Abstract
Unlike molecular solvents, imidazolium-based ionic liquids are entirely made of ions with spatial heterogeneity. There is a need for spectroscopic probes that can assess the microenvironment near the cations of these complex liquids. In this manuscript, we describe simple chemical procedures to label the C2 position of imidazolium cation with a C–D vibrational probe and show, through linear and nonlinear vibrational spectroscopies, that this C–D stretching mode can be a useful analytical tool to assess both the solvent microenvironment and solute–solvent interactions in imidazolium-based ionic liquids from the cation point of view. It is expected that this C–D vibration probe on the cation will lead to the development of innovative experimental strategies that can provide a better understanding of such ionic liquids.
Graphical Abstract
1. INTRODUCTION
Imidazolium-based ionic liquids have the potential to change current laboratory and industrial practices in chemical synthesis,1 separation,2 energy applications,3 and health care.4 However, understanding these ionic liquids on a molecular level is a challenge5–7 as these liquids are entirely made of ions with segregated polar and nonpolar domains.8 An attractive way to analyze these complex systems is by infrared spectroscopy9–11. However, there is a need for new environment-sensitive vibrational probes that can interrogate the microenvironment of these liquids, particularly from the cation point of view, to augment our understanding of these solvents. In this work, we demonstrate that a C–D vibrational label at the C2 position on the imidazolium ion can act as a sensitive spectroscopic tool to investigate the local microenvironment of imidazolium-based ionic liquids. Furthermore, we show through nonlinear vibrational spectroscopy that this C2–D infrared absorption is a suitable probe to study solute–solvent interactions. We envision that a large number of imidazolium-based ionic liquids can be labeled with the C2–D probe using the synthetic methods described in this work, allowing systematic analysis of their local environment from the perspective of the cation.
In the mid-infrared, the characteristics of the C–H stretching bands of the imidazolium cation from 3100 to 3200 cm−1 are often used to investigate the microenvironment of the ionic liquid near the cation.9–11 However, this region is spectrally congested with broad and overlapping peaks from overtones and combination bands of the imidazolium ring12–14. So, it is not surprising that there is ambiguity in the assignment of the C–H peak position stretching vibrations from the aromatic protons (C2–H, C4–H, and C5–H) in the imidazolium ring. The problem is further compounded by the fact that the infrared line shapes in ionic liquids are influenced by a subtle balance between Coulomb forces, hydrogen bonds, and dispersion forces.15 Nonetheless, some authors16–19 use the C–H vibrational bands of the cation to reinforce the idea that there are extended three-dimensional networks of hydrogen bonds that influence the solvent properties, whereas others20,21 suggest that hydrogen bonding has only a minor effect on the structure and properties of these liquids. Hydrogen bond formation is favored by the acidic nature of the hydrogen atoms of imidazolium salts, but discrepancies in assessing the hydrogen-bonded states in ionic liquids point to the fact that characterizing these interactions is not straightforward.5 To circumvent the problem of spectral congestion, many authors22–24 use external probes with a clear vibrational signature to study imidazolium-based ionic liquids. Most of these studies utilize salts of pseudohalides to directly or indirectly analyze these complex liquids. The use of salts is a problem as their solubility in ionic liquids is highly dependent on the anion–cation combination.25 So, it is unlikely that these probes can be used to study a large number of ionic liquids. Moreover, salts are external probes that are solvated, and thus the results of these studies should be viewed cautiously as they may not reflect the true characteristics of the native solvent. In short, there is a need for a better infrared spectroscopic handle to assess the microenvironment near the cation of imidazolium-based ionic liquids.
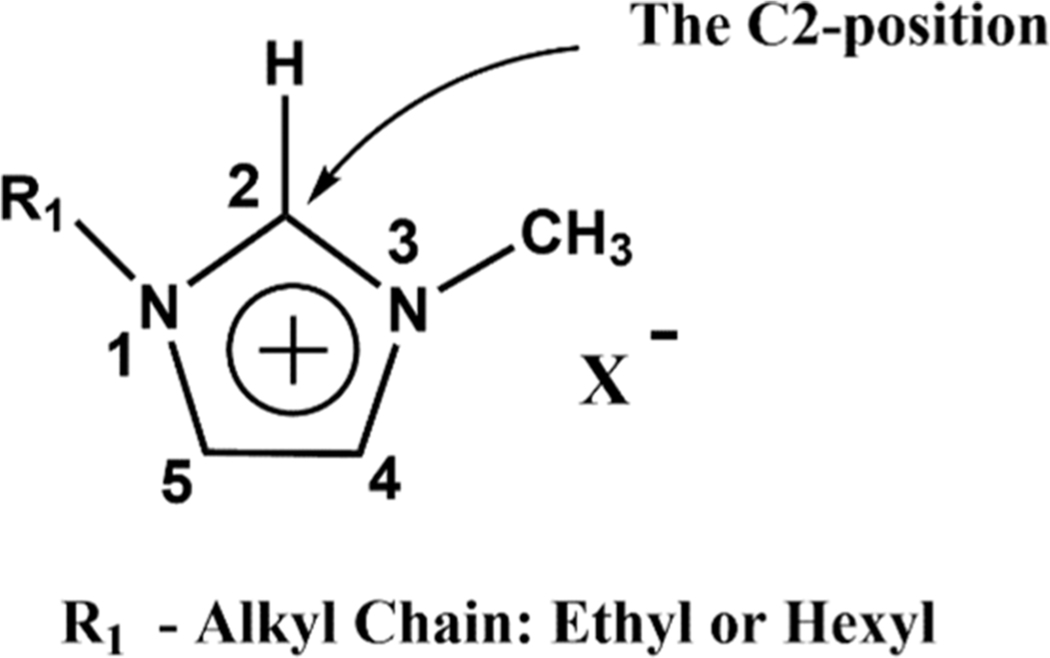
C–D vibration is a useful tool in isotope-edited Fourier transform infrared (FTIR) spectroscopy studies of proteins.26–29 The key advantage of incorporating a C–D infrared label in proteins is that its absorption appears in a region away from the protein-congested vibrational background. Such a spectral isolation technique is advantageous, as it allows the extraction of molecular-level information from a specific site within the protein. The same strategy is applied in this work to study the microenvironment near the imidazolium cation. C–D modes are stronger in ions, especially at the C2–D position, vide infra. Our plan is to convert the C2 hydrogen (see Figure 1) of the imidazolium cation to a C–D product, which we refer to as the C2–D label, to isolate the signal from the C2 position for analysis. We chose the C2 position for conversion because it plays a pivotal role in interionic interactions and on the physiochemical properties of the liquid.10 Experimental evidence, supported by computational results, shows that hydrogen bonds formed at this site are stronger than those at the C4 or C5 positions.14,30,31 Perhaps, the most persuasive evidence of the importance of this position comes from striking changes in bulk properties upon methylation of this site.32,33 It is suggested that the strong hydrogen bonds at the C2 position introduce “defects” into the Coulombic network, which affects the macroscopic properties of the solvent.19,34 Recent ultrafast vibrational studies by Ren et al.,35 employing an external vibrational probe (KSCN), showed that the cation–anion reorganization around the C2 position is related to the viscosity of the ionic liquids. Similarly, by inserting a chromophore (SeCN) at the C2 position of the cation, Yamada et al.36 were able to understand the fluctuations of local structures at this position. In short, there has been a concerted effort to probe the microenvironment at the C2 position to connect to bulk properties of the liquid.5 However, there are limitations in analyzing this position by vibrational spectroscopy. As mentioned before, the C2–H stretching region is congested and the use of external probes or inserting a probe at this location can compromise the integrity of the solvent.
Figure 1.
Typical structure of imidazolium-based ionic liquid (the C2 position is highlighted).
