (Beck‐Shimmer 2008).
Risk of bias in included studies
The details of the risk of bias for each of the trials are given in the 'Risk of bias' tables under Characteristics of included studies. The bias‐risk summary across all the trials is shown in Figure 1. The bias‐risk in individual trials is shown in Figure 2. Two trials had adequate random sequence generation (Marx 2000; Beck‐Shimmer 2008). None of the trials had adequate allocation concealment (Beck‐Shimmer 2008). One trial reported double blinding (Beck‐Shimmer 2008). Two trials were free from bias regarding incomplete outcome data (Orii 2000; Li 2004). Three trials were free from bias regarding selective reporting (Stadheim 2000; Li 2004; Beck‐Shimmer 2008). None of the trials were free from all the other risks of bias. Because none of the trials were considered as low risk of bias in all five of the afore mentioned risk of bias measurements, all the trials were considered to be at high risk of bias.
1.
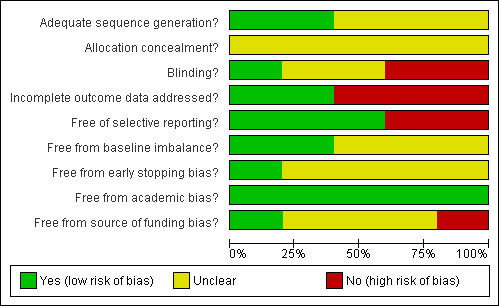
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
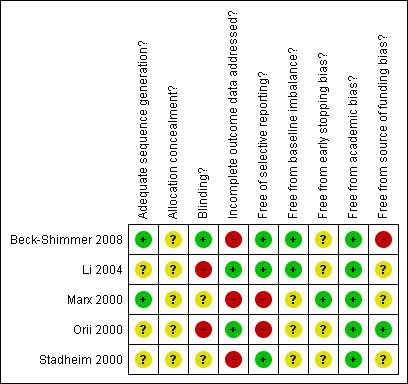
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
A meta‐analysis could not be performed for any of the outcomes as each comparison was only reported on by a single trial. Table 2 provides a summary of the results. For some of the outcomes the trials only reported whether there was a significant difference between the groups but did not provide numerical values. We obtained the mean values from graphs whenever possible and imputed the standard deviation from P values following the guidelines of the Cochrane Handbook (Higgins 2008). However, many of the reports did not report the exact P value, and we were unable to impute the standard deviation. So, these results were reported under the relevant outcomes of the relevant trials without the effect estimates.
2. Outcomes reported by single trials.
| Outcome | Type of outcome | Intervention group 1 | Intervention group 2 | P value |
| Dopexamine versus dopamine | ||||
| Blood transfusion [units] | Continuous | 1.8 (2.5) | 2.6 (3) | 0.53 |
| Prothrombin time [percentage of normal prothrombin activity] | Continuous | 60 (13) | 78 (22) | 0.03* |
| AST [IU/L]: 1st POD | Continuous | 129 (36) | 137 (109) | 0.83 |
| ALT [IU/L]: 1st POD | Continuous | 160 (53) | 190 (155) | 0.56 |
| Prostaglandin E1 versus amrinone | ||||
| Mortality | Dichotomous | 0/15 (0.0%) | 0/15 (0.0%) | Not applicable |
| Blood loss [litres] | Continuous | 0.59 (0.47) | 0.62 (0.41) | 0.85 |
| AST [IU/L]: 1st POD | Continuous | 216 (239) | 231 (152) | 0.84 |
| Prostaglandin E1 versus pentoxifylline | ||||
| Liver decompensation | Dichotomous | 0/13 (0.0%) | 0/10 (0.0%) | Not applicable |
| Ulinastatin versus gantaile | ||||
| Mortality | Dichotomous | 0/16 (0.0%) | 0/15 (0.0%) | Not applicable |
| Liver decompensation | Dichotomous | 1/16 (6.3%) | 3/15 (20.0%) | 0.33 |
| Wound infection | Dichotomous | 2/16 (12.5%) | 3/15 (20.0%) | 0.65 |
| Intra‐abdominal infection | Dichotomous | 1/16 (6.3%) | 1/15 (6.7%) | > 0.99 |
| Hospital stay [days] | Continuous | 13.3 (4) | 16.4 (8.4) | 0.19 |
| Blood loss [litres] | Continuous | 0.53 (0.29) | 0.58 (0.31) | 0.64 |
| Sevoflurane versus propofol | ||||
| Mortality | Dichotomous | 1/30 (3.3%) | 1/34 (2.9%) | > 0.99 |
| Liver decompensation | Dichotomous | 0/30 (0.0%) | 0/34 (0.0%) | Not applicable |
| Intra‐abdominal infection | Dichotomous | 0/30 (0.0%) | 3/34 (8.8%) | 0.24 |
| Postoperative bleeding | Dichotomous | 0/30 (0.0%) | 2/34 (5.9%) | 0.49 |
| Bile leak | Dichotomous | 1/30 (3.3%) | 1/34 (2.9%) | > 0.99 |
| ITU stay [days] | Continuous | 4 (16.5) | 9 (16.5) | 0.23 |
| Hospital stay [days] | Continuous | 10.93 (6.8) | 12.79 (6.8) | 0.28 |
| Bilirubin [mmoles/L]: peak postoperative levels | Continuous | 33.67 (26.9) | 44.5 (63.9) | 0.37 |
| AST [IU/L]: peak postoperative levels | Continuous | 507.5 (291.8) | 733.4 (636.5) | 0.06 |
| ALT [IU/L]: peak postoperative levels | Continuous | 463.5 (288.0) | 717.7 (497.5) | 0.01* |
For dichotomous outcomes, the numbers in column 'intervention group' and 'control group' denote number of patients with the outcome/total number of patients. The proportion of patients with the outcome is indicated in brackets.
For continuous outcomes, the numbers in column 'intervention group' and 'control group' denote the mean and standard deviation (in brackets) in each group.
* statistically significant
Dopexamine versus dopamine
Blood transfusion requirements
There was no significant difference in the blood transfusion requirements between the groups (mean difference (MD) ‐0.80 units, 95% CI ‐3.27 to 1.67).
Markers of liver function
There was a significantly higher prothrombin time as a percentage of normal activity in the dopamine group compared to the dopexamine group (MD ‐18.0 percentage of normal activity, 95% CI ‐34.06 to ‐1.94) measured on the first postoperative day (POD).
Enzyme markers of liver injury
There were no significant differences between the two groups in the AST or ALT levels on the first POD (AST: MD ‐8.0 IU/L, 95% CI ‐79.53 to 63.53; ALT: MD ‐30.0 IU/L, 95% CI ‐132.12 to 72.12).
Prostaglandin E1 versus amrinone
Mortality
There was no mortality in the trial evaluating this comparison (Orii 2000).
Blood loss
There was no significant difference in the rate of blood loss between the groups (MD ‐0.03 litres, 95% CI ‐0.35 to 0.29).
Enzyme markers of liver injury
Analyses of the data provided showed no significant differences in AST levels between the groups on the first POD (MD ‐15.0 IU/L, 95% CI ‐158.34 to 128.34). Furthermore, the trial reported no significant differences between the groups of either the AST levels on the third POD, or ALT levels on the first or third PODs.
Prostaglandin E1 versus pentoxifylline
Mortality
The only trial to evaluate this comparison reported no significant difference in perioperative mortality (Stadheim 2000).
