Abstract
Newly emerging or re-emerging arthropod-borne viruses (arboviruses) are important causes of human morbidity and mortality worldwide. Arboviruses such as Dengue (DENV), Zika (ZIKV), Chikungunya (CHIKV), and West Nile virus (WNV) have undergone extensive geographic expansion in the tropical and sub-tropical regions of the world. In the Americas the main vectors of DENV, ZIKV, and CHIKV are mosquito species adapted to urban environments, namely Aedes aegypti and Aedes albopictus, whereas the main vector of WNV is Culex quinquefasciatus. Given the widespread distribution in the Americas and high permissiveness to arbovirus infection, these mosquito species may play a key role in the epidemiology of other arboviruses normally associated with sylvatic vectors. Here, we test this hypothesis by determining the vector competence of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus to Mayaro (MAYV) virus, a sylvatic arbovirus transmitted mainly by Haemagogus janthinomys that has been causing an increasing number of outbreaks in South America, namely in Brazil. Using field mosquitoes from Brazil, female mosquitoes were experimentally infected, and their competence for infection and transmission rates of MAYV was evaluated. We found consistent infection rate for MAYV in Ae. aegypti (57.5%) and Ae. albopictus (61.6%), whereas very low rates were obtained for Cx. quinquefasciatus (2.5%). Concordantly, we observed high potential transmission ability in Ae. aegypti and Ae. albopictus (69.5% and 71.1% respectively), in contrast to Cx. quinquefasciatus, which could not transmit the MAYV. Notably, we found that very low quantities of virus present in the saliva (undetectable by RT-qPCR) were sufficiently virulent to guarantee transmission. Although Ae. aegypti and Ae. albopictus mosquitoes are not the main vectors for MAYV, our studies suggest that these mosquitoes could play a significant role in the transmission of this arbovirus, since both species showed significant vector competence for MAYV (Genotype D), under laboratory conditions.
Author summary
The present study demonstrated that Ae. aegypti and Ae. albopictus mosquitoes can be competent laboratory vectors for MAYV. In contrast, Cx. quinquefasciatus mosquitoes were refractory to MAYV. Regarding the viral dilution and nanoinjection, a higher detection sensitivity was observed after virus nanoinjection into naïve mosquitoes, indicating that only a few viral particles are required to infect mosquitoes, and these particles may not be detected by RT-qPCR before the nanoinjection procedure.
Introduction
The mosquitoes Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus are widely distributed throughout the world, especially in tropical and subtropical regions [1–3]. They are considered a serious concern to public health as vectors of several arboviruses such as DENV, ZIKV, CHIKV, and WNV [4–13]. Studies have shown that Ae. aegypti and Ae. albopictus mosquitoes exhibit laboratory vector competence for the infection and transmission of MAYV, and Cx. quinquefasciatus mosquitoes infected with MAYV have been found in Cuiabá [14–16].
The MAYV was first isolated in 1954 from rural workers in Mayaro, on the island of Trinidad [17] and, like CHIKV, it is an arbovirus of the genus Alphavirus, belonging to the family Togaviridae [18–20]. To date, three MAYV genotypes (D, L and N) have been identified. The D genotype has a wide geographical distribution, occurring in Brazil, Bolivia, Peru, Suriname, Trinidad, Tobago, Argentina, Colombia, and Venezuela [21–25]. The L genotype was isolated in Brazil and Haiti [24] and contains strains detected only in Brazil[26]. On the other hand, the N genotype, was discovered in an outbreak in 2015 in Venezuela [27].
The MAYV is transmitted primarily by the bite of female mosquitoes of the genus Hemagogus, and in nature several vertebrates can host this virus, which is detected in non-human primates, rodents, birds, sloths, and other small mammals [28].
In humans, MAYV infection usually occurs in people with a history of activities in forested areas [18–21]. Long et al. [14], described, in febrile humans, a high load of viral RNA, determined by real-time polymerase chain reaction, ranging from 5.01×102 to 2.18×105 (log10/PFU equivalents/mL). This disease has similar symptomatology to DENV and/or CHIKV, causing an acute and self-limiting febrile illness, which may be accompanied by hemorrhagic phenomena such as petechiae and gingival bleeding. Usually the wrist, ankle, hands, and feet joints are significantly affected, and symptoms may persist for several months, incapacitating the infected person [22,23,29]. In recent years in Brazil, several cases of MAYV have been registered in Pará (2008), Mato Grosso (2012), and Goiás (2014–2016) [20–22,30–34]. Although there is still no evidence of the transmission efficiency of MAYV in an urban cycle, it has the potential to establish an epidemic scenario in the Americas, similar to what occurred with ZIKV and CHIKV [35]. Using mathematical models taking into account outbreaks since 1960 and increasing global temperature, Lorenz et al. [36] predicted that MAYV would expand its area of coverage in the coming years. Thus, the importance of MAYV as a human pathogen with the potential to emerge in urban areas is strong. Therefore, the main objective of this study was to evaluate the vector competence of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus for MAYV, since these mosquitoes may be involved in the dispersion of this virus.
Materials and methods
Ethics statement
The human blood used in all experiments was obtained from a blood bank (Fundação Hemominas), according to the terms of an agreement with the Instituto René Rachou, Fiocruz/MG (OF.GPO/CCO agreement—Nr 224/16).
Mosquito species and rearing
For this study, three mosquito species were used: Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus. Ae. aegypti and Ae. albopictus mosquitoes were collected from ovitraps, whereas Cx. quinquefasciatus were collected using an entomological ladle. All collections occurred in the neighborhood of Pampulha (19.8527° S, 43.9560° W), in the city of Belo Horizonte, Brazil, in the first half of 2017. The Ae. aegypti and Ae. albopictus populations were initiated from at least 2,000 eggs for each species, whereas for Cx. quinquefasciatus, the colony was obtained with more than 3,000 larvae.
