Abstract
Purpose:
To use delayed enhancement cardiac magnetic resonance (DE-CMR) as a reference standard to evaluate the prevalence and predictors of right atrial (RA) thrombus.
Methods:
In this retrospective study, 130 cancer patients with central venous catheters undergoing CMR from August 2012–January 2018 were included. CMR (cine-CMR and DE-CMR) and echocardiography were interpreted for RA thrombus blinded to other imaging results and clinical data. RA thrombus properties including the number of discrete masses, size, total thrombus area, and perimeter were also assessed. Cine-CMR was also used to quantify cardiac structure and function as markers of RA thrombus. Student’s t-test was used to assess continuous variables; chi-square or Fisher’s exact test were used to assess categorical variables.
Results:
31/130 (24%) patients had RA thrombus on DE-CMR. Echocardiography (attained in 64% of the study population) demonstrated moderate sensitivity and specificity (75%, 90% respectively) in relation to DE-CMR; cine-CMR performance was higher (sensitivity 90%, specificity 98%). Patients with and without RA thrombus had similar right-sided structure/function and cancer diagnosis. Catheter depth approached significance in patients with RA thrombus (p = 0.05). 13% of patients with RA thrombus had concomitant pulmonary embolism within 60 days of CMR vs. 2% of patients without RA thrombus (p = 0.03). Embolic events were independent of RA thrombus size (p = 0.66).
Conclusion:
Morphologic imaging by cine-CMR and echocardiography provide limited diagnostic utility for RA thrombus as established by DE-CMR tissue characterization. Catheter-associated RA thrombus occurs independently of right-sided structure or function and is associated with clinical embolic events and catheter depth.
Keywords: Thrombus, Cardiovascular magnetic resonance, Echocardiography, central venous catheter
1. Introduction
Right atrial (RA) thrombus is an important diagnostic consideration as it provides a substrate for clinical embolic events and/or impeded right heart function, thus warranting anticoagulation therapy [1]. While cancer patients are at increased risk for RA thrombus, given that neoplastic conditions are predisposed to hypercoagulability [2], they can also be at risk for RA thrombus due to localized mechanical processes associated with the use of central venous catheters. Central venous catheters provide a nidus for thrombosis, possibly due to catheterrelated tissue properties or catheter-induced alterations in right atrial geometry/function.
Because RA thrombus impacts therapeutic decision-making and clinical risk, it is important to determine the optimal imaging strategy to detect RA thrombus. In routine clinical practice, echocardiography is often employed as a screening test for RA thrombus [3] because it is widely available and inexpensive [4,5]. Echocardiography typically identifies RA thrombus based on morphology – an approach which can be potentially misleading because neoplasms or normal anatomic structures can appear as discrete tissue prominences of similar morphology to thrombus [3,5–7]. On the other hand, cardiac magnetic resonance (CMR) enables masses to be assessed based on tissue properties rather than morphologic appearance.
Tissue characterization via delayed-enhancement CMR (DE-CMR) enables a thrombus to be discerned based on avascular composition, an intrinsic feature irrespective of shape or location [8]. DE-CMR has been shown to improve left ventricular (LV) thrombus detection vs. echocardiography and has been validated in studies in relation to histopathology as well as clinical embolic events [3–5,9–12]. DE-CMR has also been applied to assess left atrial thrombus, yielding a high diagnostic accuracy [13,14]. Despite a growing body of literature validating DE-CMR as a reference standard for thrombus, it has yet to be used to assess RA thrombus in an at-risk population.
In this study, we employed DE-CMR as a reference standard to evaluate the prevalence and predictors of RA thrombus in cancer patients with central venous catheters. Our aims were to: (1) compare imaging techniques that identify thrombus based on anatomic appearance (echocardiography, cine-CMR) with DE-CMR tissue characterization;(2) identify functional/structural predictors of catheter-associated RA thrombus; and (3) assess the relative risk of clinical embolic events among patients with and without RA thrombus.
