Highlights
-
•
Genetic differences in 30 wheat cultivars in flag-leaf photosynthesis rate were correlated with differences in grain yield under high N conditions.
-
•
Post-anthesis flag-leaf photosynthesis rate was positively associated with pre-anthesis N uptake under high N conditions.
-
•
Genetic differences in Normalized Difference Vegetation Index during late grain filling were associated with differences in grain yield under high N and low N conditions.
-
•
Cultivar grain protein deviation was associated with post-anthesis N uptake under N limitation.
Keywords: N remobilization, Leaf photosynthesis, Leaf senescence, Grain protein deviation, Wheat
Abstract
Nitrogen (N) fertilizer represents a significant cost for the grower and may also have environmental impacts through nitrate leaching and N2O (a greenhouse gas) emissions associated with denitrification. The objectives of this study were to quantify the genetic variability in N partitioning and N remobilization in Indian spring wheat cultivars and identify traits for improved grain yield and grain protein content for application in breeding N-efficient cultivars. Twenty-eight bread wheat cultivars and two durum wheat cultivars were tested in field experiments in two years in Maharashtra, India. Growth analysis was conducted at anthesis and harvest to assess above-ground dry matter (DM) and dry matter and N partitioning. Flag-leaf photosynthesis rate (Amax), flag-leaf senescence rate and canopy normalized difference vegetation index (NDVI) were also assessed. Significant N × genotype level interaction was observed for grain yield and N-use efficiency. There was a positive linear association between post-anthesis flag-leaf Amax and grain yield amongst the 30 genotypes under high N (HN) conditions. Flag-leaf Amax was positively associated with N uptake at anthesis (AGNA). Under both HN and low N (LN) conditions, higher N uptake at anthesis was associated with delayed onset of flag-leaf senescence and higher grain yield. Under N limitation, there was a genetic negative correlation between grain yield and grain protein concentration. Deviation from this negative relationship (grain protein deviation or GPD) was related to genotypic differences in post-anthesis N uptake. It is concluded that N uptake at anthesis was an important determinant of flag-leaf photosynthesis rate and grain yield under high N conditions; while post-anthesis N uptake was an important determinant of GPD of wheat grown under low to moderate N conditions in India.
1. Introduction
Nitrogen (N) fertilizer represents a significant cost for the grower and may also have environmental impacts through nitrate leaching and N2O (a greenhouse gas) emissions associated with denitrification by soil bacteria. Breeding of N-efficient cultivars is one approach to reduce N fertilizer inputs while maintaining acceptable yields and grain protein content (Foulkes et al., 2009). N-use efficiency (NUE) can be defined as the grain dry matter (DM) yield (kg DM ha−1) divided by the supply of available N from the soil and fertilizer (kg N ha−1; Moll et al., 1982). N-use efficiency can be further sub-divided into N-uptake efficiency (above-ground N uptake/N supply from soil N and fertilizer N; NUpE) and N-utilization efficiency (grain DM yield/above-ground N uptake; NUtE). The relative contributions of NUpE and NUtE to genetic variation in NUE in wheat vary according to N fertilizer management and environment as reported in previous field studies (Ortiz-Monasterio et al., 1997 ; Foulkes et al., 2007; Brancourt-Hulmel et al., 2003; Muurinen et al., 2006; Barraclough et al., 2010; Le Gouis et al., 2010; Gaju et al., 2011; Sadras and Lawson, 2013). These studies overall indicated that at low N supply the genetic variation in NUE was more usually associated with NUpE, whereas at high N supply it was more usually associated with NUtE. We previously showed genetic variation in NUE for 30 Indian wheat cultivars under high N and low N conditions was associated with variation in both NUpE and NUtE (Nehe et al., 2018). Therefore, understanding the physiological basis of variation in NUpE and NUtE may offer avenues to increase NUE in wheat grown in India and elsewhere in the world.
Physiological traits affecting genetic variation in NUpE mainly relate to the ability to maintain root activity during the grain-filling period and/or regulation of N uptake by plant N status (Foulkes et al., 2009; Hawkesford, 2014). Physiological traits reported to affect genetic variation in NUtE in wheat include flag-leaf photosynthesis rate (Gaju et al., 2016), N partitioning at anthesis (Pask et al., 2012; Gaju et al., 2014) and post-anthesis N remobilization and leaf and canopy senescence kinetics (Bogard et al., 2010; Gaju et al., 2011, 2014). Genetic variation in flag-leaf photosynthesis rate was associated with grain yield in eight spring wheat cultivars in Mexico (Fischer et al., 1998), in 18 winter wheat cultivars in China (Jiang et al., 2003) and in 18 facultative wheat cultivars in China (Zheng et al., 2011a, 2011b). Gaju et al. (2016) showed that pre-anthesis flag-leaf photosynthesis rate was positively associated with grain yield amongst 15 wheat genotypes (modern UK cultivars, synthetic-derivatives and landraces) grown in the UK. In addition, genetic variation in flag-leaf photosynthetic rate has been associated with flag-leaf N content (Austin et al., 1982) and relative chlorophyll content in wheat (Gaju et al., 2014) and leaf Rubisco content in rice (Hubbart et al., 2007).
In wheat, 35–42 % of the above-ground N accumulated at anthesis is in leaf lamina, 14–20 % in the leaf sheath, 20–31 % in the stem and 16–23 % in the ear under optimal N application (Pask et al., 2012; Barraclough et al., 2014; Gaju et al., 2014). It has been suggested that a higher capacity for storing accumulated N in the stem internodes before anthesis could enable the translocation of a larger amount of N to grains without reducing plant photosynthetic capacity (Bertheloot et al., 2008; Foulkes et al., 2009). Ears also have a capacity to store N in the pre-anthesis period in the glumes (Lopes et al., 2006). Again, this could be a mechanism of buffering leaf lamina senescence through the remobilization of N from the glumes in the post-anthesis period (Lopes et al., 2006).
Wheat crops remobilize N to grain during grain filling affecting final grain yield and grain N concentration (Barraclough et al., 2010; Gaju et al., 2011, 2014). Fifty to 95 % of grain N at harvest comes from the remobilization of N stored before anthesis (Kichey et al., 2007). N-remobilization efficiency (NRE) can be defined as the proportion of N in the crop or crop component at anthesis which is not present in the crop or crop component at harvest. In wheat various investigations have found that the leaves have a higher NRE (0.73-0.76) than the stems (0.43-0.73) and the chaff (0.56-0.73) (Zhen-Yuan et al., 1996; Barbottin et al., 2005; Pask et al., 2012; Gaju et al., 2014); and higher N remobilization has been observed under low N supply compared to high N supply (Barbottin et al., 2005; Pask et al., 2012; Gaju et al., 2014).
Previous studies reported that genetic control of N remobilization is linked to the regulation of leaf senescence (Masclaux et al., 2001; Uauy et al., 2006a, 2006b). In wheat, delayed onset of flag-leaf senescence (stay green) was associated with lower N remobilization under low N conditions in winter wheat grown in the UK and France (Derkx et al., 2012; Gaju et al., 2014). In sorghum, the stay-green trait has been related to the N supply-demand balance during grain filling (Van Oosterom et al., 2010). Developing cultivars that stay-green and photosynthesize for a longer time by modifying NRE could be advantageous to produce a higher yield. However, longer photosynthesis for same amount N available could eventually reduce the grain N concentration affecting grain N protein. In addition, a deeper root system can potentially increase the stay-green effect by taking up more post-anthesis N (Foulkes et al., 2009).
Grain yield (GY) and grain N concentration (GNC) are two major targets in wheat breeding programmes, but have been difficult to improve simultaneously due to the negative genetic relationship between GNC and GY (Slafer et al., 1990; Bogard et al., 2010). The physiological basis of this negative correlation may relate to competition between carbon and N for energy (Munier‐Jolain and Salon, 2005) and a N dilution effect by carbon-based compounds (Acreche and Slafer, 2009). Monaghan et al. (2001) suggested that deviation from the regression line between GY and grain protein concentration (grain protein deviation; GPD) could be used to identify genotypes having higher grain protein concentration than would be expected from their GY. Previous studies in wheat suggest GPD was related to post-anthesis N uptake (Bogard et al., 2010) and crop N remobilization (Slafer et al., 1990; Uauy et al., 2006a, 2006b).