Eggs/larvae were transported to the insectary of the René Rachou Institute, Fiocruz/MG, and were kept in a controlled environment, at 27 ± 2°C and ∼82% RH with a 12:12 h light/dark cycle. Larvae were maintained with fish food pellets, Tetramin tropical and GoldFish Colour. After emergence and identification, adults were kept on a 10% sucrose solution regimen, ad libitum. Adult females were fed with human blood in an artificial feeder for egg production. To simulate field conditions and minimize the effects of inbreeding and population colonization, all experiments were conducted with mosquitoes from the same geographic area and up to the third generation.
Mayaro virus culture
MAYV isolated from the serum of an infected human in Trinidad (strain TRVL 4675) in 1954 and belonging to the D genotype was originally acquired from the American Type Culture Collection (ATCC). Full-length sequences are available on GenBank [37]. To prepare the MAYV for oral feeding, a frozen virus stock (an aliquot of MAYV was kindly supplied by the Flavivirus Laboratory of the Oswaldo Cruz Instituto—IOC / Fiocruz) was passaged once through C6/36 cells (approximately 2 million cells) in Leibowitz L-15 medium supplemented with 10% fetal calf serum and maintained at 28°C. The supernatant viral stock was harvested after day 4 (for the second experiment and at 5 days for the first experiment). Both viral titers were quantified after a freeze-thaw cycle and two replicates (A and B) were used to evaluate the mosquito infection rates. For the first replicate, the viral titer was 1×109 PFU/mL, and for the second, 6×109 PFU/mL.
Mosquito infection and transmission analysis
In order to analyze infection rates and transmission rates of MAYV, batches of mosquitoes were analyzed 7 and 14 days after infection (dpi). From each blood-fed mosquito we collected and assayed two samples for MAYV: head + thorax and saliva. Mixed head and thorax samples were assayed for virus to discriminate infection, and the infection rate was defined as the number of samples with detectable virus divided by all samples tested. Samples of nanoinjected saliva were assayed for virus to discriminate transmission, and the transmission rate was defined as the number of saliva samples with detectable virus divided by all samples from mosquitoes with detectably virus in the body (head + thorax). For infection, 5-day-old adult females were allowed to ingest a mixture of viral supernatant and human blood (2:1). On the first replicate, the viral titer was 1×109 PFU/mL, and for the second, 6×109 PFU/mL, which was offered for 45 minutes through glass feeders using pig intestine as the membrane and a water jacket system with the temperature maintained at 38 °C. Immediately after feeding, fully engorged females were separated and maintained with a 10% sucrose solution until the end of the experiment.
Mosquitoes were anesthetized with CO2 and kept on an ice plate while the legs and wings were removed. Each mosquito proboscis was inserted in a 10 μL pipette tip containing a 1:1 solution of 10 μL of 30% sucrose and sterile fetal calf serum. After 30 minutes, the contents of the tips were individually collected in 0.6 mL tubes and stored at −80°C until processing.
In order to test the arboviruses absence in the blood, prior to the experiments, we performed tests for Yellow Fever (YFV), DENV, ZIKV, CHIKV, and MAYV. A group of mosquitoes was fed on the blood sample and, after 10 days post-feeding, these mosquitoes were checked by RT-qPCR. This blood test is a routine procedure in our laboratory to ensure the absence of virus in the blood.
Confirmation of infectious particles in saliva by nanoinjection
To confirm infectivity in saliva, individual samples of undiluted saliva from each mosquito species were nanoinjected into 10–15 naïve Ae. aegypti (mosquitoes that had never had contact with any viruses), using a Nanoject II (Drummond Sci) portable injector. In each mosquito, a 276 nL dose of saliva was nanoinjected intrathoracically (pleural membrane) with a pulled glass capillary. Nanoinjected mosquitoes were collected at 5 days post nanoinjection and, on average, 6 whole mosquitoes, per injected saliva, were processed and analyzed by RT-qPCR.
Virus serial dilution and nanoinjection
To try understanding our findings about the fact some negative mosquito produced positive mosquitoes after saliva nanoinjection (see previous section), we tested the sensitivity of virus detection particles through RT-qPCR. Serial dilutions (10-fold) of known virus stocks were nanoinjected into naïve mosquitoes. Replicates A and B had initial concentrations of 2.09×104 and 5.59×104 viral copies for virus stocks, respectively, and were further diluted 6 times. As a control, uninfected cell supernatant was used. Nanoinjected mosquitoes were collected at 5 days post-nanoinjection and only 5 mosquitoes (whole body), per dilution, were processed and analyzed by RT-qPCR.
Analysis of MAYV by RT-qPCR
Total RNA from mosquitoes was extracted to verify the infection rates (mosquito head+thorax), transmission rates (through saliva nanoinjection into naïve mosquitoes) or serial virus dilution and nanoinjection. The extraction of RNA viral was performed with the High Pure Viral Nucleic Acid Kit (Roche), following the manufacturer's instructions. The thermocycling conditions were as follows: reverse transcription at 50°C for 10 min, RT inactivation/initial denaturation at 95°C for 30s, 40 cycles of 95°C for 5 s and 60°C for 30s, followed by cooling at 37°C for 30s. The volume of the reaction was 10 μL (5x LightCycler Multiplex RNA Virus Master (Roche), with 1 μM primers, 0.2 μM probe, and 125 ng RNA.
