Abstract
Background
Hypertension is considered to be a serious health problem worldwide. Controlling and lowering blood pressure are of significant benefit to people with hypertension because hypertension is a risk factor for stroke, heart disease, and cardiovascular disease. Roselle, the tropical plant Hibiscus sabdariffa, also commonly called sour tea or red tea, has been used as both a thirst‐quenching drink and for medicinal purposes.
Objectives
To assess the effect of Roselle on blood pressure in people with primary hypertension.
Search methods
For this update, the Cochrane Hypertension Information Specialist searched the following databases and trials registers for randomised controlled trials (RCTs): the Cochrane Hypertension Specialised Register (to 6 August 2021), Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 7), MEDLINE Ovid (1946 to 5 August 2021), Embase Ovid (1974 to 5 August 2021), ProQuest Dissertations & Theses (to 6 August 2021), Web of Science Clarivate (to 7 August 2021), Food Science and Technology Abstracts Clarivate (to 7 August 2021), the WHO International Clinical Trials Registry Platform (to 6 August 2021), and the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (to 6 August 2021). We searched Google Scholar and OpenSIGLE. We also handsearched local and regional Chinese databases: CBM, CMCC, TCMLARS, CNKI, CMAC, and the Index to Chinese Periodical Literature (to 14 September 2020), as well as Thai databases (ThaiJO, CUIR, TDC, CMU e‐Theses, TCTR) (to 3 October 2020). There were no language or publication date restrictions.
Selection criteria
We sought RCTs evaluating the use of any forms of Roselle with placebo or no treatment in adults with hypertension. Our primary outcome was change in trough and/or peak systolic and diastolic blood pressure (SBP, DBP). Secondary outcomes were withdrawals due to adverse effects, change in pulse pressure, and change in heart rate.
Data collection and analysis
All search results were managed using Covidence and re‐checked for the number of records, inclusion and exclusion of studies with Mendeley reference management software. We used standard methodological procedures expected by Cochrane. Two review authors worked independently in parallel for screening (titles and abstracts, and full reports), data extraction, risk of bias assessment, and assessment of the certainty of the evidence using the GRADE approach. Any disagreements were resolved by discussion or by consultation with the third review author if necessary. We presented mean difference (MD) of change in SBP and DBP with their corresponding 95% confidence interval (CI).
Main results
For this update, only one RCT with a parallel‐group design involving 60 participants with type 2 diabetes mellitus fulfilled the inclusion criteria. This study investigated the effect of Roselle extract capsules (total dose of 5600 mg) compared with placebo (lactose) at eight weeks. The study was at low risk of selection bias, performance bias, and detection bias. Conversely, it was at high risk of attrition bias, reporting bias, and other bias (baseline imbalance).
We have very little confidence in the effect estimate of Roselle on change‐from‐baseline in both SBP and DBP between the two groups. The MD of change in SBP was 1.65, 95% CI −7.89 to 11.19 mmHg, 52 participants, very low‐certainty evidence. The MD of change in DBP was 4.60, 95% CI −1.38 to 10.58 mmHg, 52 participants, very low‐certainty evidence. Our secondary outcomes of withdrawals due to adverse effects, change in pulse pressure, and change in heart rate were not reported. Due to the limited available data, no secondary analyses were performed (subgroup and sensitivity analysis).
Authors' conclusions
The evidence is currently insufficient to determine the effectiveness of Roselle compared to placebo for controlling or lowering blood pressure in people with hypertension. The certainty of evidence was very low due to methodological limitations, imprecision, and indirectness. There is a need for rigorous RCTs that address the review question.
Plain language summary
Red tea (Roselle) for lowering blood pressure in adults
Key messages:
We don't know if taking Roselle lowers blood pressure in people with hypertension (high blood pressure).
We don’t know if Roselle is safe for people with hypertension to consume or if it affects heart rate or pulse pressure.
What did we want to find out?
We wanted to know if red tea (Roselle) is a safe and effective treatment for lowering blood pressure in adults with high blood pressure when compared to placebo (dummy treatment) or no treatment. Roselle contains substances known as anthocyanins which have been shown to have lowered blood pressure in studies carried out in animals and humans.
What did we do?