2. Methods
2.1. Study population
This retrospective study was approved by the institutional review board; informed consent was waived for the review of pre-existing imaging and clinical data. The population comprised 130 consecutive cancer patients with central venous catheters who underwent clinically indicated CMR with contrast at Memorial Sloan Kettering Cancer Center between August 2012 and January 2018. All consecutive patients were included in the study with demographics demonstrated in Table 1. Patient characteristics collected from the medical record included the interval between the CMR and catheter placement, catheter features (type, lumen #), cancer diagnosis, and cancer treatment modality.Medical records were also reviewed (blinded to imaging results) for pulmonary embolism within 60 days after the CMR.
Table 1.
Population characteristics.
| Overall (n = 130) | RA thrombus + (n = 31) | RA thrombus − (n =99) | P-value | |
|---|---|---|---|---|
| Age (years) | 50 ± 18 | 46 ± 15 | 51 ± 19 | 0.19 |
| Male gender | 49% (63) | 52% (16) | 48% (47) | 0.69 |
| Anthropomorphic features | ||||
| Height (m) | 1.7 ± 0.2 | 1.67 ± 0.2 | 1.64 ± 0.2 | 0.63 |
| Weight (kg) | 75.5 ± 25.8 | 82.3 ± 27.5 | 73.3 ± 25.0 | 0.09 |
| Body surface area (m2) | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.3 | 0.09 |
| Cancer diagnosisa | ||||
| Hematologic | 25% (33) | 39% (12) | 21% (21) | 0.05 |
| Gastrointestinal | 19% (25) | 16% (5) | 20% (20) | 0.62 |
| Sarcoma | 19% (24) | 10% (3) | 21% (21) | 0.15 |
| Breast | 12% (16) | 10% (3) | 13% (13) | 0.76 |
| Genitourinary | 12% (16) | 16% (5) | 11% (11) | 0.53 |
| Cancer stageb (0-I/II/III/IV) | 7% (8)/2% (2)/13% (15)/78% (89) | 4% (1)/0% (0)/15% (4)/81% (21) | 8% (7)/2% (2)/13% (11)/77% (68) | 0.39 |
| Chemotherapy regimen | ||||
| Alkylating agent | 35% (46) | 42% (13) | 33% (33) | 0.38 |
| Platinum | 30% (39) | 29% (9) | 30% (30) | 0.89 |
| Antimetabolite | 40% (52) | 33% (10) | 42% (42) | 0.37 |
| Anthracycline | 33% (43) | 23% (7) | 36% (36) | 0.16 |
| Topoisomerase inhibitor | 21% (27) | 29% (9) | 18% (18) | 0.19 |
| Mitotic inhibitor | 39% (51) | 45% (14) | 37% (37) | 0.43 |
| Radiation therapy | 30% (39) | 39% (12) | 27% (27) | 0.23 |
| Coronary artery disease | 6% (8) | 7% (2) | 6% (6) | 1.00 |
| Atherosclerosis risk factors | ||||
| Hypertension | 27% (35) | 19% (6) | 29% (29) | 0.28 |
| Hyperlipidemia | 22% (28) | 23% (7) | 21% (21) | 0.87 |
| Diabetes mellitus | 10% (13) | 7% (2) | 11% (11) | 0.73 |
| Tobacco | 34% (45) | 39% (12) | 33% (33) | 0.58 |
| Prior anticoagulant therapy | ||||
| Antiplatelet | 20% (26) | 16% (5) | 21% (21) | 0.54 |
| Heparin | 24% (31) | 29% (9) | 22% (22) | 0.44 |
| Oral anticoagulants | 7% (9) | 0% | 9% (9) | 0.11 |
| Pulmonary embolism | 5% (6) | 13% (4) | 2% (2) | 0.03 |
Abbreviations: RA, right atrial.
