Abstract
Background:
The efficacy of the recombinant, live, attenuated, tetravalent dengue vaccine (CYD-TDV) against virologically-confirmed dengue (VCD) has been documented in a phase 3 trial in Latin America (CYD15, NCT01374516). This is a descriptive secondary analysis of the efficacy and safety of CYD-TDV in participants from Colombia.
Methods:
Data from 9740 Colombian participants 9–16 years of age who were randomized 2:1 to receive CYD-TDV or placebo were assessed to describe the vaccine efficacy of CYD-TDV against VCD and severe VCD. Estimation was made of the relative risk (RR) for hospitalized VCD cases and severe hospitalized VCD cases after the first dose of CYD-TDV, as well as a description of the incidence of hospitalized dengue from the start of the study and per year of the study until study completion.
Results:
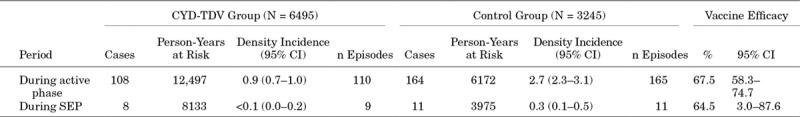
During the active phase of the trial in Colombia, the efficacy of CYD-TDV was 67.5% [95% confidence interval (CI): 58.3–74.7] against symptomatic VCD due to any serotype from injection 1 (month 0) to 25 months postinjection 1. Over 6 years, the RR across all 4 serotypes was 0.166 (95% CI: 0.09–0.29) in hospitalized VCD patients and 0.154 (95% CI: 0.04–0.50) in patients with severe hospitalized VCD.
Conclusions:
Analysis of the data from Colombia mimics the efficacy observed in CYD15 during the active surveillance follow-up (25 months), but with a sustained beneficial RR for dengue hospitalizations on the subsequent years of follow-up. In Colombia, where seroprevalence has been demonstrated to be high in several regions of the country, CYD-TDV is a useful tool to consider as part of an integrated control strategy against endemic dengue, a disease with a high economic impact on the health system.
Keywords: Recombinant, live, attenuated, tetravalentdengue vaccine, dengue vaccine, efficacy, Colombia
Dengue is one of the most prevalent mosquito-borne diseases, with an estimated 50 million infections, and a resultant 22,000 deaths worldwide each year.1 Dengue is also an increasing problem among international travelers, and with unplanned migration from other highly endemic countries.2–5 In Latin American countries, over 8.7 million cases have occurred over the past 5 years, with Honduras, Mexico, Colombia, Peru and Brazil accounting for the most severe dengue cases.1,6 In Colombia, over 415,000 cases, 1233 deaths and 6881 severe dengue cases occurred from 2013 to 2017,6–11 with most cases being reported in children below 15 years of age.12 The risk of dengue transmission in Colombia is highly variable and is affected by climate and socioeconomic factors, including imported dengue from people coming from other dengue endemic countries. In 2019, imported dengue cases from 20 countries have been reported to the Ministry of Health.13–15 While the 4 serotypes may be present in the country at any given time, serotype predominance has varied over the last 5 years. All 4 serotypes circulated between 2013 and 2016, while serotypes 1–3 have predominated since 2017. In addition, seroprevalence, a crucial consideration for vaccination, has been reported to be as high as 92.5%,16,17 with some variation according to age range and geographic areas within the country,18 including nonendemic dengue areas like Bogota (absence of dengue vector Aedes aegypti).
Outbreaks of dengue and other arboviruses in Colombia have resulted in an adverse impact on the health of the population and the economy of the country. A recent analysis found a difference in the total disability-adjusted life years between endemic and epidemic years of 1115.19
These data indicate that dengue prevention and control strategies have not been successful in reducing the spread of the virus, and that the adoption of new tools for the Integrated Vector Management and the Integrated Management Strategy20 is required to successfully control dengue and other Aedes transmitted diseases in Colombia. Furthermore, World Health Organization (WHO) has considered the availability of the dengue vaccine in its latest recommendations so that it can be taken into consideration at a regional level.20
Recombinant, live, attenuated, tetravalent dengue vaccine (CYD-TDV) is a recombinant, live, attenuated, tetravalent dengue vaccine administered according to a 3-dose schedule at 6-month intervals (0, 6 and 12 months). Vaccine efficacy (VE) studies have been conducted in several dengue endemic countries, including a phase 3 study, CYD15 (NCT01374516), conducted in 5 Latin American countries (Colombia, Brazil, Mexico, Puerto Rico and Honduras).21 The study included 3 phases: the active phase, which was an active surveillance of 25 months follow-up that captured all symptomatic dengue cases in the study participants (hospitalized or not); followed by the hospital phase, which was a passive follow-up of the participants to capture those dengue cases that merited hospitalization and a surveillance expansion period from around year 4 to the end of the study, which was reinstated to capture symptomatic dengue cases (hospitalized or not). The vaccine was administered as per schedule (3 doses at 0, 6 and 12 months), with a follow-up at month 13 including a blood sample. In CYD15, postdose 3 to month 25 VE in the Per-Protocol Analysis Set (primary endpoint) demonstrated a protection of 60.8% [95% confidence interval (CI): 52.0–68.0] against symptomatic virologically-confirmed dengue (VCD); in the intention-to-treat population (participants who had received at least 1 injection), VE was 80.3% (95% CI: 64.7–89.5) against hospitalized VCD and 95.5% (95% CI: 68.8–99.9) against severe VCD after the first injection.21 In a case–cohort posthoc study of participants of CYD15 who were dengue seropositive at baseline, the VE for symptomatic VCD was 78.1% (95% CI: 69.9–84.1).22 The objective of the current analysis is to describe the efficacy and safety of CYD-TDV in participants from Colombia, based on the data from CYD15.