Liver decompensation
None of the trial participants developed post operative liver decompensation.
Enzyme markers of liver injury
The trial reported no significant differences in AST or ALT levels on the second, third, fourth, or sixth PODs.
Ulinastatin (protease inhibitor) versus gantaile
Mortality
There was no mortality in the groups in the trial evaluating this comparison (Li 2004).
Liver decompensation
There was no significant difference in the rate of liver decompensation between the groups (P = 0.33).
Postoperative morbidity
There were no significant differences in the rate of wound infection (P = 0.65) or intra‐abdominal infection (P > 0.99).
Hospital stay
The trial reported no significant differences between the groups in the length of hospital stay (MD ‐3.10 days, 95% CI ‐7.78 to 1.58).
Blood loss
There was no significant difference in the rate of blood loss (MD ‐0.05 litres, 95% CI ‐0.26 to 0.16) between the groups.
Markers of liver function
The trial reported significantly lower bilirubin levels on the third but not the first or seventh PODs in the ulinastatin compared to the gantaile group.
Enzyme markers of liver injury
The trial reported significantly lower AST levels on the third but not the first or seventh PODs in the ulinastatin compared to the gantaile group. The ALT levels were statistically significantly lower on the first, third, and seventh PODs in the ulinastatin compared to the control group.
Sevoflurane versus propofol
Mortality
There was one death in the sevoflurane group and one death in the propofol group. Both deaths were due to sepsis. The difference in the mortality between the two groups did not amount to statistical significance (P > 0.99).
Liver decompensation
There was no liver decompensation in the groups in the trial evaluating this comparison (Beck‐Shimmer 2008).
Postoperative morbidity
There was no significant difference in the rate of postoperative intra‐abdominal infections (P = 0.24), bile leak (P > 0.99), or bleeding (P = 0.49) between the two groups.
ITU and hospital stay
There was no significant difference in the length of ITU (MD ‐5.00 days, 95% CI ‐13.08 to 3.08) or hospital (MD ‐1.86 days, 95% CI ‐5.20 to 1.48) stay between the groups.
Markers of liver function
There was no significant difference in the peak postoperative bilirubin levels between the groups (MD ‐10.80 micromoles/L, 95% CI ‐34.34 to 12.74).
Enzyme markers of liver injury
There was no significant difference in the peak postoperative AST levels (MD ‐225.82 IU/L, 95% CI ‐463.88 to 12.24). The peak postoperative ALT levels were significantly lower in the sevoflurane group (MD ‐254.18 IU/L, 95% CI ‐450.59 to ‐57.77).
Discussion
In this review we included all the trials that fulfilled the inclusion criteria regardless of the pharmacological drug tested. This resulted in identification of nine different drugs in five different comparisons. The primary outcomes for analysis in this review were postoperative mortality and liver decompensation or failure. The remaining outcomes were secondary outcomes.
Dopexamine versus dopamine
Dopexamine and dopamine are catecholamines that act as vasodilator drugs, improving liver blood flow (Kullmann 1983; Lokhandwala 1992) and have been used with an intention of improving liver function following hemi‐hepatectomies (Kinoshita 1989).
There were no significant differences between the groups regarding the primary outcomes. There were no significant differences between the groups in the postoperative levels of liver enzymes. The prothrombin time showed a significant improvement in the dopamine group as compared with the dopexamine group. This isolated difference in the prothrombin time was hypothetically explained by the authors as a sign of a more marked activation of the haemostasis system in the dopexamine group leading to consumption of clotting factors in this group. The doses used for both of the study medication were considered equipotent (Marx 2000) and there were no reported adverse events at these doses.
Based on these results there is no evidence of a superior protective effect of dopexamine over dopamine or vice versa in elective liver resections with vascular occlusion. Further trials comparing dopamine versus placebo and dopexamine versus placebo are warranted. Such trials are lacking (Abu‐Amara 2009).
Prostaglandin E1 versus amrinone
Prostaglandin E1 is an eicosanoid that acts through various pathways to ameliorate ischaemia reperfusion injury. Prostaglandin E1 activates potassium ATP channels, inhibits cellular calcium influx, and attenuates neutrophil infiltration into tissues subjected to ischaemia reperfusion injury (Schror 1988; Hide 1995; Yamamoto 1999). Amrinone is a selective phosphodiesterase III inhibitor leading to increased cyclic adenosine 5'‐monophosphate (cAMP) levels.
There were no reported mortalities in either group. Furthermore, there were no significant differences in the postoperative liver enzyme levels or rate of blood loss between the groups. All the participants in this trial had cirrhotic livers and underwent minor liver resection. The amrinone dose used was very low, less than half the dose compared to that used in heart failure patients (Goenen 1985). The prostaglandin E1 dose was chosen based on the dose used in other trials (Sugawara 1998). None of the groups reported any adverse events.
Based on these results there is no evidence of a superior protective effect of amrinone over prostaglandin E1 or vice versa in cirrhotic patients undergoing elective liver resections performed under vascular occlusion.
Prostaglandin E1 versus pentoxifylline
Pentoxifylline inhibits TNF‐α gene transcription leading to a reduction in hepatocyte apoptosis following ischaemia reperfusion (Rüdiger 2002; El‐Ghoneimi 2007).
There was no difference in the perioperative mortality or liver enzymes between the groups. There were no liver decompensations postoperatively. The trial drugs were administered pre and postoperatively. Fourty‐six per cent of participants in the pentoxifylline group developed nausea or vomiting, or both, and failed to receive all the doses of the study medication.
Based on these results there is no evidence of a superior protective effect of using pentoxifylline over prostaglandin E1 or vice versa in elective resections performed under vascular occlusion. We lack trials showing that prostaglandin E1 or pentoxifylline is better than placebo (Abu‐Amara 2009).
Ulinastatin (protease inhibitor) versus gantaile
Ulinastatin is a protease inhibitor that can also inhibit the adhesion of white blood cells and release of cytokines and has been shown to protect from ischaemia reperfusion injury in experimental studies (Okuhama 1999). Gantaile is a Chinese herbal medicine described as a 'liver protectant' that is commonly used in chronic liver disease. However, systematic reviews have not found any beneficial effects of gantaile in these conditions (Liu 2001a; Liu 2001b).
There was no reported mortality in this clinical trial. There were no significant differences in the proportion of patients with liver decompensation, postoperative morbidity or in the length of hospital stay between the two groups. The AST and bilirubin levels were only transiently significantly lower in the ulinastatin group compared with controls on the third POD. However, the ALT levels were consistently lower postoperatively from the first to the seventh POD in the ulinastatin group. The authors of this trial did not allude as to how they chose the time or dose of ulinastatin administration. There were no reported adverse events of the study medication.
Based on these results, it seems ulinastatin may offer a potential protective role over and above the effects of gantaile in elective liver resections performed under vascular occlusion. Further trials of good methodological quality are required of ulinastatin versus placebo in order to assess the absolute effects of this agent in the setting of liver ischaemia reperfusion injury. Such trials are lacking (Abu‐Amara 2009).