MAYV infection/transmission rates in mosquitoes was quantified by RT-qPCR using a LightCycler 96 (Roche). A multiplex assay was performed according to previous studies, with the MAYV primers MAYVF 5′-GTG GTC GCA CAG TGA ATC TTT C-3′ and MAYVR 5′-CAA ATG TCC ACC AGG CGA AG-3 and the May-Probe 5′-FAM/ATG GTG GTA GGC TAT CCG ACA GGT C/3lABkFQ-3′ (14). For the mosquito control we used the ribosomal gene S17 (RPS17) primers 17S-F 5′-TCC GTG GTA TCT CCA TCA AGC T-3′ and 17S-R 5′-CAC TTC CGG CAC GTA GTT GTC-3′ and the probe 5′-HEX/CAG GAG GAG GAA CGT GAG CGC AG/3BHQ2-3’ (33). All samples were tested in duplicate for MAYV, and the viral genome was determined by comparison with a standard curve using serial dilutions of the target gene cloned into the pGEMT-Easy plasmid (Promega).
Data analysis
Mosquito infection rate was analyzed with both Person omnibus normality and D’Agostino tests. Fisher’s exact test was then used to assess differences in viral prevalence. Comparisons were significant for P values lower than 0.05, and viral load data were compared through the Mann-Whitney U test. All analyses were performed by using Prism V 7.4 (GraphPad).
Results
MAYV infection in Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus
Our analysis of different post-infection time points showed that Ae. aegypti and Ae. albopictus became positive for MAYV. In addition, in both replicates (Fig 1A and 1B) there was increased infection rate at 14 dpi compared with 7 dpi in Ae. aegypti and Ae. albopictus. On the other hand, Cx. quinquefasciatus mosquitoes were shown to be refractory to MAYV.
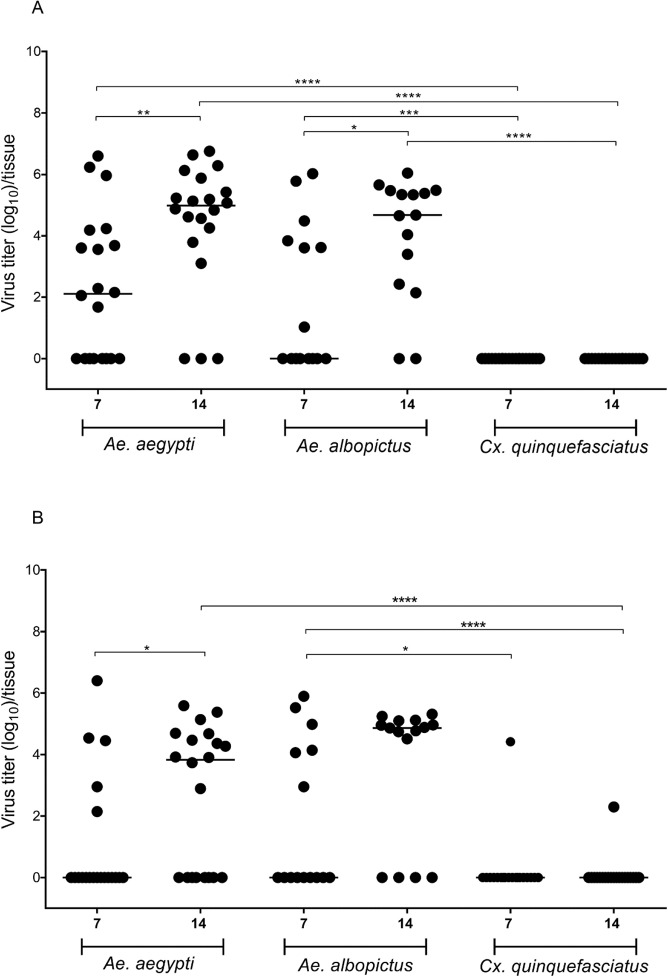
Fig 1.
Mosquito viral infection rate (replicates A and B). Each point represents a single head+thorax of adult female, and the black lines indicate the median copy number of the Mayaro virus in each group. The viral titer in the infective blood meal was 1×109 PFU/mL and, and 6×109 PFU/mL respectively for the replicates A and B. The asterisks represent P < 0.05 after the Mann-Whitney U-Test.
In replicate A (Fig 1) at 7 dpi, we observed differences in the infection rate between Cx. quinquefasciatus and Ae. aegypti (p < 0.0001) or Ae. albopictus (p = 0.0010), but no difference was observed between Ae. aegypti and Ae. albopictus. Considering the infection rate at 14 dpi, the same pattern was observed: Cx. quinquefasciatus was significantly different from Ae. aegypti (p < 0.0001) and Ae. albopictus (p < 0.0001). Comparisons at 7 and 14 dpi for the same mosquito species showed differences for Ae. aegypti (p = 0.0043) and Ae. albopictus (p = 0.0230), clearly showing increasing infection over time.
For replicate B (Fig 1) at 7 dpi, we only observed differences in the infection rate between Cx. quinquefasciatus and Ae. albopictus (p = 0.0207). At 14 dpi, we observed significantly different infection rate between Cx. quinquefasciatus and both Ae. aegypti (p < 0.0001) and Ae. albopictus (p < 0.0001). When we compared infection rates at the two time points (7 and 14 dpi), the only mosquito species that showed a difference was Ae. aegypti (p = 0.0262); however, a nonsignificant increasing infection rate trend was observed for Ae. albopictus.
In general, our combined results from both 7 and 14 dpi show higher susceptibility to MAYV in Ae. albopictus (61.6%), followed by Ae. aegypti (57.5%). However, Cx. quinquefasciatus mosquitoes may be considered refractory to MAYV infection (with only a 2.5% infection rate). The infection rates of MAYV, in each species, in both experiments, and at different dpi are shown in Table 1.
Table 1. Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus orally infected with Mayaro virus.