In India wheat provides 50 % of the calories consumed, contributing substantially to national food security (ICAR- IIWBR, 2019). Global wheat production in 2018 was 735 M t and in India was 99 M t (FAO, 2020). India has the second highest global wheat production after China (FAO, 2020). Previously we reported on associations between grain yield, NUpE, NUtE and whole-crop NRE for 30 Indian cultivars in the Peninsular zone of India. In the present paper for the same set of cultivars we report on genetic variation in N partitioning for plant components, flag-leaf photosynthesis rate, post-anthesis N remobilization for plant components and flag-leaf and canopy senescence parameters and associations with grain yield and grain protein deviation. The specific objective was to identify the main processes that determine NUE, grain yield and grain protein deviation under optimal and N-limiting conditions in Indian wheat cultivars. Two field experiments were carried out examining 30 Indian cultivars grown under high and low N conditions at Agharkar Research Institute, Pune, India in two seasons.
2. Materials and methods
2.1. Experimental design and plot management
Two field experiments were conducted at the Agharkar Research Institute farm, near Pune, India (16° 30′ N, 74° 40′ E, altitude: 557.5 m) in 2013-14 and 2015-16. The full experimental details were described by Nehe et al. (2018). In summary, the site has a temperate climate with mean annual rainfall of 457 mm and mean annual minimum temperature of 9 °C and maximum temperature of 36 °C. Soil mineral analysis before planting showed N, P, K was 197, 25, 578 in 2013-14 and 142, 156, 230 kg ha−1 in 2015–16, respectively. The soil type was a black cotton soil or Vertisol.
The experiments used a split-plot design with two replications in 2013-14 and three replications in 2015-16; the main plot treatment was two levels of N fertilizer application and the sub-plot treatment was 30 elite Indian wheat cultivars. The sub-plot size was 2.5 × 1.0 m in 2013-14 and 4.5 × 1.5 m in 2015-16. Two levels of N fertilizer treatment, optimum or high N (HN) and low N (LN), were applied. The high N fertilizer amount was 120 kg N ha−1 in both years: 60 kg N ha−1 was applied at sowing and 60 kg N ha−1 at onset of stem extension (GS30, Zadoks et al., 1974). In the low N treatment, 0 kg N ha−1 was applied in 2013-14 and 40 kg N ha−1 in 2015-16 at sowing. All N fertilizer was applied as granules of urea (34.5 % N) and each split was applied on the same calendar date for the 30 cultivars. All other crop inputs, including pesticide, herbicide and fungicide inputs, and potassium, phosphate and sulphur fertilizers, were applied at levels to prevent non-N nutrients or pests, weeds and diseases from limiting yield. The sowing date was 26 November in 2013 and 2 November in 2015. The seed rate was 240 seeds m−2 in both experiments.
Thirty elite Indian spring wheat cultivars were selected to be representative of the most widely grown cultivars in the main wheat breeding zones in India in recent years. All the cultivars were semi-dwarfs except two tall cultivars, Kharchia-65 and BH-1146. There were two durum wheat (Triticum turgidum subsp. durum) cultivars (PDW- 314 and HI- 8498); the remaining 28 cultivars were bread wheat (Triticum aestivum L.) cultivars. Information about the pedigree, year of release and breeding zone origin of the cultivars is given in Supplementary Table 1. In each year, the crop was irrigated using a gravity-based flood irrigation system at 15 day intervals or when irrigation was needed to avoid water stress.
2.2. Crop measurements
2.2.1. Developmental stages and plant height
Regular monitoring of crop growth stages was done following the decimal code of Zadoks (Zadoks et al., 1974). In both years, date of anthesis (GS65, AD) and physiological maturity (GS89, 50 % of peduncle yellow) was recorded in each sub-plot. Plant height was measured between GS71 and GS89 from the ground level to the tip of ear using a ruler for five randomly selected fertile shoots (those with an ear) per sub-plot in 2014 and three fertile shoots per sub-plot in 2016.
2.2.2. Dry matter and plant N% analysis
For dry matter and N analysis, in 2014 five fertile shoots at anthesis and 10 fertile shoots at physiological maturity were randomly selected per sub-plot and cut at ground level. In 2016, samples at anthesis were taken by cutting shoots at ground level in four 25 cm row-lengths giving a sampled area of 0.25 m2 per sub-plot. The fresh weight was recorded and a sub-sample of 10 shoots was taken and the fresh weight recorded. At physiological maturity, 12 fertile shoots were randomly selected per sub-plot and cut at ground level.
Shoots were separated into three components: (i) ear, (ii) flag-leaf lamina and (iii) stem, leaf sheath and remaining leaf lamina. Dry weight of each component was recorded after drying for 48 h at 70 °C. Dried ears at harvest were threshed by hand and grain dry weight measured and chaff dry weight was calculated by difference. The number of fertile and infertile shoots was counted in a 1 m row length at physiological maturity in 2014 and at anthesis and at physiological maturity in 2016.
After the samples for DM and N partitioning had been taken at physiological maturity, the remainder of each sub-plot was bulk harvested by hand. The ends of the plots (to 0.25 m) were removed as discard and the rest of the plot was harvested as a bulk by cutting at ground level. The grain was threshed using a threshing machine and weighed and a sample of approximately 50 g grain taken for moisture content analysis. Harvest index (grain DM / above-ground DM) was calculated based on the measurements on the 10- or 12-shoot samples at physiological maturity. Grain number (GN) from the 10- or 12-shoot samples was counted manually and the thousand grain weight calculated (TGW). From these measurements, above-ground DM per m2, grains per ear and ears per m2 were calculated.
In both years, N concentration of plant components was analyzed by the Dumas method (Dumas, 1831). N% determination was carried out for: i) flag-leaf lamina, ii) remaining leaf lamina and stem and leaf sheath and iii) ears at anthesis (GS65); and for i) flag-leaf lamina ii) remaining leaf lamina and stem and leaf sheath and chaff and iii) grain at harvest. The dried samples were firstly milled into fine powder (particle size <200 μm) using a mixer-grinder mill with mechanical modification for small quantity samples. Milled samples were then re-dried at 80 °C for 48 h and weighed to 0.00001 g precision, encapsulated in tin capsules and analyzed for N%. Nitrogen Nutrition Index (NNI) was estimated according to the ratio of the actual above-ground crop N% at anthesis and the critical N% (N%ct), where N%ct was estimated according to the ‘critical dilution curve’ described by Justes et al. (1994).
N partitioning index (NPI) at anthesis was calculated as the proportion of above-ground N at anthesis in the crop component at anthesis. Post-anthesis N-remobilization efficiency (NRE) was calculated as the proportion of N in the crop component at anthesis which is not present in the crop component at harvest:
| NRE = (NA – NH)/NA | (1) |
where NRE is the N-remobilization efficiency of the crop component, NA is the amount of N in the crop component at anthesis and NH is the amount of N in the crop component at harvest.
Post-anthesis N uptake (PANU) was calculated as the difference between above-ground N at harvest (AGNH) and at anthesis (AGNA). In 2014, AGNA was calculated by multiplying the above-ground N per fertile shoot at GS65 by the ears m−2 measured at harvest, assuming ears m−2measured at harvest was representative of values at anthesis. (In 2016, when we carried out a direct count of ears per m2 in a 1 m row length at anthesis and harvest, there was a strong correlation between measurements at anthesis and harvest amongst the 30 cultivars under HN (r = 0.74, P < 0.001) and LN (r = 0.63, P < 0.001) conditions (data not shown). In 2016, AGNA was calculated by scaling up the above-ground N in the 5-shoot sample based on the FW ratio of the 5-shoot sample and the 0.25 m2 sample at anthesis. The N harvest index (NHI) was calculated as the proportion of above-ground N in the grain at harvest. Grain protein concentration (GPC) was calculated as the grain N concentration multiplied by 5.7. The grain yield – grain protein linear relationship and its residual deviation (grain protein deviation, GPD) were calculated for each replicate in each environment. Genotypic GPD values were then obtained for each N treatment and season combination by averaging GPD values.