Other cancer etiologies were lung (7% [N = 9]), CNS (2% [N = 3]), skin/ melanoma (2% [N = 2]), endocrine (1% [N = 1]), and mesothelioma (1%[n = 1]).
Stage reported for patients with solid cancers (n = 114).
2.2. Image acquisition
CMR was performed using a commercial 1.5 T (90%) or 3 T (10%) scanner with dedicated phased array surface coils (General Electric, Waukesha WI). Exams were performed in accordance with an established pre-protocol for mass assessment [7], for which imaging consisted of two components – cine-CMR and DE-CMR. Cine-CMR was performed using a steady-state free precession sequence; images were acquired in contiguous LV/right ventricular (RV) short axis (aortic valve annulus through ventricular apices) and long axis (2-, 3-, sequential 4-chamber) orientations. Following cine-CMR, gadolinium (0.2 mmol/kg) was intravenously administered. DE-CMR was subsequently acquired in orientations matched to cine-CMR using inversion times tailored to null viable myocardium (~300 msec). “Long TI” DE-CME (600 msec) was performed for further assessment of thrombus tissue properties.
Standard 2D transthoracic echocardiography was performed using commercial equipment (Vivid E9 [GE Medical Systems, Milwaukee, WI] or iE33 [Philips Medical Systems, Andover, MA]). In accordance with consensus (American Society of Echocardiography) guidelines [15]: Non-contrast images were acquired in standard parasternal, apical, and subcostal views, corresponding to the long and short axis orientations acquired via CMR. In accordance with clinical practice, dedicated focused views of right atrium mass were obtained on a per-patient basis tailored to maximize border definition and spatial resolution.
2.3. Image analyses
2.3.1. Right atrial thrombus
DE-CMR and cine-CMR were retrieved from imaging databases, and each were interpreted for RA thrombus by an experienced reader (JWW) blinded to the results of other technique; high reproducibility for the diagnosis of thrombus has been previously reported [4]. Meanwhile, echocardiography analysis was performed via a dedicated, level III experienced reader (DG), who was blinded to clinical data and CMR results.
RA thrombus was defined via DE-CMR using established criteria, i.e., as a mass in the right atrium with avascular tissue [11,12]. On standard DE-CMR (TI = 300 msec), thrombus has a typical gray appearance. To confirm avascularity, additional DE-CMR (TI = 600 msec) was performed where vascular masses appeared hyperintense with enhancement (gray) and thrombi appeared hypointense without enhancement (black) [12].
On both cine-CMR and echocardiography, RA thrombus was identified using established anatomic criteria, i.e., based on anatomic appearance and defined as a right atrial mass that was distinct from the adjacent atrial wall and crista terminalis. RA thrombus was echodense on echocardiography, and signal intensity was similar to myocardium on cine-CMR.
Additional analyses were performed to assess RA thrombus properties, including the number of discrete masses, size (measured on a 4-chamber view by drawing the longest axis for the length and then taking the longest perpendicular distance to determine the width), total thrombus area, and perimeter (as determined on the 4-chamber view). Catheter depth was assessed by measuring the distance from the catheter tip to the cavoatrial junction on CT or if not available, x-ray.
2.3.2. Cardiac structure/function
To further assess the potential structural markers of RA thrombus, cardiac chamber size as well as LV and RV systolic function were quantified on cine-CMR. LV and RV planimetry were performed according to established methods previously shown to be highly reproducible in the literature. The right atrial area was measured at the ventricular end-diastole and end-systole in 4-chamber orientation; chamber boundaries were defined in relation to the tricuspid valve annulus.
2.4. Statistical methods
Comparisons between patients with or without RA thrombus were performed using the Student’s t-test (expressed as mean ± standard deviation) with the Levene’s test for equality of variance to confirm homogeneity for continuous variables. Categorical variables were compared using the chi-square test or, when fewer than five expected outcomes were expected per cell, Fisher’s exact test. Univariate and multivariable logistic regression analyses were performed to evaluate associations between imaging parameters and presence of thrombus. Two-sided p < 0.05 was considered indicative of statistical significance. Statistical calculations were performed using SPSS 24 (SPSS Inc., Chicago, IL).
