Summary:
The interplay between viral infection and Alzheimer’s disease (AD) has long been an area of interest but proving causality has been elusive. Several recent studies have renewed the debate concerning the role of herpesviruses, and human herpesvirus 6 (HHV-6) in particular, in AD. We screened for HHV-6 detection across three independent AD brain repositories, using (1) RNAseq datasets and (2) DNA samples extracted from AD and non-AD control brains. The RNAseq data were screened for pathogens against taxon references from over 25,000 microbes, including 118 human viruses, while DNA samples were probed for PCR reactivity to HHV-6A and HHV-6B. HHV-6 demonstrated little specificity to AD brains over controls by either method, while other viruses such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV) were detected at comparable levels. These direct methods of viral detection do not suggest an association between HHV-6 and AD.
Keywords: Herpesvirus, Alzheimer’s disease, Human herpesvirus 6
In Brief:
Allnutt et al. screened 3 independent Alzheimer’s disease (AD) cohorts for pathogens including 118 human viruses, using RNAseq and PCR from 711 AD and non-AD control brains. No differences in viral detection between AD and non-AD controls were observed.
Introduction:
Alzheimer’s disease (AD) is a neurological disease characterized by extensive accumulation of the amyloid-beta (Aβ) peptide, deterioration of brain structures, neuroinflammation, and ultimately dramatic cognitive deficits (Holmes, 2013; Regen et al.,2017). The primary models of AD focus on amyloid pathology (Brody et al., 2017; Fessel, 2018; Moir and Tanzi, 2019; Walsh et al., 2002), although there has been renewed interest in potential infectious triggers of AD (Eimer et al., 2018; Goldschmidt-Clermont et al., 2019; Qin and Li, 2019; Readhead et al., 2018). Many pathogens have been associated with the disease, including Chlamydia pneumoniae (Balin and Hudson, 2014) and spirochaetes (Miklossy, 2015), but most frequently, human herpesviruses such as human herpesvirus 6A (HHV-6A), HHV-6B, and herpes simplex virus 1 (HSV-1) have been linked to AD pathogenesis (Ball, 1982; Haas and Lathe, 2018; Itzhaki et al., 1997; Lin et al., 2002; Liu et al., 2018; Rizzo et al., 2019; Romeo et al., 2019a; Romeo et al., 2019b; Wozniak et al., 2009).
Both HSV-1 and HHV-6 are active within the CNS, with HSV-1 causing HSV encephalitis, and HHV-6, particularly HHV-6A, being linked to various CNS diseases, including multiple sclerosis, epilepsy, and encephalitis (Leibovitch and Jacobson, 2018; Piacentini et al., 2014). Studies comparing AD and non-AD controls have detected increased HHV-6 DNA (Lin et al., 2002) as well as increased co-localization of HSV-1 DNA with Aβ plaques (Wozniak et al., 2009) in AD brains. Compared to non-AD controls, AD samples have been found to have decreased HHV-6 IgG titer in blood (Haas and Lathe, 2018) and increased risk of AD development in the presence of HSV-1 in brain (Itzhaki et al., 1997) or plasma (Lovheim et al.,2018). In addition, several groups have identified overlap between AD genetic risk factors and genes affected by viral infection, such as a receptor involved in spreading HSV-1 (Liu et al., 2018) and a human leukocyte antigen (HLA) subtype associated with increased susceptibility to HHV-6A infection (Rizzo et al., 2019).
Two recent publications (Eimer et al., 2018; Readhead et al., 2018) in particular have brought a renewed focus on the role of HHV-6 and HSV-1 in the pathogenesis of AD. Extensive analysis of RNA-seq and whole-genome sequencing data available through several independent brain banks were used to identify associations between HHV-6A/HHV-7 and AD, citing an increase in both viral RNA and DNA in AD brains over controls (Readhead et al., 2018). Furthermore, the authors observed that host genes that are upregulated by viral infection extensively overlapped with AD risk genes (Readhead et al., 2018). Supporting the association between HHV-6, HSV-1, and AD, another study suggested that the aggregation of Aα could be stimulated by the presence of either HHV-6 or HSV-1 (Eimer et al., 2018). Amyloid plaques formed around these viruses, providing an antiviral protective effect both in vitro and in a 5xFAD mouse model (Eimer et al., 2018). Collectively, these studies provide a mechanistic framework for the role of herpesviruses in AD.
Given the renewed interest in infectious triggers in AD with the recent focus on HHV-6, combined with our long-standing experience in studies on the association of neurologic disorders and this virus, ie., multiple sclerosis (Leibovitch et al., 2019; Virtanen et al., 2014), epilepsy (Bartolini et al., 2018), glioblastomas (Lin et al., 2016), and post-transplant encephalitis (Yao et al., 2009), it was of interest to us to explore the observation that HHV-6A and/or HHV-6B could be involved in the pathogenesis of AD. We approached this question in two ways: (1) a reanalysis of the bioinformatic data using RNAseq data sets from the Mount Sinai Brain Bank (MSBB) and the Religious Orders Study/Memory and Aging Project (ROSMAP); and (2) a direct interrogation of brain material for HHV-6A and HHV-6B PCR reactivity from AD patients and controls from which DNA had been extracted and which we had access to from ROSMAP (Bennett et al., 2012a; Bennett et al., 2012b) and the Johns Hopkins Brain Resource Center, which includes brain autopsies from the NIA Baltimore Longitudinal Study of Aging (Blauwendraat et al., 2019). The Broad Institute PathSeq tool, which screens over 25,000 microbes, including 118 human viruses, was used for RNAseq analysis (Walker et al., 2018). PCR detection for HHV-6A and HHV-6B utilized a digital droplet PCR (ddPCR) platform, which is a highly sensitive and precise novel PCR methodology that we have used extensively (Leibovitch et al., 2014). The findings of this study, using the complementary methods of PathSeq and ddPCR on samples from three independent repositories, demonstrated little difference in viral detection between AD and non-AD controls.