We searched for studies that compared Roselle to placebo or no treatment in people with hypertension.
What did we find?
We included one study with 60 participants with type 2 diabetes and hypertension. Participants consumed either a capsule of pure Roselle extract or a placebo containing lactose for eight weeks. We are not sure if Roselle has an effect on blood pressure and the study did not report on the safety of Roselle or on changes to heart rate.
What are the main limitations of the evidence?
We found only one trial which included a small number of participants, all of whom had diabetes. More studies with various types of participants and different ways of taking Roselle (forms, amounts, and time of day, length of use) are needed.
How up to date is the evidence?
The review updates our previous review. We searched for randomised controlled trials (studies in which participants are randomly assigned to one of two or more treatment groups) in core databases up to August 2021, and searched local and regional Chinese and Thai databases by hand up to October 2020.
Summary of findings
Summary of findings 1. Roselle extract compared to placebo for hypertension in adults.
| Roselle extract compared to placebo for hypertension in adults | ||||||
| Patient or population: hypertension in adults with type 2 diabetes mellitus Setting: 5 public health centres in Yogyakarta, Indonesia Duration of follow‐up periods: 8 weeks of treatment Intervention: Roselle extract Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Roselle extract | |||||
| Change** in SBP at week 8 (mmHg) | The mean change in SBP at week 8 was −5.69 mmHg. at baseline mean 146.8 (SD 20), at week 8 mean 141.11 (SD 20.63) |
MD 1.65 higher (7.89 lower to 11.19 higher) | Not applicable | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | Change in SBP is highly skewed for both groups. |
| Change** in DBP at week 8 (mmHg) | The mean change in DBP at week 8 was 0.13 mmHg. at baseline mean 81.47 (SD 9.71), at week 8 mean 81.61 (SD 8.29) |
MD 4.6 higher (1.38 lower to 10.58 higher) | Not applicable | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1, 2, 3 | Change in DBP is highly skewed for both groups. |
| Withdrawals due to adverse effects | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Change** in pulse pressure (mmHg) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Change** in heart rate (beats/min) | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Change‐from‐baseline outcomes calculated by end measurement minus baseline measurement. CI: confidence interval; DBP: diastolic blood pressure; MD: mean difference; RCT: randomised controlled trial; SBP: systolic blood pressure; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded three levels due to methodological limitations: attrition bias (overall dropout rate 13.4%, reason for dropout not provided for one withdrawal in the intervention group, and unequal dropout rates between study arms (20% vs 6.6%)); selective outcome reporting; and imbalance in participant characteristics at baseline between groups. 2Downgraded two levels due to imprecision: few participants (total sample size included was 52 participants; less than 400 (rule of thumb)); for SBP, 95% CI included no effects and also appreciable benefit and harm (minimally important difference (MID) of 5 mmHg), for DBP 95% CI included no effects and appreciable harm (MID of 5 mmHg). 3Downgraded one level due to indirectness: the evidence was restricted to participants aged 50 and over.
Background
Description of the condition
Hypertension, or high blood pressure, is defined in most major guidelines as systolic blood pressure (SBP) of 140 mmHg or greater and/or diastolic blood pressure (DBP) of 90 mmHg or greater in the office or clinic (Unger 2020). Hypertension is a serious medical condition, as it contributes to a number of comorbidities: coronary artery disease (CAD), stroke, chronic kidney disease (CKD), heart failure (HF), and chronic obstructive pulmonary disease (COPD) (Unger 2020; WHO 2020). It is also the leading cause of premature death worldwide, accounting for 10.4 million deaths per year (GBD 2018). Of adults aged 18 years and over, the global hypertension prevalence was around 22% in 2014 (WHO 2016). The number of hypertension adults grew from 594 million in 1975 to 1.13 billion in 2015, with the increment occurring to a great extent in low‐ and middle‐income countries. These estimates of the global increment of the number of hypertensive adults are a net impact of increment due to the growth and ageing of the population (NCD‐RisC 2017). Additionally, various factors are associated with hypertension including high sodium intake, alcohol consumption, and physical inactivity (WHO 2020).