2.2.3. Flag-leaf photosynthesis rate and stomatal conductance
Post-anthesis flag-leaf photosynthesis measurements were taken on two dates (7 and 23 January) in 2016 under HN conditions. Light-saturated photosynthetic rate (Amax) and stomatal conductance (gs) of the flag-leaf were measured using a Li-Cor LI-6400XT Portable Photosynthesis System (Licor Biosciences, Lincoln, NE, USA). In each sub-plot, a single reading on the flag-leaf of two randomly selected fertile shoots was taken. The instrument was adjusted for humidity at 50–60 % within the cuvette and the settings for the Amax readings were: photosynthetically active radiation (PAR) 1500 μ mol m−2s−1, sample chamber CO2 concentration 360 μl l−1 and flow rate 400 μ mol s−1. The measurements were taken between 10.00 and 15.00.
2.2.4. NDVI measurements
The Normalized Difference Vegetation Index (NDVI) was measured in each experiment in each sub-plot approximately every seven days from anthesis (GS65) until late grain-filling using a FieldScout CM 1000 NDVI Meter (Spectrum Technologies, Aurora, IL, USA). The measurements were taken between 10.00 and 15.00 on clear sunny days. The NDVI meter sensor was held 50 cm above the crop. NDVI was calculated from measurements of light reflectance in the red and near infra-red (NIR) regions of the spectrum as:
| NDVI = (R840 – R660)/(R840 + R660) | (2) |
where R660 and R840 are the reflectance at 660 and 840 nm, respectively.
NDVI average values from 0 to 400 °C d post-anthesis (GS65) (NDVI pre-tt400) and from 400 to 800 °C d (NDVI post-tt400) post-GS65 were used as indicators of canopy greenness.
2.2.5. Flag-leaf senescence parameters
Senescence kinetics of the flag leaf were assessed visually for main shoots on five tagged plants in 2014 and for the whole flag-leaf layer in 2016 by recording the percentage green area senesced using a standard diagnostic key based on a scale of 0–10 (100 % senesced), as described by Gaju et al. (2011). Assessment was carried out weekly after anthesis until full flag-leaf senescence. The data were then fitted against thermal time from anthesis (GS65; base temperature of 0 °C) using a modified version of an equation with five parameters consisting of a monomolecular and a logistic function (Génard et al., 1999) as described by Gaju et al. (2011). The onset of post-anthesis senescence (VSOnset; °Cd) was defined as the onset of the rapid phase of senescence and the end of post-anthesis senescence (VSEnd; °Cd) as the thermal time when the visual senescence score was 9.5.
2.2.6. Environmental measurements
Meteorological data were collected daily for rainfall, humidity and minimum and maximum temperature from a meteorological station located 100 m from the field site.
2.3. Statistical analysis
Analysis of variance (ANOVA) procedures for a split-plot design were used to analyze N and cultivar effects and test their interaction with year using GenStat version 18 (www.genstat.com; VSN International Ltd, Hemel Hempsted, UK), where replicates were regarded as random effects and cultivars as fixed effects. A cross-season ANOVA was applied to analyze N and cultivar effects across years and the interaction with year, assuming N treatments and cultivars were fixed effects and replicates and seasons were random effects. Linear regressions were calculated to quantify associations between traits using the 2-year cultivar means using Genstat version 18.1 (VSN International, Hemel Hempstead UK). Bi-plot procedures to test associations between traits were carried out using the R software Version 3.0-2 (http://www.R-project.org/). The GPC–GY linear relationship and its residuals (grain protein deviation, GPD) were calculated for each replicate in each N treatment using Genstat version 18.1.
3. Results
3.1. N accumulation and N partitioning at anthesis
At anthesis, flag-leaf lamina N per m2 increased from 0.76 g m−2 under LN to 1.66 g m−2 under HN conditions (P < 0.001; Table 1). The increase under HN did not differ significantly amongst the cultivars. Stem (stem plus leaf sheath) and remaining lamina N per m2 increased from 4.64 g m−2 under LN to 9.1 g m−2 under HN conditions (P < 0.001), with the increase ranging amongst cultivars from 1.74 (HI-8498) to 7.90 g N m−2 (HD-2967) (P < 0.01). Ear N at anthesis increased from 2.85 g N m−2 under LN to 4.31 g N m-2 under HN (P < 0.001), with increases ranging amongst cultivars from 0.34 (HI-8489) to 3.25 g N m−2 (HD-2967) (P < 0.001). For flag-leaf N and the stem-and-remaining leaf N per m2 the year × N × cultivar interaction was not significant. However, for ear N per m2 the three-way interaction was significant (P < 0.01) indicating cultivar responses to N supply depended on year.
Table 1.
Flag-leaf N (FL NA), stem and remaining leaf N (StL NA) and ear N (Ear NA) accumulation; flag-leaf N partitioning index (FL NPI), stem and remaining leaf N partitioning index (StL NPI) and ear N partitioning index (Ear NPI) at anthesis (GS65); and flag-leaf N (FL NH), stem and remaining leaf N and chaff (StL NH) and grain N (Grain NH) accumulation at harvest in 30 wheat cultivars under high N (HN) and low N (LN) conditions. Values represent means in 2014 and 2016.
| FL NA (g N m−2) | StL NA (g N m−2) | Ear NA (g N m−2) | FL NPI | StL NPI | Ear NPI | FL NH (g N m−2) | StL NH (g N m−2) | Grain NH (g N m−2) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HN | Min | 1.10 | 5.78 | 2.91 | 0.08 | 0.54 | 0.21 | 0.20 | 1.30 | 9.1 |
| Max | 2.23 | 11.97 | 6.13 | 0.16 | 0.68 | 0.34 | 0.43 | 2.79 | 16.1 | |
| Mean | 1.66 | 9.10 | 4.31 | 0.11 | 0.61 | 0.28 | 0.29 | 1.88 | 13.0 | |
| LN | Min | 0.50 | 3.61 | 2.06 | 0.07 | 0.52 | 0.28 | 0.07 | 0.53 | 5.3 |
| Max | 1.04 | 6.33 | 4.23 | 0.13 | 0.63 | 0.41 | 0.17 | 1.00 | 8.3 | |
| Mean | 0.76 | 4.64 | 2.85 | 0.09 | 0.56 | 0.35 | 0.11 | 0.80 | 7.1 | |
| SED | ||||||||||
| N (df 3) | 0.07 *** | 0.56 ** | 0.15 ** | 0.004 ** | 0.01 * | 0.01 ** | 0.01 *** | 0.06 *** | 0.41 *** | |
| Gen (df 174) | 0.16 *** | 0.84 *** | 0.35 *** | 0.01*** | 0.02 *** | 0.02 *** | 0.03 *** | 0.22 *** | 0.87 *** | |
| N*Gen (df 174) | 0.23 ns | 1.29 ** | 0.51 *** | 0.01 ns | 0.03 ns | 0.03 ns | 0.04 *** | 0.32 ** | 1.30 * | |
| Y*N*Gen (df 174) | 0.33 ns | 1.85 ns | 0.76 ** | 0.016 ns | 0.042 ns | 0.04 ns | 0.06 *** | 0.46 ns | 1.96 ns | |
Significance at the 5% (P = 0.05) level. **1% (P = 0.01) level. ***0.1 % (P = 0.001) level. SED = standard error of the differences of the means.
Under HN conditions, there was a positive association between above-ground DM at anthesis and above-ground N at anthesis (r = 0.85, P < 0.001), and also a positive association between DM accumulation (g m−2) and N uptake (g m−2) in each plant component (flag leaf r = 0.45, P < 0.001; stem and remaining leaf r = 0.79, P < 0.001) and ear (r = 0.81; P < 0.001; Supplementary Table 2). The N partitioning index (NPI) was strongly correlated with the DM partitioning index for the respective components (r > 0.90 for all three components, P < 0.001). The NPI was also correlated with the N uptake in each plant component (g m−2), for the flag-leaf (r = 0.35, P < 0.10), stem and remaining leaf (r = 0.47, P < 0.01) and ear (r = 0.53, P < 0.01). Generally, similar associations were observed between DM and N partitioning indices and N accumulation in the plant components at anthesis under LN conditions as under HN conditions. However, flag-leaf N uptake was more strongly associated with flag-leaf NPI and less strongly with aboveground DM under LN conditions than under HN conditions.