Sevoflurane versus propofol
Sevoflurane is a volatile anaesthetic agent typically used for maintenance of anaesthesia. Sevoflurane has been shown to reduce liver ischaemia reperfusion injury by various mechanism including improving hepatic blood flow, decreasing oxidative stress and inflammatory cytokine release, and increasing ATP content (Bedirli 2008). Propofol is an intravenous anaesthetic agent typically used during anaesthesia induction. Propofol attenuates liver ischaemia reperfusion injury by decreasing oxygen free radical release and subsequent reduction in apoptosis (Chan 2008; Wang 2008). Both agents are well tolerated and very commonly used in general anaesthesia. There is evidence that sevoflurane protects against liver ischaemia reperfusion injury in experimental studies (Schmidt 2007). There is also evidence that other volatile anaesthetic agents such as isoflurane posses cardioprotective properties in the setting of coronary artery bypass graft surgery (Belhomme 1999).
With the exception of a significant decrease in peak postoperative ALT levels in the sevoflurane group compared with the propofol group, none of the other primary or secondary outcomes of interest showed a significant difference between the groups.
Based on these results there is no evidence of a superior protective effect of sevoflurane over propofol or vice versa in elective liver resections with vascular occlusion. Further trials comparing different volatile anaesthetic agents versus controls are warranted to assess the absolute effects of these agents in the setting of liver ischaemia reperfusion injury. Such trials are lacking (Abu‐Amara 2009).
Authors' conclusions
Implications for practice.
Ulinastatin may have a protective effect against ischaemia reperfusion injury relative to gantaile in elective liver resections performed under vascular occlusion. We have no data supporting that either ulinastatin or gantaile offer any benefits compared with placebo. The absolute benefits of these agents remain unknown. None of the pharmacological agents can be recommended for routine clinical practice.
Implications for research.
Further trials are needed to clarify the role of ulinastatin in ischaemia reperfusion injury sustained during elective liver resections under vascular occlusion.
Considering that none of the agents have proven useful in decreasing ischaemia reperfusion injury, such trials should include a group of patients who do not receive any active intervention (a placebo) in order to determine the pharmacological agent's absolute effects in liver ischaemia reperfusion injury.
Development of composite outcomes may be useful for design of trials in liver resection surgery because of the low potential for the pharmacological interventions to decrease perioperative mortality.
The trials need to be conducted and reported according to the CONSORT Statement (www.consort‐statement.org) (Moher 2001).
Notes
The original protocol for this review encompassed all randomised trials that assessed one or more pharmacological interventions versus another pharmacological intervention or no pharmacological intervention (REF to the Protocol!). In order to reduce the length and improve the readability of the full review emanating from the protocol, two separate reviews are being published instead of one.
This review only evaluates pharmacological interventions versus other pharmacological interventions. The other review (Abu‐Amara 2009) evaluates only trials comparing pharmacological interventions versus no pharmacological intervention (placebo or control). These changes are based on the recommendations of CHBG Editors.
Acknowledgements
Yiping Wang for translation of the Chinese article. The CHBG for their assistance in the preparation of this review.
Peer Reviewers: Jesus Prieto, Spain; Vijay P Khatri, USA. Contact Editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategy
| Database | Period of Search | Search Strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | Issue 4, 2008 | (((ischaemia OR ischaemia OR ischemic OR ischaemic OR reperfusion) AND (injury OR injuries OR damage OR damages)) OR gabexate mesilate OR steroid OR steroids OR corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR allopurinol OR prostaglandin OR prostaglandins OR amrinone OR dopexamine OR antioxidant OR antioxidants OR prostanoid OR prostanoids OR thiol OR bucillamine OR n‐acetylcysteine) AND ((liver OR hepatic OR hepato) AND (resection OR segmentectomy) OR hepatectomy) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | Issue 4, 2008 | #1 (ischaemia OR ischaemia OR ischemic OR ischaemic OR reperfusion) AND (injury OR injuries OR damage OR damages) #2 MeSH descriptor Reperfusion Injury explode all trees #3 gabexate mesilate OR steroid OR steroids OR corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR allopurinol OR prostaglandin OR prostaglandins OR amrinone OR dopexamine OR antioxidant OR antioxidants OR prostanoid OR prostanoids OR thiol OR bucillamine OR n‐acetylcysteine #4 MeSH descriptor Glucocorticoids explode all trees #5 MeSH descriptor Allopurinol explode all trees #6 MeSH descriptor Prostaglandins explode all trees #7 MeSH descriptor Antioxidants explode all trees #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 liver OR hepatic OR hepato #10 MeSH descriptor Liver explode all trees #11 (#9 OR #10) #12 resection OR segmentectomy #13 (#11 AND #12) #14 hepatectomy#15MeSH descriptor Hepatectomy explode all trees#16(#13 OR #14 OR #15) #17 (#8 AND #16) |
| MEDLINE (Pubmed) | January 1951 to January 2009 | (((ischaemia OR ischaemia OR ischemic OR ischaemic OR reperfusion) AND (injury OR injuries OR damage OR damages)) OR "Reperfusion Injury"[Mesh] OR (gabexate mesilate OR steroid OR steroids OR corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR "Glucocorticoids"[Mesh] OR allopurinol OR "Allopurinol"[Mesh] OR prostaglandin OR prostaglandins OR "Prostaglandins"[Mesh] OR amrinone OR dopexamine OR antioxidant OR antioxidants OR "Antioxidants"[Mesh] OR prostanoid OR prostanoids OR thiol OR bucillamine OR n‐acetylcysteine)) AND (((liver OR hepatic OR hepato OR "liver"[MeSH]) AND (resection OR segmentectomy)) OR hepatectomy OR "hepatectomy"[MeSH]) AND (((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh])))) |
| EMBASE (OvidSP) | Janurary 1974 to January 2009 | 1 (ischaemia or ischaemia or ischemic or ischaemic or reperfusion).af. 2 (injury or injuries or damage or damages).af. 3 1 and 2 4 exp Reperfusion Injury/ 5 (gabexate mesilate or steroid or steroids or corticosteroid or corticosteroids or glucocorticoid or glucocorticoids or allopurinol or prostaglandin or prostaglandins or amrinone or dopexamine or antioxidant or antioxidants or prostanoid or prostanoids or thiol or bucillamine or n‐acetylcysteine).af. 6 exp Glucocorticoid/ 7 exp Allopurinol/ 8 exp Prostaglandin E1/ 9 exp Prostaglandin E2/ 10 exp Antioxidant/ 11 6 or 3 or 7 or 9 or 8 or 4 or 10 or 5 12 (liver or hepatic or hepato).af. 13 (resection or segmentectomy).af. 14 13 and 12 15 hepatectomy.af. 16 exp Liver Resection/ 17 16 or 15 or 14 18 11 and 17 19 exp CROSSOVER PROCEDURE/ 20 exp DOUBLE BLIND PROCEDURE/ 21 exp SINGLE BLIND PROCEDURE/ 22 exp RANDOMIZED CONTROLLED TRIAL/ 23 (((RANDOM* or FACTORIAL* or CROSSOVER* or CROSS) and OVER*) or PLACEBO* or (DOUBL* and BLIND*) or (SINGL* and BLIND*) or ASSIGN* or ALLOCAT* or VOLUNTEER*).af. 24 22 or 21 or 23 or 19 or 20 25 18 and 24 |
| Science Citation Index Expanded (http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame) | January 1945 to January 2009 | #1 TS=((ischaemia OR ischaemia OR ischemic OR ischaemic OR reperfusion) AND (injury OR injuries OR damage OR damages)) #2 TS=(gabexate mesilate OR steroid OR steroids OR corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR allopurinol OR prostaglandin OR prostaglandins OR amrinone OR dopexamine OR antioxidant OR antioxidants OR prostanoid OR prostanoids OR thiol OR bucillamine OR n‐acetylcysteine) #3 #2 OR #1 #4 TS=((liver OR hepatic OR hepato) AND (resection OR segmentectomy) OR hepatectomy) #5 TS=(random* OR blind* OR placebo* OR meta‐analysis) #6 #5 AND #4 AND #3 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beck‐Shimmer 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Switzerland.