Results:
Disease Demographics and Repositories Analyzed
RNAseq datasets were available from both MSBB and ROSMAP using the AMP-AD Knowledge Portal on SYNAPSE (MSBB synapse ID: syn3157743, ROSMAP: syn3388564) (Table 1) and were filtered into the PathSeq pipeline from the Broad Institute to screen for pathogens (Walker et al., 2018). In the MSBB repository, RNAseq data were available from 301 individuals from which up to four different brain regions from each individual were screened, for a total of 1027 specimens (Table 1). The four brain regions included: Brodmann area 10 (BM10), the anterior prefrontal cortex; Brodmann area 22 (BM22), the superior temporal gyrus; Brodmann area 36 (BM36), the parahippocampal gyrus; and Brodmann area 44 (BM44), the inferior frontal gyrus (Readhead et al., 2018; Wang et al., 2018). From the ROSMAP repository, RNAseq data were available from 600 brain samples from the dorsolateral prefrontal cortex (Bennett et al., 2012a; Bennett et al., 2012b) (Table 1). For both MSBB and ROSMAP repositories, brains were classified with respect to AD disease status using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropathology classification, which ranks individuals in categories of “No AD,” “Possible AD”, “Probable AD”, and “Definite AD,” (Table 1) (Bennett et al., 2012a; Bennett et al., 2012b; Wang et al., 2018). In addition to RNAseq data, we were fortunate to be able to obtain extracted DNA for ddPCR analysis from 364 brains also from the ROSMAP brain repository (although DNA from these brains did not have corresponding RNASeq information). DNA extracted from an additional 344 brains were also made available to us from a third brain repository, the Johns Hopkins Brain Resource Center, comprising 243 AD brains and 101 non-AD controls (Blauwendraat et al., 2019). These DNA samples were extracted for genetic analysis of neurodegenerative diseases in a well-characterized clinicopathological cohort (Blauwendraat et al., 2019). RNAseq data were also not available for this cohort.
Table 1. Disease demographics and repositories analyzed.
Two data types were analyzed from three different brain banks: (1) RNAseq data from AD and non-AD control brains were analyzed via PathSeq from both MSBB (n=301) and ROSMAP (n=600) cohorts, while (2) DNA extracted from AD and non-AD control brains from both ROSMAP (n=364) and JHBRC (n=344) was assessed for PCR reactivity.
| Mount Sinai Brain Bank (MSBB) | Religious Orders Study/Memory and Aging Project (ROSMAP) | Johns Hopkins Brain Resource Center (JHBRC) | |||||
|---|---|---|---|---|---|---|---|
| RNAseq | RNAseq | DNA ddPCR | DNA ddPCR | ||||
| n=301a | n=600 | n=364 | n=344 | ||||
| Definite ADb | 135 | Definite ADb | 173 | AD | 267 | AD | 243 |
| Multiple System Atrophy (MSA) | 12 | ||||||
| Probable ADb | 42 | Probable ADb | 207 | ||||
| Possible AD b | 38 | Possible ADb | 62 | Non-AD controls | 97 | Progressive Supranuclear Palsy (PSP) | 20 |
| Non-AD controlsb | 86 | Non-AD controlsb | 158 | ||||
| Non-AD controls | 69 | ||||||
Up to 4 brain regions screened per individual (BM10, BM22, BM36, BM44). Total # specimens=1027
AD classification coding: Neuropathology categories as measured by CERAD (Wang et al., 2018; Bennett et al., 2012a; Bennett et al., 2012b)
Detection of HHV-6 in MSBB and ROSMAP RNAseq data sets
RNAseq data from the MSBB cohort were available from multiple brain regions for 301 total individuals. Clinical data for each individual included neocortical plaque density across 5 regions (# of plaques/mm2) and Clinical Dementia Rating (CDR) score (Wang et al., 2018), which stratifies clinical symptoms of dementia by severity. When individuals were arranged by a compounded Plaque*CDR score (disease severity score), it was possible to easily visualize the correlation between PathSeq results and clinical and neuropathological disease (Figure 1A). The PathSeq score, based on the number of reads that align with taxon references, indicates the amount of evidence that a taxon is present in a given sample (Walker et al., 2018). These scores, defined by the Broad Institute (https://software.broadinstitute.org/gatk/documentation/tooldocs/current/org_broadinstitute_hellbender_tools_spark_pathseq_PathSeqScoreSpark.php), are used to “detect and quantify microbe abundance. Alignments with sufficient identity score (e.g. 90% of read length) estimate read counts and the relative abundance of microorganisms present in the sample at each level of the taxonomic tree… Reads with a single valid alignment add a score of 1 to the corresponding species or strain. For reads with N hits, a score of 1/N is distributed to each organism. Scores are totaled for each taxon by summing the scores across all reads and the scores of any descendent taxa.” Pathogen load is correlated with score as score is based on RNA read counts.
Figure 1. Detection of HHV-6 in MSBB and ROSMAP RNAseq datasets.
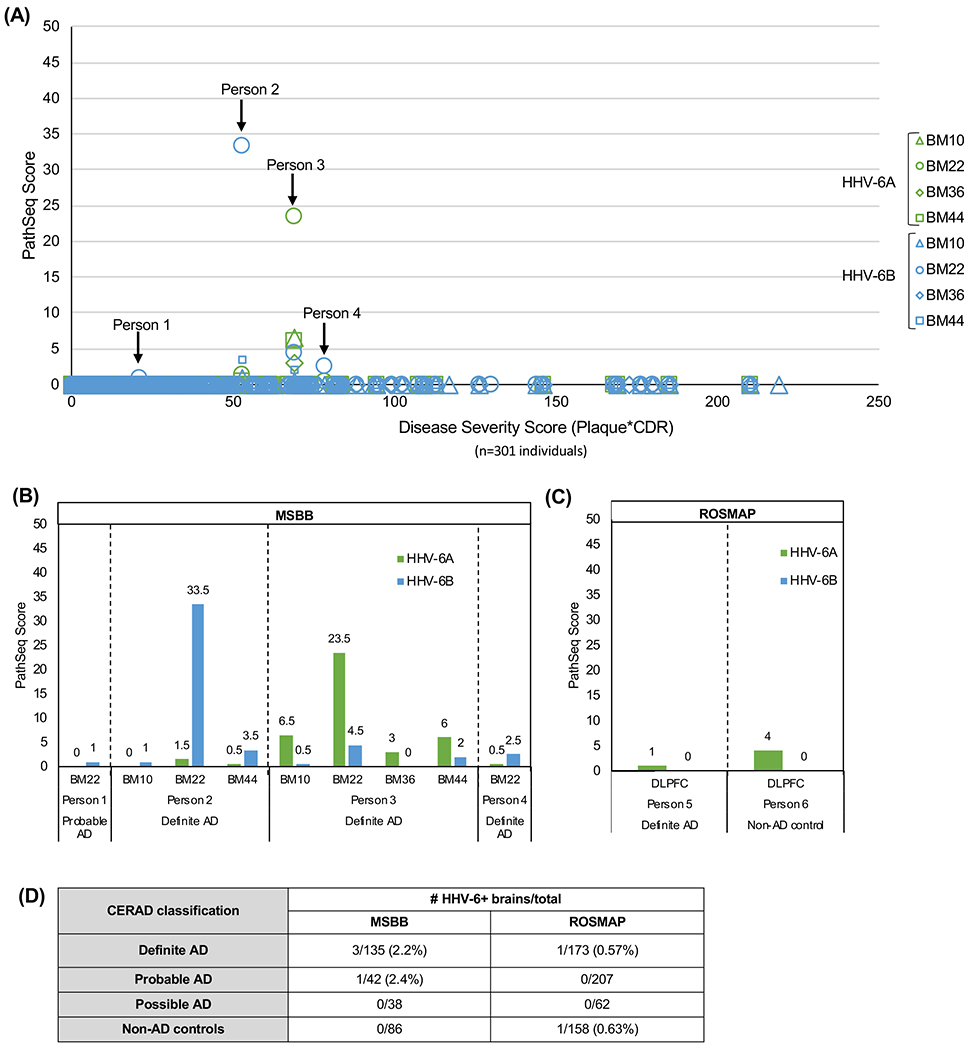
(A) HHV-6A and -6B PathSeq scores are displayed in order of increasing disease severity score (calculated as neuritic plaque density*Clinical Dementia Rating score). (B) HHV-6 was detected in 9 specimens from 4 individuals in the MSBB cohort, with varying PathSeq scores. (C) HHV-6A was detected in 2 individuals in the ROSMAP cohort, both with extremely low PathSeq scores, while no HHV-6B was detected. (D) Brains with HHV-6 in one or more brain regions are classified by CERAD neuropathology, with HHV-6 detected in individuals with definite and probable AD in addition to 1 non-AD control brain.