METHODS
The CYD15 participants from Colombia came from 9 study centers located in Armenia, La Tebaida, Montenegro, Calarcá, Girardot, Yopal, Aguazul, Acacías and Bucaramanga, selected based on endemicity level and incidence of dengue in the area. The study design has been previously described.21 Briefly, healthy children 9–16 years of age were randomized 2:1 (vaccine:placebo) to receive 3 injections of CYD-TDV or placebo, at months 0, 6 and 12. The investigators, participants, parents and the sponsor were not informed of group allocation. Of the participants, 10% were also randomly assigned into an immunogenicity subset. The study protocol and the informed consent were approved by the Ministry of Health and the corresponding ethics committees before trial initiation.
Posthoc Case–Cohort Study for Dengue Serostatus at Baseline
Data from each efficacy trial were analyzed in a case–cohort study, including a randomly selected subcohort of around 10% of the entire population, as described by Sridhar et al.22 Baseline dengue serostatus was determined based on measured plaque reduction neutralization test (PRNT50), with a cut off threshold for seropositivity ≥10 or predicted when missing. For participants in the posthoc case–cohort analysis, missing PRNT50 serostatus at baseline was imputed using month 13 anti-non-structural protein-1 (NS1) titers [cutoff thresholds for positivity of 9 enzyme-linked immunosorbent assay (ELISA) units per milliliter] as a continuous variable and other variables as predictors using multiple imputation (MI) as previously described.22
Identification of Cases
Active follow-up of the participants occurred from the first injection until month 25; active surveillance of hospitalized and nonhospitalized febrile cases (temperature ≥38°C for ≥2 consecutive days) was conducted. Blood samples were obtained from any participant with an acute febrile illness so as to confirm the presence of dengue. This acute sample was obtained within 5 days after the onset of fever. The reverse transcriptase-polymerase chain reaction was performed to test for VCD, and ELISA for the presence of the dengue NS1 antigen on the acute samples. After this active follow-up stage, a 4-year, long-term safety follow-up took place with a passive surveillance of hospitalized VCD cases (hospital phase) from years 3–6 of the study; the current subanalysis does not include data from year 6 of the study. Additionally, the subjects who consented were also offered to enter an active surveillance expansion phase (SEP) at any time from year 4, to detect all cases of VCD, hospitalized or not, occurring up to the end of the study (year 6). An Independent Data Monitoring Committee performed a periodic review of safety data and dengue cases, including the evaluation of dengue severity.
Vaccine Efficacy
VE from 28 days postinjection 1 and postinjection 2 to the end of the active phase was evaluated on a different analysis set defined as subjects who received at least 1 or 2 doses. The great majority of cases, however, received the 3 scheduled doses. Estimated VE from 28 days postinjection 3 to the end of the active phase was calculated on the modified full analysis set for efficacy (FASE). The subjects of the immunogenicity and reactogenicity subset who received at least 1 injection, who did not have severe noncompliance and who had a blood sample drawn and a result available after this injection were included in the full analysis set for immunogenicity.
For safety analyses, subjects were analyzed according to whether or not they actually received at least 1 injection of CYD-TDV vaccine (“as treated”).
Statistical Analysis
The current analysis was performed on the FASE comprising the participants who received at least 1 injection, which accounted for 99.9% of the randomized subjects. Overall, there were 20,854 participants in the FASE (13,914 in the CYD-TDV group and 6940 in the control group). Of these, 9740 (46.7%) participants were from Colombia (6495 in the CYD-TDV group and 3245 in the control group).
The objective of this secondary analysis was to describe the efficacy and safety of CYD-TDV in Colombia from June 2011 to March 2018. It was restricted to the primary objective of the protocol, that is, VE against any symptomatic VCD, regardless of severity. The primary objective was to assess efficacy from 28 days after dose 1 (month 13) to month 25. The endpoint was the occurrence of symptomatic VCD cases. Efficacy after at least 1 dose was a secondary objective. The analysis by country such as this one was a descriptive posthoc analysis. Data on severe dengue and dengue hospitalization are limited due to the relatively low number of hospitalized and/or severe VCD cases, especially in the hospital phase; nevertheless, VE was inferred against this outcome from the relative risk (RR).