The initial viral titer was determined by plaque-forming units (PFU/mL). Infected/total mosquito numbers are shown parenthesis.
| MAYV | Titer (PFU/mL) | Days post- infection | Ae. aegypti | Ae. albopictus | Cx. quinquefasciatus |
|---|---|---|---|---|---|
| Infection rate % | |||||
| Replicate A | 1×109 | 7 | 60 (12/20) | 46.6 (7/15) | 0 (0/20) |
| 14 | 85 (17/20) | 86.6 (13/15) | 0 (0/20) | ||
| Replicate B | 6×109 | 7 | 25 (5/20) | 40 (6/15) | 5 (1/20) |
| 14 | 60 (12/20) | 73.3 (11/15) | 5 (1/20) | ||
Amplification of MAYV in mosquitoes after nanoinjection
To verify the occurrence of MAYV in mosquito saliva, we added a virus amplification step in live Ae. aegypti. Using preamplification by nanoinjection in mosquitoes, MAYV virus was detected in 69.5% (n = 69) of the individual saliva samples from orally infected Ae. aegypti with detectable amount of virus in their bodies at days 7 and 14 after infection. Likewise, for Ae. albopictus 71.1% (n = 59) of the mosquitoes with detectable amounts of virus in their bodies also expelled virus in saliva. These results show that orally infected Ae. aegypti and Ae. albopictus from Brazil could transmit the virus in their saliva and thus are competent laboratory vectors of MAYV.
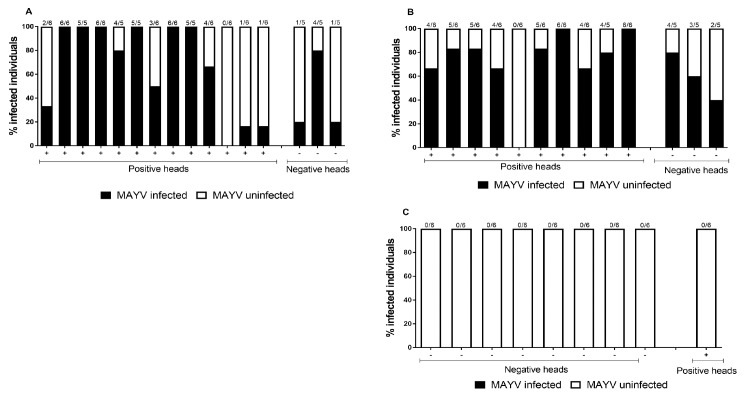
In contrast, none of the 54 nanoinjected mosquitoes with Cx. quinquefasciatus saliva were able to become infected. We also nanoinjected saliva from negative Ae. aegypti and Ae. albopictus mosquitoes (through RT-qPCR) and, surprisingly, some samples were able to infect naïve mosquitoes. Saliva samples from 3 negative Ae. aegypti (Fig 2A) were nanoinjected into 15 mosquitoes, and 6 (40%) became infected with MAYV. Saliva from 3 negative Ae. albopictus (Fig 2B) was nanoinjected in 15 mosquitoes, and 9 (60%) became infected with MAYV.
Fig 2. Nanoinjection of saliva from three infected mosquito species into naïve Aedes aegypti mosquitoes.
Saliva samples were collected from Aedes aegypti (A), Aedes albopictus (B), and Culex quinquefasciatus (C), which were previously infected with MAYV (at 14 dpi), followed by injection into naïve mosquitoes. Mosquitoes that became infected are shown in black and uninfected are depicted in white. Each bar represents a single saliva sample, and the number of transmission rate mosquitoes nanoinjected mosquitoes is given at the top of each bar.
Virus serial dilution and nanoinjection
In order to try to understand why negative mosquitoes (through RT-qPCR) are able to produce infectious saliva, we performed a series of virus dilutions and injections into naïve mosquitoes, followed by RT-qPCR detection. We found that some viral samples, upon dilution, are not detected through RT-qPCR but are able to infect naïve mosquitoes, using the nanoinjection methodology. Our results showed that RT-qPCR had a detection limit of around 10 copies of the MAYV genome.
Discussion
To our knowledge, this is the first study to examine the vector competence of Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus mosquitoes for MAYV (genotype D) in Brazil. Our results show that Ae. aegypti mosquitoes can become infected with MAYV, and in general, these mosquitoes present high infection rates at 14 dpi, averaging 9.6×104 to 4.7×104 viral genome copies per mosquito for replicates A and B, respectively (Fig 1).
Burstolin et al., 2018 [38] evaluated two strains of MAYV; the genotype L strain isolated from Hg. janthinomys mosquitoes in Para, Brazil, in March 1991, and the genotype D strain originally isolated from a monkey in Para, Brazil, in May 1978. These authors observed in Ae. aegypti that the genotype L exhibited significantly higher infection rates (86.2% 7 dpi and 51.7% 14 dpi) when compared with the genotype D strain (7.1% at 7 dpi and 0% at 14 dpi). In the present study, using the genotype D strain, we observed higher infection rates (Fig 1 replicates A and B together): 42.5% at 7 dpi and 72.5% at 14 dpi. However, we have to take into consideration that the genetic background of the vector can strongly influence its susceptibility to the virus [39], as well as the relationship between viral titer and infection rate. When we try to look at the relationship between viral titer and percentage of infection rate, we see that Diop et al., 2019 [40] using MAYV genotype L with a viral titer of 1×106 focus forming unit (FFU/mL) obtained 53.8% infection rate in Ae. aegypti, which was like our infection rate (57.5%). Burstolin et al., 2018 [38] with a viral titer of 1×107 FFU/mL obtained an infection rate of 86.2% (on 7dpi) and 51.7% (14 dpi) in Ae. aegypti. Yet, in our experiments, even with a viral titer 2 logs above, we obtained 60% infection rate at 7dpi and 85% at 14dpi for replicate A and 25% at 7dpi and 60% at 14dpi for replicate B (Fig 1).
Our results showed an increasing infection rate over time and overall lower infection rate compared to Burstolin et al., 2018 [38], which even with lower viral titer obtained more infected mosquitoes, but with decreasing infection rate over time. Furthermore, these authors noted significantly higher titers in mosquito bodies (7 and 14 dpi), which in most cases were greater than 1×106 viral genome copies per mosquito.