Key: BM10= Brodmann area 10, anterior prefrontal cortex; BM22= Brodmann area 22, superior temporal gyrus; BM36=Brodmann area 36, parahippocampal gyrus; BM44= Brodmann area 44, inferior frontal gyrus. DLPFC=dorsolateral prefrontal cortex
PathSeq scores for HHV-6 within the MSBB cohort were fairly low, with the highest score being 33.5 and most scores remaining between 0 and 10. All of the positive reads for HHV-6 fell within samples from just 4 out of 301 individuals, with HHV-6 found in multiple brain regions for 2 of those individuals (Figure 1B). There appears to be little difference in the frequency of detection between HHV-6A and HHV-6B. Of the four individuals in which any HHV-6 signal was detected, three of four had both HHV-6A and HHV-6B and one individual (Person 1) had only HHV-6B with a low Pathseq score of 1.0 (and the lowest disease severity score) (Figure 1A, 1B). Person 2 had a modest PathSeq score of 33.5 in the BM22 brain region for HHV-6B and Person 3 had a likewise modest PathSeq score of 23.5 for HHV-6A also in BM22 (Figure 1A, 1B). There was no correlation between disease severity and PathSeq score (Figure 1A, R2=0.0016).
In addition to the MSBB cohort, RNAseq data were also available from ROSMAP. The dataset included 600 individuals of varying AD classification (Table 1). Similar to MSBB, PathSeq analysis demonstrated low frequency of HHV-6 detection (with low PathSeq scores) in ROSMAP, with HHV-6A detected in only two brains in this cohort (Figure 1C). No HHV-6B was detected. Both ROSMAP and MSBB classified samples according to CERAD neuropathology guidelines (Bennett et al., 2012a; Bennett et al., 2012b; Wang et al., 2018). As shown in Figure 1D, HHV-6A was detected in only 1 of 173 brains with definite AD and only 1 of 158 non-AD, aged controls in the ROSMAP dataset (Figure 1D). In the MSBB cohort, HHV-6A and HHV-6B were detected in 3 of 135 brains with definite AD, and HHV-6B was detected in an additional 1 of 42 brains with probable AD (Figure 1D).
Specificity of viral detection in MSBB and ROSMAP RNAseq data sets
In addition to HHV-6A and HHV-6B, several other CNS-associated viruses were also detected by PathSeq in both MSBB and ROSMAP (Figure 2). These included John Cunningham virus (JCV) (Bartsch et al., 2019), human immunodeficiency virus 1 (HIV) (Nath, 2015), Epstein-Barr virus (EBV) (Leibovitch and Jacobson, 2018) and cytomegalovirus (CMV) (Bookstaver et al., 2017), although PathSeq scores for each were all less than 7 (Figure S1). The frequency of detection for any virus was extremely low and was not significantly different between AD (nor between sub-classifications of AD) and non-AD brains (Figure 2, Table S1). For example, in the MSBB and ROSMAP brain cohorts, 6.0% and 0.9%, respectively, of brain specimens with definite, probable, or possible AD contained EBV reads compared with 5.8% and 1.3% of brains from non-AD controls (Figure 2). With a particular focus on HHV-6 (including both HHV-6A and HHV-6B), in the MSBB and ROSMAP brain cohorts, 3.3% and 0.2%, respectively, of brain specimens with definite, probable, or possible AD contained HHV-6 reads compared with 0% and 0.6% of brains from non-AD, aged controls (Figure 2). Again, no statistical difference was found in the frequency of any one virus between diseased and control brains and there was no evidence for virus specificity.
Figure 2. CNS-related virus detection across both MSBB and ROSMAP.
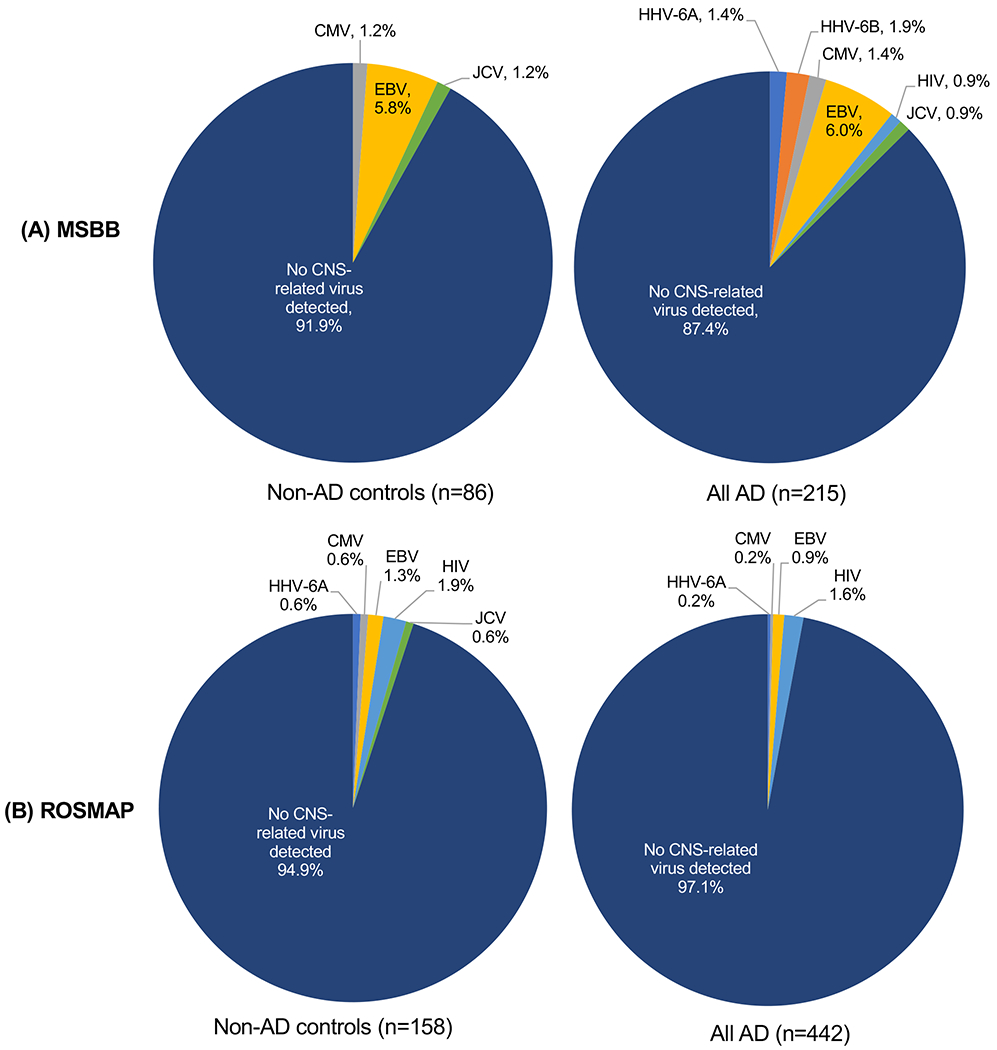
All CNS-related viruses detected by PathSeq analysis were distributed across AD classifications in both (A) MSBB and (B) ROSMAP. All CNS-related viruses detected in brains from the definite, probable, and possible AD neuropathology categories are compared to non-AD controls for both cohorts. See also Supplemental Figure S1.