Statistical Analysis According to Serostatus at Baseline
The hazard ratios (HRs) of VCD were estimated on the basis of serostatus measured or imputed at baseline (month 0) by PRNT50; the MI and NS1 month 13 threshold 9 approach to assess the risk of hospitalization was calculated using a weighted Cox (Prentice method) model with a Wald-based 95% CI calculated using the variance estimator by Barlow.23–25 For the MI, the estimate is computed from 10 iterations and combined with the use of Rubin variance rule. The VE (%) and 95% CIs were derived as 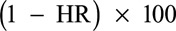 .
.
Statistical analyses were performed using SAS version 9.2 for the original CYD15 analysis and SAS version 9.4 for NS1 analysis (SAS Institute, Cary, NC).
Serious adverse events (SAEs) and deaths were reported in both study groups.
RESULTS
In the posthoc case–cohort study, 959 participants (645 in the CYD-TDV group and 314 in control group) from Colombia were part of the subcohort for analysis according to dengue serostatus at baseline. The proportion of participants in this subcohort who were dengue seropositive at baseline was similar in the CYD-TDV and control groups (91.5% in the CYD-TDV group and 92.0% in the control group) (Table 1).
TABLE 1.
Dengue Serostatus at Baseline in Colombia
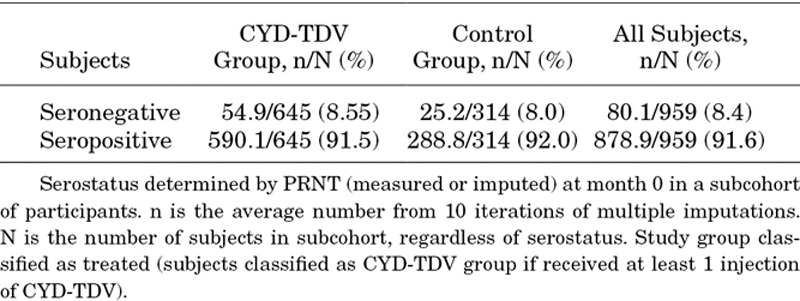
During the active phase (years 1–2), 2798 febrile episodes were reported in the CYD-TDV group compared with 1486 in the control group. During the hospital phase (years 3–6), acute blood samples of fever cases were collected for a total of 74 episodes. During the SEP (years 4–6), a total of 862 febrile episodes were reported, 568 in the CYD-TDV group and 621 in the control group.
In the FASE, from 28 days postinjection 1 to the end of the active phase, there were 105 cases of symptomatic VCD in the CYD-TDV group with a density incidence of 0.9 (95% CI: 0.7–1.1) and 161 cases in the control group with a density incidence of 2.7 (95% CI: 2.3–3.2). VE against symptomatic VCD after dose 1 + 28 days for each serotype was as follows: serotype 1, 62.6% (95% CI: 43.2–75.5); serotype 2, 67.2% (95% CI: 41.3–82.0); serotype 3: 73.2% (95% CI: 59.3–82.7) and serotype 4, 77.7% (95% CI: 20.2–95.0). From 28 days postinjection 2 to the end of the active phase, there were 89 symptomatic VCD cases in the CYD-TDV group with an incidence of 1.0 (95% CI: 0.8–1.2) and 127 cases in the control group with an incidence of 2.9 (95% CI: 2.4–3.4), resulting in a postdose 2 VE against VCD due to any serotype of 65.2% (CI: 54.0–73.7). The postdose 2 VE for each serotype was as follows: serotype 1, 60.3% (95% CI: 36.6–75.3); serotype 2, 61.3% (95% CI: 23.7–80.7); serotype 3, 71.4% (95% CI: 54.7–82.2) and serotype 4, 81.2% (95% CI: 21.5–96.8). Estimated VE from 28 days postdose 3 to the end of the active phase shows that there were 74 symptomatic VCD cases with a density incidence of 1.3 (95% CI: 1.0–1.6) in the CYD-TDV group and 108 cases with a density incidence of 3.8 (95% CI: 3.1–4.5) in the control group, resulting in a postdose 3 VE against VCD due to any serotype of 66.0% (95% CI: 53.9–75.1). The postdose 3 VE for each serotype was as follows: serotype 1, 57.8% (95% CI: 31.2–74.3), serotype 2, 56.2% (95% CI: 4.3–80.2); serotype 3, 73.5% (95% CI: 55.5–84.5) and serotype 4, 81.2% (95% CI: 21.5–96.8).
During the active phase, VE against symptomatic VCD due to any of the 4 serotypes, regardless of dengue serostatus at baseline, was 67.5% (95% CI: 58.3–74.7) and remained similar during the SEP, 64.5% (95% CI: 3.0–87.6) (Table 2).