3. Results
3.1. Population characteristics
The population comprised 130 cancer patients with central catheters, among whom nearly one fourth (24%) had RA thrombus based on DE-CMR tissue characterization. Table 1 details the clinical characteristics of the study population, stratified based on the presence or absence of RA thrombus. Patients with and without RA thrombus were similar regarding cancer etiology, treatment regimen, and other clinical characteristics. Nearly all patients with solid cancer (91%) had advanced (stage III or IV) cancer, of which the etiology varied widely. Presence of pulmonary embolism within 60 days of CMR was significantly associated (p = 0.03) in patients with RA thrombus (13%) vs patients without RA thrombus (2%).
3.2. Cardiac structure and function
Table 2 demonstrates the characteristics of central venous catheters between patients with and without RA thrombus. The central venous line was placed on the right in 100 patients and on the left in 29 patients, and one patient had bilateral catheters. The depth of central venous catheters (2.3 ± 1.8 vs. 1.6 ± 1.7 cm from the superior vena cava/right atrial junction, p = 0.05) and duration of placement (12.4 ± 17.1 vs. 11.8 ± 14.5 months, p = 0.85) were similar between patients with and without RA thrombus. However, the presence of RA thrombus was significantly associated with a catheter depth > 2.0 cm from the cavoatrial junction (p = 0.02). The side of catheter placement and number of lumens were not significantly associated with RA thrombus (p = 0.94, 0.65, and 0.24 for right, left, and bilateral placement, respectively, and 0.06 for more than one lumen).
Table 2.
Catheter features and cardiac remodeling in relation to right atrial thrombus.
| Overall (n = 130) | RA thrombus + (n = 31) | RA thrombus − (n = 99) | P-value | ||
|---|---|---|---|---|---|
| Catheter features | |||||
| Chronicity (months after insertion) | 11.9 ± 15.0 | 12.4 ± 17.1 | 11.8 ± 14.5 | 0.85 | |
| Catheter type | |||||
| Mediport | 86% (112) | 87% (27) | 86% (85) | 1.00 | |
| Hickman catheter | 3% (4) | 3% (1) | 3% (3) | 1.00 | |
| Leukophoresis catheter | 5% (6) | 10% (3) | 3% (3) | 0.15 | |
| PICC | 7% (9) | 7% (2) | 7% (7) | 1.00 | |
| Side | |||||
| Right IJ | 77% (100) | 77% (24) | 77% (76) | 0.94 | |
| Left IJ | 22% (29) | 19% (6) | 23% (23) | 0.65 | |
| Bilateral | 1% (1) | 3% (1) | 0% (0) | 0.24 | |
| Lumen # | |||||
| 1 | 82% (106) | 68% (21) | 86% (85) | 0.02 | |
| ≥2 | 18% (23) | 29% (9) | 14% (14) | 0.06 | |
| Depth | |||||
| Distance from SVC-RA junction (cm) | 1.8 ± 1.8 | 2.3 ± 1.8 | 1.6 ± 1.7 | 0.05 | |
| ≥2 cm | 46% (60) | 65% (20) | 40% (40) | 0.02 | |
| Right sided structure/function Right atrial (RA) | |||||
| RA area | (cm2) | 18.2 ± 5.4 | 18.4 ± 6.7 | 18.2 ± 4.9 | 0.88 |
| (cm2/m2) | 10.1 ± 2.6 | 9.6 ± 2.9 | 10.3 ± 2.5 | 0.20 | |
| Tricuspid regurgitation (≥moderate) | 5% (6) | 3% (1) | 5% (5) | 1.00 | |
| Right ventricle (RV) | |||||
| RV ejection fraction (%) | 54.9 ± 8.9 | 55.5 ± 9.1 | 54.7 ± 8.9 | 0.65 | |
| RV stroke volume (ml) | 69.0 ± 24.0 | 75.6 ± 30.0 | 66.9 ± 21.7 | 0.08 | |
| RV end-diastolic volume | (ml) | 127.7 ± 45.5 | 138.0 ± 55.5 | 124.5 ± 41.6 | 0.15 |
| (ml/m2) | 69.7 ± 19.6 | 71.2 ± 20.9 | 69.2 ± 19.3 | 0.63 | |
| RV end-systolic volume | (ml) | 58.9 ± 26.2 | 62.4 ± 30.0 | 57.8 ± 24.9 | 0.41 |
| (ml/m2) | 32.2 ± 12.6 | 32.1 ± 12.5 | 32.2 ± 12.6 | 0.98 | |
Abbreviations: IJ, internal jugular; RA, right atrium; RV, right ventricle; SVC/RA, superior vena cava/right atrial junction.