CMV= cytomegalovirus, EBV= Epstein-Barr virus, HIV= human immunodeficiency virus, JCV= John Cunningham virus
HHV-6 screening using ddPCR
To further explore the association between HHV-6 and AD, DNA from available brain sections were subjected to PCR amplification of HHV-6A and HHV-6B specific regions (Leibovitch et al., 2014). A total of 708 brain sections were used from 2 brain repositories including 344 brains from a Johns Hopkins brain resource center collection and 364 brains from the ROSMAP brain bank consisting of AD and non-AD controls (Table 1). The frequency of detection of HHV-6 was low in both cohorts (Figure 3). Only one individual in the ROSMAP non-AD control cohort was positive for HHV-6A (Figure 3). In addition, the frequency of HHV-6B detection was not significantly different between AD and non-AD controls for either the ROSMAP (χ2 p=0.55) or JHBRC (χ2 p=0.76) cohorts (Figure 3). The magnitude of HHV-6 PCR reactivity was also low and did not vary between AD and non-AD controls for either ROSMAP (p=0.39) or JHBRC (p=0.40), with the majority of positive brains demonstrating a viral load of fewer than 600 copies of HHV-6/106 cells (Figure 3). Of interest was the observation that within the 708 brain samples tested for HHV-6 PCR reactivity, 5 brains had PCR reactivity at approximately 1 copy per cell (Figure S2A), suggesting that these individuals had chromosomally integrated HHV-6 (ciHHV-6) (Flamand, 2018). CiHHV-6A was detected in 3 brains from the ROSMAP cohort, 1 with AD and 2 non-AD controls, while ciHHV-6B was detected in 2 AD brains from JHBRC (Figure S2B). This observation of ciHHV-6 in 5 of 708 (0.71%) brains is consistent with the known prevalence of ciHHV-6, which is present in less than 1% of the healthy human population (Flamand, 2018).
Figure 3. HHV-6 detection via ddPCR across disease statuses.
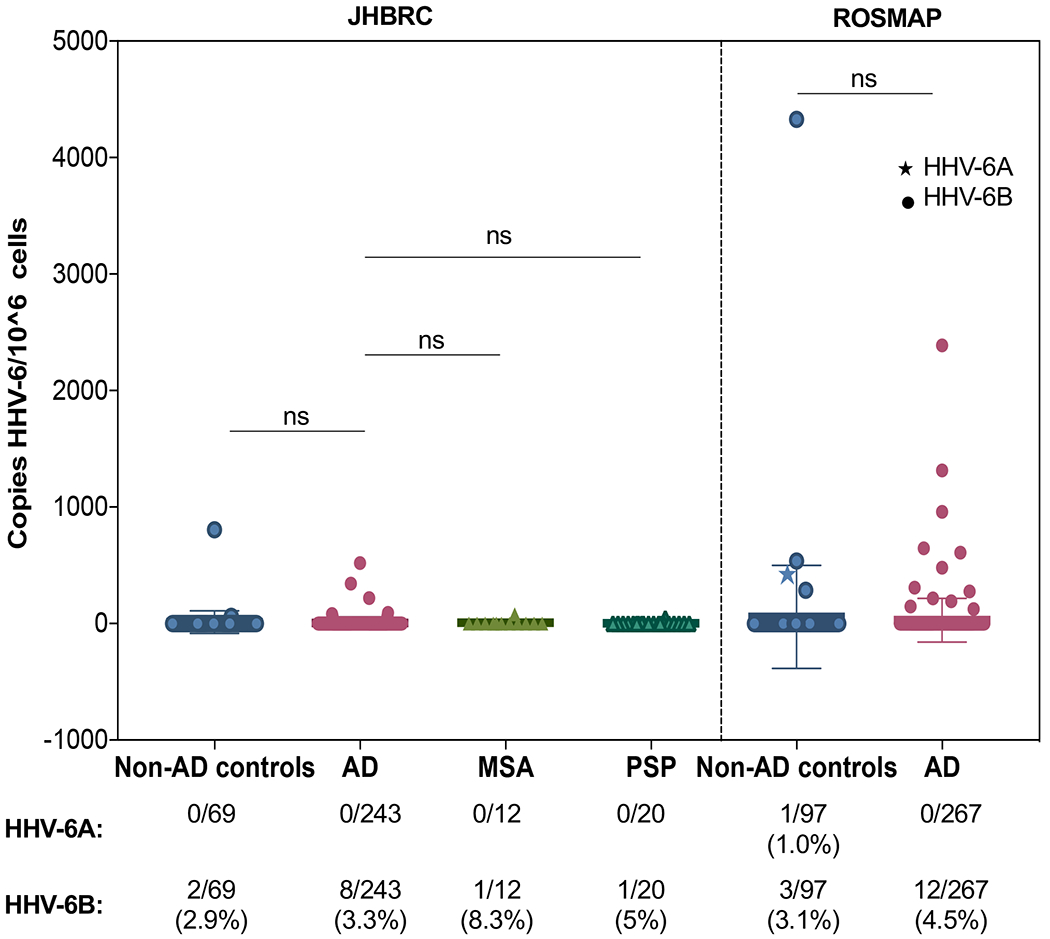
The HHV-6B viral load, in copies per 106 cells, is compared between AD and non-AD samples in both the ROSMAP and JHBRC cohorts. Viral load did not differ significantly across groups (One-way ANOVA p=0.41). The frequency of detection of HHV-6A and HHV-6B did not differ between AD and non-AD samples (χ2 p=0.81), with frequencies all remaining well below 10% in each disease cohort. Data are represented as mean ± SD.