TABLE 2.
Vaccine Efficacy Against Symptomatic Virologically-Confirmed Dengue Due to Any or Each of the 4 Serotypes Regardless of Dengue Serostatus at Baseline: Full Analysis Set
In Colombia, all clinically severe dengue cases were hospitalized. As shown in Table 3, the RR of hospitalized clinically severe VCD in the CYD-TDV group throughout the entire study period was 0.154 (95% CI: 0.04–0.50), corresponding to an inferred protection of 84.6%.
TABLE 3.
Risk of Hospitalized Clinically Severe Virologically-Confirmed Dengue Cases in Colombia
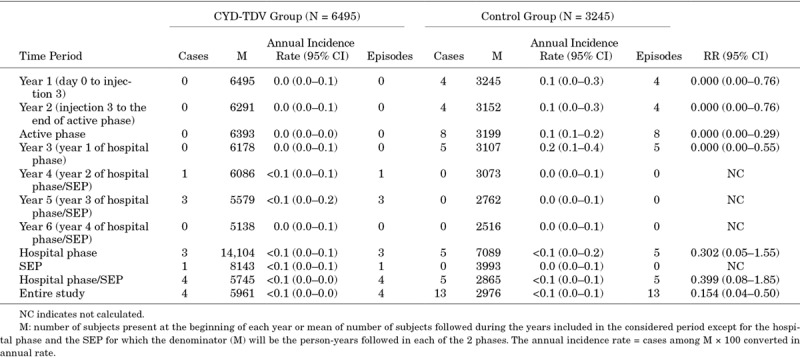
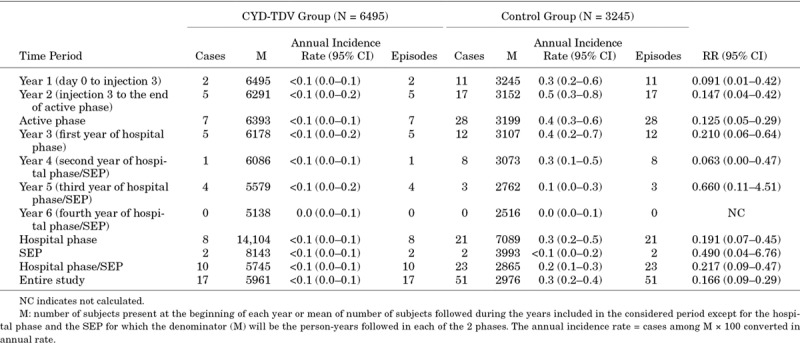
As shown in Table 4, over the entire study, a total of 17 cases of hospitalized VCD were reported in the CYD-TDV group and 51 in the control group, with a RR of 0.166, corresponding to an inferred protection against hospitalized dengue (regardless of their severity assessment) of 83.4%. An increase was not observed during the study in the CYD-TDV group for hospitalizations or hospitalized severe VCD cases alone. No hospitalized clinically severe cases were reported during the last year of the study.
TABLE 4.
Risk of Hospitalized Virologically-Confirmed Dengue Cases in Colombia
VE and Risk of Hospitalization According to Dengue Serostatus at Baseline
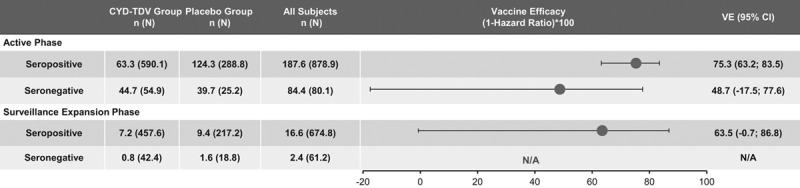
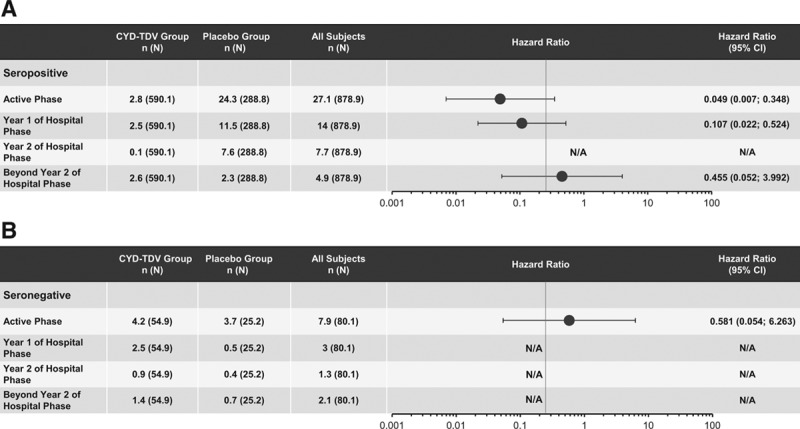
Results based on the data from the case–cohort in the posthoc NS1 analyses showed that VE against symptomatic VCD during the active phase in dengue-seropositive participants was 75.3% (95% CI: 63.2–83.5) (Fig. 1). In those dengue seronegative at baseline, the VE was lower, 48.7% (95% CI: –17.5 to 77.6); however, the lower bound of CI was negative. This VE persisted during the SEP in dengue-seropositive participants [63.5% (95% CI: –0.7 to 86.8)], but with a lower bound of CI below zero (Fig. 1). With regards to the risk of hospitalization in dengue-seropositive participants, a HR of 0.049 was observed in the active phase, whereas in seronegative participants, it was 0.581. VE against dengue hospitalizations during the active phase was inferred [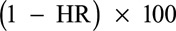 ] as 95.1% and 41.9% in seropositive and seronegative participants, respectively (Fig. 2). Likewise, VE was calculated after each dose regardless of the serostatus at baseline; in dengue-seropositive participants from month 0 to dose 2, the VE was 80.4% (95% CI: 51.8–92.1), from dose 2 to dose 3, 77.7% (95% CI: 45.6–90.9) and from dose 3 to the end of the active phase, 73.4% (95% CI: 57.6–83.3) (Fig. 3A). In seronegative participants, the VE from month 0 to dose 2, from dose 2 to dose 3 and from dose 3 to the end of the active phase, the VE was 48.2%, 37.0% and 50.7%, respectively, but in all cases, the lower bound of CI was negative (Fig. 3B).