Number randomised: initially 70, post randomisation drop outs 6, final participants number 64.
Mean age: 56 years.
Females: 29 (45.3%).
Cirrhotic livers: 0
Steatotic livers: 30 (46.9%). Inclusion criteria: Elective liver resection with inflow occlusion for at least 30 minutes. Exclusion criteria: 1. Age < 18 years old. 2. Liver cirrhosis. 3. Additional ablation therapies (cryosurgery or radiofrequency). 4. Living donors. 5. Liver resections without inflow occlusion. |
|
| Interventions | Participants were randomly assigned to two groups: Intervention group 1: during the 30 minutes before commencing liver vascular occlusion, propofol anaesthesia was stopped and replaced by sevoflurane. On commencing the vascular occlusion sevoflurane was stopped and propofol infusion was recommenced (n = 30). Intervention group 2: propofol infusion was continually administered (not stopped during the 30 minutes preceding induction of liver ischaemia) from the start to the end of the operation (n = 34). |
|
| Outcomes | The outcomes reported were mortality, liver decompensation, intra‐abdominal infections, bile leak, postoperative bleeding, ITU stay, hospital stay, markers of liver function (bilirubin), and enzyme markers of liver injury. | |
| Notes | We contacted the authors for information regarding blinding of participants and trial investigators, and post operative complications (questions sent January 2009; replies received February 2009). The authors provided this information. Reasons for drop‐outs: intervention group 1: delay of than 30 minutes between commencing sevoflurane anaesthesia and commencement of liver vascular occlusion (4); intervention group 2: liver vascular occlusion not required (2). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "The randomization sequence without any stratification was generated by computer and sealed". |
| Allocation concealment? | Unclear risk | Quote: "consecutively numbered envelopes provided concealment of random allocation". |
| Blinding? All outcomes | Low risk | Quote: "Each patient was operated under the supervision of 1 of 2 blinded, experienced hepatobiliary surgeons". "The patients were blinded" (author replies). "The anaesthetists who administered sevoflurane were not involved in further care of the patients including administration of anaesthesia" (author replies). |
| Incomplete outcome data addressed? outcomes 2‐4 weeks | High risk | Comment: post‐randomisation drop‐outs could be related to outcomes. |
| Free of selective reporting? | Low risk | Comment: important outcomes reported. |
| Free from baseline imbalance? | Low risk | Comment: no baseline imbalance in important characteristics. |
| Free from academic bias? | Low risk | Comment: no previous published studies of the same comparison by the trial authors. |
| Free from source of funding bias? | High risk | Quote: "The study was supported by Swiss National Science Foundation grant 3200B0‐109906 (to P.A.C.) and 3200B0‐109558 (to B.B.S.); Abbott AG, Baar Switzerland." |
Li 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: China.
Number randomised: initially 31, post randomisation drop outs 0, final number of study participants 31.
Mean age: 50.2 years.
Females: 5 (16.1%).
Cirrhotic livers: 27 (87.1%).
Steatotic livers: not stated. Inclusion criteria: 1. Elective hepatic resection. 2. Portal triad clamping. 3. Diagnosis of primary hepatocellular carcinoma. |
|
| Interventions | Participants were randomly assigned to two groups: Intervention group 1: administered 10000 IU of ulinastatin during the operation, and twice a day with combination of vitamin K1 and glucose for 5 consecutive days after the operation (n = 16). Intervention group 2: administered gantaile (a Chinese herbal medicine used as a 'common liver protectant') the dose for which was not given, vitamin K1, and glucose (n = 15). |
|
| Outcomes | The outcomes reported were mortality, liver decompensation, wound infection, intra‐abdominal infection, hospital stay, blood loss, markers of liver function, and enzyme markers of liver injury. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Quote: "sealed envelope". |
| Blinding? All outcomes | High risk | Comment: trial not double blinded. |
| Incomplete outcome data addressed? outcomes 2‐4 weeks | Low risk | Comment: no post randomisation drop‐outs. |
| Free of selective reporting? | Low risk | Comment: all important outcomes reported. |
| Free from baseline imbalance? | Low risk | Comment: no baseline imbalance in important characteristics. |
| Free from academic bias? | Low risk | Comment: no previous published studies of the same comparison by the trial authors. |
Marx 2000.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany.
Number randomised: initially 20, post randomisation drop outs 1, final participants number 19.
Mean age: 58 years.
Females: 8 (42.1%).
Cirrhotic livers: not stated.
Steatotic livers: not stated. Inclusion criteria: Hemihepatectomy. Exclusion criteria: 1. Congestive heart failure. 2. Valvular heart disease. 3. On beta blockers or mono‐oxidase blockers. 4. Liver dysfunction (AST > 80 IU/L, ALT > 80 IU/L). 5. Renal insufficiency (creatinine > 1.5 mg/dl). 6. American Society of Anesthesiologists (ASA) score III to V. |
|
| Interventions | Participants were randomly assigned to two groups: Intervention group 1: administered dopexamine at 0.5 mcg/kg/min (after induction of anaesthesia until 16 hours postoperatively) (n = 9). Intervention group 2: administered dopamine at 2.5 mcg/kg/min (after induction of anaesthesia until 16 hours postoperatively) (n = 10). |
|
| Outcomes | The outcomes reported were blood transfusion requirements, markers of liver function, and enzyme markers of liver injury. | |
| Notes | We contacted the authors for information regarding random sequence generation and allocation concealment (questions sent January 2008; replies received January 2008). The authors provided this information. Reason for drop‐out: intervention group 1: catheter in hepatic vein cut inadvertently (1). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "computer generated" (author replies). |
| Allocation concealment? | Unclear risk | Quote: "sealed envelope" (author replies). |
| Incomplete outcome data addressed? outcomes 2‐4 weeks | High risk | Comment: post‐randomisation drop‐outs could be related to outcomes. |
| Free of selective reporting? | High risk | Comment: important outcomes not reported. |
| Free from early stopping bias? | Low risk | Comment: sample size calculation provided and trial not stopped early. |
| Free from academic bias? | Low risk | Comment: no previous published studies of the same comparison by the trial authors. |
Orii 2000.
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan.
Number randomised: initially 45, post randomisation drop outs 0, final participants number 45.
Mean age: 66 years.
Females: 8 (17.8%).
Cirrhotic livers: 45 (100%).