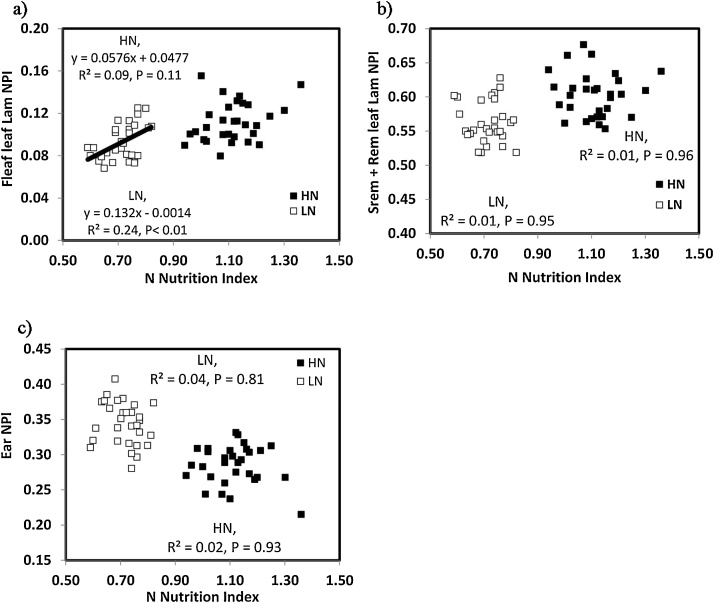
In our experiments we used the N nutrition index (NNI) at anthesis as an indicator of the crop N stress incurred by the cultivars in the N treatments (Justes et al., 1994). Averaging across cultivars and years, with increasing N supply the NNI increased from 0.71 under LN to 1.11 under HN conditions. There was no evidence for a relationship between NNI and N partitioning index across cultivars for the stem-and-remaining-leaf NPI or ear NPI. However, flag-leaf NPI was positively associated with NNI under LN (r2 = 0.24, P < 0.01) with a trend for a positive association under HN conditions (r2 = 0.09, P = 0.09; Fig. 1).
Fig. 1.
N partitioning index (NPI) at anthesis (GS65) for (a) flag-leaf lamina, (b) stem-and-remaining leaf lamina and (c) ear versus N nutrition index at anthesis for 30 wheat cultivars grown under high N (HN) and low N (LN) conditions. Values represent means across 2014 and 2016.
The flag-leaf NPI decreased with N deficiency from 0.11 (HN) to 0.09 (LN) (P < 0.01); genotypes overall ranged from 0.08 (DBW-16) to 0.14 (HW 2044); but the N × cultivar interaction was not significant (Table 1). Stem-and-remaining-leaf NPI decreased with N deficiency from 0.61 (HN) to 0.56 (LN) (P < 0.001); and ranged amongst cultivars from 0.54 (RAJ-4328 and KRL-213) to 0.68 (DBW-16) under HN and 0.52 (MACS-6222) to 0.63 (DBW-16) under LN conditions (P < 0.001); there was a N × cultivar interaction (P = 0.08). In contrast, ear NPI increased with N deficiency from 0.28 (HN) to 0.35 (LN) ranging overall amongst cultivars from 0.25 (BH-1466) to 0.37 (MACS-2496; P < 0.001); there was no N × cultivar interaction. Ear NPI increased with later anthesis date under both HN (r2 = 0.10, P < 0.10) and LN (r2 = 0.15, P < 0.05) conditions. In contrast, flag-leaf NPI decreased with later anthesis date under HN (r2 = 0.58) and LN (r2 = 0.36) conditions (P < 0.05). There was no change in stem-and-remaining-leaf NPI with anthesis date.
3.2. Flag-leaf photosynthesis and stomatal conductance under HN conditions
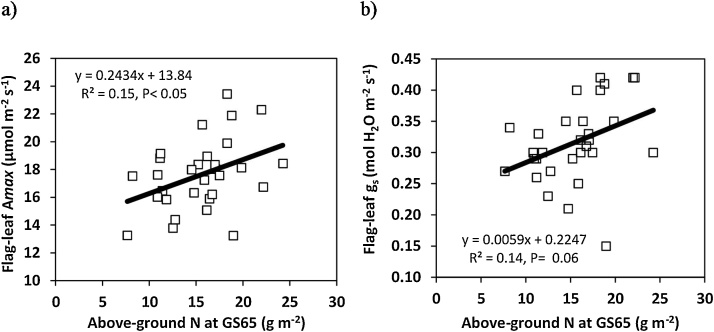
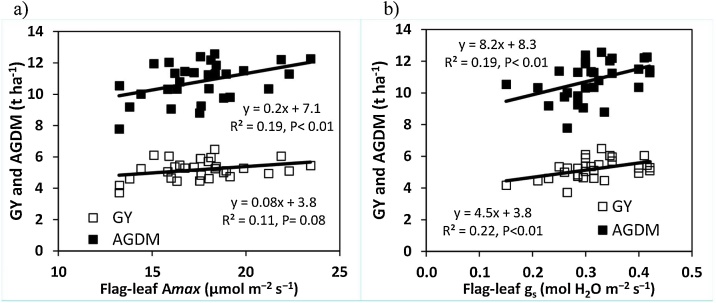
In 2016, light-saturated flag-leaf photosynthesis rate (Amax) and stomatal conductance (gs) were measured post-anthesis under HN conditions. Averaging across assessment dates, flag-leaf Amax ranged amongst cultivars from 13.2 to 23.4 μmol m−2 s-1 (P < 0.001) and was positively linearly related amongst genotypes with grain yield (r2 = 0.11, P = 0.08), above-ground DM at harvest (AGDMH) (r2 = 0.19, P < 0.05) and above-ground N at anthesis (AGNA; r2 = 0.15; P < 0.05; Fig. 2). Flag-leaf gs ranged from 0.15 - 0.42 mol m−2 s−1 (P < 0.001) and also showed a positive linear association with grain yield (r2 = 0.22, P < 0.01), AGDMH (r2 = 0.19, P < 0.05) and AGNA (r2 = 0.15; P < 0.05; Fig. 2, Fig. 3). There was no association amongst cultivars between flag-leaf Amax or gs and flag-leaf specific leaf N (flag-leaf N per unit green area; SLNA) at anthesis or with flag-leaf area (data not shown).
Fig. 2.
Linear relationship between a) post-anthesis flag-leaf photosynthesis rate (Amax) and above-ground N at anthesis and b) post-anthesis flag-leaf stomatal conductance (gs) and above-ground N at anthesis for 30 wheat cultivars under high N conditions in 2016.
Fig. 3.
Relationship between grain yield (GY, 100 % DM) and aboveground DM (AGDM) and a) post-anthesis flag-leaf photosynthetic rate (Amax) and b) post-anthesis flag-leaf stomatal conductance (gs) for 30 wheat cultivars under high N conditions in 2016.
3.3. N-remobilization efficiency and association with flag-leaf senescence and harvest traits
In these experiments grain yield ranged from 3.72 - 6.48 t ha−1 under HN and 2.95 - 4.51 t ha−1 under LN conditions (P < 0.001; Supplementary Table 3). The grain N% ranged amongst genotypes from 2.12–2.98 under HN and 1.62–2.30 under low N conditions (P < 0.001). Grains per m-2 ranged from 8,783 - 20,056 under HN and 7138 - 12,845 under LN conditions P < 0.001); there was a correlation between grains m-2 and grain yield under HN (r = 0.69, P < 0.001) and LN (r = 0.77, P < 0.001) conditions. The N × genotype interaction was not statistically significant for grain yield, grains per m2 or grain N%.