] as 95.1% and 41.9% in seropositive and seronegative participants, respectively (Fig. 2). Likewise, VE was calculated after each dose regardless of the serostatus at baseline; in dengue-seropositive participants from month 0 to dose 2, the VE was 80.4% (95% CI: 51.8–92.1), from dose 2 to dose 3, 77.7% (95% CI: 45.6–90.9) and from dose 3 to the end of the active phase, 73.4% (95% CI: 57.6–83.3) (Fig. 3A). In seronegative participants, the VE from month 0 to dose 2, from dose 2 to dose 3 and from dose 3 to the end of the active phase, the VE was 48.2%, 37.0% and 50.7%, respectively, but in all cases, the lower bound of CI was negative (Fig. 3B).
FIGURE 1.
Vaccine efficacy against symptomatic VCD due to any of the 4 serotypes by study phase and dengue serostatus at baseline in Colombia. Serostatus determined by PRNT (measured or imputed) at Month 0. n and N are average numbers from 10 iterations of multiple imputations. Study group classified as randomized (Subjects classified according to the injection assigned at randomization). N indicates total number of subjects selected in sub-cohort; n, number of subjects fulfilling the item listed; N/A, not available.
FIGURE 2.
Risk of dengue hospitalization occurring after Month 0 by time period, in participants who were seropositive (A) or seronegative (B) at baseline in Colombia. Serostatus determined by PRNT (measured or imputed) at Month 0. n and N are average numbers from 10 iterations of multiple imputations. Study group as treated (Subjects classified as CYD-TDV group if received at least 1 injection of CYD-TDV vaccine). Beyond Year 2 Hospital phase means until the end of the study. N indicates total number of subjets selected in sub-cohort; n, number of subjects fulfilling the item listed.
FIGURE 3.
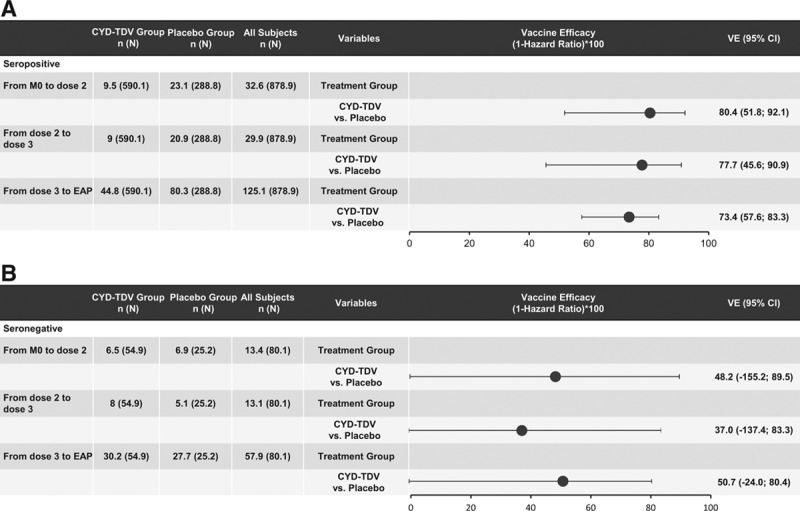
Vaccine efficacy against symptomatic VCD between doses in participants who were seropositive (A) or seronegative (B) at baseline in Colombia. Serostatus Determined By Prnt (Measured Or Imputed) At Month 0 In A Subcohort Of Participants. N And N Are Average Numbers From 10 Iterations Of Multiple Imputations. Study Group Classified As Randomized (Subjects Classified According To The Injection Assigned At Randomization). Eap Indicates End Of The Active Phase; N: Total Number Of Subjets Selected In Sub-Cohort; N, Number Of Subjects Fulfilling The Item Listed.