Yet, our results show that although our viral titer is high, fewer mosquitoes passed the range of 1×106 viral genome copies per mosquito. However, we would like to point out that such comparisons are difficult to make since the genotype/isolate and evaluation used by Burstolin et al., 2018 [38] and Diop et al., 2019 [40] were different from what we used.
A previous study by Pereira et al. [41] showed that Ae. aegypti mosquitoes from Rio de Janeiro were highly susceptible to MAYV, presenting a higher number of viral genome copies per mosquito than those obtained in this study. However, this difference may be related to the viral input at the time of infection or even the genetics of the mosquitoes used in our study. Regarding genetic variability, Gokhale et al. [42] suggested that for CHIKV, which is a similar virus to MAYV, the vector genetic background strongly influence the susceptibility to the virus [43].
MAYV cases have been reported in the North, Northeast and Center-West regions of Brazil [21,22,30–33]. Ae. aegypti mosquitoes naturally infected with MAYV have been found in Cuiabá, Mato Grosso [15], but, at this time, they cannot be incriminated as mosquito species for this virus. Thus, to evaluate viral transmission rate, MAYV-infected saliva of Ae. aegypti was nanoinjected into naïve mosquitoes, with 69% of mosquitoes becoming infected. Other laboratory studies also confirmed the ability of this vector to transmit MAYV [14,16,41]. Therefore, our results confirm that this mosquito species has great potential for infection/transmission rates of MAYV and, therefore, could play an important role in the transmission of this virus, if it becomes urbanized.
Ae. albopictus mosquitoes were found to have a similar infection rate to Ae. aegypti, showing high susceptibility to MAYV. At 14 dpi, significant numbers of viral particles were observed in this species. So far, there are few studies showing the relationship between MAYV and Ae. albopictus.
Smith and Francy [44], evaluated the vector efficiency of a Brazilian Ae. albopictus mosquito line fed on viremic hamster blood for MAYV and found that the infection rate ranged from 9% to 16%. The authors classified this strain as being relatively refractory to MAYV infection but suggested that it may become more susceptible, serving as a secondary vector in an outbreak or as a bridge vector between MAYV transmission cycles. Wiggins et al. [16] observed in an oral infection experiment that Ae. albopictus mosquitoes had a significantly higher infection rate than Ae. aegypti mosquitoes, a similar pattern observed in our study.
Diop et al., 2019 [40] using a viral titer of 1×106 FFU/mL (MAYV genotype L) obtained in mosquitoes Ae. albopictus 76.6% of infection rate. The authors also report the increased expression levels of thioester containing protein 22 (TEP22) and Niemann–Pick type C1 (NPC1- gene responsible for facilitating mosquito infection when infected with Dengue) gene transcripts were observed in infected Ae. albopictus. In our results, although with a different genotype, we had less infected mosquitoes when fed high viral titer (61.6%—MAYV genotype D), this fact may indicate that for MAYV the viral titer may not be primarily responsible for the success of the infection, but secondary factors such as the viral genotype, the expression of genes related to the mosquito immune system or even the genetic background of the vector.
Infection rates were significantly higher in Ae. albopictus (85%–100%) than in Ae. aegypti (67–82%). The same mosquito species may present differentiated vector competence at different sites, since different genotype/genotype interactions between the virus and vector may occur. As an example, it has been demonstrated that the re-emergence of the CHIKV may have been facilitated by the genetic adaptation of the virus to the Ae. albopictus vector [45,46].
In 2016, the first imported case of MAYV in a French citizen was reported in an area where the Ae. albopictus mosquito is well established [47], thus highlighting the need to better understand the vector competence of this mosquito, as well as its possible role in the transmission of MAYV.
To evaluate viral transmission rate in our experiments, Ae. albopictus mosquito saliva submitted to MAYV infection was nanoinjected into naïve mosquitoes, resulting in a high rate of infectivity. Smith and Francy [44], noted in their study that approximately half (5/11) of the mosquitoes infected with hamster viremic blood were able to transmit MAYV when their saliva was tested in capillary tubes. Wiggins et al. [16] observed that Ae. albopictus mosquitoes exhibited low transmission rate of MAYV in saliva expectorates. However, these authors used a different methodology.
We also attempted to study whether saliva originating from negative mosquitoes samples were able to infect naïve mosquitoes. Unexpectedly, negative mosquitoes samples from Ae. aegypti and Ae. albopictus were able to infect other mosquitoes through their saliva. Previous experiments in our group have shown the same effect for DENV with a negative thorax and positive saliva, but it resulted in a lower infection rate.
Furthermore, some studies have described similar results in Ae. aegypti and Cx. quinquefasciatus with ZIKV, DENV, and WNV [48–50]. One important hypothesis to consider is that over the course of infection, viral decline occurs in other tissues, but the salivary glands/saliva remains positive for transmission. Furthermore, we suspect that mosquito salivation may, in some cases, deplete the glands of almost all viral particles, which could not be detected (in the head) through RTq-PCR.
To better understand these findings, we investigated the relationship between the amounts of (detectable) particles required for mosquito infection. It was possible to observe that samples classified as negative for RT-qPCR were able to infect other mosquitoes, indicating that only a few viral particles are necessary to initiate infection. In addition, it was observed that regardless of the nanoinjected viral doses they produced between 5.6×105 to 1.4×106 viral genome copies per mosquito respectively for replicates A and B (S1 Fig). Therefore, nanoinjection of the viral saliva/dilution in the mosquito acts as a model that amplifies the particles and facilitates later detection [51]. This may explain why saliva from heads classified as negative produced positive mosquitoes upon injection. We believe that only a few viral particles, which cannot be detected through RT-qPCR, are required to infect a mosquito. We suggest that for a broader understanding of this finding, complementary studies using immunofluorescence assays to detect transmission through saliva originating from PCR-negative heads should be undertaken.