Steatotic livers: not stated. Inclusion criteria: 1. Liver resection. 2. Bilirubin < 2 mg/dl. 3. Indocyanin green (ICG) elimination rate > 0.06 U/min. 4. Cirrhotics with hepatocellular carcinoma. 5. Tumour limited to one segment. 6. Pringle's manoeuvre used to induce liver ischaemia. Exclusion criteria: 1. Ascites. 2. ASA score III to V. |
|
| Interventions | Participants were randomly assigned to three groups: Intervention group 1: administered an intravenous infusion of amrinone at a rate of 4 mcg/kg/min through a central venous catheter. The infusion was initiated at the start and terminated at the end of surgery (n = 15). Intervention group 2: administered an intravenous infusion of PGE1 at a rate of 0.02 mcg/kg/min through a central venous catheter. The infusion was initiated at the start and terminated at the end of surgery (n = 15). |
|
| Outcomes | The outcomes reported were mortality, blood loss, and enzyme markers of liver injury. | |
| Notes | We contacted the authors for information regarding random sequence generation and allocation concealment (questions sent January 2008). There was no reply from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding? All outcomes | High risk | Comment: not a double blind trial. |
| Incomplete outcome data addressed? outcomes 2‐4 weeks | Low risk | Comment: no post randomisation drop‐outs. |
| Free of selective reporting? | High risk | Comment: not all important outcomes reported. |
| Free from academic bias? | Low risk | Comment: no previous published studies of the same comparison by the trial authors. |
| Free from source of funding bias? | Low risk | Quote: "This work was supported in part by a Grand‐in‐aid for Scientific Research from the Ministry of Education, Science and Culture of Japan". |
Stadheim 2000.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA.
Number randomised: initially not stated, post randomisation drop outs not stated, final participants number 23.
Mean age: not stated.
Females: not stated.
Cirrhotic livers: not stated.
Steatotic livers: not stated. Inclusion criteria: 1. Major liver resection. 2. ECOG (Eastern Cooperative Oncology Group) performance status (0 to 2). 3. Primary liver cancer or metastatic liver cancer confined to liver. |
|
| Interventions | Participants were randomly assigned to three groups: Intervention group 1: administered preoperative and postoperative misoprostol (a synthetic prostaglandin E1 analogue) (n = 13). Intervention group 2: administered preoperative and postoperative pentoxifylline (a xanthine derivative) (n = 10). |
|
| Outcomes | The outcomes reported were mortality, liver decompensation, and enzyme markers of liver injury. | |
| Notes | We contacted the authors for information regarding random sequence generation and allocation concealment (questions sent January 2008). There was no reply from the authors. Reasons for drop‐outs: liver vascular occlusion not required and inoperable tumour. The number of drop‐outs across each intervention group not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data addressed? outcomes 2‐4 weeks | High risk | Comment: post‐randomisation drop‐outs could be related to outcomes. |
| Free of selective reporting? | Low risk | Comment: all important outcomes reported. |
| Free from academic bias? | Low risk | Comment: no previous published studies of the same comparison by the trial authors. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aldrighetti 2006 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Baek 1999 | Not a randomised clinical trial. |
| Bartels 2004 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Cerwenka 1998 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Cerwenka 1999 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Dunschede 2006 | Quasi‐randomised trial (allocation by alternation). This trial did not report on adverse effects due to the pharmacological intervention. |
| Garcia 1994 | Not a randomised clinical trial. |
| Hanazaki 2000 | Not a randomised clinical trial. |
| Hassanain 2008 | A case report and the randomised clinical trial to which it refers has not been published and the majority of the participants had hepatectomy without liver blood supply occlusion. |
| Hayakawa 1994 | Not a randomised clinical trial. |
| Inagaki 1999 | Hepatectomy performed without liver blood supply occlusion. |
| Inagaki Kurokaw 1999 | Not a randomised clinical trial. |
| Kaiho 2008 | Not a randomised clinical trial. |
| Katsuramaki 1996 | Not a randomised clinical trial. |
| Kawano 2005 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Kim 2002 | The authors state that randomisation was achieved by randomly allocating one patient to one group and matched patients to the other groups. We do not consider this as a randomised or quasi‐randomised trial. |
| Kim 2006 | The authors state that randomisation was achieved by randomly allocating one patient to one group and matched patients to the other groups. We do not consider this as a randomised or quasi‐randomised trial. |
| Kim 2007 | The authors state that randomisation was achieved by randomly allocating one patient to one group and matched patients to the other groups. We do not consider this as a randomised or quasi‐randomised trial. |
| Kostopanagiotou 2006 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Muratore 2003 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Nakagawa 1991 | Not a randomised clinical trial. |
| Nakayama 1995 | Hepatectomy performed without liver blood supply occlusion. |
| Schemmer 2008 | Protocol of a trial. No results available. |
| Schmidt 2007 | Hepatectomy performed without liver blood supply occlusion. |
| Settaf 2001 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Sheth 2005 | Hepatectomy performed without liver blood supply occlusion. |
| Shimada 1994 | Hepatectomy performed without liver blood supply occlusion. |
| Shimada 1996 | Not a randomised clinical trial; hepatectomy performed without blood supply occlusion. |
| Shirabe 1996 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Suehiro 2008 | Not a randomised clinical trial. |
| Sugawara 1998 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Tang 2007 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Une 1995 | Decision to administer intervention drug was made by physicians; hepatectomy performed without liver blood supply occlusion. |
| Vriens 2002 | This trial compared a pharmacological agent versus no pharmacological agent. |
| Wada 1995 | Hepatectomy performed without liver blood supply occlusion. |
| Yamashita 2001 | This trial compared a pharmacological agent versus no pharmacological agent. |
Differences between protocol and review
In the Background section, the number of examples of pharmacological agents that were discussed in the protocol have been reduced, and those that remained have been placed under the appropriate comparison in the discussion. This was done to improve the readability and consistency of the review as too many examples were included in the protocol.
In the Types of interventions section of the methods, only pharmacological interventions versus other pharmacological interventions were included in the review. Pharmacological interventions versus no pharmacological interventions are assessed in a separate review (Abu‐Amara 2009). This was done to reduce the length and improve the readability of the review based on the recommendations of CHBG Editors.
The outcomes were reordered to give more importance to clinically relevant outcomes.
The 'Risk of bias' assessment has been modified in line with The CHBG module (Gluud 2009; Gurusamy 2009d).
In the protocol we stated that we would calculate the risk ratio with 95% confidence intervals for dichotomous outcomes. However, there was only one trial included under each comparison and this is why we performed Fisher's exact test for the dichotomous outcomes.
Contributions of authors
M Abu‐Amara wrote the review after identifying the trials, extracting data, and performing the statistical analysis. K Gurusamy independently identified trials, extracted data from the trials, and helped in the preparation of the manuscript. G Glantzounis, B Fuller, and BR Davidson critically commented on the review and made suggestions to improve the review.
Sources of support
Internal sources
none, Not specified.
External sources
none, Not specified.
Declarations of interest
None.