In terms of safety, 15.0% (977/6495) of participants in the CYD-TDV group experienced at least 1 SAE and 16.2% (527/3245) in the control group during the entire study, most SAEs being due to infectious diseases. There were 29 deaths (20 in the CYD-TDV group and 9 in the control group). None of the deaths and some of the SAEs were related to CYD-TDV (respiratory, thoracic and mediastinal disorders and asthma); the deaths were due to traffic accident (n = 9), violence (ie, gunshot wound, stabbing, homicide) (n = 8), intentional self-poisoning and exposure to other unspecified chemicals and noxious substances (n = 3), intentional self harm by hanging (n = 2), death due to fall from a roof (n = 1), renal failure due to systemic peri-nuclear antineutrophil cytoplasmic antibodies vasculitis of an autoimmune etiology (n = 1), intracranial hemorrhage secondary to ruptured aneurysm (n = 1), autoimmune encephalitis due to antibodies against N-methyl-D-aspartate receptors (n = 1), unattended death (body found in woods after disappearance) (n = 1), acute respiratory failure (n = 1) and septic shock secondary to compression of chest trauma (n = 1).
DISCUSSION
This descriptive subanalysis was based on the data from a phase 3 study of CYD-TDV initially conducted across 5 countries in Latin America. Colombia was one of these countries and accounted for almost half of the overall study population. The results observed in the Colombian study population corroborate the findings from CYD15,21 where VE against symptomatic VCD due to any of the 4 serotypes from month 0 to month 25 of the active phase was 67.5% (95% CI: 58.3–74.7). The high flavivirus seroprevalence in several regions of Colombia,16,17 indicates that Colombia has highly dengue endemic areas. Some of these areas were selected for the study sites and thus is not surprising that this descriptive analysis focusing on Colombia demonstrated that CYD-TDV presents good efficacy against severe VCD from the first dose which perdures at similar levels throughout the study period.
As shown by the previously described endemicity of dengue in Colombia,16,17 supportive data are relevant for public health considerations given that tools to fight dengue are currently insufficient or not consistently or sustainably implemented in the country. The results from this subanalysis illustrate the potential of CYD-TDV as an additional tool in the prevention and control of dengue in Colombia, as shown by the observed efficacy and the high level of protection against serious and severe dengue outcomes over time.
Efficacy data were also analyzed as part of a separate posthoc analysis to measure the effect of serostatus on the safety and efficacy of CYD-TDV, as reported by Sridhar et al.22 In individuals previously exposed to dengue before vaccination, CYD-TDV protected against severe VCD and hospitalization for VCD for 6 years compared with individuals not previously exposed to dengue before vaccination, where there could be higher a risk of these outbreaks. It should be noted that the seroprevalence of dengue was 92.2% in Colombia,21 suggesting that the vaccine would represent a public health tool for the prevention of dengue in similar endemic areas. The VE against symptomatic VCD and hospitalized cases, according to serostatus at baseline, was also observed in the seropositive participants from the subgroup of the posthoc case–control study at levels even higher than the ones reported in the original study, regardless of the baseline dengue serostatus. The VE against hospitalized VCD declined beyond 2 years of the hospital phase but still persisted (54.5%) to the end of the study.