When evaluating the vector competence of Cx. quinquefasciatus, only two mosquitoes were found to be positive for MAYV, and no mosquito became infected after injection with saliva from these Cx. quinquefasciatus mosquitoes submitted to MAYV infection. Corroborating our data, Brustolin et al. [38], also tested the vector competence of Cx. quinquefasciatus and observed that this mosquito had either poor or null infection and transmission rates for MAYV.
Cx. quinquefasciatus is quite abundant in Brazil, and to date there is only a single record of this mosquito harboring MAYV in Cuiabá [15]. This mosquito is, however, a vector of Wuchereria bancrofti in Brazil, an etiologic agent of lymphatic filariasis in humans [52], and was recently incriminated in ZIKV transmission in the metropolitan region of Pernambuco [8]. Guo et al. [48], have also demonstrated the vector competence of Cx. quinquefasciatus for ZIKV in China. In contrast, several other studies have demonstrated the lack of ability of this mosquito to infect and transmit ZIKV [53–55]. These results confirm that the vector competence of the same mosquito species can vary geographically, emphasizing the importance of studying the vector competence of different mosquitoes and from different localities.
In conclusion, our studies show that, under laboratory conditions, Ae. aegypti and Ae. albopictus can be infected and potentially transmit MAYV (genotype D), although Cx. quinquefasciatus exhibited poor vector competence. To our knowledge this is the first report of a study involving the vector competence of a particular MAYV genotype, done simultaneously with three mosquito species. Ae. aegypti and Ae. albopictus are widely distributed throughout the Americas [3,56], and although they are not the main vectors for MAYV, they can potentially play a significant role in the transmission rate of this virus. We suggest that further studies should be conducted to demonstrate the vector competence of the same species towards other MAYV genotypes and even isolates, since distinct isolates of the same virus can behave differently on the same vector species. Furthermore, studies to demonstrate the co-infections with other arboviruses, as well as ecological studies on the recent YFY outbreaks, considering that Hg. janthinomys is a common vector for both viruses.
Supporting information
A and B represent different replicates. Each viral dilution (represented by green dots) was nanoinjected into naïve mosquitoes, followed by virus detection through RT-qPCR (red dots). Samples 8A and 8B are mock controls. Each red spot represents a single female mosquito.
(DOCX)
Acknowledgments
We wish to thank you Dra. Ana Maria Bispo de Filippis (IOC—FIOCRUZ) for donating the MAYV isolate. We are in debt to Dr. Marco Antônio Silva Campos and Dr. Alexandre de Magalhães Vieira Machado and their team, who provided viral culture infrastructure in the Laboratório de Imunologia de Doenças Virais (IRR—FIOCRUZ). We are grateful to all members from the Group Mosquitos Vetores: Endossimbiontes e Interação Patógeno-Vetor, (IRR—FIOCRUZ), especially for Dr. Alvaro Gil Araujo Ferreira for his critical reading of the manuscript. We are grateful to Belo Horizonte municipality and the Universidade Federal de Minas Gerais who helped to collect mosquito samples.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, FAPEMIG, CNPq (LAM) and, indirectly, by the World Mosquito Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett [Internet]. 2005;8(5):558–74. Available from: 10.1111/j.1461-0248.2005.00755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ministério da Saúde B. Guia de vigilância do Culex quinquefasciatus. 2011. 80 p.
- 3.Kraemer MUG, Sinka ME, Duda KA, Mylne AQN, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. Albopictus. Elife. 2015;4(JUNE2015):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9(1):71–86. 10.1017/S1466252307001430 [DOI] [PubMed] [Google Scholar]
- 5.Ng LC, Hapuarachchi HC. Tracing the path of Chikungunya virus-Evolution and adaptation. Infect Genet Evol [Internet]. 2010;10(7):876–85. Available from: 10.1016/j.meegid.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Dibo MR, Menezes RMT De, Ghirardelli CP, Mendonça AL, Chiaravalloti Neto F. The presence of Culicidae species in medium-sized cities in the State of São Paulo, Brazil and the risk of West Nile fever and other arbovirus infection. Rev Soc Bras Med Trop. 2011;44(4):496–503. 10.1590/s0037-86822011000400019 [DOI] [PubMed] [Google Scholar]
- 7.Staples JE, Fischer M. Chikungunya at the Door—Déjà Vu All Over Again? N Engl J Med [Internet]. 2014;371(10):885–7. Available from: 10.1056/NEJMp1408509 [DOI] [PubMed] [Google Scholar]
- 8.Guedes DRD, Paiva MHS, Donato MMA, Barbosa PP, Krokovsky L, Rocha SWS, et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg Microbes Infect. 2017;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ottesen EA, Ramachandran C. Lymphatic Filariasis Infection and Disease: Control Strategies. Parasitol Today. 1995;11(4):129–31. [Google Scholar]
- 10.Gardner CL, Ryman KD, Genetics M. HHS Public Access. Clin Lab Med. 2015;30(1):237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika Virus in Gabon (Central Africa) - 2007: A New Threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samy AM, Elaagip AH, Kenawy MA, Ayres CFJ, Peterson AT, Soliman DE. Climate Change Influences on the Global Potential Distribution of the Mosquito Culex quinquefasciatus, Vector of West Nile Virus and Lymphatic Filariasis. PLoS One [Internet]. 2016;11(10):e0163863 Available from: 10.1371/journal.pone.0163863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabuto E, Rebelo MT. The Asian tiger mosquito, Aedes albopictus (Skuse, 1894), a vector of dengue, chikungunya and zika, reaches Portugal. bioRxiv [Internet]. 2017;192575 Available from: https://www.biorxiv.org/content/early/2017/09/22/192575 [DOI] [PubMed] [Google Scholar]