Patients with and without RA thrombus had similar right-sided structure and function based on indexed right atrial end-diastolic area (9.6 ± 2.9 vs. 10.3 ± 2.5 cm2/m2, p = 0.20), RV ejection fraction (55.5 ± 9.1 vs. 54.7 ± 8.9%, p = 0.65), indexed RV end-diastolic volume (71.2 ± 20.9 vs. 69.2 ± 19.3 ml/m2, p = 0.63), indexed RV end-systolic volume (32.1 ± 12.5 vs. 32.2 ± 12.6 ml/m2, p = 0.98), and RV stroke volume (75.6 ± 30.0 vs. 66.9 ± 21.7 ml/m2, p = 0.08). Clinical embolic events were independent of RA thrombus size (2.1 ± 2.3 cm2 vs. 1.7 ± 1.2 cm2, p = 0.66).
3.3. Thrombus identification by DE-CMR
Among patients with RA thrombus, 74% (n = 23) had a solitary RA thrombus (26% multiple). RA thrombus had an average area of 1.6 ± 1.2 cm2 and maximal diameter of 1.5 ± 0.6 cm. Table 3 demonstrates that on average the RA thrombus missed by echocardiography were smaller. Three patients had a lesion in the right atrium with enhancement on DE-CMR consistent with a mass.
Table 3.
Characteristics of right atrial thrombus detected by transthoracic echocardiogram.
| Overall (n = 24) | Right atrial thrombus detected by echocardiography (n = 18) | Right atrial thrombus not detected by echocardiography (n= 6) | P-value | |
|---|---|---|---|---|
| Thrombus size | ||||
| Maximal diameter (cm) | 1.5 ± 0.6 | 1.5 ± 0.6 | 1.4 ± 0.7 | 0.79 |
| Orthogonal diameter (cm) | 1.2 ± 0.9 | 1.3 ± 0.9 | 0.7 ± 0.4 | 0.09 |
| Area (cm2) | 1.6 ± 1.2 | 1.9 ± 1.0 | 1.3 ± 0.6 | 0.12 |
3.4. Diagnostic performance of anatomical imaging
Table 4 summarizes the diagnostic performance of Cine-CMR and echocardiography compared with the reference standard of DE-CMR. Transthoracic echocardiography was acquired in 83 patients (64% of the study population), on average 3.0 [interquartile range 1.0–7.0] days from CMR. Echocardiography demonstrated moderate sensitivity (75%) and specificity (90%) in relation to DE-CMR. Cine-CMR yielded higher sensitivity (90%) and specificity (98%) vs. the reference standard of DE-CMR. Cine-CMR was more accurate (96%) when compared to echocardiography (86%).
Table 4.