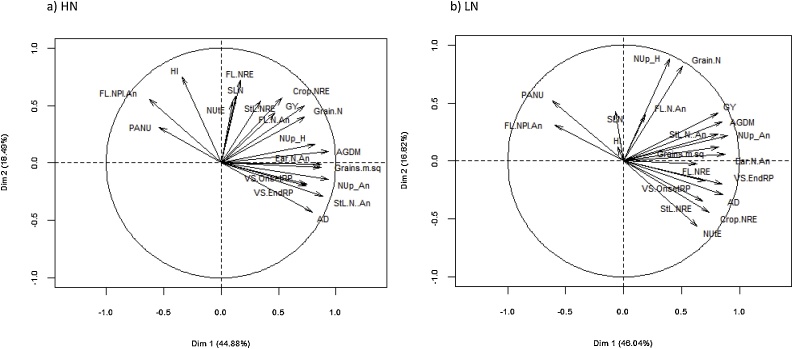
Under HN conditions, N-remobilization efficiency was higher in the flag leaf (0.82) than the remaining stem and leaf lamina (0.78). For both plant components, NRE was increased under LN compared to HN conditions (P < 0.01; Table 2). For flag-leaf NRE, genotypes ranged overall from 0.71 - 0.87 and for stem-and-remaining-leaf NRE from 0.71 - 0.84 (P < 0.01), but there was no N × cultivar interaction. Bi-plots showing associations between N-remobilization traits, N uptake, senescence-related traits and grain yield and yield components under HN and LN conditions are presented in Fig. 4. Under HN conditions, the first two principal components (PCs) explained 62.9 % of phenotypic variation (PC1, 43.4 % and PC2, 19.5 %). Grain yield showed a positive association with above-ground N at anthesis and flag-leaf NRE and stem-and remaining-leaf NRE. Higher above-ground N at anthesis was associated with later anthesis date and delayed onset and end of flag-leaf senescence. In addition, higher flag-leaf N uptake at anthesis was associated with delayed onset and end of flag-leaf senescence. Higher flag-leaf NRE and stem-and-remaining-leaf NRE were also associated with delayed flag-leaf senescence and with higher NUtE. Post-anthesis N uptake (PANU) showed a negative association with above-ground N at anthesis. In addition, the bi-plot showed that higher grain sink strength as indicated by grains m−2 was positively associated with above-ground N at anthesis and harvest and grain N uptake per m2 (P < 0.001).
Table 2.
Genetic ranges in N-remobilization efficiency for the flag-leaf lamina, stem and remaining leaf lamina and whole crop in 30 wheat cultivars under HN and LN conditions. Values represent means across years 2014 and 2016.
| Crop NRE | Flag-leaf Lam NRE | Stem + Rem Lam NRE | ||||
|---|---|---|---|---|---|---|
| HN | LN | HN | LN | HN | LN | |
| Mean | 0.79 | 0.84 | 0.82 | 0.85 | 0.78 | 0.82 |
| Min | 0.74 | 0.78 | 0.71 | 0.78 | 0.71 | 0.75 |
| Max | 0.84 | 0.87 | 0.87 | 0.89 | 0.84 | 0.86 |
| SED | ||||||
| N (df 3) | 0.004 ** | 0.004 *** | 0.010 * | |||
| Gen (df 180) | 0.020 *** | 0.020 ** | 0.021 * | |||
| N × Gen (df 180) | 0.030 ns | 0.028 ns | 0.030 ns | |||
| Y × N×Gen (df 180) | 0.040 ns | 0.042 ns | 0.050 ns | |||
* Significance at P = 0.05, ** P=0.01, ***0.1 % P = 0.001 levels; ns, non-significant. SED = standard error of the differences of the means.
Fig. 4.
Bi-plots for grain yield (GY), above-ground dry matter (AGDM) at harvest, harvest index (HI), grains m−2 (Grains.m.sq), flag-leaf N remobilization efficiency (FL NRE), stem and remaining leaf lamina NRE (StL NRE), N-remobilization efficiency (Crop NRE), N-utilization efficiency (NUtE), N-uptake at harvest (NUp_H), N-uptake at anthesis (NUp_An), post-anthesis N-uptake (PANU), flag-leaf N uptake anthesis (FL.N.An), stem and remaining leaf lamina N uptake at anthesis (StL N.An), grain N uptake (Grain.N), flag-leaf specific N (SLN), onset of flag-leaf senescence (VSOnset), end of flag-leaf senescence (VSEnd), post-anthesis N-uptake (PANU), and days sowing to anthesis (AD) for 30 wheat cultivars under (a) high N (HN) and (b) low N (LN) conditions. Values based on mean of 2014 and 2016.
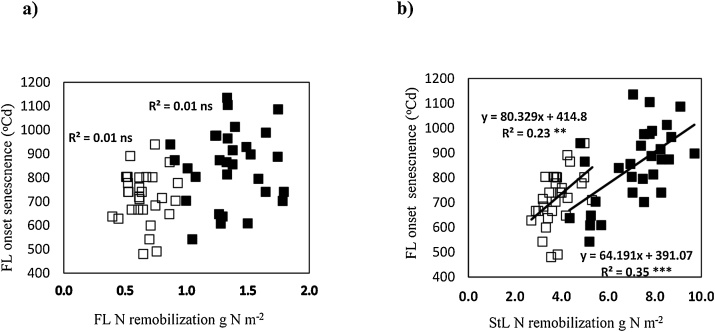
Under LN conditions, the first two PCs explained 62.7 % of phenotypic variation (PC1, 45.1 % and PC2, 17.6 %). Grain yield showed a positive association with above-ground N at anthesis and its components (stem-and-remaining-leaf N and ear N) and with above-ground N at harvest. Higher flag-leaf NRE and stem-and-remaining-leaf NRE were each associated with delayed onset and end of flag-leaf senescence. Higher flag-leaf NRE and stem-and-remaining leaf NRE were also associated with higher NUtE, later anthesis date and lower PANU. Later anthesis date was associated with delayed onset and end of flag-leaf senescence. The amount of N remobilization from the stem and remaining leaf sheath (N g m−2) showed a positive association with the onset of flag-leaf senescence under HN (r2 = 0.35, P < 0.001) and LN (r2 = 0.23, P < 0.01) conditions. (Fig. 5). As under HN conditions, grains m−2 was positively associated with above-ground N at anthesis and harvest and grain N uptake per m−2 (P < 0.001). In addition, higher grains m−2 was associated with increased crop NRE (r = 0.39, P < 0.05).
Fig. 5.
Association between onset of flag-leaf senescence (thermal time post anthesis (GS65) base temp. 0 °C) and a) flag-leaf N remobilization and b) stem and remaining leaf lamina N remobilization for 30 wheat cultivars under high N (◼) and low N (□) conditions. Values represent means in 2014 and 2016.
3.4. Post-anthesis NDVI and association with grain yield
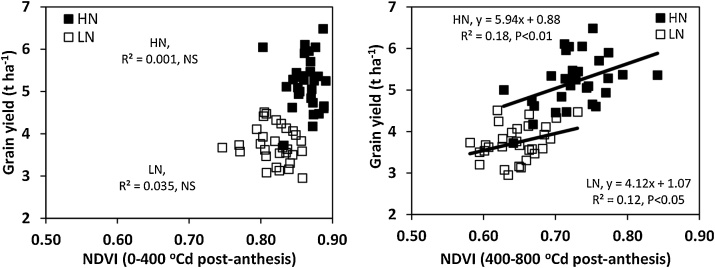
Averaged across years, NDVI during early grain filling was higher under HN (0.87) than under LN conditions (0.82) (P < 0.05) and ranged amongst cultivars from 0.80 to 0.89 and from 0.75 to 0.86, respectively (P < 0.001) (Fig. 6; Supplementary Table 4). Decreases in NDVI under LN ranged amongst cultivars from -0.02 to 0.10 (P < 0.05). NDVI during late grain filling was again higher under HN (0.73) than under LN conditions (0.65) (P < 0.05) ranging amongst cultivars from 0.63 to 0.84 under HN and from 0.58 to 0.84 under LN conditions (P < 0.001). Decreases in NDVI with N limitation ranged amongst cultivars from -0.04 to 0.17 (P < 0.05). During early grain filling there was no association amongst cultivars between NDVI and grain yield under either HN or LN conditions. However, during late grain filling there was a positive linear association between NDVI and grain yield under both HN (r2 = 0.18, P < 0.05) and low N conditions (r2 = 0.12, P < 0.05; Fig. 6).