According to the revised strategic advisory group of experts recommendations on the use of the dengue vaccine published in April 2018,26,27 a “pre-vaccination screening strategy”, whereby only dengue-seropositive persons are vaccinated, would be the preferred option for the countries considering vaccination as part of their dengue control program. These data validate the WHO recommendations to offer the vaccine where there will be the greatest benefit, that is, populations in areas with high dengue seroprevalence and circulation of the 4 serotypes. Seroprevalence has been reported to be between 79.5% and 97.8% in endemic areas of the country but can be lower (50.8%) as reported by Carabali et al, in Medellin.16,17,28,29 In our study, serostatus at baseline was measured (PRNT50 at month 0) or imputed (ELISA NS1 immunoglobulin G at month 13), using tests that are not validated or registered to assess dengue baseline serostatus. Currently, classification of serostatus for implementation of the CYD-TDV vaccine relies on the use of commercially available serologic tests which may perform differently in terms of sensitivity and specificity. For large dengue vaccination programs, a highly specific test would be required to minimize the individual risk and the inadvertent use of the vaccine in seronegative persons, by reducing the number of false-positive test results.30,31 Also, the test needs to have a high sensitivity to maximize individual and population benefit by identifying a high proportion of previously exposed persons who will benefit from vaccination. The positive predictive value of a test would be a unifying indicator for an acceptable safety profile of a prevaccination screening strategy. The required sensitivity to achieve a high positive predictive value will depend on the seroprevalence in the population in which the test is conducted and on the test specificity. Furthermore, it is argued that an additional reasonable criterion for the selection of a diagnostic test is that those who are deemed ineligible for vaccination as a result of the test should be at a lower risk of hospitalized or severe dengue disease if they are left unvaccinated than if they are vaccinated.32 High sensitivity ensures a low proportion of misclassifications among those who test negative, particularly in high prevalence settings. In a documented population with level seroprevalence setting of >80%, as in the specific setting of CYD15 study in Colombia, the vaccine could be used without individual prevaccination testing at 9 years of age, following WHO recommendations.27 In such a high seroprevalence setting, if the government opts for prevaccination screening strategy, a test with very high sensitivity would be preferred over a test with a high specificity. Several reviews of the currently available tests or conventional ELISA assays to determine serostatus have been made, showing the high specificity but variable sensitivity among the rapid tests analyzed and the need to improve these.33,34 In the meantime, serologic tests are a useful tool to comply with WHO recommendations for dengue vaccination in endemic settings. Currently, there are limitations for the use of CYD-TDV vaccine in travelers from nonendemic to dengue endemic areas.35 It will be necessary to determine that those travelers have a laboratory-confirmed dengue illness in the past, and also have a risk to be exposed to dengue, to assess if vaccinating with CYD-TDV would be justified.27,36
ACKNOWLEDGMENTS
Editorial assistance was provided by Nwoza Eshun, inScience Communications, Springer Healthcare Ltd, United Kingdom. Funding for this assistance was provided by Sanofi Pasteur.
The CYD15 teams in sites in Colombia: Dr. Victor Sierra, Yopal; Dr. Edith Rodriguez, Aguazul; Dr. Hector Velasquez, Acacias; Dr. Jaime Carrillo, Girardot; Dr. Victor Osorio, La Tebaida; Dr. Hector Pachón, Montenegro; Dr. Reinaldo López, Calarcá; Dr. Erwin Pardo, Armenia and Dr. Luis Villar, Bucaramanga.
Footnotes
This study was funded by Sanofi Pasteur.
H.R. and MF has participated in several clinical studies for pharmaceutical companies such as GSK, Merck and Sanofi Pasteur; TM, SP, OH, FN, and BZ are employees of Sanofi Pasteur; JJ and MC are employees of Sanofi and G.C. and J.Q. have participated in several clinical studies for Sanofi Pasteur.
REFERENCES
- 1.Castrillón JC, Castaño JC, Urcuqui S. [Dengue in Colombia: ten years of database records]. Rev Chilena Infectol. 2015;32:142–149. [DOI] [PubMed] [Google Scholar]
- 2.Masyeni S, Yohan B, Somia IKA, et al. Dengue infection in international travellers visiting Bali, Indonesia. J Travel Med. 2018;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddell A, Babiker ZO. Imported dengue fever in East London: a 6-year retrospective observational study. J Travel Med. 2017;24. [DOI] [PubMed] [Google Scholar]
- 4.Halstead S, Wilder-Smith A. Severe dengue in travellers: pathogenesis, risk and clinical management. J Travel Med. 2019;26. [DOI] [PubMed] [Google Scholar]
- 5.Jentes ES, Lash RR, Johansson MA, et al. Evidence-based risk assessment and communication: a new global dengue-risk map for travellers and clinicians. J Travel Med. 2016;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organización Panamericana de la Salud. Casos Reportados de Dengue en las Americas: Pan American Health Organization. Available at: http://www.paho.org/data/index.php/es/temas/indicadores-dengue/dengue-nacional/9-dengue-pais-ano.html?showall=&start=1.
- 7.Instituto Nacional De Salud. Epidemiological week number 51, 2013. Boletín Epidemiológico Semanal 2013. Available at: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2013%20Boletin%20epidemiologico%20Semana%2051.pdf.
- 8.Instituto Nacional De Salud. Epidemiological week number 52, 2014. Boletín Epidemiológico Semanal 2014. Available at: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2014%20Boletin%20epidemiologico%20semana%2052.pdf.
- 9.Instituto Nacional De Salud. Epidemiological week number 52, 2015. Boletín Epidemiológico Semanal 2015. Available at: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2015%20Boletin%20epidemiologico%20Semana%2052.pdf.
- 10.Instituto Nacional De Salud. Epidemiological week number 52, 2016. Boletín Epidemiológico Semanal 2016. Available at: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2016%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2052%20-.pdf.
- 11.Instituto Nacional De Salud. Epidemiological week number 52, 2017. Boletín Epidemiológico Semanal 2017. Available at: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2017%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2052.pdf.