New
References
References to studies included in this review
Beck‐Shimmer 2008 {published data only}
- Beck‐Shimmer B, Breitenstein S, Urech S, Conno E, Wittlinger M, Puhan M, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Annals of Surgery 2008;248:909‐18. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li SQ, Liang LJ. [Protection of liver function with protease inhibitor from ischemia‐reperfusion injury in hepatocellular carcinoma patients undergoing hepatectomy after hepatic inflow occlusion]. Chinese Journal of Bases and Clinics in General Surgery 2004;11(1):61‐4. [Google Scholar]
Marx 2000 {published data only}
- Holtje M, Mahr KH, Bornscheuer A, Marx G, Stamme C, Rueckoldt H, et al. [Circulatory function and oxygenation during hemihepatectomy. Dopamine versus dopexamine]. Der Anaesthesist 1999;48(4):224‐30. [DOI] [PubMed] [Google Scholar]
- Marx G, Leuwer M, Holtje M, Bornscheuer A, Herrmann H, Mahr KH, et al. Low‐dose dopexamine in patients undergoing hemihepatectomy: an evaluation of effects on reduction of hepatic dysfunction and ischaemic liver injury. Acta Anaesthesiologica Scandinavica 2000;44(4):410‐16. [DOI] [PubMed] [Google Scholar]
Orii 2000 {published data only}
- Orii R, Sugawara Y, Hayashida M, Yamada Y, Chang K, Takayama T, et al. Effects of amrinone on ischaemia‐reperfusion injury in cirrhotic patients undergoing hepatectomy: A comparative study with prostaglandin E1. British Journal of Anaesthesia 2000;85(3):389‐95. [DOI] [PubMed] [Google Scholar]
Stadheim 2000 {published data only}
- Stadheim LM, Gores GJ, Lee TC, Nagorney DM. Normothermic ischemia‐reperfusion injury during hepatic resection: A prospective randomized placebo controlled study of pretreatment with misoprostol or pentoxifylline. Hepatology 2000;32(4):250A. [Google Scholar]
References to studies excluded from this review
Aldrighetti 2006 {published data only}
- Aldrighetti L, Pulitano C, Arru M, Finazzi R, Catena M, Soldini L, et al. Impact of preoperative steroids administration on ischemia‐reperfusion injury and systemic responses in liver surgery: A prospective randomized study. Liver Transplantation 2006;12(6):941‐9. [DOI] [PubMed] [Google Scholar]
- Finnazi R, Pulitano C, Aldrighetti L, Arru M, Catena M, Milani F, et al. Benefit of preoperative corticosteroid therapy on the hepatic ischaemia‐reperfusion injury and cytokine response in patients undergoing hepatic resection: a prospective randomized trial. Journal of Hepatolology 2005;42(Suppl 2):49. [Google Scholar]
- Pulitano C, Aldrighetti L, Arru M, Finazzi R, Catena M, Guzzetti E, et al. Preoperative methylprednisolone administration maintains coagulation homeostasis in patients undergoing liver resection: importance of inflammatory cytokine modulation. Shock 2007;28:401‐5. [DOI] [PubMed] [Google Scholar]
- Pulitanò C, Aldrighetti L, Arru M, Finazzi R, Soldini L, Catena M, et al. Prospective randomized study of the benefits of preoperative corticosteroid administration on hepatic ischemia‐reperfusion injury and cytokine response in patients undergoing hepatic resection. HPB 2007;9(3):183‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulitanò C, Aldrighetti L, Finazzi R, Arru M, Catena M, Ferla G. Inihibition of cytokine response by methylprednisolone attenuates antithrombin reduction following hepatic resection. Thrombosis and Haemostasis 2005;93(6):1199‐200. [DOI] [PubMed] [Google Scholar]
Baek 1999 {published data only}
- Baek Y, Nakano H, Kumada K, Nagasaki H, Kigawa G, Sasaki J, et al. Administration of prostaglandin E1 reduces post‐operative hepatocellular damage and restores hepatic integrity in patients undergoing hepatectomy. Hepato‐Gastroenterology 1999;46(27):1836‐41. [PubMed] [Google Scholar]
Bartels 2004 {published data only}
- Bartels M, Biesatski HK, Engelhart K, Sendlhofer G, Rehak P, Nagel E. Pilot study on the effect of parenteral vitamin E on ischemia and reperfusion induced liver injury: a double blind, randomized, placebo‐controlled trial. Clinical Nutrition 2004;23(6):1360‐70. [DOI] [PubMed] [Google Scholar]
Cerwenka 1998 {published data only}
- Cerwenka H, Bacher H, Werkgartner G, El‐Shabrawi A, Quehenberger F, Hauser H, et al. Antioxidant treatment during liver resection for alleviation of ischemia‐reperfusion injury. Hepato‐Gastroenterology 1998;45(21):777‐82. [PubMed] [Google Scholar]
Cerwenka 1999 {published data only}
- Cerwenka H, Khoschsorur G, Bacher H, Werkgartner G, El‐Shabrawi A, Quehenberger F, et al. Normothermic liver ischemia and antioxidant treatment during hepatic resections. Free Radical Research 1999;30(6):463‐9. [DOI] [PubMed] [Google Scholar]
Dunschede 2006 {published data only}
- Dunschede F, Erbes K, Kircher A, Westermann S, Seifert J, Schad A, et al. Reduction of ischemia reperfusion injury after liver resection and hepatic inflow occlusion by (alpha)‐lipoic acid in humans. World Journal of Gastroenterology 2006;12(42):6812‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 1994 {published data only}
- Garcia‐Valdecasas JC, Rull R, Rimola A, Grande L, Beltran J, Angas J, et al. The role of systemic prostaglandins during human liver transplantation. Annals of the New York Academy of Sciences 1994;723:473‐5. [PubMed] [Google Scholar]
Hanazaki 2000 {published data only}
- Hanazaki K, Kajikawa S, Fujimori Y, Nakata S, Shimozawa N, Koide N, et al. Effects of prostaglandin E1 administration during hepatectomy for cirrhotic hepatocellular carcinoma. Hepato‐Gastroenterology 2000;47(32):461‐4. [PubMed] [Google Scholar]
Hassanain 2008 {published data only}
- Hassanain M, Schricker T, Metrakos P, Carvalho G, Vrochides D, Lettermann R. Hepatic protection by perioperative metabolic support?. Nutrition 2008;24:1217‐9. [DOI] [PubMed] [Google Scholar]
Hayakawa 1994 {published data only}
- Hayakawa J, Yoshida G, Usuda Y. Effect of prostaglandin E1 on arterial ketone body ratio in hepatectomy. Journal of Anesthesia 1994;8(2):167‐71. [DOI] [PubMed] [Google Scholar]
Inagaki 1999 {published data only}
- Inagaki H, Nonami T, Kurokawa T, Takeuchi Y, Okuda N, Nakao A, et al. Effects of nafamostat mesilate, a synthetic protease inhibitor, on immunity and coagulation after hepatic resection. Hepato‐Gastroenterology 1999;46(30):3223‐8. [PubMed] [Google Scholar]
Inagaki Kurokaw 1999 {published data only}
- Inagaki H, Kurokawa T, Nonami T, Miwa T, Nakao A, Takagi H. The effect of intraportal administration of prostaglandin E1 on liver blood flow and liver function. Hepato‐Gastroenterology 1999;46(29):2909‐13. [PubMed] [Google Scholar]
Kaiho 2008 {published data only}
- Kaiho T, Tsuchiya S, Yanagisawa S, Takeuchi O, Togawa A, Okamoto R, et al. Effect of the herbal medicine Inchin‐Ko‐To for serum bilirubin in hepatectomized patients. Hepato‐Gastroenterology 2008;55:150‐4. [PubMed] [Google Scholar]
Katsuramaki 1996 {published data only}
- Katsuramaki T, Mukaiya M, Yamashiro K, Kimura H, Denno R, Hirata K. Beneficial effects of administering intraportal prostaglandin E1 postoperatively to hepatectomy patients with massive intraoperative blood loss. Surgery Today 1996;26:895‐9. [DOI] [PubMed] [Google Scholar]
Kawano 2005 {published data only}
- Kawano T, Hosokawa N, Maruta T, Maruta N, Takasaki M. Reevaluation of protective effects of alprostadil on hepatic function in patients undergoing hepatectomy. Masui. The Japanese Journal of Anesthesiology 2005;54(9):982‐91. [PubMed] [Google Scholar]
Kim 2002 {published data only}
- Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. Hepatocyte protection by a protease inhibitor against ischemia/reperfusion injury of human liver. Journal of the American College of Surgeons 2002;195(1):41‐50. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim YI, Chung HJ, Song KE, Hwang YJ, Lee JW, Lee YJ, et al. Evaluation of a protease inhibitor in the prevention of ischemia and reperfusion injury in hepatectomy under intermittent Pringle maneuver. American Journal of Surgery 2006;191(1):72‐6. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim YI, Fujita S, Hwang YJ, Chun JM, Song KE, Chun BY. Successful intermittent application of the Pringle maneuver for 30 minutes during human hepatectomy: a clinical randomized study with use of a protease inhibitor. Hepato‐Gastroenterology 2007;54:2055‐60. [PubMed] [Google Scholar]
Kostopanagiotou 2006 {published data only}
- Kostopanagiotou G, Pandazi AK, Andreadou I, Markantonis SL, Niokou D, Teloudis A, et al. Effects of mannitol in the prevention of lipid peroxidation during liver resection with hepatic vascular exclusion. Journal of Clinical Anesthesia 2006;18(8):570‐4. [DOI] [PubMed] [Google Scholar]
Muratore 2003 {published data only}
- Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. The British Journal of Surgery 2003;90(1):17‐22. [DOI] [PubMed] [Google Scholar]
Nakagawa 1991 {published data only}
- Nakagawa I, Izum H, Fujii K, Kurokawa H, Kusunoki S, Yuge O. Comparison of the effect of PGE1 and dopamine on arterial blood ketone body ratio in hepatectomized patients. Hiroshima Journal of Anesthesia 1991;27(4):367‐71. [Google Scholar]
Nakayama 1995 {published data only}
- Nakayama M, Kanaya N, Fujita S, Kawana S, Namiki A. [Effect of prostaglandin E1 on arterial ketone body ratio during and after hepatectomy]. Masui. The Japanese Journal of Anesthesiology 1995;44(9):1224‐7. [PubMed] [Google Scholar]
Schemmer 2008 {published data only}
- Schemmer P, Nickkholgh A, Schneider H, Sobirey M, Weigand M, Koch M, et al. PORTAL: Pilot study on the safety and tolerance of preoperative melatonin application in patients undergoing major liver resection: A double‐blind randomized placebo‐controlled trial. BMC Surgery 2008;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 2007 {published data only}
- Schmidt SC, Hamann S, Langrehr JM, Hoflich C, Mittler J, Jacob D, et al. Preoperative high‐dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. Journal of Hepato‐Biliary‐Pancreatic Surgery 2007;14:484‐92. [DOI] [PubMed] [Google Scholar]
Settaf 2001 {published data only}
- Settaf A, Zaim N, Bellouch M, Tillement JP, Morin D. Trimetazidine alleviates ischemia‐reperfusion injury induced by vascular clamping of the liver. Therapie 2001;56(5):569‐74. [PubMed] [Google Scholar]
Sheth 2005 {published data only}
- Sheth H, Glantzounis G, Hafez T, Quaglia A, Duncan J, Davidson BR. A randomised double‐blind controlled clinical trial to assess the effects of prophylactic N‐acetylcysteine on liver injury during liver resection. The British Journal of Surgery 2005;92(10):1307. [Google Scholar]
- Sheth H, Glantzounis G, Hafez T, Quaglia A, Duncan J, Davidson BR. Does perioperative N‐aceylcysteine prevent ischaemia reperfusion injury during liver resection?: A prospectively randomised double blind clinical trial. Gut 2005;54:A39. [Google Scholar]
Shimada 1994 {published data only}
- Shimada M, Matsumata T, Taketomi A, Shirabe K, Yamamoto K, Sugimachi K. The role of prostaglandins in hepatic resection. Prostaglandins Leukotrienes and Essential Fatty Acids 1994;50(2):65‐8. [DOI] [PubMed] [Google Scholar]
Shimada 1996 {published data only}
- Shimada M, Saitoh A, Kano T, Takenaka K, Sugimachi K. The effect of a perioperative steroid pulse on surgical stress in hepatic resection. International Surgery 1996;81(1):49‐51. [PubMed] [Google Scholar]
Shirabe 1996 {published data only}
- Shirabe K, Takenaka K, Yamamoto K, Kitamura M, Itasaka H, Matsumata T, et al. The role of prostanoid in hepatic damage during hepatectomy. Hepato‐Gastroenterology 1996;43(9):596‐601. [PubMed] [Google Scholar]
Suehiro 2008 {published data only}
- Suehiro T, Shimura T, Okamura K, Okada T, Okada K, Hashimoto S, et al. The effect of hyperbaric oxygen treatment on postoperative morbidity of left lobe donor in living donor adult liver transplantation. Hepato‐Gastroenterology 2008;55:1014‐9. [PubMed] [Google Scholar]
Sugawara 1998 {published data only}
- Sugawara Y, Kubota K, Ogura T, Esumi H, Inoue K, Takayama T, et al. Protective effect of prostaglandin E1 against ischemia/reperfusion‐induced liver injury: results of a prospective, randomized study in cirrhotic patients undergoing subsegmentectomy. Journal of Hepatology 1998;29(6):969‐76. [DOI] [PubMed] [Google Scholar]
Tang 2007 {published data only}
- Tang L, Tian F, Tao W, Cui J. Hepatocellular glycogen in alleviation of liver ischemia‐reperfusion injury during partial hepatectomy. World Journal of Surgery 2007;31(10):2039‐43. [DOI] [PubMed] [Google Scholar]
- Tang LJ, Tian FZ, Gao XM. Hepatocellular glycogen in alleviation of liver ischemia reperfusion injury. Hepatobiliary & Pancreatic Diseases International 2002;1(4):532‐5. [PubMed] [Google Scholar]
Une 1995 {published data only}
- Une Y, Shimamura T, Uchino J. Effect of prostaglandin E(1) on hepatic function after hepatectomy in patients with chronic liver disease. Clinical Therapeutics 1995;17(6):1118‐25. [DOI] [PubMed] [Google Scholar]
Vriens 2002 {published data only}
- Vriens MR, Marinelli A, Harinck HIJ, Zwinderman KH, Velde CJH. The role of allopurinol in human liver ischemia/reperfusion injury: A prospective randomized clinical trial. Hepato‐Gastroenterology 2002;49(46):1069‐73. [PubMed] [Google Scholar]
Wada 1995 {published data only}
- Wada Y, Zaima M, Mori K, Egawa H, Higashiyama H, Iwata S, et al. Effect of gabexate mesilate on thrombin and plasmin generation after hepatic resection in cirrhotic patients. European Surgical Research 1995;27(1):57‐62. [DOI] [PubMed] [Google Scholar]
Yamashita 2001 {published data only}
- Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, et al. Effects of preoperative steroid administration on surgical stress in hepatic resection ‐ Prospective randomised trial. Archives of Surgery 2001;136(3):328‐33. [DOI] [PubMed] [Google Scholar]
Additional references
Abu‐Amara 2009
- Abu‐Amara M, Gurusamy KS, Hori S, Glantzounis G, Fuller B, Davidson BR. Pharmacological interventions versus no pharmacological interventions for ischaemia reperfusion injury in liver resection surgery performed under vascular control. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007472] [DOI] [PubMed] [Google Scholar]
Azoulay 2005
- Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Annals of Surgery 2005;241(2):277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azoulay 2006
- Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. Journal of the American College of Surgeons 2006;202(2):203‐11. [DOI] [PubMed] [Google Scholar]
Bedirli 2008
- Bedirli N, Ofluoglu E, Kerem M, Utebey G, Alper M, Yilmazer D, et al. Hepatic energy metabolism and the differential protective effects of sevoflurane and isoflurane anesthesia in a rat hepatic ischemia‐reperfusion injury model. Anesthesia and Analgesia 2008;106:830‐7. [DOI] [PubMed] [Google Scholar]
Belghiti 1993
- Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Annals of Surgery 1993;218(6):748‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belhomme 1999