Fig. 6.
Linear regression of grain yield (100 % DM) on a) mean NDVI during early grain filling (0-400 °Cd (base temp. 0 °C) post-GS65) and (b) mean NDVI during late grain filling (400-800 °C d post-GS65) for 30 wheat cultivars under high N (HN) and low N (LN) conditions. Values represent means in 2014 and 2016.
3.5. Association between grain protein deviation, N uptake and N remobilization
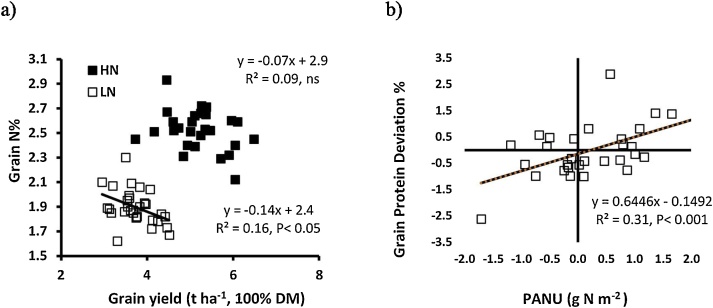
There was a negative linear relationship amongst cultivars between grain yield and grain N% under LN conditions (r2 = 0.16; P < 0.05), but no association under HN conditions (Fig. 7a.). Grain N% was positively associated with post-anthesis N uptake (PANU) under LN conditions (r2 = 0.33, P < 0.01), but there was no association under HN conditions. The grain protein deviation was calculated as the residual from the GY versus grain protein concentration (GPC) linear regression, positive deviations representing higher GPC relative to grain yield than predicted according the overall negative linear relationship between grain yield and GPC. The GPD was positively associated with post-anthesis N uptake (r2 = 0.31; P < 0.001; Fig. 7b) under LN conditions. There was a trend for a negative linear association between GPD and stem-and-remaining-leaf NRE (r2 = 0.12, P = 0.07), but there was no association with flag-leaf NRE under LN conditions.
Fig. 7.
Linear regressions of a) grain N% on grain yield under high N and low N conditions; and b) grain protein deviation on post-anthesis N uptake (PANU) for 30 wheat cultivars under low N conditions. Values represent means in 2014 and 2016.
4. Discussion
Firstly, we will discuss the physiological basis of genetic variation in N partitioning and N remobilization and their responses to N supply. We will then consider their associations with senescence timing, grain yield and grain protein deviation and the implications for breeding more N-efficient crops.
4.1. Genetic variation in N partitioning in response to N availability
In these experiments later anthesis date favoured N uptake at anthesis under both HN and LN conditions. It is feasible that greater above-ground N with later anthesis was associated with larger root biomass favouring N uptake. Alternatively, a longer duration for N accumulation may have increased N uptake. As N stress increased (as indicated by NNI) genotypes partitioned proportionally less N to the flag-leaf under LN conditions; and overall N limitation increased the proportion of N partitioned to the ear and decreased the proportion partitioned to the flag leaf. Present results therefore are generally consistent with previous reports of N limitation favouring ear N partitioning over lamina N partitioning in wheat cultivars (Pask et al., 2012; Gaju et al., 2014).
The cultivar range in ear N partitioning at anthesis was relatively large in the present study (0.20-0.34 H N and 0.28-0.41 L N). These ranges were wider than those reported by Gaju et al. (2014) for ear NPI at anthesis for 16 winter wheat cultivars of 0.21-0.27 under LN and 0.23-0.33 under HN and by Pask et al. (2012) for four winter wheat cultivars (0.18-0.22 under HN and 0.23-0.28 under LN). The reason for the greater genetic variation in the present study may be partly that germplasm was selected from a wide range of wheat breeding zones across India whereas in previous studies genotypes were selected from cultivars grown within a more restricted geographical range in the UK and northern France.
4.2. Genetic variation in N-remobilization efficiency and response to N availability
Present results indicated flag-leaf NRE and stem-and-leaf NRE increased slightly with N deficiency. Similar effects of N limitation increasing crop NRE (Kichey et al., 2007) and stem NRE and lamina NRE (Gaju et al., 2016) were previously reported. In the UK, Pask (2009) reported that crop NRE for winter wheat cv. Istabraq was not affected by N limitation, whereas in New Zealand under N limitation leaf-lamina NRE decreased, leaf-sheath NRE was unchanged and stem NRE increased. The reason for the increase in FL NRE and stem-and-leaf NRE with N limitation in the present study may be partly associated with a decrease in post-anthesis N uptake under LN conditions. Moreover, there was a strong negative association amongst the cultivars between PANU and each of flag-leaf NRE and stem-and-leaf NRE under LN conditions, but no association under HN conditions. This suggested that grain N was supplied to a greater extent by remobilized N from leaves and stems under N limitation than under HN conditions. Overall cultivars showed similar genetic ranges in flag-leaf lamina NRE (0.75-0.88) and stem-and-leaf NRE (0.73-0.85) in the present study. Gaju et al. (2014) and Pask (2009) reported generally similar ranges for lamina NRE (0.72-0.81) to our results for FL NRE. However, Gaju et al. (2014) observed lower values for stem NRE at 0.48-0.61 compared to our results for stem-and-leaf NRE; and Pask et al. (2012) also observed a lower value for the true-stem NRE of 0.48 for cultivar Istabraq compared to our results. Present results therefore indicate that stem NRE for the spring wheat cultivars in India was higher than that observed for winter wheat in NW Europe. The relatively low PANU in our experiments may have decreased N supply (source) relative to the grain N sink demand increasing stem N remobilization efficiency compared to the studies on wheat cultivars in Europe.
4.3. Genetic variation in flag-leaf photosynthesis and association with yield
Present findings showed a positive association between post-anthesis flag-leaf Amax and grain yield amongst the 30 genotypes under high N conditions. Associations between leaf photosynthetic rate and grain yield progress were previously reported in the last decades, e.g. in eight spring wheat cultivars in Mexico (Fischer et al., 1998), in 18 winter wheat cultivars in China (Jiang et al., 2003) and in 18 facultative wheat cultivars in China (Zheng et al., 2011a, 2011b). As far as we are aware this is the first time an association between flag-leaf Amax and grain yield has been shown in spring wheat grown under irrigated conditions in India. Driever et al. (2014), however, reported that for 64 wheat cultivars grown in the UK flag-leaf photosynthesis in the pre-anthesis phase was not well correlated with above-ground biomass or grain yield. Genetic variation in flag-leaf photosynthesis rate in previous work has been associated with flag-leaf N content (Austin et al., 1982) and chlorophyll content (Gaju et al., 2016) in wheat and leaf Rubisco content in rice (Hubbart et al., 2007). However, in our study there was no association between flag-leaf Amax and flag-leaf specific leaf N (SLN; N per unit area flag leaf) at anthesis. Flag-leaf SLN in the present study ranged from 2.22 to 2.44 g m−2 (data not shown). Pask et al. (2012) reported a break point for the relationship between SLN and radiation-use efficiency (RUE) of ca. 2 g m−2 in wheat, above which RUE did not increase with increasing SLN. Therefore, in the present study the genetic range in flag-leaf SLN was on the plateau of the SLN - RUE relationship possibly explaining why there was no association between genetic differences in flag-leaf SLN and flag-leaf Amax.