- 12.Villar LA, Rojas DP, Besada-Lombana S, et al. Epidemiological trends of dengue disease in Colombia (2000-2011): a systematic review. PLoS Negl Trop Dis. 2015;9:e0003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattar S, Morales V, Cassab A, et al. Effect of climate variables on dengue incidence in a tropical Caribbean municipality of Colombia, Cerete, 2003-2008. Int J Infect Dis. 2013;17:e358–e359. [DOI] [PubMed] [Google Scholar]
- 14.Instituto Nacional de Salud DdVyAdreSP. Boletín Epidemiológico Semanal 2019. Available at: https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019_Boletin_epidemiologico_semana_44.pdf.
- 15.Londoño-Rentería B, Cárdenas JC, Giovanni JE, et al. Aedes aegypti anti-salivary gland antibody concentration and dengue virus exposure history in healthy individuals living in an endemic area in Colombia. Biomedica. 2015;35:572–581. [DOI] [PubMed] [Google Scholar]
- 16.Dayan G, Arredondo JL, Carrasquilla G, et al. Prospective cohort study with active surveillance for fever in four dengue endemic countries in Latin America. Am J Trop Med Hyg. 2015;93:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiménez MM, Arias J, Carrasquilla G. Seroprevalence of dengue infection in the municipalities of Armenia, Calarcá, La Tebaida and Montenegro in Quindío, 2014. Biomedica. 2017;37:34–41. [DOI] [PubMed] [Google Scholar]
- 18.Velandia-Romero ML, Coronel-Ruiz C, Castro-Bonilla L, et al. Prevalence of dengue antibodies in healthy children and adults in different Colombian endemic areas. Int J Infect Dis. 2019;91:9–16. [DOI] [PubMed] [Google Scholar]
- 19.Castro Rodríguez R, Carrasquilla G, Porras A, et al. The burden of dengue and the financial cost to Colombia, 2010-2012. Am J Trop Med Hyg. 2016;94:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Organización Panamericana de la Salud. Estrategia de Gestión Integrada para la prevención y control del dengue en la Región de las Américas 2017 July 2018. Available at: http://iris.paho.org/xmlui/bitstream/handle/123456789/34859/OPSCHA17039_spa.pdf?sequence=8&isAllowed=y.
- 21.Villar L, Dayan GH, Arredondo-García JL, et al. ; CYD15 Study Group. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. [DOI] [PubMed] [Google Scholar]
- 22.Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med. 2018;379:327–340. [DOI] [PubMed] [Google Scholar]
- 23.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 24.Prentice R. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 25.Rubin D. Multiple Imputation for Nonresponsein Surveys. 1987New York: John Wiley. [Google Scholar]
- 26.Dengue vaccine: WHO position paper – September 2018. Wkly Epidemiol Rec. 2018;93:457–76. [Google Scholar]
- 27.Dengue vaccine: WHO position paper, september 2018 – recommendations. Vaccine. 2019;37:4848–9. [DOI] [PubMed] [Google Scholar]
- 28.Carabali M, Lim JK, Velez DC, et al. Dengue virus serological prevalence and seroconversion rates in children and adults in Medellin, Colombia: implications for vaccine introduction. Int J Infect Dis. 2017;58:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.L’Azou M, Assoukpa J, Fanouillere K, et al. Dengue seroprevalence: data from the clinical development of a tetravalent dengue vaccine in 14 countries (2005-2014). Trans R Soc Trop Med Hyg. 2018;112:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilder-Smith A, Smith PG, Luo R, et al. Pre-vaccination screening strategies for the use of the CYD-TDV dengue vaccine: a meeting report. Vaccine. 2019;37:5137–5146. [DOI] [PubMed] [Google Scholar]
- 31.Wilder-Smith A, Hombach J, Ferguson N, et al. Deliberations of the Strategic Advisory Group of experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis. 2019;19:e31–e38. [DOI] [PubMed] [Google Scholar]
- 32.Flasche S, Smith PG. Sensitivity and negative predictive value for a rapid dengue test. Lancet Infect Dis. 2019;19:465–466. [DOI] [PubMed] [Google Scholar]
- 33.Luo R, Fongwen N, Kelly-Cirino C, et al. Rapid diagnostic tests for determining dengue serostatus: a systematic review and key informant interviews. Clin Microbiol Infect. 2019;25:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonaparte M, Zheng L, Garg S, et al. Evaluation of rapid diagnostic tests and conventional enzyme-linked immunosorbent assays to determine prior dengue infection. J Travel Med. 2019. [DOI] [PubMed] [Google Scholar]
- 35.Sanofi Pasteur. DENGVAXIA®: Full Prescribing Information 2019 December 2019. Available at: https://www.fda.gov/media/124379/download.
- 36.Wilder-Smith A. Serostatus-dependent performance of the first licensed dengue vaccine: implications for travellers. Journal of Travel Medicine. 2018;25. [DOI] [PubMed] [Google Scholar]