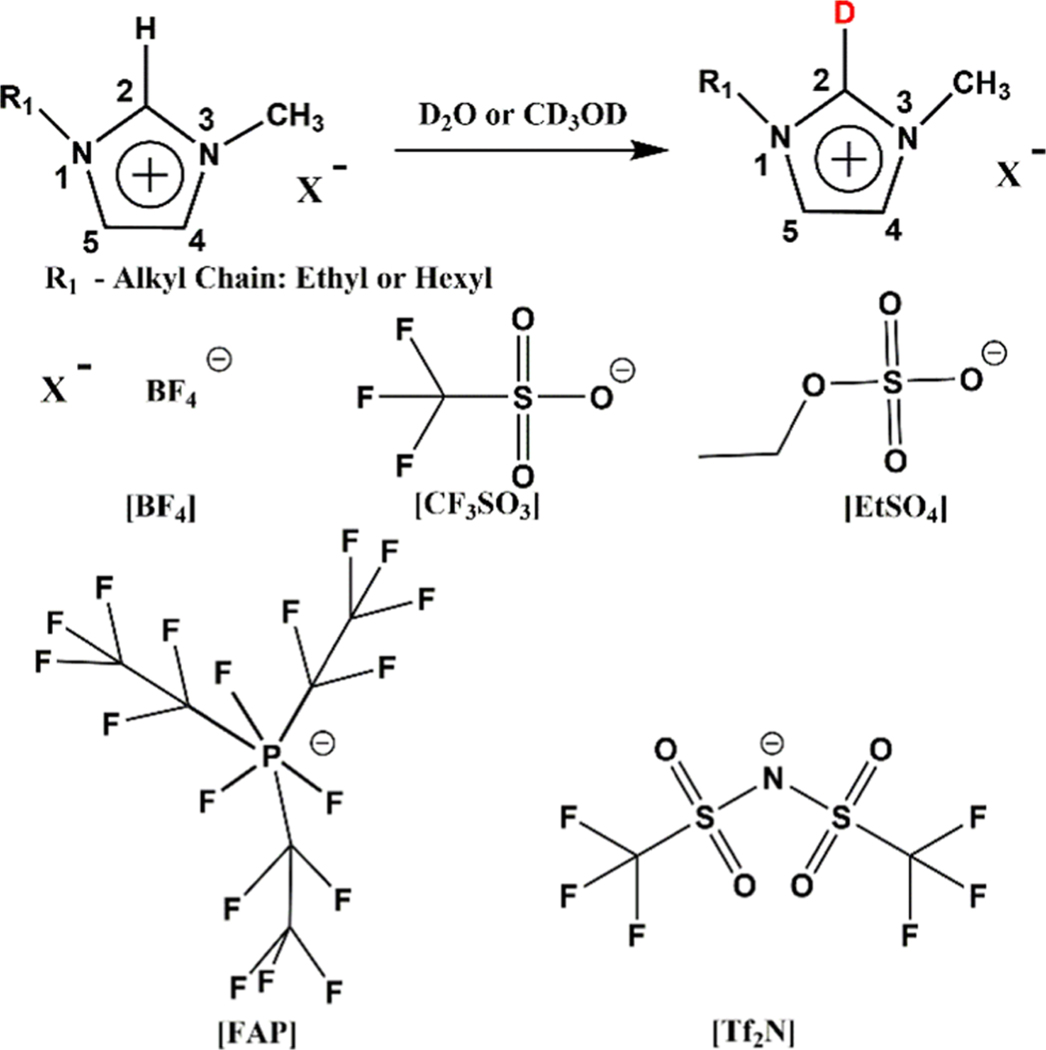
Using C2–D vibration as a spectroscopic handle is beneficial in ionic liquid studies, as this chemical transformation is unlikely to change the physicochemical properties of the solvent. Results from recent studies indicate that the C2–D vibration from the imidazolium cation of ionic liquids is strong and appears in a clear region (∼2300 cm−1).9,12,13,37,38 Based on these reports, we hypothesize that C2–D vibration on the imidazolium cation can be a sensitive reporter of the complex ionic liquid microenvironment and can reveal the nature of solute–ionic liquid interactions on a molecular level. To test this hypothesis, we introduced the C–D vibrational label at the C2 position of the imidazolium cation by heating the liquids with either heavy water (D2O) or anhydrous tetradeutero-methanol (CD3OD) (Scheme 1). Ten distinct room-temperature ionic liquids are studied here, where either 1ethyl-3-methylimidazolium ([EMIM]) or 1-hexyl-3-methylimidazolium ([HMIM]) is the cation and tetrafluoroborate ([BF4]), ethylsulfate ([EtSO4]), triflate ([CF3SO3]), tris(pentafluoroethyl)trifluorophosphate ([FAP]), or bis(trifluoromethylsulfonyl) ([Tf2N]) is the anion. Our results demonstrate that the C2–D label can be a sensitive probe that can be used to interrogate ionic liquid on a molecular level by both linear and nonlinear vibrational spectroscopies.
Scheme 1.
Imidazolium-Based Ionic Liquid Selectively Labeled at the C2 Position Using Deuterated Solvents
2. EXPERIMENTAL SECTION
2.1. Reagents and Materials.
All reagents were purchased from Sigma-Aldrich, Fisher Scientific, or Iolitec. All ionic liquids had mass fraction purities higher than 99+ and were dried under high vacuum (<0.001 torrs) and at 80 °C for a minimum period of 24 h before labeling them with a deuterium atom to reduce the water content.39,40 The deuterating agents, D2O or CD3OD, also had low water content (≤50 ppm of water).
2.2. General Procedure for Synthesis and Isolation of C2–D Labeled Ionic Liquids.
The synthesis of deuterated ionic liquids (Scheme 1) was performed by modifying existing protocols, which are described elsewhere.41–43 All procedures were done in a nitrogen atmosphere using standard air-free techniques.44,45 Briefly, the dried ionic liquid was transferred to the reaction vessel under nitrogen. D2O or CD3OD was added to the liquid in a 30:1 molar ratio. This ionic liquid–D2O or ionic liquid–CD3OD mixture was stirred at 60 °C for 24 h under nitrogen.
2.2.1. Workup Procedure for Ionic Liquid–D2O Mixture.
After 24 h, the reaction in D2O was cooled to 0 °C. Dry methylene chloride was added to the reaction mixture. The solution was shaken and allowed to equilibrate for an hour. The heavier and immiscible methylene chloride separated from heavy water and settled in the bottom. This layer was collected in a clean container under nitrogen. This extraction process with methylene chloride was repeated for several times. The collected methylene chloride layers were removed under reduced pressure to obtain the desired C2–D labeled ionic liquid. This method was used for all EMIM-based liquids reported in this work for D2O-mediated H/D exchange.
For hexyl-based ionic liquids reacting in D2O, cooling to 0 °C resulted in the separation of the reaction mixture into two distinct phases. The denser ionic liquid settled in the bottom and was removed under nitrogen. The liquid was redissolved in D2O at room temperature and extracted with methylene chloride in a similar way as mentioned above to obtain the C2–D labeled ionic liquid.
2.2.2. Workup Procedure for Ionic Liquid–CD3OD Mixture.
The workup procedure for ionic liquid–CD3OD mixture involved cooling the mixture to room temperature and removing the solvent by rotovaping. The product was again dissolved in anhydrous methylene chloride to remove trace water. The solvent, methylene chloride, was removed under reduced pressure to obtain the purified C2–D labeled ionic liquid.
The synthesized products were characterized by NMR measurements using a Bruker 300 MHz high-resolution spectrometer (see the Supporting Information, S1). All products were synthesized at least three times and were stored under high vacuum.
2.3. FTIR Studies.
FTIR of ionic liquids was performed using a Bruker Tensor II spectrometer equipped with an MCT detector. The selected sample, in vacuum storage, was first heated to 80 °C under high vacuum. After 24 h of vacuum drying, the storage container containing the ionic liquid was cooled and backfilled with nitrogen. Approximately, 10 μL of the ionic liquid sample was taken and placed between two CaF2 windows separated by a 25 μm spacer and sealed in a gastight dismountable liquid cell holder (Pike Technologies).