4.4. Relationship between N accumulation, N remobilization and senescence duration
Our results indicated that under HN conditions higher above-ground N at anthesis was associated with the stay-green trait (delayed start and end of flag-leaf senescence). Higher flag-leaf NRE and stem-and-remaining-leaf NRE were also associated with delayed flag-leaf senescence. The latter finding is in contrast to findings in winter wheat cultivars in NW Europe were higher NRE was associated with more rapid senescence (Gaju et al., 2016). Present results indicated that flag-leaf NRE and stem-and-remaining-leaf NRE were both strongly correlated with N accumulation at anthesis, and imply that NRE in our experiments was likely a source-driven trait (Martre et al., 2003). Thus, higher N accumulation (N supply) at anthesis favoured higher post-anthesis N remobilization and higher NRE. Previous evidence in wheat indicates grain N accumulation is principally driven by the availability of N from the sources (Triboi and Triboi-Blondel, 2002; Martre et al., 2003), defined as the total non-structural crop N at anthesis. This was demonstrated in experiments by Martre et al. (2003) on four wheat cultivars in which the N source–sink balance was manipulated by removing the top half of the ear at anthesis. This manipulation resulted in a significant increase in the grain N concentration, indicating grain N accumulation was regulated by the source and not by the activity of the grain (sink regulated). Our results showed that there was a positive association between the amount of stem-and-remaining-leaf N (g N m−2) remobilized and the onset of flag-leaf senescence, i.e. greater stem N remobilization was associated with delayed senescence. This association has not been demonstrated previously in wheat as far as we are aware. This implied that N remobilization from the stem and lower leaves likely buffered flag-leaf N during grain filling thus maintaining flag-leaf green area and photosynthetic capacity.
In our experiments onset of flag-leaf senescence was positively associated with grain yield under both HN and LN conditions (Nehe et al., 2018), i.e. delayed senescence increased grain yield. An association between grain yield and flag-leaf senescence timing would be expected under LN conditions where grain growth is source limited (Gaju et al., 2011); the reasons for the association under HN conditions are not certain but may partly relate low PANU in our study at only 13.3 kg N ha−1. This meant grain N was supplied mainly from N remobilization as mentioned above, which may have contributed to accelerated senescence under HN conditions. The positive association between NDVI during late grain filling and grain yield also indicates that grain growth was likely source limited under HN conditions in these experiments. These results could also be partly explained by the importance of climatic factors in determining the balance between senescence duration and grain yield. The impact of senescence on grain yield in our experiments may have be heightened under climatic conditions where short intervals of heat stress may have occurred during the grain-filling period at the field site in Maharashtra.
4.5. Relationship between N remobilization, PANU and GPD
Grain yield was negatively associated with grain N% under LN conditions, but there was no association under HN conditions. PANU was also positively associated with grain N% under LN conditions. These results indicated that higher PANU favoured high grain N% under N limitation. To investigate traits that would minimize the trade-off between GY and grain protein concentration, we calculated the grain protein deviation (a more positive deviation representing higher grain yield relative to GPC than predicted according the overall negative linear relationship between grain yield and GPC) under LN conditions. For the first time we have shown that the GPD was positively associated with PANU in spring wheat under irrigated conditions in India. The positive association with PANU may indicate that higher PANU buffered N remobilization from the leaves and stem thus maintaining post-anthesis photosynthetic capacity and grain yield. Bogard et al. (2010) also showed an association between GPD and the PANU in 27 winter wheat cultivars in northern France. The impact of PANU on GPD for winter wheat in the UK was also demonstrated by Monaghan et al. (2001). The genetic differences in GPD we observed might therefore be related to differences in rooting depth and/or the ability to maintain root activity during the grain-filling period influencing PANU.
Our results showed no significant association between flag-leaf NRE or stem-and-remaining-leaf NRE and GPD. Other studies have shown that increasing N remobilization by accelerating senescence (Uauy et al., 2006a, 2006b) or increasing N accumulation before anthesis while maintaining high remobilization efficiency (Slafer et al., 1990) may increase GPC without reducing GY. However, similar to the present study Bogard et al. (2010) found no association between N remobilization and GPD. Overall our results suggested that in the Peninsular zone of India the main physiological trait underlying GPD related to post-anthesis N uptake. As mentioned above, rooting traits such a steeper nodal root angle which are related to deeper roots (Manschadi et al., 2008, 2010) may therefore be relevant to higher GPD.
4.6. Conclusions
Our results showed that identifying genotypes with improved N uptake at anthesis and flag-leaf photosynthetic rate will be important in future strategies to increase GY in wheat breeding programmes in India under optimal N levels. Our results also indicated that identifying genotypes with improved post-anthesis N uptake and stay-green traits will be important to increase grain yield and GPD in wheat breeding programmes in India under low to moderate N levels. Field screening for physiological traits such as crop N uptake and stay-green properties in wheat can be laborious and time-consuming. Present results suggested NDVI offers promise as a ground-based spectral reflectance index for screening large numbers of genotypes for senescence and grain yield in Indian wheat cultivars. NDVI provides an objective measure of senescence and there is scope for the deployment of spectral reflectance indices using unmanned aerial vehicles for high-throughput stay-green phenotyping in breeders’ plots (Araus and Cairns, 2014; Shi et al., 2016). Further studies are required combining genetics and physiology to identify the location of QTLs for senescence-timing traits and PANU linked to NUE and to better understand the way in which alleles associated with these QTL may promote substantial differences in NUE and GPD in spring wheat.
CRediT authorship contribution statement
A.S. Nehe: Investigation, Formal analysis, Writing - review & editing. S. Misra: Conceptualization, Writing - review & editing. E.H. Murchie: Conceptualization, Writing - review & editing. K. Chinnathambi: Investigation. B. Singh Tyagi: Conceptualization, Methodology, Writing - review & editing. M.J. Foulkes: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgements
This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/ J011827/1] and by the Indian government Department of Biotechnology (DBT). We thank Dr Bagwan Juned Hanif for assistance in managing the field experiments at Agharkar Research Institute, Pune and Dr Jaswant Singh of Nottingham University, UK for assistance with preparing and distributing the seed for the field experiments.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.fcr.2020.107778.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Acreche M.M., Slafer G.A. Grain weight, radiation interception and use efficiency as affected by sink-strength in Mediterranean wheats released from 1940 to 2005. Field Crops Res. 2009;110:98–105. [Google Scholar]
- Austin R.B., Morgan C.M., Bhagwat S.G. Flag leaf photosynthesis of Triticum aestivum and related diploid and tetraploid species. Ann. Bot. 1982;49:177–189. doi: 10.1093/oxfordjournals.aob.a086238. [DOI] [Google Scholar]
- Barbottin A., Lecompte C., Bouchard C., Jeuffroy M.H. Nitrogen remobilization during grain filling in wheat’. Crop Sci. 2005;45:1141–1150. [Google Scholar]
- Barraclough P.B. Nitrogen efficiency of wheat: genotypic and environmental variation and prospects for improvement. Eur. J. Agron. 2010;33(1):1–11. doi: 10.1016/J.EJA.2010.01.005. Elsevier. [DOI] [Google Scholar]