Transthoracic echocardiography and cine-CMR performance for right atrial thrombus.
| Modality | Sensitivity | Specificity | Accuracy | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| Cine-CMRa | 90% (28/31) | 98% (97/99) | 96% (125/130) | 93% (28/30) | 97% (97/100) |
| Transthoracic echob | 75% (18/24) | 90% (53/59) | 86% (71/83) | 75% (18/24) | 90% (53/59) |
Echo within 14 days of MRI are available in 64% (n = 83) of study population.
RA mass classified as neoplasm in 3 cases (of whom 1 had echocardiography within a 14-day study window).
4. Discussion
The incidence of catheter-associated RA thrombus has been reported to be 8–13% in the oncologic population [16]. Ogren et al. demonstrated that among patients dying from or with pulmonary embolism, 7% had RA thrombus as a potential cause [17]. In the case of catheter-associated RA thrombus, overall mortality has been reported up to 27% [18]. In this study, we compared echocardiography with CMR for thrombus detection and attempted to identify the predictors of catheter-associated RA thrombus. Our study demonstrates that DE-CMR is superior to morphologic imaging by cine-CMR and echo for detection of RA thrombus. In addition, catheter-associated RA thrombus occurs independently of right-sided structure or function, catheter depth, and is associated with clinical embolic events.
This is the first study to compare RA thrombus detection by echocardiography versus CMR. There is no universal gold standard to evaluate thrombus. Thus, there are limitations for using any imaging as a reference standard. Our study used DE-CMR as a reference standard which is based on prior research using pathology and thrombo-embolic event data confirming that DE-CMR is highly accurate for thrombus characterization [9,19]. Standard cine-CMR yielded improved sensitivity (90 vs. 75%) and specificity (98 vs. 90%) than echocardiography based on the reference standard of DE-CMR.
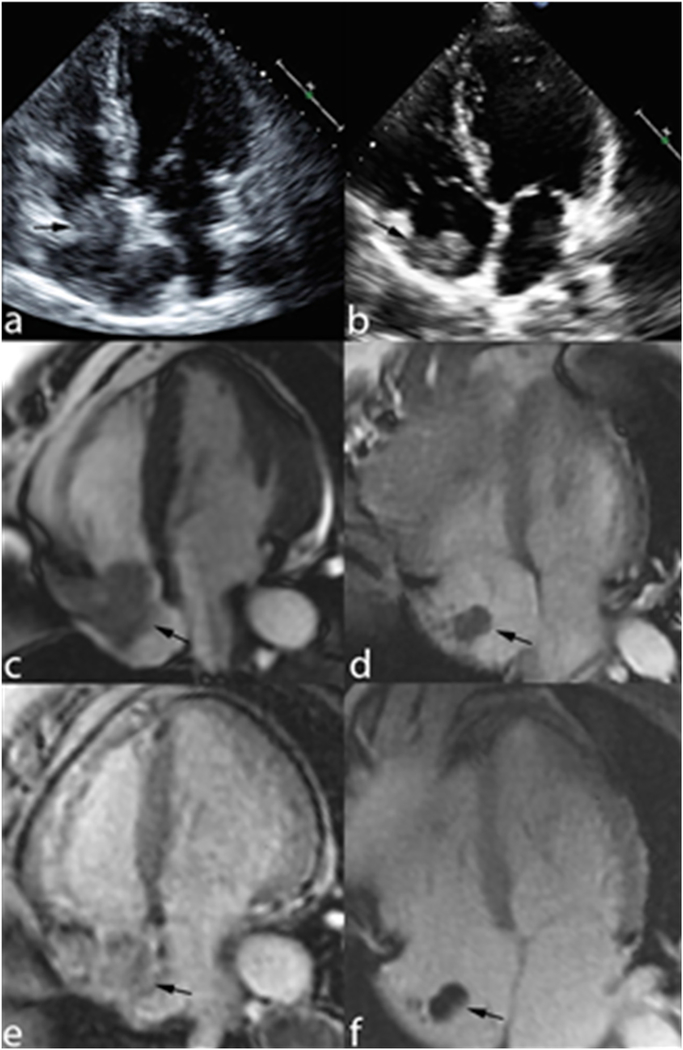
An additional advantage of CMR over echocardiography is the accurate characterization of masses associated with catheters. When a hyperechoic lesion is detected in the RA on echocardiography, its appearance is non-specific and may represent neoplasm or RA thrombus (Fig. 1A, B). Even though standard cine CMR is more specific and accurate for the detection of RA thrombus, the appearance of mass versus RA thrombus remains similar in appearance (Fig. 1C, D). However, with a “long TI” DE-CMR, a right atrial mass be characterized as neoplasm or RA thrombus. On “long TI” DE-CMR, RA thrombus characterization consists of a non-enhancing lesion that appears black whereas a neoplasm would exhibit contrast uptake and appear gray (Fig. 1E, F). With these concepts in mind, once a right atrial mass has been identified (e.g. by echocardiography), a DE-CMR should be obtained for further assessment - especially in an oncologic population in which risk for thrombus is increased but cardiac metastases are always a consideration.