- 14.Long KC, Ziegler SA, Thangamani S, Hausser NL, Kochel TJ, Higgs S, et al. Experimental transmission of Mayaro virus by Aedes aegypti. Am J Trop Med Hyg. 2011;85(4):750–7. 10.4269/ajtmh.2011.11-0359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira Serra O, Fernandes Cardoso B, Maria Ribeiro AL, dos Santos FAL, Dezengrini Slhessarenko R. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2016;111(1):20–9. 10.1590/0074-02760150270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiggins K, Eastmond B, Alto BW. Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes. Med Vet Entomol. 2018;1–7. [DOI] [PubMed] [Google Scholar]
- 17.Casals J, Whitman L. Mayaro virus: a new human disease agent. I. Relationship to other arbor viruses. Am J Trop Med Hyg. 1957;6(6):1004–11. 10.4269/ajtmh.1957.6.1004 [DOI] [PubMed] [Google Scholar]
- 18.Vasconcelos PFC, Travassos da Rosa APA, Degallier N, Travassos da Rosa JFS, Pinheiro FP. Clinical and ecoepidemiological situation of human arboviruses in Brazilian Amazonia. Cienc Cult [Internet]. 1992;44:117–24. Available from: http://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_6/b_fdi_33-34/38273.pdf [Google Scholar]
- 19.Coimbra TL, Santos CL, Suzuki a, Petrella SM, Bisordi I, Nagamori a H, et al. Mayaro virus: imported cases of human infection in Sao Paulo State, Brazil. Rev Inst Med Trop Sao Paulo [Internet]. 2007;49(4):221–4. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17823750 10.1590/s0036-46652007000400005 [DOI] [PubMed] [Google Scholar]
- 20.Azevedo RSS, Silva EVP, Carvalho VL, Rodrigues SG, Nunes Neto JP, Monteiro HAO, et al. Mayaro fever virus, Brazilian amazon. Emerg Infect Dis. 2009;15(11):1830–2. 10.3201/eid1511.090461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinheiro F, Freiras R, Rosa -Tracassos J, Yvone G, Wyller M, Leduc J. An outbreak of mayaro virus disease. Am J Trop Med Hyg. 1981;30(3):674–81. 10.4269/ajtmh.1981.30.674 [DOI] [PubMed] [Google Scholar]
- 22.Mourão MPG, Bastos M de S, de Figueiredo RP, Gimaque JBL, dos Santos Galusso E, Kramer VM, et al. Mayaro Fever in the City of Manaus, Brazil, 2007–2008. Vector-Borne Zoonotic Dis [Internet]. 2012;12(1):42–6. Available from: http://www.liebertonline.com/doi/abs/10.1089/vbz.2011.0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halsey ES, Siles C, Guevara C, Vilcarromero S, Jhonston EJ, Ramal C, et al. Mayaro virus infection, Amazon Basin region, Peru, 2010–2013. Emerg Infect Dis. 2013;19(11):1839–42. 10.3201/eid1911.130777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lednicky J, Madsen V, Rochars B De, Elbadry M, Loeb J, Telisma T, et al. Mayaro Virus in Child with Acute. 2016;22(11):2015–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forshey BM, Guevara C, Laguna-torres VA, Cespedes M, Vargas J, Aguayo N, et al. Arboviral Etiologies of Acute Febrile Illnesses in Western South America, 2000–2007. 2010;4(8):2000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powers Ann M, Russell KL, Olson J, Vasconcelos PFC, Travassos A, Rosa DA, et al. Genetic relationships among mayaro and una viruses suggest distinct patterns of transmission. 2006;75(3):461–9. [PubMed] [Google Scholar]
- 27.Auguste AJ, Liria J, Forrester NL, Giambalvo D, Moncada M, Long KC, et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an. 2015;21(10);13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thoisy B, Gardon J, Salas R, Morvan J, Kazanji M. Mayaro Virus in wild mammals, Frensch Guiana. Emerg Infect Dis. 2003;9(10);12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slegers CAD, Keuter M, Günther S, Schmidt-Chanasit J, van der Ven AJ, de Mast Q. Persisting arthralgia due to Mayaro virus infection in a traveler from Brazil: Is there a risk for attendants to the 2014 FIFA World Cup? J Clin Virol [Internet]. 2014;60(3):317–9. Available from: 10.1016/j.jcv.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo RMP, Thatcher BD, De Lima ML, Almeida TC, Alecrim WD, De Farias Guerra MV. Doenças exantemáticas e primeira epidemia de dengue ocorrida em Manaus, Amazonas, no período de 1998–1999. Rev Soc Bras Med Trop. 2004;37(6):476–9. 10.1590/s0037-86822004000600009 [DOI] [PubMed] [Google Scholar]
- 31.Tavares-Neto J, Freitas-Carvalho J, Teixeira Nunes MR, Rocha G, Guerreiro Rodrigues S, Damasceno E, et al. Pesquisa de anticorpos contra arbovírus e o vírus vacinal da febre amarela em uma amostra da população de Rio Branco, antes e três meses após a vacina 17D. Rev Soc Bras Med Trop. 2004;37(1):1–6. 10.1590/s0037-86822004000100001 [DOI] [PubMed] [Google Scholar]
- 32.Silva-Nunes M da, Malafronte R dos S, Luz B de A, Souza EA de, Martins LC, Rodrigues SG, et al. The Acre Project: the epidemiology of malaria and arthropod-borne virus infections in a rural Amazonian population. Cad Saude Publica [Internet]. 2006;22(6):1325–34. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2006000600021&lng=en&tlng=en 10.1590/s0102-311x2006000600021 [DOI] [PubMed] [Google Scholar]