Description of the intervention
One of the global targets for non‐communicable diseases is to achieve 25% hypertension control by 2025 (WHO 2016; WHO 2020). In order to lower individual blood pressure in people with primary hypertension aged 18 and over, there are various pharmacological classes of antihypertensive drugs available, the use of which depends on patient age and clinical conditions (e.g. grade of hypertension, type 2 diabetes mellitus (T2DM)) (NICE 2019; Williams 2018). The major pharmacological classes of antihypertensive drugs are beta‐blockers, thiazide and thiazide‐like diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, and calcium channel blockers. However, with treatment success comes possible undesirable side effects, such as metabolic syndrome, hypokalaemia, metabolic alkalosis, and ankle oedema (Laurent 2017). Further information for adverse events was presented elsewhere (Chen 2018; Garjon 2020; Wright 2018).
Medication adherence is a very important factor to achieve successful treatment outcomes in terms of improving hypertension control as well as reducing complications of hypertension (WHO 2003). It was estimated that non‐adherence to antihypertensive medications occurs in 10% to 80% of hypertensive patients (Unger 2020). For adult hypertensive patients, the overall non‐adherence to antihypertensive medications was 45.2%, 95% confidence interval 34.4% to 56.1% (Abegaz 2017). In low‐ and middle‐income countries, rates of non‐adherence to antihypertensive medications using the Morisky Medication Adherence Scale (MMAS) was 63.4%, 95% confidence interval 38.8% to 87.9% (Nielsen 2017).
Various potential factors associated with non‐adherence to antihypertensive medications have been previously investigated and presented in the literature. These factors can be classified according to the five dimensions proposed by the World Health Organization: social and economic factors, healthcare team and system‐related factors, conditions‐related factors, therapy‐related factors, and patient‐related factors (WHO 2003). Of these five dimensions, side effects of medication is included in the last two dimensions: therapy‐related factors and patient‐related factors.
Lifestyle modification approach is the first line of antihypertensive treatment (Unger 2020). As modifications in lifestyle can enhance the effects of antihypertensive treatment, improving adherence and long‐term blood pressure controlling, hypertensive patients grade 2 or 3 should receive antihypertensive drugs alongside a healthy lifestyle (NICE 2019; Unger 2020). Lifestyle modifications include salt reduction, healthy diet and drinks, moderation of alcohol consumption, weight reduction, smoking cessation, regular physical activity, reducing stress and inducing mindfulness, complementary, alternative, or traditional medicines, and reducing exposure to air pollution and cold temperature (Unger 2020). Of these lifestyle modifications, Roselle (Hibiscus sabdariffa Linne, a member of the family Malvacae), also known as red sorrel, is included in healthy diet and drinks, as well as alternative or traditional medicines.
Roselle is a tropical plant originally native to India to Malaysia and widely grown in tropical and subtropical regions around the world, such as Central and West Africa and South‐East Asia. Roselle is also known as Karkade, Bissap, sour tea, and red tea (Ali 2005; Mozaffari‐Khosravi 2009; Riaz 2018; Thiagarajah 2019). It has been used as traditional culinary (foods, food colouring agents, beverages), as well as a traditional therapeutic for a number of conditions. For instance, various parts of Roselle (flower, leaves, calyx, and corolla) are widely consumed as a beverage in China, Taiwan, and Thailand as a thirst‐quenching drink. The pharmacological evidence of Roselle, such as antihypertensive, antihyperlipidaemic, anti‐inflammatory, antimicrobial effect, diuretic, uricosuric effect and hyper‐uricaemia, treatment of anaemia, have been documented (Ali 2005; Herrera‐Arellano 2004; Riaz 2018; Thiagarajah 2019; Wright 2007). Consumption of a typical amount of Roselle is generally considered to be safe for most people (Da‐Costa‐Rocha 2014; Hopkins 2013; Riaz 2018). Evidence from animal and human studies found no changes in liver function (aspartate aminotransferase (AST) and alanine aminotransferase (ALT) liver enzymes) and kidney function (blood urea nitrogen and urea). However, hepatotoxicity was observed with very high doses of Roselle (300 mg/kg/day over a three‐month period) (Hopkins 2013).