Fig. 1.
The patient on the left (A, C, E) has a right atrial neoplasm and the patient on the right (B, D, F) has a right atrial (RA) thrombus. A and B demonstrate echocardiography images in the four-chamber view. Both neoplasm and RA thrombus have similar echotecture. C + D demonstrate cine-CMR in the four-chamber view, again the neoplasm and RA thrombus have similar signal. Only on “long TI” DE-CMR can we see the difference between the two patients. E demonstrates contrast uptake and appears gray consistent with neoplasm and F demonstrations a non-enhancing lesion that appears black consistent with RA thrombus.
Secondly, this study demonstrates that the formation of catheter-associated RA thrombus was independent of catheter placement, right heart function, right heart size, and patient characteristics (age, gender, weight, height, body surface area, cancer diagnosis, prior thromboembolism, and anticoagulation therapy).
Thirdly, in our population, catheter-associated RA thrombus was related to an increased incidence of pulmonary embolism. Among patients with RA thrombus, 13% had pulmonary embolism whereas among patients without RA thrombus, 2% had pulmonary embolism within 60 days after the exam (p = 0.03). These results are in agreement with Stavroulopoulos et al., where in a hemodialysis cohort, the incidence of pulmonary embolism associated with catheter-associated RA thrombus was found to be 14% with a mortality rate of 18.3% [20]. This suggests that patients with RA thrombus are at higher risk for PE and should be place on anticoagulation therapy.
Several limitations in this study should be recognized. First, this was a small cohort of patients referred for clinical indications which may bias the data, in particular the incidence of pulmonary embolism in the RA thrombus group. Also, 32% of patients underwent echocardiography and MRI for suspected right atrial mass on a prior CT.
5. Conclusions
In conclusion, morphologic imaging by cine-CMR and echocardiography provide limited diagnostic utility for RA thrombus as established by DE-CMR tissue characterization. Catheter-associated RA thrombus occurs independently of right-sided structure or function and is associated with clinical embolic events and catheter depth.
Acknowledgements
The authors would like to thank Joanne Chin for assisting with the preparation of the manuscript.
Funding information
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The funding source had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Declaration of competing interest
None.