- 33.Zuchi N, Silva Heinen LB, Santos MAM, Pereira FC, Slhessarenko RD. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem Inst Oswaldo Cruz. 2014;109(6):820–3. 10.1590/0074-0276140108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunini S, Fraça D, Silva J, Rezza G. High Frequency of Mayaro Virus IgM among Febrile Patients, Central Brazil—Volume 23, Number 6—June 2017—Emerging Infectious Disease journal—CDC. Emerg Infect Dis [Internet]. 2017;23(6):1025–6. Available from: http://wwwnc.cdc.gov/eid/article/23/6/16-0929_article.htm 10.3201/eid2306.160929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas D, Esposito A, Antonio B. Review article Will Mayaro virus be responsible for the next outbreak of an arthropod-borne virus in Brazil? Brazilian J Infect Dis [Internet]. 2017;21(5):540–4. Available from: 10.1016/j.bjid.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz C, Azevedo TS, Virginio F, Aguiar BS, Chiaravalloti-Neto F, Suesdek L. Impact of environmental factors on neglected emerging arboviral diseases. PLoS Negl Trop Dis. 2017;11(9):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantor AM, Lin J, Wang A, Thompson DC, Franz AWE. Vector / Pathogen / Host Interaction, Transmission Infection Pattern of Mayaro Virus in Aedes aegypti (Diptera: Culicidae) and Transmission Potential of the Virus in Mixed Infections With Chikungunya Virus. 2019;56(1):832–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell. 2009;139(7):1268–78. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 39.Pereira TN, Rocha MN, Sucupira PHF, Carvalho FD, Moreira LA. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci Rep [Internet]. 2018;8(1):1–9. Available from: 10.1038/s41598-017-17765-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrington LB, Chau B, Tran N, Thanh N, Le H, Thi T, et al. Field- and clinically derived estimates of Wolbachia—mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. 2018;115(2):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brustolin M, Pujhari S, Henderson CA, Rasgon JL. Anopheles mosquitoes may drive invasion and transmission of Mayaro virus across geographically diverse regions. 2018;(7)1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, et al. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. 2009;11(3)1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diop F, Alout H, Diagne CT, Baronti C, Vargas REM, De AN. Di ff erential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus. 2019:12 (4)1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gokhale MD, Paingankar MS, Sudeep AB, Parashar D. Chikungunya virus susceptibility & variation in populations of Aedes aegypti (Diptera: Culicidae) mosquito from India. Indian J Med Res. 2015;142(12):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasconcelos PFC, Calisher CH. Emergence of Human Arboviral Diseases in the Americas, 2000–2016. Vector-Borne Zoonotic Dis [Internet]. 2016;16(5):295–301. Available from: http://online.liebertpub.com/doi/10.1089/vbz.2016.1952 [DOI] [PubMed] [Google Scholar]
- 46.Smith GC, Francy DB. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J Am Mosq Control Assoc [Internet]. 1991;7(1):89–93. Available from: http://europepmc.org/abstract/MED/1646286 [PubMed] [Google Scholar]
- 47.Tsetsarkin KA, McGee CE, Higgs S. Chikungunya virus adaptation to Aedes albopictus mosquitoes does not correlate with acquisition of cholesterol dependence or decreased pH threshold for fusion reaction. Virol J. 2011;8(3):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):1895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llagonne-Barets M, Icard V, Leparc-Goffart I, Prat C, Perpoint T, André P, et al. A case of Mayaro virus infection imported from French Guiana. J Clin Virol [Internet]. 2016;77:(12)66–8. Available from: 10.1016/j.jcv.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 50.Guo XX, Li CX, Deng YQ, Xing D, Liu QM, Wu Q, et al. Culex pipiens quinquefasciatus: A potential vector to transmit Zika virus. Emerg Microbes Infect. 2016;5(9):e102–5. 10.1038/emi.2016.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smartt CT, Richards SL, Anderson SL, Erickson JS. West Nile Virus Infection Alters Midgut Gene Expression in Culex pipiens quinquefasciatus Say (Diptera: Culicidae). 2009;81(2):258–63. [PMC free article] [PubMed] [Google Scholar]
- 52.Tsujimoto H, Hanley KA, Sundararajan A, Devitt NP, Schilkey D, Hansen IA. Dengue virus serotype 2 infection alters midgut and carcass gene expression in the Asian tiger mosquito, Aedes albopictus. 2017;12(3)1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demarquay J-N. Note on a Tumour of the Scrotal Sac Containing a Milky Fluid (Galactocele of Vidal) and Enclosing Small Wormlike Beings That Can be Considered as Hematoid Helminths in the Embryos Stage’. Trop Med Parasitoloqy Class Investig. 1863;11(7):374–7. [Google Scholar]
- 54.Fernandes RS, Campos SS, Ribeiro PS, Raphael LMS, Bonaldo MC, Lourenço-de-Oliveira R. Culex quinquefasciatus from areas with the highest incidence of microcephaly associated with Zika virus infections in the northeast region of Brazil are refractory to the virus. Mem Inst Oswaldo Cruz. 2017;112(8):577–9. 10.1590/0074-02760170145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amraoui F, Atyame-Nten C, Vega-Rúa A, Lourenço-De-Oliveira R, Vazeille M, Failloux AB. Culex mosquitoes are experimentally unable to transmit zika virus. Eurosurveillance. 2016;21(35):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duchemin JB, Mee PT, Lynch SE, Vedururu R, Trinidad L, Paradkar P. Zika vector transmission risk in temperate Australia: A vector competence study. Virol J. 2017;14(1):1–10. 10.1186/s12985-016-0669-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A and B represent different replicates. Each viral dilution (represented by green dots) was nanoinjected into naïve mosquitoes, followed by virus detection through RT-qPCR (red dots). Samples 8A and 8B are mock controls. Each red spot represents a single female mosquito.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.