FTIR spectra were collected over 16 scans with a resolution of 2 cm−1 at regions from 4000 to 1000 cm−1. The instrument was continuously purged with nitrogen to remove ambient CO2 and water from the instrument during measurements. Analysis of the spectra was done with OPUS software.
2.4. Dual-Frequency Two-Dimensional Infrared (2D-IR) Spectroscopy.
An in-depth description of the fully automated dual-frequency three-pulse echo 2D-IR instrument used in this work is presented elsewhere.46 Briefly, a Ti:sapphire laser-producing 1.5 W power at a 1 kHz repetition rate, an 800 nm wavelength, and an 80 fs pulse duration (Libra, Coherent) was used to pump a computer-controlled dual-optical parametric amplifier (OPA, Palitra-duo, Quantronix). Two separate pairs of signal and Idler pairs generated by this OPA were directed to two computer-controlled difference frequency generation units (NIR Quantronix) to generate mid-IR pulses tunable in the frequency range from 500 to 5000 cm−1 with a pulse energy ranging from 1.0 to 10 μJ. The two mid-IR beams with distinct center frequencies are passed through different optical pathways to satisfy phase-matching conditions at the sample.47 The heterodyned signal was measured using a multichannel MCT array detector (IR-6400, Infrared Associates) attached to a monochromator (Triax-190, HORIBA).
The 2D-IR spectra were collected by scanning the delay between the first two mid-IR pulses, τ (both of the same central frequency), at a fixed waiting time, T, which is the delay between the second and third pulses. The heterodyned signal in the frequency range of interest (λ → ωt) is recorded for every τ. Fourier transformation along τ results in the ωτ axis gives rise to the 2D-IR spectrum at a given waiting time. A typical 2D-IR spectrum took about 20 s to acquire and contained ∼30 points along the τ direction, which was scanned in 5 μm steps (undersampled).
We used [EMIM][FAP] as a model in our 2D-IR studies as it can absorb a large amount of gas.48 The sample was prepared by taking 1 mL of the C2–D labeled [EMIM][FAP] in a glass vial. The liquid was vacuum-dried for at least 2 h at 80 °C in the vial and then cooled down to room temperature. Pure greenhouse gas (N2O or 13CO2) was introduced to this vacuum-sealed liquid till the pressure was ∼1 atm inside the vial. Approximately, 10 μL of this liquid was placed between two CaF2 windows separated by a 25 μm spacer for 2D-IR studies and sealed in a gastight dismountable liquid cell holder.
2.5. Computational Methods.
Calculations were done at the ω-B97XD/def2-TZVP level of theory using the SMD implicit solvation model (ε = 10) to mimic the ionic liquid environment. We used the Gaussian G16 suite of molecular electronic structure programs for our computations. Optimizations were carried out using “tight” convergence criteria. Several (at least five) starting structures with anions in different geometries around the cation were considered for calculations. The structures either converged to very similar geometries (with near-identical C–D frequencies) or, in some cases, failed to converge.
3. RESULT AND DISCUSSION
Our results show that the C2–D labeled imidazolium-based ionic liquids with short (ethyl) and long (hexyl) alkyl side chains can be produced in a straightforward way from the deuterated solvents, D2O and CD3OD, independently by modifying the existing protocols41–43 To the best of our knowledge, a C2–D label on long-side-chain imidazolium cation with [EtSO4], [CF3SO3], [FAP], or [Tf2N] as the anion has not been reported before in the literature. For the reactions to be successful, it is essential to use anhydrous reagents, maintain an inert atmosphere, and conduct the reaction at an elevated temperature. A significant difference between the current work and previously reported studies9,12,38 is that the products were isolated from the reaction mixture using methylene chloride and dried before spectroscopic measurements. All C2–D products were liquids at room temperature.
To quantify the conversion, we use the well-documented disappearance of proton signal in NMR spectroscopy upon deuteration to calculate the extent of deuteration.49,50 Specifically, the degree of deuteration was determined by integrating the residual C2–H proton signal in the 8–10 ppm range of the imidazolium cation in the spectrum, using the methyl protons at the C3 position as the internal reference.43,51,52 Our results clearly show that the signal associated with the C2 proton is significantly reduced after deuteration (see the Supporting Information, S1). The NMR signal from C4 and C5 protons does not change. Quantifying the residual peak area of the C2 proton shows that the conversion to C2–D product can range from 50 to >90% after allowing the reaction to run for 24 h in D2O at an elevated temperature (>50 °C). Formation of the C2–D products in CD3OD was poor when compared to that in D2O. Our studies also indicate that a catalytic amount of anhydrous sodium deuteroxide or potassium carbonate added to the reaction mixture improves the conversion in both of the deuterating solvents. Though we can exclusively control deuterium insertion in the C2 position, it is possible to deuterate at the C4 and C5 positions of the cation by modifying the reaction conditions (see the Supporting Information, S2).
Even though the conversion is high in D2O, it is difficult to extract the C2–D labeled ionic product because of its solubility in heavy water. One way to recover the product is to use methylene chloride to extract the ionic liquid from the aqueous phase, but the yield is poor.42,43 Our explorations show that for EMIM-based products, the yield improves (<30 vs >70%) if the liquid is extracted at a lower temperature. On the other hand, C2–D labeled HMIM-based ionic liquids become immiscible in D2O below ∼4 °C and thus can be directly extracted from D2O, which simplifies the separation process and improves the yield. Reactions in CD3OD do not require additional purification steps and thus the yield is high. Taken together, we show that the C2–D labeled imidazolium-based ionic liquids can be conveniently synthesized and extracted in a straightforward way using either CD3OD or D2O.
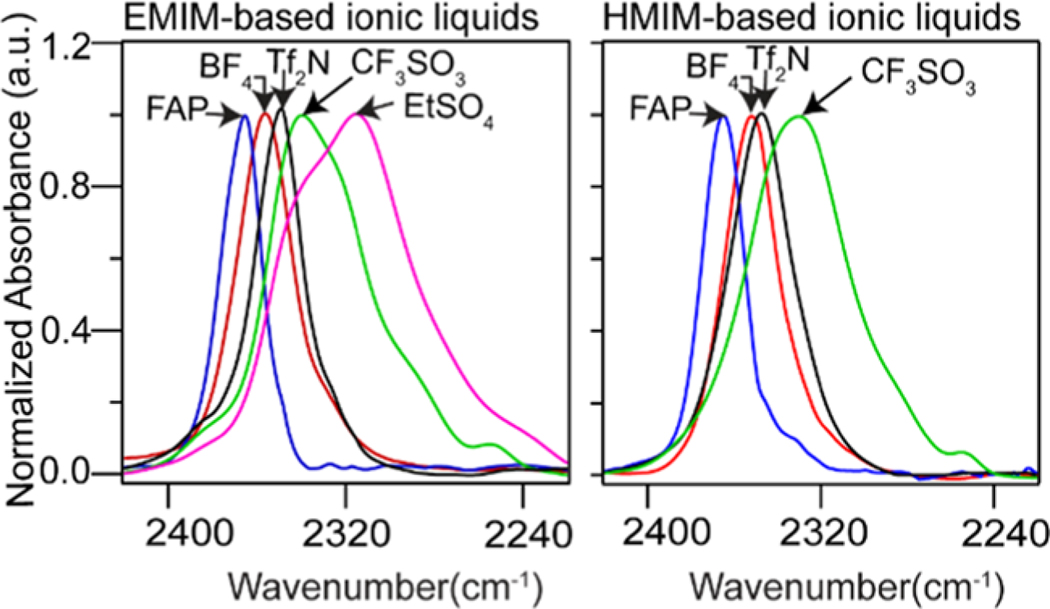
Infrared measurements of vacuum-dried C2–D labeled ionic liquids (water content <800 ppm) show the appearance of a strikingly new absorption feature in the infrared transparent region ranging from 2300 to 2400 cm−1. The new signature is assigned to νC–D stretching mode of the imidazolium cation in line with previous literature reports.9,37 We observed that the line shape of the C–D band of the labeled ionic liquids was the same, irrespective of the reagent, D2O or anhydrous CD3OD, used for their synthesis. Unlike their neutral C–D counterparts, imidazolium cations exhibit relatively high molar absorptivity of the C–D band (∼150 M−1 cm−1) (see the Supporting Information, S3). The presence of such a strong absorption band has been indicated in earlier works.37,53 As C2–H is converted to C2–D, we observe changes in spectral features in the region between 3100 and 3200 cm−1 but they are difficult to analyze systematically. It is clear from Figure 2 and Table 1 that the peak position of the C–D stretch on the cation is different for each investigated ionic liquid. We observe that in ionic liquids with anions without oxygen or nitrogen in their structure, [FAP] and [BF4], the peaks are blue-shifted when compared to those in [Tf2N], [EtSO4], and [CF3SO3]. The peak position is also observed to be affected by the size of the cation. There is a noticeable red shift of the C–D stretch from [EMIM] [CF3SO3] to [HMIM] [CF3SO3], [EMIM] [Tf2N] to [HMIM] [Tf2N], and [EMIM][BF4] to [HMIM] [BF4]. Like the peak position, the line width of the C–D mode is sensitive to the local solvent structure. In ionic liquids with sulfur oxyanions, the line shapes are broad (>30 cm−1, FWHM) with multiple peaks, whereas liquids with the [FAP], [BF4], or [Tf2N] anion are narrow (<20 cm−1, FWHM). The unambiguous changes in the infrared line shapes are clear indicators that the C–D vibrational mode is a sensitive reporter of the microenvironment near the C2 position of the cation of imidazolium-based ionic liquids.