Discussion:
While many studies have reported evidence of an association between HHV-6, HSV-1, and AD (Eimer et al., 2018; Haas and Lathe, 2018; Itzhaki et al., 1997; Lin et al., 2002; Liu et al., 2018; Lovheim et al., 2018; Readhead et al., 2018; Rizzo et al., 2019; Romeo et al., 2019b; Wozniak et al., 2009), these results have been tempered by contemporaneous studies which found little or no association (Agostini et al., 2016; Hemling et al., 2003), and an apparent lack of specificity in the viruses that are identified. Other viruses such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), Kaposi’s sarcoma-associated herpesvirus (KSHV), have also been associated with AD development and progression (Carbone et al., 2014; Lovheim et al., 2018; Qin and Li, 2019; Talwar et al., 2019) through techniques such as PCR analysis of brain or plasma (Carbone et al., 2014; Lovheim et al., 2018), and bioinformatic analysis (Talwar et al., 2019).
The breadth of viral species which have been associated with AD is also reflected in our analysis of the MSBB and ROSMAP RNAseq datasets. Transcripts from viruses such as EBV and CMV were detected at frequencies and PathSeq scores comparable to HHV-6, and our study did not suggest major differences in HHV-6 expression between AD and non-AD control brains. Moreover, in the MSBB cohort, all instances of HHV-6 detection occurred in brains from only 4 (out of 301) individuals and 2 (out of 605) individuals in the ROSMAP cohort. Even amongst brains with detectable HHV-6 RNA, the PathSeq scores were low. While PathSeq scores of 0.5 or 1 here are scored as positive, a higher score indicates more robust evidence that a taxon is present, based on the number of reads that aligned to taxon references. The Broad Institute that developed the PathSeq tool in GATK has reported positive reads in the thousands (Walker et al., 2018). Indeed, PathSeq was recently used to identify viruses from brains obtained from 2 cases with fatal encephalopathies (one with JCV and the other with Dengue virus) in which PathSeq scores were 5000 or more (K. Johnson, manuscripts submitted). However, analysis of bulk RNAseq project data that aims for traditional gene expression profiling and does not include pathogen detection and screening as a project aim can render misleading, false-negative results. The location and the time at which RNA was collected and the depth of sequencing in conjunction with a biased transcriptome background can each lead to reduced viral detection.
While the DNA sequence data from MSBB and ROSMAP were not available for download at the time of analysis to support these results, ddPCR of DNA from both the ROSMAP and JHBRC cohorts did not reveal any difference in frequency of HHV-6 detection between AD and non-AD control groups. The frequency of HHV-6B detection in each brain bank remained very low, regardless of disease status. In addition, only one sample out of the 708 tested was positive for HHV-6A DNA, and this sample fell into the non-AD control category, again, not supporting any significant associations with HHV-6A and AD. While previous studies have found higher frequencies of HHV-6 DNA in brains, this frequency varies widely across the literature, with percentages of detection anywhere between 2 and 70% (Cermelli et al., 2003; Chan et al., 2001; Chapenko et al., 2016; Hemling et al., 2003; Lin et al., 2002; Wipfler et al., 2018). Such discrepancies could be related to differences in PCR methodologies, different PCR primer/pair sequences and different brain regions analyzed. The majority of these studies used DNA collected from multiple brain regions, while the DNA in the present study were collected from the dorsolateral prefrontal cortex.
These results add to the controversial nature of the evidence for an association between HHV-6 and AD. However, our results do not preclude the validity of the associations found by previous studies. While in the present study we directly analyzed the frequency of the virus in AD as compared to control brains by PCR, other authors used more indirect measures, such as anti-HHV-6 IgG levels in serum (Agostini et al., 2016; Haas and Lathe, 2018), or the larger network of genes that relate HHV-6 to AD risk loci (Readhead et al., 2018; Rizzo et al., 2019). In addition, RNAseq analysis methods can differ at any stage of processing of raw read files from quality inspection, adaptor clipping, quality trimming, rRNA read filtering, reference mapping, to feature enumeration. Despite the source data being exactly the same, a different method or rule at any single step can produce different end results. Analyses of the same MSBB and ROSMAP RNAseq data may vary in the length of kmer used, the method to derive the dictionary of kmers that differentiate host reference sequences from non-host, and in the end how those dictionaries compare between methods. Currently, there is no standard generalized kmer library for this purpose used across authors. Rather, they are author devised by methods and rules they chose, contributing to the variation in pathogen detection between analyses of the same datasets.
Our results suggest that if viruses (and HHV-6 in particular) do play a role in AD pathogenesis, then the agents may no longer be present in a form that can be PCR amplified or sufficiently expressed. Rather, these virus(es) may be associated with an earlier ‘triggering’ event or be present at copy numbers below the limit of laboratory detection. Early triggering events may include nucleation of amyloid beta (Aβ), that has recently been reported to have antimicrobial activity against bacteria, fungi, and viruses including HHV-6 (Bourgade et al., 2015; Bourgade et al., 2016; Eimer et al., 2018; Kumar et al., 2016; Soscia et al., 2010; Spitzer et al., 2016; White et al., 2014). Nucleation of Aβ enveloping, for example, a herpesvirus virion (Eimer et al., 2018) may be important for AD development but would not necessarily result in the detection of the complete virus thus providing a potential explanation for the lack of easily identifiable pathogen in the brains of AD patients.
The findings of this study, using the complementary methods of PathSeq and ddPCR on samples from three independent repositories, do not support an association between HHV-6 and Alzheimer’s disease, but also does not rule it out. Clearly, the observations in this report need to be extended to larger collaborative studies using multiple bioinformatic analysis pipelines as well as laboratory-based tools. Future studies on the role of the HHV-6 virus in the progression and development of AD should focus on the mechanisms by which this virus could play an important role while remaining largely undetected in large-scale analyses of brain material.
STAR Methods:
Lead Contact and Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Steven Jacobson (jacobsons@ninds.nih.gov). This study did not generate new unique reagents.
Experimental Model and Subject Details
A gender breakdown of all subjects included in each of the datasets included in this study is described in Supplemental Table 2.
Method Details
Screening for Virus using RNA-Seq Data.