- Belhomme D, Peynet J, Louzy M, Launay JM, Kitakaze M, Menasché P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation 1999;100(Suppl 19):II340‐4. [DOI] [PubMed] [Google Scholar]
Chan 2008
- Chan KC, Lin CJ, Lee PH, Chen CF, Lai YL, Sun WZ, et al. Propofol attenuates the decrease of dynamic compliance and water content in the lung by decreasing oxidative radicals released from the reperfused liver. Anesthesia and Analgesia 2008;107:1284‐9. [DOI] [PubMed] [Google Scholar]
Chouker 2005
- Chouker A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, et al. Beneficial effects of ischemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Archives of Surgery 2005;140(2):129‐36. [DOI] [PubMed] [Google Scholar]
Couinaud 1999
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Digestive Surgery 1999;16(6):459‐67. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test.. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Ghoneimi 2007
- El‐Ghoneimi A, Cursio R, Schmid‐Alliana A, Tovey M, Lasfar A, Michiels JF, et al. Pentoxifylline inhibits liver expression of tumor necrosis factor alpha mRNA following normothermic ischemia‐reperfusion. HPB 2007;9:112‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galaris 2006
- Galaris D, Barbouti A, Korantzopoulos P. Oxidative stress in hepatic ischemia‐reperfusion injury: the role of antioxidants and iron chelating compounds. Current Pharmaceutical Design 2006;12(23):2875‐90. [DOI] [PubMed] [Google Scholar]
Georgiev 2006
- Georgiev P, Dahm F, Graf R, Clavien PA. Blocking the path to death: anti‐apoptotic molecules in ischemia/reperfusion injury of the liver. Current Pharmaceutical Design 2006;12(23):2911‐21. [DOI] [PubMed] [Google Scholar]
Gluud 2009
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2009, Issue 3. Art. No.: LIVER.
Goenen 1985
- Goenen M, Pedemonte O, Baele P, Col J. Amrinone in the management of low cardiac output after open heart surgery. American Journal of Cardiology 1985;56:33B‐38B. [DOI] [PubMed] [Google Scholar]
Gurusamy 2009a
- Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006409.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009b
- Gurusamy KS, Kumar Y, Ramamoorthy R, Sharma D, Davidson BR. Vascular occlusion for elective liver resections. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007530] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009c
- Gurusamy KS, Kumar Y, Pamecha V, Sharma D, Davidson BR. Ischaemic pre‐conditioning for elective liver resections performed under vascular occlusion. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007629] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009d
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. The British Journal of Surgery 2009;96(4):342‐9. [PUBMED: 19283747] [DOI] [PubMed] [Google Scholar]
HES 2005
- Hospital Episode Statistics. Main operations. 3 character: 2004‐05. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=205 (accessed 11 August 2009).
Hide 1995
- Hide EJ, Ney P, Piper J, Thiemermann C, Vane JR. Reduction by prostaglandin E1 or prostaglandin E0 of myocardial infarct size in the rabbit by activation of ATP‐sensitive potassium channels. British Journal of Pharmacology 1995;116:2435‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
Ibrahim 2006
- Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplantation 2006;12(6):950‐7. [DOI] [PubMed] [Google Scholar]
Kim 1996
- Kim YI, Hiratsuka K, Kitano S, Joo DH, Kamada N, Sugimachi K. Simple in situ hypothermia reduced ischaemic injury to human liver during hepatectomy. European Journal of Surgery 1996;162(9):717‐21. [PubMed] [Google Scholar]
Kinoshita 1989
- Kinoshita H, Yamazaki O, Sakai K, Hirohashi K, Kubo S, Iwasa R, et al. Measurement of portal blood flow in man by continuous local thermodilution method. III. Effects of dopamine on systemic and portal hemodynamics after hepatectomy. Nippon Geka Gakkai Zasshi 1989;90:556–65. [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Koti 2003
- Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Digestive Surgery 2003;20(5):383‐96. [DOI] [PubMed] [Google Scholar]
Kullmann 1983
- Kullmann R, Breull WR, Reinsberg J, Wassermann K, Konopatzki A. Dopamine produces vasodilation in specific regions and layers of the rabbit gastrointestinal tract. Life Sciences 1983;32:2115–22. [DOI] [PubMed] [Google Scholar]
Kupiec 2005
- Kupiec‐Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplantation Proceedings 2005;37(4):1653‐6. [DOI] [PubMed] [Google Scholar]
Liu 2001a
- Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for asymptomatic carriers of hepatitis B virus infection. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002231] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2001b
- Liu JP, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003183] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lokhandwala 1992
- Lokhandwala MF, Jandhyala BS. Effects of dopaminergic agonists on organ blood flow and function. Clinical Intensive Care. Clinical Intensive Care 1992;3 (Suppl.1):12–6. [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG (for the CONSORT Group). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Okuhama 1999
- Okuhama Y, Shiraishi M, Higa T, Tomori H, Taira K, Mamadi T, et al. Protective effects of ulinastatin against ischemia‐reperfusion injury. Journal of Surgical Research 1999;82(1):34‐42. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Rüdiger 2002
- Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 2002;122(1):202‐10. [DOI] [PubMed] [Google Scholar]
Schmidt 2007
- Schmidt R, Tritschler E, Hoetzel A, Loop T, Humar M, Halverscheid L, et al. Heme oxygenase‐1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Annals of Surgery 2007;245:931‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schror 1988
- Schror K, Thiemermann C, Ney P. Protection of the ischemic myocardium from reperfusion injury by prostaglandin E1 inhibition of ischemia‐induced neutrophil activation. Naunyn‐Schmiedeberg's Archives of Pharmacology 1988;338:268‐74. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shimada 1998
- Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. The British Journal of Surgery 1998;85(2):195‐8. [DOI] [PubMed] [Google Scholar]
Smyrniotis 2006
- Smyrniotis V, Theodoraki K, Arkadopoulos N, Fragulidis G, Condi‐Pafiti A, Plemenou‐Fragou M, et al. Ischemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. American Journal of Surgery 2006;192(5):669‐74. [DOI] [PubMed] [Google Scholar]
Strasberg 2000
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB Surgery 2000;2(3):333‐9. [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang H, Xue Z, Wang Q, Feng X, Shen Z. Propofol protects hepatic L02 cells from hydrogen peroxide‐induced apoptosis via activation of extracellular signal‐regulated kinases pathway. Anesthesia and Analgesia 2008;107:534‐40. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman GD, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamamoto 1999
- Yamamoto T, Habuchi Y, Tanaka H, Suto F, Morikawa J, Kashima K, et al. EP receptor‐mediated inhibition by prostaglandin E(1) of cardiac L‐type Ca(2+) current of rabbits. American Journal of Physiology 1999;277:H1369‐H74. [DOI] [PubMed] [Google Scholar]
Yoshimura 2004
- Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World Journal of Surgery 2004;28(10):982‐6. [DOI] [PubMed] [Google Scholar]