Figure 2.
FTIR clearly shows the dependence of C2–D vibration on anion identity.
Table 1.
Changes in C2–D Stretch Line Shape with Change in Anion of the Imidazolium-Based Ionic Liquid with a 2 cm−1 Frequency Resolution
Infrared line shapes are influenced by the local solvent microenvironment.54,55 From our experimental observations, it is clear that the peak position of the C–D stretch is influenced by the chemical identity of the anion, irrespective of the size of the cation. An immediate question arises: How does the anion control the C–D vibration frequency at the C2 position? A logical explanation, apart from nonspecific electrostatics, is that the hydrogen bonding between the cation and the anion influences the C–D infrared line shape. This is a rational hypothesis as the hydrogen at the C2 position of the imidazolium cation is acidic and likely to form a hydrogen bond with the anion.18,56,57 To understand the impact of hydrogen bonding on the C–D stretching mode, we carry out harmonic vibrational analysis calculations using the ω-B97XD/def2-TZVP level of theory on an EMIM-based ionic liquid considered in this study. In this computational study, we constructed dimers involving H-bond acceptor sites on the anion with the deuterium atom at the C2 position of the cation.
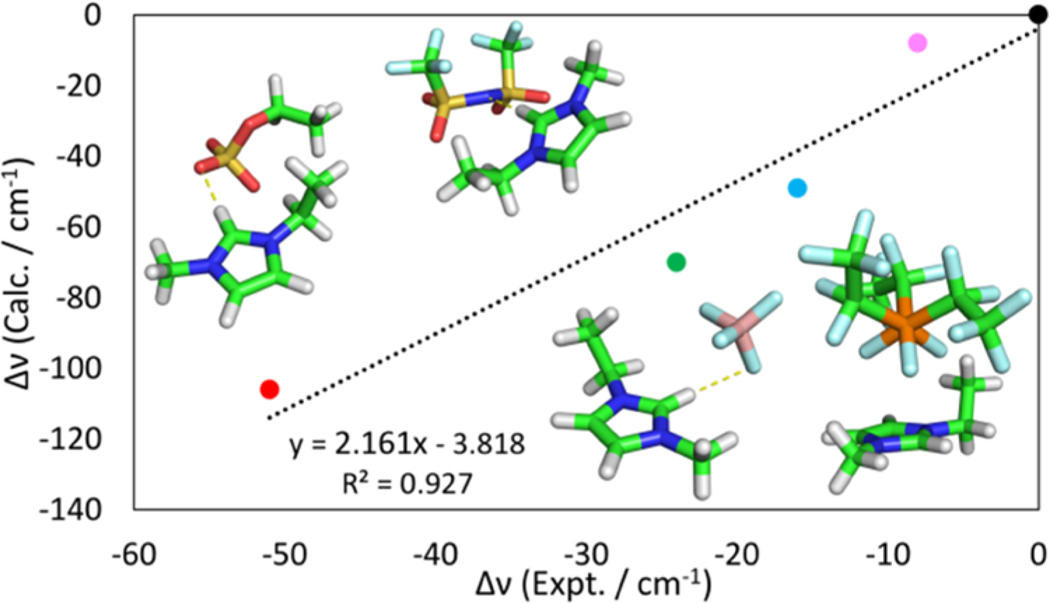
Figure 3 shows a correlation between calculated and experimental C–D vibrational frequency shifts, relative to those of [EMIM][FAP], as well as optimized complex structures for four of the five dimers. The graph shows that there is a very good correlation between experimental and calculated frequency shifts, with a coefficient of determination (R2 value) of 0.927. The correlation indicates that the formation of dimers plays an important role in vibrational red shifts that we observe experimentally. We also observe a good correlation between hydrogen bond distance and the magnitude of red shifts (R2 = 0.963, see the Supporting Information Figure SI4a). Analysis of the structures shows that there is a relationship of the H-bond length and C–D···Y (Y = F, N, or O) angle with the vibrational red shifts. Indeed, the largest red shift occurs for [EMIM][EtSO4], the structure with the shortest D···Y distance and a near-linear C–D···Y angle. In contrast, the large FAP anion forms a stacked structure with the EMIM cation in which no H-bond is formed with the EMIM cations. The frequency computed for the cation alone is 2463 cm −1, while that for the [EMIM][FAP] complex is 2461 cm −1. This indicates that stacking does not result in appreciable changes in the C2–D stretching frequency. This is not surprising, as there has generally been little evidence suggesting that stacking interactions have a strong influence on C–H stretch frequencies, although they may have a more significant impact on out-of-plane vibrations.58 It is important to note that there are multiple O-atom sites in the asymmetric anions [EtSO4] and [CF3SO3]. For such anions, multiple conformers are possible,59 which broadens the C–D line shapes, as experimentally observed in our study.
Figure 3.
Correlation between experimental and calculated harmonic red shifts in EMIM–anion dimers. EtSO4, red; CF3SO3, green; Tf2N, blue; BF4, pink; FAP, black.
Although the correlation between calculated and experimental peak positions of C–D is acceptable, the DFT-D calculation tends to overestimate the vibrational red shift substantially, as indicated by the slope of the correlation plot (m = 2.2). There are two primary reasons for this overestimation. First, density-functional-theory (DFT)-based harmonic methods fail to describe both correlation and anharmonicity effects and thus tend to overestimate vibrational red shifts.60–62 Second, the model used here uses a single pair of molecular ions to represent dynamics occurring in the bulk solution. Of course, in bulk, any given cation might interact with several anions and perhaps other cations, with all of these interactions affecting the C2–D vibrational frequency in some way. Here, we make the assumption that the C2–D···anion interaction has the strongest and most direct impact on the C2–D stretching frequency, which is typical of how red- (and blue-) shifting hydrogen bonds are generally investigated computationally.63 The strong correlation between spectroscopic and computational results points to the validity of this approximation. The computational results could not reproduce the observed red shift of the C–D stretch from EMIM to HMIM (short-chain to long-side-chain cation) for a given anion as the hexyl side chains resulted in very “floppy” systems whose geometries were difficult to optimize in our calculations. However, optimized structures of the EMIM and HMIM cations without the associated anions show that the C–D vibrational frequencies are effectively identical at the level of approximation used in these computations. As such, we assume that EMIM and HMIM cations at the C2 position interact similarly with the anion. Taken together, our studies suggest that it is reasonable to assume that the hydrogen bond formed between the cation and the anions is a crucial contributor that dictates the position of the central C–D vibrational frequency in the examined imidazolium-based ionic liquids. However, it is important to clarify that the C–D line shape contains more information about molecular structure and dynamics of the ionic liquid than what is attributed to hydrogen-bonding interactions in our studies. Indeed, the Gaussian fitting of the C2–D line shape of the ionic liquid, [EMIM] [EtSO4] (see the Supporting Information, S4), shows the presence of multiple hidden peaks, which can arise due to other factors including but not limited to multiple microenvironments within the liquid and Fermi resonances. On a broad scale, our studies suggest that the clear and strong C–D infrared signature is a good reporter of the molecular-level environment near the cation of imidazolium-based ionic liquids.