- Barraclough P., Lopez-Bellido R., Hawkesford M. Genotypic variation in the uptake, partitioning and remobilisation of nitrogen during grain-filling in wheat. Field Crops Res. 2014;156:242–248. doi: 10.1016/J.FCR.2013.10.004. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertheloot J., Martre P., Andrieu B. Dynamics of light and nitrogen distribution during grain filling within wheat canopy. Plant Physiol. 2008;148 doi: 10.1104/pp.108.124156. pp. 1707 LP – 1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogard M., Allard V., Brancourt-Hulmel M., Heumez E., Machet J.M., Jeuffroy M.H., Gate P., Martre P., Le Gouis J. Deviation from the grain protein concentration–grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J. Exp. Bot. 2010;61:4303–4312. doi: 10.1093/jxb/erq238. [DOI] [PubMed] [Google Scholar]
- Driever S.M., Lawson T., Andralojc P.J., Raines C.A., Parry M.A.J. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014;65:4959–4973. doi: 10.1093/jxb/eru253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J.B.A. Procedes de I’analyse organique. Ann. Chim. Phys. 1831;47:198–205. [Google Scholar]
- FAO . 2020. FAO Statistics of World Wheat Production. [Google Scholar]
- Fischer R.A., Rees D., Sayre K.D., Lu Z.M., Condon A.G., Larque-Saavedra A. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies science. Crop Sci. 1998;38:1467–1475. [Google Scholar]
- Foulkes M.J., Sylvester-Bradley R., Weightman R., Snape J.W. Identifying physiological traits associated with improved drought resistance in winter wheat. Field Crops Res. 2007;103:11–24. [Google Scholar]
- Foulkes M.J., Hawkesford M.J., Barraclough P.B., Holdsworth M.J., Kerr S., Kightley S., Shewry P.R. Identifying traits to improve the nitrogen economy of wheat: advances and future prospects. Field Crops Res. 2009;114:329–342. doi: 10.1016/J.FCR.2009.09.005. [DOI] [Google Scholar]
- Gaju O., Allard V., Martre P., Snape J.W., Heumez E., Le Gouis J., Moreau D., Bogard M., Griffiths S., Orford S., Hubbart S., Foulkes M.J. Identification of traits to improve the nitrogen-use efficiency of wheat genotypes’. Field Crops Res. 2011;123:139–152. doi: 10.1016/J.FCR.2011.05.010. [DOI] [Google Scholar]
- Gaju O., Allard V., Martre P., Le Gouis J., Moreau D., Bogard M., Hubbart S., Foulkes M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crops Res. 2014;155:213–223. doi: 10.1016/J.FCR.2013.09.003. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaju O., De Silva J., Carvalho Hawkesford M.J., Griffiths S., Greenland A., Foulkes M.J. Leaf photosynthesis and associations with grain yield, biomass and nitrogen-use efficiency in landraces, synthetic-derived lines and cultivars in wheat. Field Crops Res. 2016;193:1–15. doi: 10.1016/J.FCR.2016.04.018. [DOI] [Google Scholar]
- Génard M., Reich M., Lobit P., Besset J. Correlations between sugar and acid content and peach growth. J. Hortic. Sci. Biotechnol. 1999;74:772–776. [Google Scholar]
- Hawkesford M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014:276–283. doi: 10.1016/J.JCS.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbart S., Peng S., Horton P., Chen Y., Murchie E.H. Trends in leaf photosynthesis in historical rice varieties developed in the Philippines since 1966. J. Exp. Bot. 2007;58:3429–3438. doi: 10.1093/jxb/erm192. [DOI] [PubMed] [Google Scholar]
- ICAR- IIWBR . 2019. ICAR- IIWBR – Indian Institute of Wheat and Barley Research.https://www.iiwbr.org/ Available at: [Google Scholar]
- Jiang G.M., Sun J.Z., Liuy H.Q., Qu C.M., Wang K.J., Guo R.J., Bsai B.Z., Gao L.M., Kunang T.Y. Changes in the rate of photosynthesis accompanying the yield increase in wheat cultivars released in the past 50 years. J. Plant Res. 2003;116:347–354. doi: 10.1007/s10265-003-0115-5. [DOI] [PubMed] [Google Scholar]
- Justes E.M.B., Meynard J.-M., Machet J.-M., Thelier-Huche L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994;74:397–407. [Google Scholar]
- Kichey T., Hirel B., Heumez E., Dubois F., Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Res. 2007;102:22–32. doi: 10.1016/J.FCR.2007.01.002. [DOI] [Google Scholar]
- Le Gouis J. Genetic improvement for increased nitrogen use efficiency in wheat. Asp. Appl. Biol. 2010;105:151–158. [Google Scholar]
- Lopes M.S., Cortadellas N., Kichey T., Dubois F., Habash D.Z., Araus J.L. Wheat nitrogen metabolism during grain filling: comparative role of glumes and the flag leaf. Planta. 2006;225:165–181. doi: 10.1007/s00425-006-0338-5. [DOI] [PubMed] [Google Scholar]
- Manschadi A.M., Hammer G.L., Christopher J.T., deVoil P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.) Plant Soil. 2008;303:115–129. [Google Scholar]
- Manschadi A.M., Christopher J.T., Hammer G.L., de Voil P. Experimental and modelling studies of drought‐adaptive root architectural traits in wheat (Triticum aestivum L.) Plant Biosyst. 2010;144:458–462. [Google Scholar]
- Martre P., Porter J.R., Jamieson P.D., Tribo ¨ı E. Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiol. 2003;133:1959–1967. doi: 10.1104/pp.103.030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclaux C., Quillere I., Gallais A., Hirel B. The challenge of remobilisation in plant nitrogen economy. A survey of physio-agronomic and molecular approaches. Ann. Appl. Biol. 2001;138:69–81. doi: 10.1111/j.1744-7348.2001.tb00086.x. [DOI] [Google Scholar]
- Moll R.H., Kamprath E.J., Jackson W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982;74:562–564. doi: 10.2134/agronj1982.00021962007400030037x. [DOI] [Google Scholar]
- Monaghan J.M., Snape J.W., Chojecki A.J.S., Kettlewell P.S. The use of grain protein deviation for identifying wheat cultivars with high grain protein concentration and yield. Euphytica. 2001;122:309–317. doi: 10.1023/A:1012961703208. [DOI] [Google Scholar]
- Munier‐Jolain N.G., Salon C. Are the carbon costs of seed production related to the quantitative and qualitative performance? An appraisal for legumes and other crops. Plant Cell Environ. 2005;28:1388–1395. [Google Scholar]
- Nehe A.S., Misra S., Murchie E.H., Chinnathambia K., Foulkes M.J. Genetic variation in N-use efficiency and associated traits in Indian wheat cultivars. Field Crops Res. 2018:152–162. doi: 10.1016/j.fcr.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask A. University of Nottingham; UK: 2009. Optimising Nitrogen Storage in Wheat Canopies for Genetic Reduction in Fertiliser Nitrogen Inputs. PhD thesis. [Google Scholar]
- Pask A.J.D., Sylvester-Bradley R., Jamieson P.D., Foulkes M.J. Quantifying how winter wheat crops accumulate and use nitrogen reserves during growth. Field Crops Res. 2012;126:104–118. doi: 10.1016/J.FCR.2011.09.021. [DOI] [Google Scholar]
- Shi Y., Thomasson J.A., Murray S.C., Pugh N.A., Rooney W.L., Shafian S., Rajan N., Rouze G., Morgan C.L., Neely H.L., Rana A., Bagavathiannan M.V., Henrickson J., Bowden E., Valasek J., Olsenholler J., Bishop M.P., Sheridan R., Putman E.B., Popescu S., Burks T., Cope D., Ibrahim A., McCutchen B.F., Baltensperger D.D., Avant R.V., Vidrine M., Yang C. Unmanned aerial vehicles for high-throughput phenotyping and agronomic research. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slafer G.A., Andrade F.H., Satorre E.H. Genetic-improvement effects on pre-anthesis physiological attributes related to wheat grain-yield. Field Crops Res. 1990;23:255–263. [Google Scholar]
- Triboi E., Triboi-Blondel A.-M. Productivity and grain or seed composition: a new approach to an old problem. Eur. J. Agron. 2002;16:163–186. [Google Scholar]
- Uauy C., Distelfeld A., Fahima T., Blechl A., Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C., Brevis J.C., Dubcovsky J. The high grain protein content gene Gpc-B1 accelerates senescence and has pleiotropic effects on protein content in wheat. J. Exp. Bot. 2006;57:2785–2794. doi: 10.1093/jxb/erl047. [DOI] [PubMed] [Google Scholar]
- van Oosterom E.J., Chapman S.C., Borrell A.K., Broad I.J., Hammer G.L. Functional dynamics of the nitrogen balance of sorghum. II. Grain filling period. Field Crops Res. 2010;115:29–38. [Google Scholar]
- Zadoks J.C., Chang T.T., Konzak C.F. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- Zheng T.C., Zhang X.K., Yin G.H., Wang L.N., Han Y.L., Chen L., Huang F., Tang J.W., Xia X.C., He Z.H. Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crops Res. 2011;122:225–233. doi: 10.1016/J.FCR.2011.03.015. [DOI] [Google Scholar]
- Zheng W. High frequency of abnormal high molecular weight glutenin alleles in Chinese wheat landraces of the Yangtze-River region. J. Cereal Sci. 2011;54(3):401–408. doi: 10.1016/J.JCS.2011.08.004. Academic Press. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.