The “PathSeq” tool developed by the BROAD Institute was used (http://software.broadinstitute.org/pathseq/). Two different RNA-Seq data sets available for download from the Synapse database (https://www.synapse.org/) were screened. The first data set consisted of RNA-Seq data generated as part of the Mount Sinai Brain Bank (MSBB) study (https://www.synapse.org/#!Synapse:syn3159438). For this study, RNA was collected post-mortem from up to five different regions of the brain (Brodmann Areas 10, 22, 36 and 44) for more than 200 individuals diagnosed with Alzheimer’s Disease (AD) then sequenced (https://www.synapse.org/#!Synapse:syn20801188). The second data set consisted of RNA-Seq data generated as part of the Religious Orders Study and Memory and Aging Project (ROSMAP). For this study/project, RNA was collected post-mortem from the gray matter of the dorsolateral prefrontal cortex for 605 individuals diagnosed with AD (https://www.synapse.org/#!Synapse:syn3219045) then sequenced (https://www.synapse.org/#!Synapse:syn3388564). Sequence files downloaded from the MSBB study were the “unmapped” reads in .fastq format. Sequence files downloaded from the ROSMAP study were those in .bam format. Prior to running the “PathSeq” tool on these data, the MSBB files were converted into uBAM format using the “FastqToSam” command supported in the picard tool suite (https://broadinstitute.github.io/picard/). While, ROSMAP files were similarly converted using the “view -b -f 4” command supported in the samtools suite (http://samtools.sourceforge.net/) followed by use of the “RevertSam” command supported in the picard tool suite. For running the “PathSeq” tool, the “PathSeqPipelineSpark” command supported in the gatk tool suite (https://software.broadinstitute.org/gatk/download/) was passed under default parameters (https://gatkforums.broadinstitute.org/gatk/discussion/10913/how-to-run-the-pathseq-pipeline) using pre-built references (https://software.broadinstitute.org/gatk/download/bundle). The total number of microbes screened for upon running the “PathSeq” tool was 25,917; including 118 human viruses. Detection scores for these viruses per sequence file output from the “PathSeq” tool were then imported into R (https://cran.r-proiect.org/), collated by study/project, visually inspected, and summarized using standard supported commands.
Screening for Virus using droplet digital PCR (ddPCR).
DNA extracted from brain material was collected from two different repositories. HHV-6A and -6B viral load was quantified using droplet digital PCR (ddPCR) as previously described (Leibovitch et al., 2014). Briefly, the HHV-6 primer and probe sequences used (Key Resources Table) were designed based on the reference genomes NC_001664 for HHV-6A, strain U1102, and NC_000898 for HHV-6B, strain Z29. Primers amplify the U57 region encoding the major capsid protein. Ribonuclease P protein subunit P30 (RPP30) was used as a reference gene, as all diploid cells contain two copies of the gene. Each probe was fluorescently labeled, with the HHV-6A probe FAM-MGBNFQ-labeled, while both the HHV-6B and RPP30 probes were VIC-MGBNFQ-labeled. For each DNA sample, the primers and probes were triplexed, with the final concentrations of 900 nM per primer and 250 nM per probe for HHV-6A and HHV-6B, while the final concentrations of RPP30 were 450 nM per primer and 125 nM per probe. DdPCR procedures and analysis of results followed those previously described by Leibovitch et al. (2014). Positive samples were confirmed by an independent re-test.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| MSBB RNAseq | https://www.synapse.org/#!Synapse:syn20801188 | syn20801188 |
| ROSMAP RNAseq | https://www.synapse.org/#!Synapse:syn3388564 | syn3388564 |
| PathSeq Analysis of MSBB and ROSMAP Data | This paper | DOI: 10.17632/mw2ngtsh6h.1 |
| Oligonucleotides | ||
| HHV-6A U57 Probe: FAM-CTGGAACTGTATAATAGG-MGBNFQ | Leibovitch, 2014, PLoS One | |
| HHV-6B U57 Probe: VIC-CTGGAGCTGTACAACAG -MGBNFQ | Leibovitch, 2014, PLoS One | |
| HHV-6AB U57 Forward Primer: CCGTGGGATCGTCTAAAATTATAGATGT | Leibovitch, 2014, PLoS One | |
| HHV-6AB Reverse Primer: CCACACTAGTCCGGACGGATAA | Leibovitch, 2014, PLoS One | |
| RPP30 Probe: VIC-CTGACCTGAAGGCTCT-MGBNFQ | Hindson, 2011, Anal Chem. | |
| RPP30 Forward Primer: GATTTGGACCTGCGAGCG | Hindson, 2011, Anal Chem. | |
| RPP30 Reverse Primer: GCGGCTGTCTCCACAAGT | Hindson, 2011, Anal Chem. | |
| Software and Algorithms | ||
| R | https://cran.r-project.org/ | |
| gatK | https://software.broadinstitute.org/gatk/download/ | RRID:SCR_001876 |
| PathSeqPipelineSpark | https://gatkforums.broadinstitute.org/gatk/discussion/10913/how-to-run-the-pathseq-pipeline | RRID:SCR_005203 |
| samtools | http://samtools.sourceforge.net/ | RRID:SCR_002105 |
Quantification and Statistical Analysis
Statistical Analysis of PathSeq Results
Each brain in which one or more brain regions was positive for any of the 6 CNS-related viruses detected (HHV-6A, HHV-6B, CMV, EBV, HIV, or JCV) was counted in a contingency table, and a Chi-square test for independence was performed to determine if there was any relationship between viral detection and AD classification. A separate chi-square analysis was performed for each virus in both MSBB and ROSMAP.
Quantification of ddPCR Results.
Fluorescence data for each well were analyzed using QuantaSoft software, version 1.7.4.0917 (Bio-Rad, Hercules, CA). Droplet positivity was determined by fluorescence intensity; only droplets above a manually determined minimum amplitude threshold were counted as positive. For a given sample, target copies per μl were calculated by averaging over all replicate wells, and cellular DNA input was calculated by halving the number of RPP30 copies, as there are two copies of RPP30 per diploid cell. PBMC data are represented as viral copies per 106 cells.
Statistical Analysis of ddPCR Results
In both JHBRC and ROSMAP, each non-AD control sample set was compared to AD samples using an unpaired t-test of the viral loads of all samples tested. In addition to this analysis of the magnitude of viral load, the frequency of HHV-6 detection was compared between AD and non-AD controls using a chi-squared test.
Data and Code Availability
PathSeq code is available from the Broad Institute at: http://software.broadinstitute.org/pathseq/. RNAseq datasets from MSBB and ROSMAP are available for download at https://www.synapse.org/#!Synapse:syn3159438 (MSBB) and https://www.synapse.org/#!Synapse:syn3388564 (ROSMAP). All PathSeq results from this study have been captured in a single excel workbook available at Mendelev Data (DOI: 10.17632/mw2ngtsh6h.1).
Supplementary Material
Highlights:
RNAseq data from 2 Alzheimer’s disease (AD) cohorts were screened for 118 viruses.