Apart from revealing the nature of ionic liquid microenvironment, the C–D label developed in this work can provide a spectroscopic handle that can directly report solute–solvent interactions from the cation perspective. To demonstrate this point, we dissolve the greenhouse gases, N2O and CO2, as model solutes in the C2–D labeled [EMIM][FAP] ionic liquid. The capability of ionic liquids to dissolve greenhouse gases is generating much attention, but it is still not clear which interactions affect the uptake of the gases. Studies64–67 so far indicate that anions play a significant role in the gas uptake in imidazolium-based ionic liquids with the cations playing a secondary role. There is no direct evidence of cation–gas interactions.
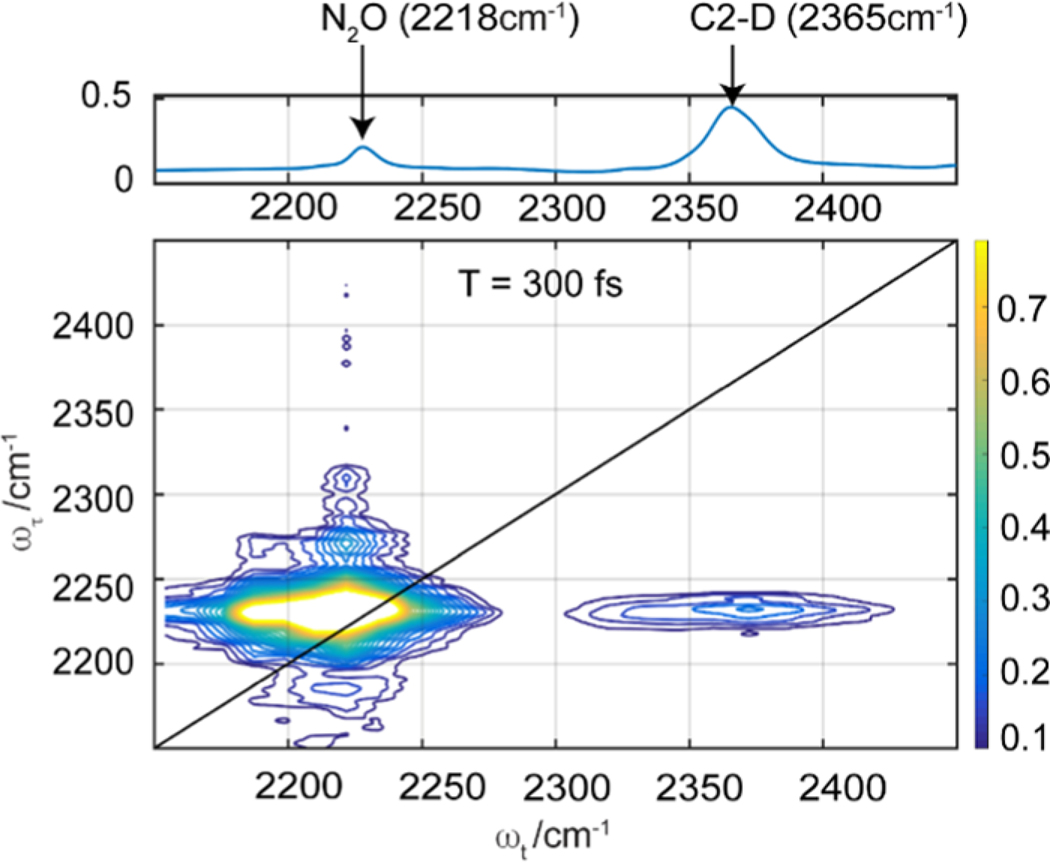
N2O dissolved in the C2–D labeled [EMIM][FAP] ionic liquid has a clear asymmetric fundamental mode at 2218 cm−1, approximately 137 cm−1 red-shifted from the C–D stretch at the C2 position of the imidazolium cation of the ionic liquid (Figure 4, top panel). From the linear FTIR spectrum, it is not clear whether there is any interaction between the cation and the gas. To tease out the relevant molecular information about specific interactions, we use the dual-frequency two-dimensional infrared (2D-IR) technique.68–70 This method is proven to reveal interactions between the solute and the solvent modes that have a large spectral separation.71 As the separation between the N2O and C–D modes is large, the dual-frequency 2D-IR technique is suitable to study the interactions between the two modes. Unlike conventional 2D-IR, where strong diagonal signals obscure cross-peaks, the dual-frequency 2D-IR spectrometer used in the experiment has the capability to enhance cross-peaks through a beam stabilization scheme, which sets the phase-matching geometry for the beams at the sample.46 In our 2D-IR experiment, two excitation ultrafast pulses centered at the solute, N2O, asymmetric vibrational mode creates a time-dependent transient grating within the sample. After a time delay (T), a third pulse centered at the solvent C2–D center frequency interacts with this grating to generate a vibrational echo. The echo is spatially and temporally overlapped with a local oscillator to generate a 2D-IR plot. It is clear from the resulting 2D-IR plot at time delay T = 300 fs (Figure 4) that there is a cross-peak between the C–D mode of the cation and the asymmetric N2O stretching vibration at approximately (2218, 2365 cm−1). The cross-peak appears at early times and decreases in intensity as the time delay increases (see the Supporting Information, S5). The stronger N2O diagonal peak conceals the C–D diagonal peak. This hidden C–D band can be observed by scanning this region using the conventional 2D-IR technique. We see a similar cross-peak between 13CO2 and the C–2D vibration mode of the liquid, [EMIM][FAP] (see the Supporting Information, S6).
Figure 4.
Absolute-value 2D-IR snapshot of N2O dissolved in C2–D labeled [EMIM][FAP] shows a clear cross-peak indicating an interaction between the gas and the cation.
The presence of cross-peaks in the 2D-IR spectrum is model-free and gives unambiguous proof of coupling between the cation of the ionic liquid and the solute molecules. The existence of the cross-peak is not a concentration-dependent effect as these gases were dissolved at approximately 1 atm pressure. Under this experimental condition, the concentration of the dissolved gas is below 10 mM for N2O gas and below 100 mM for CO2,48 clearly indicating that even in dilute solution, gas–cation interactions exist in this imidazolium-based ionic liquid (see the Supporting Information S7). These results are important as the interaction of C2 hydrogen of the cation with the dissolved gases is largely ignored65,66 due to the lack of experimental proof. In light of this new discovery, pressure-dependent studies on gas solubility should be revisited to ascertain the role of the cation in gas solubility. On a broad scale, these studies show that C–D vibration on the cation, despite having a weak signal, has the potential to reveal hidden features when investigated with dual-frequency 2D-IR spectroscopy, which is in line with studies done by others.72,73 Features like cross-peaks can provide deeper insights into solute–solvent interactions in such complex liquids, particularly from the cation point of view.
Ionic liquids are green solvents whose physiochemical properties can be tuned by customizing the organic cation and the cation–anion pairings. However, characterizing these liquids is challenging due to their complex inhomogeneous microenvironments. Vibrational spectroscopy is a well-established technique that can provide information about these liquids on a molecular level.11–14 However, analyzing infrared line shape is difficult, particularly from the cation of imidazolium-based ionic liquids due to spectral congestion and a lack of experimental strategies to isolate these vibrations. Placing a C–D label in the C2 position of the imidazolium cation, as seen in our experiments, transfers the absorption from a cluttered zone to a transparent region where the line shape can be unambiguously analyzed by both linear and nonlinear vibrational spectroscopies. The deuteration is nonperturbative, ensuring that the information is representative of the native liquid. Such a small intrinsic marker is better than large perturbing external solvatochromic probes30,74 to study solute–ionic liquid interactions.