References
- [1].Asmarats L, Fernandez-Palomeque C, Martinez-Riutort JM, Bethencourt A. Right atrial thrombosis associated with hemodialysis catheter: first description of recurrence in a poorly understood problem. J Thromb Thrombolysis 2015;39(2):254–7. [DOI] [PubMed] [Google Scholar]
- [2].Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia 2002;4(6):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weinsaft JW, Kim J, Medicherla CB, Ma CL, Codella NC, Kukar N, et al. Echocardiographic algorithm for post-myocardial infarction LV thrombus: a gate-keeper for thrombus evaluation by delayed enhancement CMR. JACC Cardiovasc Imaging 2016;9(5):505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Weinsaft JW, Kim RJ, Ross M, Krauser D, Manoushagian S, LaBounty TM, et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc Imaging 2009;2(8):969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011;4(7):702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Malik SB, Kwan D, Shah AB, Hsu JY. The right atrium: gateway to the heart—-anatomic and pathologic imaging findings. Radiographics 2015;35(1):14–31. [DOI] [PubMed] [Google Scholar]
- [7].Chan AT, Plodkowski AJ, Pun SC, Lakhman Y, Halpenny DF, Kim J, et al. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson 2017;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Goyal P, Weinsaft JW. Cardiovascular magnetic resonance imaging for assessment of cardiac thrombus. Methodist Debakey Cardiovasc J 2013;9(3):132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathologic characteristics of left ventricular thrombus: a comparison of contrast enhanced magnetic resonance imaging, transthoracic echocardiography and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006;152(1):75–84. [DOI] [PubMed] [Google Scholar]
- [10].Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation 2002;106(23):2873–6. [DOI] [PubMed] [Google Scholar]
- [11].Srichai MB, Junor C, Rodriguez LL, Stillman AE, Grimm RA, Lieber ML, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am Heart J 2006;152(1):75–84. [DOI] [PubMed] [Google Scholar]
- [12].Weinsaft JW, Kim HW, Shah DJ, Klem I, Crowley AL, Brosnan R, et al. Detection of left ventricular thrombus by delayed-enhancement cardiovascular magnetic resonance prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008;52(2):148–57. [DOI] [PubMed] [Google Scholar]
- [13].Hong YJ, Hur J, Kim YJ, Lee HJ, Nam JE, Kim HY, et al. The usefulness of delayed contrast-enhanced cardiovascular magnetic resonance imaging in differentiating cardiac tumors from thrombi in stroke patients. Int J Cardiovasc Imaging 2011;27(Suppl. 1):89–95. [DOI] [PubMed] [Google Scholar]
- [14].Kitkungvan D, Nabi F, Ghosn MG, Dave AS, Quinones M, Zoghbi WA, et al. Detection of LA and LAA thrombus by CMR in patients referred for pulmonary vein isolation. JACC Cardiovasc Imaging 2016;9(7):809–18. [DOI] [PubMed] [Google Scholar]
- [15].Lang RM, Biereg M, Devereux RM, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–63. [DOI] [PubMed] [Google Scholar]
- [16].Gilon D, Schechter D, Rein AJ, Gimmon Z, Or R, Rozenman Y, et al. Right atrial thrombi are related to indwelling central venous catheter position: insights into time course and possible mechanism of formation. Am Heart J 1998;135(3):457–62. [DOI] [PubMed] [Google Scholar]
- [17].Ogren M, Bergqvist D, Eriksson H, Lindblad B, Sternby NH. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23 796 consecutive autopsies. Eur Heart J 2005;26(11):1108–14. [DOI] [PubMed] [Google Scholar]
- [18].Negulescu O, Coco M, Croll J, Mokrzycki MH. Large atrial thrombus formation associated with tunneled cuffed hemodialysis catheters. Clin Nephrol 2003;59(1):40–6. [DOI] [PubMed] [Google Scholar]
- [19].Weinsaft J, Kim H, Shah DJ, Klem I, Crowley A, Brosnan R, et al. Detection of left ventricular thrombus by delayed-enhancement CMR: prevalence and markers in patients with systolic dysfunction. J Am Coll Cardiol 2008;52(2):148–57. [DOI] [PubMed] [Google Scholar]
- [20].Stavroulopoulos A, Aresti V, Zounis C. Right atrial thrombi complicating haemodialysis catheters. A meta-analysis of reported cases and a proposal of a management algorithm. Nephrol Dial Transpl 2012;27(7):2936–44. [DOI] [PubMed] [Google Scholar]