DNA from 711 AD and control brains was analyzed for PCR reactivity to HHV-6A/-6B.
HHV-6 demonstrated little specificity to AD brains over controls by either method.
These complimentary methods do not support strong association between HHV-6 and AD.
Acknowledgements:
This work was supported by the Intramural Research Program, National Institute of Neurological Disorders and Stroke, National Institutes of Health.
DNA samples from ROSMAP were provided by the Rush Alzheimer’s Disease Center and can be requested along with data from www.radc.rush.edu. DNA from the JHBRC cohort were provided by Dr. Sonja Scholz in collaboration with the Johns Hopkins Department of Neurology. This research was supported in part by the Intramural Research Program of the NIH National Institute of Neurological Disorders and Stroke (project number: ZIA-NS003154). We would like to thank the NIA Baltimore Longitudinal Study of Aging for contributing tissue samples to the Johns Hopkins Alzheimer’s Disease Research Center. The results published here are in whole or in part based on data obtained from the AMP-AD Knowledge Portal (doi:10.7303/syn2580853, doi:10.1038/s41593-018-0154-9). Study data were provided by the Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago. Data collection was supported through funding by NIA grants P30AG10161, R01AG15819, R01AG17917, R01AG30146, R01AG36836, U01AG32984, U01AG46152, and U01AG61356 in addition to JHU ADRC (P50AG05146), the Illinois Department of Public Health, the Translational Genomics Research Institute, and the Intramural Research Program of the National Institute on Aging, NIH. These data were generated from postmortem brain tissue collected through the Mount Sinai VA Medical Center Brain Bank and were provided by Dr. Eric Schadt from Mount Sinai School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: The authors declare no competing interests.
References:
- Agostini S, Mancuso R, Baglio F, Cabinio M, Hernis A, Guerini FR, Calabrese E, Nemni R, and Clerici M (2016). Lack of evidence for a role of HHV-6 in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 49, 229–235. [DOI] [PubMed] [Google Scholar]
- Balin BJ, and Hudson AP (2014). Etiology and pathogenesis of late-onset Alzheimer’s disease. Curr Allergy Asthma Rep 14, 417. [DOI] [PubMed] [Google Scholar]
- Ball MJ (1982). “Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved?”. Can J Neurol Sci 9, 303–306. [DOI] [PubMed] [Google Scholar]
- Bartolini L, Piras E, Sullivan K, Gillen S, Bumbut A, Lin CM, Leibovitch EC, Graves JS, Waubant EL, Chamberlain JM, et al. (2018). Detection of HHV-6 and EBV and Cytokine Levels in Saliva From Children With Seizures: Results of a Multi-Center Cross-Sectional Study. Front Neurol 9, 834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Rempe T, Leypoldt F, Riedel C, Jansen O, Berg D, and Deuschl G (2019). The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol 26, 566–e541. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, and Wilson RS (2012a). Overview and findings from the religious orders study. Curr Alzheimer Res 9, 628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, and Wilson RS (2012b). Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauwendraat C, Pletnikova O, Geiger JT, Murphy NA, Abramzon Y, Rudow G, Mamais A, Sabir MS, Crain B, Ahmed S, et al. (2019). Genetic analysis of neurodegenerative diseases in a pathology cohort. Neurobiol Aging 76, 214 e211–214 e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookstaver PB, Mohorn PL, Shah A, Tesh LD, Quidley AM, Kothari R, Bland CM, and Weissman S (2017). Management of Viral Central Nervous System Infections: A Primer for Clinicians. J Cent Nerv Syst Dis 9, 1179573517703342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgade K, Garneau H, Giroux G, Le Page AY, Bocti C, Dupuis G, Frost EH, and Fulop T Jr. (2015). beta-Amyloid peptides display protective activity against the human Alzheimer’s disease-associated herpes simplex virus-1. Biogerontology 16, 85–98. [DOI] [PubMed] [Google Scholar]
- Bourgade K, Le Page A, Bocti C, Witkowski JM, Dupuis G, Frost EH, and Fulop T Jr. (2016). Protective Effect of Amyloid-beta Peptides Against Herpes Simplex Virus-1 Infection in a Neuronal Cell Culture Model. J Alzheimers Dis 50, 1227–1241. [DOI] [PubMed] [Google Scholar]
- Brody DL, Jiang H, Wildburger N, and Esparza TJ (2017). Non-canonical soluble amyloid-beta aggregates and plaque buffering: controversies and future directions for target discovery in Alzheimer’s disease. Alzheimers Res Ther 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Lazzarotto T, Ianni M, Porcellini E, Forti P, Masliah E, Gabrielli L, and Licastro F (2014). Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging 35, 122–129. [DOI] [PubMed] [Google Scholar]
- Cermelli C, Berti R, Soldan SS, Mayne M, D’Ambrosia J M, Ludwin SK, and Jacobson S (2003). High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J Infect Dis 187, 1377–1387. [DOI] [PubMed] [Google Scholar]
- Chan PK, Ng HK, Hui M, and Cheng AF (2001). Prevalence and distribution of human herpesvirus 6 variants A and B in adult human brain. J Med Virol 64, 42–46. [DOI] [PubMed] [Google Scholar]
- Chapenko S, Roga S, Skuja S, Rasa S, Cistjakovs M, Svirskis S, Zaserska Z, Groma V, and Murovska M (2016). Detection frequency of human herpesviruses-6A, -6B, and -7 genomic sequences in central nervous system DNA samples from post-mortem individuals with unspecified encephalopathy. J Neurovirol 22, 488–497. [DOI] [PubMed] [Google Scholar]
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, and Collin F (2018). Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14, 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, Rodriguez AS, Mitchell T, Washicosky KJ, Gyorgy B, Breakefield XO, Tanzi RE, and Moir RD (2018). Alzheimer’s Disease-Associated beta-Amyloid Is Rapidly Seeded by Herpesviridae to Protect against Brain Infection. Neuron 99, 56–63.e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel J (2018). Amyloid is essential but insufficient for Alzheimer causation: addition of subcellular cofactors is required for dementia. Int J Geriatr Psychiatry 33, e14–e21. [DOI] [PubMed] [Google Scholar]
- Flamand L (2018). Chromosomal Integration by Human Herpesviruses 6A and 6B. Adv Exp Med Biol 1045, 209–226. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont PJ, Volinsky FG, LaRosa SP, Gilbert JR, and Pericak-Vance MA (2019). Time for Well-Powered Controlled Prospective Studies to test a Causal Role for Herpes Viruses in Alzheimer Using Anti-Herpes Drugs. J Gerontol A Biol Sci Med Sci. [DOI] [PubMed] [Google Scholar]
- Haas JG, and Lathe R (2018). Microbes and Alzheimer’s Disease: New Findings Call for a Paradigm Change. Trends Neurosci 41, 570–573. [DOI] [PubMed] [Google Scholar]
- Hemling N, Roytta M, Rinne J, Pollanen P, Broberg E, Tapio V, Vahlberg T, and Hukkanen V (2003). Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann Neurol 54, 267–271. [DOI] [PubMed] [Google Scholar]