The clear and strong environment-sensitive C–D absorption profile at the C2 position provides an opportunity to detect organized structural domains that are predicted to exist in these liquids. For example, the C–D probe can be used to test for the existence of three regions near the melting point: the charge-rich region, the symmetry-breaking region, and the hydrophobic region in the vicinity of the cation, as predicted by the quantitative structure–property relationship models.75 Similarly, the counterintuitive presence of a new class of H-bonded complexes comprising cation–cation pairing within the ionic liquid can be potentially detected by temperature-dependent experiment with the C–D probe. If such structures exist, the C2–D absorption will intensify with a decrease in temperature, red-shift, and show the emergence of new peaks.76 Examining how the linear spectra of the C2–D band change with the type of cation and on the chosen anion in these temperature dependence experiments can provide insight into the connection between microscopic changes and bulk properties like viscosity. In short, the C2–D probe can become an innovative molecular tool to assess the structural heterogeneity in ionic liquid. The amenability of the C–D probe to be interrogated by 2D-IR spectroscopy can provide the ability to tease out the structure and motions associated with ion pairs. Recent work by Ren35 showed that the lifetime of caged structures in these liquids can be determined by 2D-IR spectroscopy. Similar observations are possible using the C2–D labels. Observing dynamics at the C2 position for different anions can provide a means to systematically study the structural fluctuations near the cation, which can enhance our existing knowledge.36 Moreover, 2D-IR can show hidden components such as the presence of multiple conformers and the contribution of dynamics to the C2–D linear line shape. As the C–D is in a clear zone, dilution experiments with cosolvents may reveal the presence of contact ion pairs (CIPs) or tight ion pairs, solvent-separated ion pairs or loose ion pairs, and solvent-shared ion pairs when these mixtures are analyzed by 2D-IR technique.77 A striking application of the C2–D band can be toward the understanding of the role of the C2 position in gas solubility and chemical reactivity in this medium. In particular, for chemical reactions, solute-specific solvation23,30 is suggested to exist, the presence that can directly impact the reaction outcomes because the interaction of the cation with the solute has to compete with the interaction with the anion.5 The detection of a solute–solvent complex using the cross-peak between C2–D and a vibration mode of a chemical reagent by 2D-IR spectroscopy can provide the missing connection of observed selectivity and enhanced rates that are seen in ionic liquids for many chemical reactions. Taken together, the C–D stretch can prove itself to be an important tool to investigate imidazolium-based ionic liquids from the point of view of the cation.
4. CONCLUSIONS
Our work shows that C2–D labels can be selectively inserted into the cation of many room-temperature imidazolium-based ionic liquids by carefully controlling the deuteration reaction. The C2–D probe is observed to have a strong and isolated infrared signature in these liquids. Analysis of this C2–D label shows that this probe has the potential to act as a sensitive reporter of the ionic liquid microenvironment. Our studies also indicate that the C2–D label is compatible with the two-dimensional vibrational technique and thus can provide new information about the solute–solvent interactions in these liquids. We envision that this probe, if utilized rationally, can significantly improve our knowledge of the ionic liquid environment and provide an experimental handle to scrutinize theoretical models.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported, in part, by the resources provided by Louisiana Cancer Research Consortium through its seed grant. The authors also thank Dr. Igor V. Rubtsov, Professor, Tulane University, for providing his facilities for this study and insightful comments. K.R. gratefully acknowledges support from the Army Research Office (W911NF-18-1-0458) and the National Science Foundation (CHE-1832167).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpca.9b02387.
NMR characterizations of ionic liquids are presented along with representative infrared spectrum; results from the DFT calculations are tabulated for ionic liquids; and nonlinear vibrational measurements (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Hallett JP; Welton T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev 2011, 111, 3508–3576. [DOI] [PubMed] [Google Scholar]
- (2).Han X; Armstrong DW Ionic Liquids in Separations. Acc. Chem. Res 2007, 40, 1079–1086. [DOI] [PubMed] [Google Scholar]
- (3).Wishart JF Energy Applications of Ionic Liquids. Energy Environ. Sci 2009, 2, 956–961. [Google Scholar]
- (4).Egorova KS; Gordeev EG; Ananikov VP Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev 2017, 117, 7132–7189. [DOI] [PubMed] [Google Scholar]
- (5).Weingärtner H. Understanding Ionic Liquids at the Molecular Level: Facts, Problems, and Controversies. Angew. Chem., Int. Ed 2008, 47, 654–670. [DOI] [PubMed] [Google Scholar]
- (6).Shi R; Wang Y. Dual Ionic and Organic Nature of Ionic Liquids. Sci. Rep 2016, 6, No. 19644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hayes R; Warr GG; Atkin R. Structure and Nanostructure in Ionic Liquids. Chem. Rev 2015, 115, 6357–6426. [DOI] [PubMed] [Google Scholar]
- (8).Canongia Lopes JN; Costa Gomes MF; Padua AAH Nonpolar, Polar, and Associating Solutes in Ionic Liquids. J. Phys. Chem. B 2006, 110, 16816–16818. [DOI] [PubMed] [Google Scholar]
- (9).Jeon Y; Sung J; Seo C; Lim H; Cheong H; Kang M; Moon B; Ouchi Y; Kim D. Structures of Ionic Liquids with Different Anions Studied by Infrared Vibration Spectroscopy. J. Phys. Chem. B 2008, 112, 4735–4740. [DOI] [PubMed] [Google Scholar]
- (10).Noack K; Schulz PS; Paape N; Kiefer J; Wasserscheid P; Leipertz A. The Role of the C2 Position in Interionic Interactions of Imidazolium Based Ionic Liquids: A Vibrational and NMR Spectroscopic Study. Phys. Chem. Chem. Phys 2010, 12, 14153–14161. [DOI] [PubMed] [Google Scholar]
- (11).Paschoal VH; Faria LFO; Ribeiro MCC Vibrational Spectroscopy of Ionic Liquids. Chem. Rev 2017, 117, 7053–7112. [DOI] [PubMed] [Google Scholar]
- (12).Grondin J; Lassegues JC; Cavagnat D; Buffeteau T; Johansson P; Holomb R. Revisited Vibrational Assignments of Imidazolium-Based Ionic Liquids. J. Raman Spectrosc 2011, 42, 733–743. [Google Scholar]
- (13).Lassegues JC; Grondin J; Cavagnat D; Johansson P. New Interpretation of the CH Stretching Vibrations in Imidazolium-Based Ionic Liquids. J. Phys. Chem. A 2009, 113, 6419–6421. [DOI] [PubMed] [Google Scholar]
- (14).Roth C; Chatzipapadopoulos S; Kerle D; Friedriszik F; Lütgens MS; Kühn O; Ludwig R. Hydrogen Bonding in Ionic Liquids Probed by Linear and Nonlinear Vibrational Spectroscopy. New J. Phys 2012, 14, No. 105026. [Google Scholar]
- (15).Fumino K; Ludwig R. Analyzing the Interaction Energies between Cation and Anion in Ionic Liquids: The Subtle Balance between Coulomb Forces and Hydrogen Bonding. J. Mol. Liq 2014, 192, 94–102. [Google Scholar]
- (16).Dupont J. On the Solid, Liquid and Solution Structural Organization of Imidazolium Ionic Liquids . J. Braz. Chem. Soc 2004, 15, 341–350. [Google Scholar]
- (17).Dupont J; Suarez PAZ Physico-Chemical Processes in Imidazolium Ionic Liquids. Phys. Chem. Chem. Phys 2006, 8, 2441–2452. [DOI] [PubMed] [Google Scholar]
- (18).Dong K; Zhang S; Wang J. Understanding the Hydrogen Bonds in Ionic Liquids and Their Roles in Properties and Reactions. Chem. Commun 2016, 52, 6744–6764. [DOI] [PubMed] [Google Scholar]
- (19).Fumino K; Peppel T; Geppert-Rybczynska M; Zaitsau DH Lehmann JK; Verevkin SP;ckerling M; Ludwig R. The Influence of Hydrogen Bonding on the Physical Properties of Ionic Liquids. Phys. Chem. Chem. Phys 2011, 13, 14064–14075. [DOI] [PubMed] [Google Scholar]
- (20).Schröder C; Rudas T; Steinhauser O. Simulation Studies of Ionic Liquids: Orientational Correlations and Static Dielectric Properties. J. Chem. Phys 2006, 125, No. 244506. [DOI] [PubMed] [Google Scholar]
- (21).Dahl K; Sando GM; Fox DM; Sutto TE; Owrutsky JC Vibrational Spectroscopy and Dynamics of Small Anions in Ionic Liquid Solutions. J. Chem. Phys 2005, 123, No. 084504. [DOI] [PubMed] [Google Scholar]
- (22).Ren Z; Brinzer T; Dutta S; Garrett-Roe S. Thiocyanate as a Local Probe of Ultrafast Structure and Dynamics in Imidazolium-Based Ionic Liquids: Water-Induced Heterogeneity and Cation-Induced Ion Pairing. J. Phys. Chem. B 2015, 119, 4699–4712. [DOI] [PubMed] [Google Scholar]
- (23).Dutta S; Ren Z; Brinzer T; Garrett-Roe S. Two-Dimensional Ultrafast Vibrational Spectroscopy of Azides in Ionic Liquids Reveals Solute-Specific Solvation. Phys. Chem. Chem. Phys 2015, 17, 26575–26579. [DOI] [PubMed] [Google Scholar]
- (24).Shin JY; Yamada SA; Fayer MD Dynamics of a Room Temperature Lonic Liquid in Supported Ionic Liquid Membranes vs the Bulk Liquid: 2D IR and Polarized IR Pump-Probe Experiments. J. Am. Chem. Soc 2017, 139, 311–323. [DOI] [PubMed] [Google Scholar]
- (25).Pereiro AB; Araujo JMM; Oliveira FS; Esperança JMSS; Canongia Lopes JN; Marrucho IM; Rebelo LPN Solubility of Inorganic Salts in Pure Ionic Liquids. J. Chem. Thermodyn 2012, 55, 29–36. [Google Scholar]
- (26).Buchner GS; Kubelka J. Isotope-Edited Infrared Spectroscopy. Methods Mol. Biol 2012, 895, 347–358. [DOI] [PubMed] [Google Scholar]
- (27).Arkin IT Isotope-Edited IR Spectroscopy for the Study of Membrane Proteins. Curr. Opin. Chem. Biol 2006, 10, 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ma J; Pazos IM; Zhang W; Culik RM; Gai F. Site-Specific Infrared Probes of Proteins. Annu. Rev. Phys. Chem 2015, 66, 357–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Cremeens ME; Fujisaki H; Zhang Y; Zimmermann J; Sagle LB; Matsuda S; Dawson PE; Straub JE; Romesberg FE Efforts toward Developing Direct Probes of Protein Dynamics. J. Am. Chem. Soc 2006, 128, 6028–6029. [DOI] [PubMed] [Google Scholar]
- (30).Crowhurst L; Mawdsley PR; Perez-Arlandis JM; Salter PA; Welton T. Solvent–Solute Interactions in Ionic Liquids. Phys. Chem. Chem. Phys 2003, 5, 2790–2794. [Google Scholar]
- (31).Thar J; Brehmy M; Seitsonen AP; Kirchner B. Unexpected Hydrogen Bond Dynamics in Imidazolium-Based Ionic Liquids. J. Phys. Chem. B 2009, 113, 15129–15132. [DOI] [PubMed] [Google Scholar]
- (32).Bonhôte P; Dias A-P; Papageorgiou N; Kalyanasundaram KM Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem 1996, 35, 1168–1178. [DOI] [PubMed] [Google Scholar]
- (33).Yoshida Y; Baba O; Saito G. Ionic Liquids Based on Dicyanamide Anion: Influence of Structural Variations in Cationic Structures on Ionic Conductivity. J. Phys. Chem. B 2007, 111, 4742–4749. [DOI] [PubMed] [Google Scholar]
- (34).Zhang Y; Maginn EJ The Effect of C2 Substitution on Melting Point and Liquid Phase Dynamics of Imidazolium Based-Ionic Liquids: Insights from Molecular Dynamics Simulations. Phys. Chem. Chem. Phys 2012, 14, 12157–12164. [DOI] [PubMed] [Google Scholar]
- (35).Ren Z; Ivanova AS; Couchot-Vore D; Garrett-Roe S. Ultrafast Structure and Dynamics in Ionic Liquids: 2D-IR Spectroscopy Probes the Molecular Origin of Viscosity. J. Phys. Chem. Lett 2014, 5, 1541–1546. [DOI] [PubMed] [Google Scholar]
- (36).Yamada SA; Bailey HE; Tamimi A; Li C; Fayer MD Dynamics in a Room-Temperature Ionic Liquid from the Cation Perspective: 2D IR Vibrational Echo Spectroscopy. J. Am. Chem. Soc 2017, 139, 2408–2420. [DOI] [PubMed] [Google Scholar]
- (37).Katsyuba SA; Zvereva EE; Vidiš A; Dyson PJ Application of Density Functional Theory and Vibrational Spectroscopy toward the Rational Design of Ionic Liquids. J. Phys. Chem. A 2007, 111, 352–370. [DOI] [PubMed] [Google Scholar]
- (38).Cha S; Kim D. Anion Exchange in Ionic Liquid Mixtures. Phys. Chem. Chem. Phys 2015, 17, 29786–29792. [DOI] [PubMed] [Google Scholar]
- (39).Rodríguez H; Brennecke JF Temperature and Composition Dependence of the Density and Viscosity of Binary Mixtures of Water + Ionic Liquid. J. Chem. Eng. Data 2006, 51, 2145–2155. [Google Scholar]
- (40).Martins MAR; Neves CMSS; Kurnia KA; Carvalho PJ; Rocha MAA; Santos LMNBF; Pinho SP; Freire MG Densities, Viscosities and Derived Thermophysical Properties of Water-Saturated Imidazolium-Based Ionic Liquids. Fluid Phase Equilib. 2016, 407, 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Handy ST; Okello M. The 2-Position of Imidazolium Ionic Liquids: Substitution and Exchange. J. Org. Chem 2005, 70, 1915–1918. [DOI] [PubMed] [Google Scholar]
- (42).Kanakubo M; Ikeda T; Aizawa T; Nanjo H; Kameda Y; Amo Y; Usuki T. Liquid Structure of 1-Butyl-3-Methylimidazolium Hexafluorophosphate by Neutron Diffraction with H/D Isotopic Substitution Method. Anal. Sci 2008, 24, 1373–1376. [DOI] [PubMed] [Google Scholar]
- (43).Giernoth R; Bankmann D. Transition-Metal-Free Synthesis of Perdeuterated Imidazolium Ionic Liquids by Alkylation and H/D Exchange. Eur. J. Org. Chem 2008, No. 17, 2881–2886. [Google Scholar]