- Holmes C (2013). Review: systemic inflammation and Alzheimer’s disease. Neuropathol Appl Neurobiol 39, 51–68. [DOI] [PubMed] [Google Scholar]
- Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, and Jamieson GA (1997). Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet 349, 241–244. [DOI] [PubMed] [Google Scholar]
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, and Moir RD (2016). Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med 8, 340ra372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch EC, Brunetto GS, Caruso B, Fenton K, Ohayon J, Reich DS, and Jacobson S (2014). Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS One 9, e92328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch EC, and Jacobson S (2018). Viruses in chronic progressive neurologic disease. Mult Scler 24, 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovitch EC, Lin CM, Billioux BJ, Graves J, Waubant E, and Jacobson S (2019). Prevalence of salivary human herpesviruses in pediatric multiple sclerosis cases and controls. Mult Scler 25, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT, Leibovitch EC, Almira-Suarez MI, and Jacobson S (2016). Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect Agent Cancer 11, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, and Itzhaki RF (2002). Herpesviruses in brain and Alzheimer’s disease. J Pathol 197, 395–402. [DOI] [PubMed] [Google Scholar]
- Liu C, Chyr J, Zhao W, Xu Y, Ji Z, Tan H, Soto C, Zhou X, and , f.t.A.s.D.N.I. (2018). Genome-Wide Association and Mechanistic Studies Indicate That Immune Response Contributes to Alzheimer’s Disease Development. Frontiers in Genetics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovheim H, Olsson J, Weidung B, Johansson A, Eriksson S, Hallmans G, and Elgh F (2018). Interaction between Cytomegalovirus and Herpes Simplex Virus Type 1 Associated with the Risk of Alzheimer’s Disease Development. J Alzheimers Dis 61, 939–945. [DOI] [PubMed] [Google Scholar]
- Miklossy J (2015). Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front Aging Neurosci 7, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD, and Tanzi RE (2019). Low Evolutionary Selection Pressure in Senescence Does Not Explain the Persistence of Abeta in the Vertebrate Genome. Front Aging Neurosci 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A (2015). Neurologic Complications of Human Immunodeficiency Virus Infection. Continuum (Minneap Minn) 21, 1557–1576. [DOI] [PubMed] [Google Scholar]
- Piacentini R, De Chiara G, Li Puma DD, Ripoli C, Marcocci ME, Garaci E, Palamara AT, and Grassi C (2014). HSV-1 and Alzheimer’s disease: more than a hypothesis. Front Pharmacol 5, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, and Li Y (2019). Herpesviral infections and antimicrobial protection for Alzheimer’s disease: Implications for prevention and treatment. J Med Virol. [DOI] [PubMed] [Google Scholar]
- Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, et al. (2018). Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus. Neuron 99, 64–82 e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regen F, Hellmann-Regen J, Costantini E, and Reale M (2017). Neuroinflammation and Alzheimer’s Disease: Implications for Microglial Activation. Curr Alzheimer Res 14, 1140–1148. [DOI] [PubMed] [Google Scholar]
- Rizzo R, Bortolotti D, Gentili V, Rotola A, Bolzani S, Caselli E, Tola MR, and Di Luca D (2019). KIR2DS2/KIR2DL2/HLA-C1 Haplotype Is Associated with Alzheimer’s Disease: Implication for the Role of Herpesvirus Infections. J Alzheimers Dis 67, 1379–1389. [DOI] [PubMed] [Google Scholar]
- Romeo MA, Faggioni A, and Cirone M (2019a). Could autophagy dysregulation link neurotropic viruses to Alzheimer’s disease? Neural Regen Res 14, 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo MA, Masuelli L, Gaeta A, Nazzari C, Granato M, Gilardini Montani MS, Faggioni A, and Cirone M (2019b). Impact of HHV-6A and HHV-6B lytic infection on autophagy and endoplasmic reticulum stress. J Gen Virol 100, 89–98. [DOI] [PubMed] [Google Scholar]
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, and Moir RD (2010). The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One 5, e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer P, Condic M, Herrmann M, Oberstein TJ, Scharin-Mehlmann M, Gilbert DF, Friedrich O, Gromer T, Kornhuber J, Lang R, and Maler JM (2016). Amyloidogenic amyloid-beta-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci Rep 6, 32228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar P, Gupta R, Kushwaha S, Agarwal R, Saso L, Kukreti S, and Kukreti R (2019). Viral Induced Oxidative and Inflammatory Response in Alzheimer’s Disease Pathogenesis with Identification of Potential Drug Candidates: A Systematic Review using Systems Biology Approach. Curr Neuropharmacol 17, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen JO, Wohler J, Fenton K, Reich DS, and Jacobson S (2014). Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult Scler 20, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MA, Pedamallu CS, Ojesina AI, Bullman S, Sharpe T, Whelan CW, and Meyerson M (2018). GATK PathSeq: a customizable computational tool for the discovery and identification of microbial sequences in libraries from eukaryotic hosts. Bioinformatics 34, 4287–4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, and Selkoe DJ (2002). Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans 30, 552–557. [DOI] [PubMed] [Google Scholar]
- Wang M, Beckmann ND, Roussos P, Wang E, Zhou X, Wang Q, Ming C, Neff R, Ma W, Fullard JF, et al. (2018). The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease. Sci Data 5, 180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MR, Kandel R, Tripathi S, Condon D, Qi L, Taubenberger J, and Hartshorn KL (2014). Alzheimer’s associated beta-amyloid protein inhibits influenza A virus and modulates viral interactions with phagocytes. PLoS One 9, e101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipfler P, Dunn N, Beiki O, Trinka E, and Fogdell-Hahn A (2018). The Viral Hypothesis of Mesial Temporal Lobe Epilepsy - Is Human Herpes Virus-6 the Missing Link? A systematic review and meta-analysis. Seizure 54, 33–40. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Mee AP, and Itzhaki RF (2009). Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol 217, 131–138. [DOI] [PubMed] [Google Scholar]
- Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, and Jacobson S (2009). Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol 65, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
PathSeq code is available from the Broad Institute at: http://software.broadinstitute.org/pathseq/. RNAseq datasets from MSBB and ROSMAP are available for download at https://www.synapse.org/#!Synapse:syn3159438 (MSBB) and https://www.synapse.org/#!Synapse:syn3388564 (ROSMAP). All PathSeq results from this study have been captured in a single excel workbook available at Mendelev Data (DOI: 10.17632/mw2ngtsh6h.1).