- (44).Shriver DF; Drezdzon MA The Manipulation of Air-Sensitive Compounds, 2nd ed.; Shriver DF, Ed.; A Wiley-Interscience Pub., 1986. [Google Scholar]
- (45).Leonard J; Procter G; Lygo B. Advanced Practical Organic Chemistry, 3rd ed.; CRC Press, 2013. [Google Scholar]
- (46).Leger JD; Nyby CM; Varner C; Tang J; Rubtsova NI; Yue Y; Kireev VV; Burtsev VD; Qasim LN; Rubtsov GI; et al. Fully Automated Dual-Frequency Three-Pulse-Echo 2DIR Spectrometer Accessing Spectral Range from 800 to 4000 Wavenumbers. Rev. Sci. Instrum 2014, 85, No. 083109. [DOI] [PubMed] [Google Scholar]
- (47).Nyby CM; Leger JD; Tang J; Varner C; Kireev VV; Rubtsov IV Mid-IR Beam Direction Stabilization Scheme for Vibrational Spectroscopy, Including Dual-Frequency 2DIR. Opt. Express 2014, 22, 6801–6809. [DOI] [PubMed] [Google Scholar]
- (48).Althuluth M; Mota-Martinez MT; Kroon MC; Peters CJ Solubility of Carbon Dioxide in the Ionic Liquid 1-Ethyl-3Methylimidazolium Tris(Pentafluoroethyl)Trifluorophosphate. J. Chem. Eng. Data 2012, 57, 3422–3425. [Google Scholar]
- (49).Wolfsberg M; Alexander Van Hook W; Paneth P; Rebelo LPN Isotope Effects: In the Chemical, Geological, and Bio Sciences; Springer Pub., 2009. [Google Scholar]
- (50).Pavia DL; Lampman GM; Kriz GS Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; Harcourt College Pub., 2009. [Google Scholar]
- (51).Murray CJ; Duffin KL Determination of Rates of Proton Exchange of Thiamine Hydrochloride by 1H NMR Spectroscopy: A Bioorganic Experiment for the Undergraduate Laboratory. J. Chem. Educ 1991, 68, 683–684. [Google Scholar]
- (52).Bradbury JH; Chapman BE; Pellegrino FA Hydrogen-Deuterium Exchange Kinetics of the C-2 Protons of Imidazole and Histidine Compounds. J. Am. Chem. Soc 1973, 95, 6139–6140. [DOI] [PubMed] [Google Scholar]
- (53).Dieter KM; Dymek CJ; Heimer NE; Rovang JW; Wilkes JS Ionic Structure and Interactions in 1-Methyl-3Ethylimidazolium Chloride-AlCl3 Molten Salts. J. Am. Chem. Soc 1988, 110, 2722–2726. [Google Scholar]
- (54).Kim H; Cho M. Infrared Probes for Studying the Structure and Dynamics of Biomolecules. Chem. Rev 2013, 113, 5817–5847. [DOI] [PubMed] [Google Scholar]
- (55).Levinson NM; Fried SD; Boxer SG Solvent-Induced Infrared Frequency Shifts in Aromatic Nitriles Are Quantitatively Described by the Vibrational Stark Effect. J. Phys. Chem. B 2012, 116, 10470–10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Hunt PA; Ashworth CR; Matthews RP Hydrogen Bonding in Ionic Liquids. Chem. Soc. Rev 2015, 44, 1257–1288. [DOI] [PubMed] [Google Scholar]
- (57).Dong K; Zhang S; Wang D; Yao X. Hydrogen Bonds in Imidazolium Ionic Liquids. J. Phys. Chem. A 2006, 110, 9775–9782. [DOI] [PubMed] [Google Scholar]
- (58).Fornaro T; Biczysko M; Monti S; Barone V. Dispersion Corrected DFT Approaches for Anharmonic Vibrational Frequency Calculations: Nucleobases and Their Dimers. Phys. Chem. Chem. Phys 2014, 16, 10112–10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Hunt PA; Gould IR; Kirchner B. The Structure of Imidazolium-Based Ionic Liquids: Insights from Ion-Pair Interactions. Aust. J. Chem 2007, 60, 9–14. [Google Scholar]
- (60).Heger M; Suhm MA; Mata RA Communication: Towards the Binding Energy and Vibrational Red Shift of the Simplest Organic Hydrogen Bond: Harmonic Constraints for Methanol Dimer. J. Chem. Phys 2014, 141, No. 101105. [DOI] [PubMed] [Google Scholar]
- (61).Kollipost F; Papendorf K; Lee YFP; Lee YFP; Suhm MA Alcohol Dimers-How Much Diagonal OH Anharmonicity? Phys. Chem. Chem. Phys 2014, 16, 15948–15956. [DOI] [PubMed] [Google Scholar]
- (62).Larsen RW; Zielke P; Suhm MA Hydrogen-Bonded OH Stretching Modes of Methanol Clusters: A Combined IR and Raman Isotopomer Study. J. Chem. Phys 2007, 126, No. 194307. [DOI] [PubMed] [Google Scholar]
- (63).Joseph J; Jemmis ED Red-, Blue-, or No-Shift in Hydrogen Bonds: A Unified Explanation. J. Am. Chem. Soc 2007, 129, 4620–4632. [DOI] [PubMed] [Google Scholar]
- (64).Camper D; Scovazzo P; Koval C; Noble R. Gas Solubilities in Room-Temperate Ionic Liquids. Ind. Eng. Chem. Res 2004, 3049–3054. [Google Scholar]
- (65).Lei Z; Dai C; Chen B. Gas Solubility in Ionic Liquids. Chem. Rev 2014, 114, 1289–1326. [DOI] [PubMed] [Google Scholar]
- (66).Ramdin M; de Loos TW; Vlugt TJH State-of-the-Art of CO2 Capture with Ionic Liquids. Ind. Eng. Chem. Res 2012, 51, 8149–8177. [Google Scholar]
- (67).Coutinho JAP; Carvalho PJ; Kurnia KA Dispelling Some Myths about the CO2 Solubility in Ionic Liquids. Phys. Chem. Chem. Phys 2016, 18, 14757–14771. [DOI] [PubMed] [Google Scholar]
- (68).Rubtsov IV; Kumar K; Hochstrasser RM Dual-Frequency 2D IR Photon Echo of a Hydrogen Bond. Chem. Phys. Lett 2005, 402, 439–443. [Google Scholar]
- (69).Rubtsov I; Wang J; Hochstrasser RM Dual-Frequency 2-D-IR Spectroscopy in Peptides. Proc. Natl. Acad. Sci. U.S.A 2003, 100, 5601–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Kim YS; Hochstrasser RM Applications of 2D IR Spectroscopy to Peptides, Proteins, and Hydrogen-Bond Dynamics. J. Phys. Chem. B 2009, 113, 8231–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Kuo CH; Vorobyev DY; Chen J; Hochstrasser RM Correlation of the Vibrations of the Aqueous Azide Ion with the O H Modes of Bound Water Molecules. J. Phys. Chem. B 2007, 111, 14028–14033. [DOI] [PubMed] [Google Scholar]
- (72).Kurochkin DV; Naraharisetty SRG; Rubtsov IV Dual-Frequency 2D IR on Interaction of Weak and Strong IR Modes. J. Phys. Chem. A 2005, 109, 10799–10802. [DOI] [PubMed] [Google Scholar]
- (73).Kumar K; Sinks LE; Wang J; Kim YS; Hochstrasser RM Coupling between C-D and CO Motions Using Dual-Frequency 2D IR Photon Echo Spectroscopy. Chem. Phys. Lett 2006, 432, 122–127. [Google Scholar]
- (74).Paul A; Mandal PK; Samanta A. On the Optical Properties of the Imidazolium Ionic Liquids. J. Phys. Chem. B 2005, 109, 9148–9153. [DOI] [PubMed] [Google Scholar]
- (75).López-Martin I; Burello E; Davey PN; Seddon KR; Rothenberg G. Anion and Cation Effects on Imidazolium Salt Melting Points: A Descriptor Modelling Study. ChemPhysChem 2007, 8, 690–695. [DOI] [PubMed] [Google Scholar]
- (76).Knorr A; Ludwig R. Cation-Cation Clusters in Ionic Liquids: Cooperative Hydrogen Bonding Overcomes like-Charge Repulsion. Sci. Rep 2015, 5, No. 17505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Sun Z; Zhang W; Ji M; Hartsock R; Gaffney KJ Contact Ion Pair Formation between Hard Acids and Soft Bases in Aqueous Solutions Observed with 2DIR Spectroscopy. J. Phys. Chem. B 2013, 117, 15306–15312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.