How the intervention might work
Roselle is also known as an importance source of nutritional composition, bioactive compounds, and colouring agents, being rich in anthocyanins and water‐soluble pigments (Da‐Costa‐Rocha 2014; Jabeur 2017; Wu 2018). The red anthocyanin pigments in the calyxes are used as food colouring agents (Ali 2005). Anthocyanins are associated with hypotensive effects; the direct and indirect mechanisms of anthocyanins were presented in a previous study (Amin 2020), which found that anthocyanins reduce synthesis of vaso‐constricting molecules through the inhibition of angiotensin II converting enzyme, thus preventing vaso‐constriction and hypertension (Amin 2020; Vendrame 2019; Wahabi 2010). A recent study found an inverse association between dietary anthocyanin intake of 24 mg/day and blood pressure in adults aged 50 years and over (Igwe 2019).
Previous studies have investigated the capabilities of Roselle on blood pressure. Three studies conducted in animals showed the following results. Mojiminiyi 2007, a study conducted in hypertensive and normotensive rats, found that an aqueous extract of the calyx of Roselle possesses antihypertensive, hypotensive, and negative chronotropic effects (Roselle was administered as dissolved in normal saline). Odigie 2003 performed a study on renovascular hypertensive rats, which suggested that the aqueous extract of Roselle petal exhibited antihypertensive and cardioprotective properties. Onyenekwe 1999 reported that Roselle calyx infusion was found to significantly lower both systolic and diastolic pressure in spontaneously hypertensive and normotensive Wistar‐Kyoto rats.
In humans, Herrera‐Arellano 2007 assessed the effects of dried extract of Roselle calyxes on patients with stage I or II hypertension aged 25 to 61 years. The results showed that the dried extract of Roselle calyxes decreased blood pressure from 146/98 mmHg to 130/86 mmHg. The authors concluded that Roselle exerted important antihypertensive effectiveness with a wide margin of tolerability and safety.
Information from a non‐Cochrane systematic review, Thavorn 2006, and a mini‐review, Ernst 2005, confirmed the modest antihypertensive effects observed in the study of Herrera‐Arellano 2004 (dry calyx from Roselle significantly decreased both systolic and diastolic blood pressure of hypertensive patients) and found a marked effect (11% reduction) on both systolic and diastolic blood pressure in patients with moderate essential hypertension (Haji 1999). Wahabi 2010 reported that Roselle appeared to be effective in reducing blood pressure when compared with black tea but not when compared with the ACE inhibitors captopril and lisinopril, whilst Walton 2016 reported that Roselle appeared to be as effective as captopril. It should be noted that the evidence from these studies was based on diverse populations and comparisons.
Why it is important to do this review
Antihypertensive drugs can cause undesirable side effects which may negatively affect adherence to antihypertensive treatment. Roselle has demonstrated potential hypotensive effects due to its bioactive compounds, anthocyanins, with rare side effects. In addition, Roselle may be included in a healthy diet as a lifestyle modification that may improve adherence and long‐term blood pressure control. The effectiveness of Roselle in treating or lowering blood pressure has been documented in mixed populations (e.g. normal and hypertensive patients) and using various comparisons (e.g. different type of tea, berry). It is therefore necessary to study the effect of Roselle (Hibicus sabdariffa Linne) on blood pressure to determine both its desirable and undesirable effects because hypertension is the first and most important modifiable risk factor for cardiovascular disease. The findings could support the use of Roselle as an alternative therapy for primary hypertension patients. This is the first update of a Cochrane Review published in 2010 (Ngamjarus 2010).
Objectives
To determine the effect of Roselle (Hibicus sabdariffa Linne) on blood pressure in people with primary hypertension.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing the use of any form of Roselle with placebo or no intervention in participants with hypertension, with a minimum study duration of 3 weeks and a maximum study duration of 12 weeks.
Types of participants
Adults (18 years of age or older) who had at least 140 mmHg systolic blood pressure (SBP) or at least 90 mmHg diastolic blood pressure (DBP). At least two blood pressure measurements were needed at baseline to qualify patients as being hypertensive. Pregnant women were excluded.
Types of interventions
Any form of Roselle compared to placebo or no treatment.
Types of outcome measures
The outcomes of interest are listed below.
Primary outcomes
Change in trough (13 to 26 hours after the dose) and/or peak (1 to 12 hours after the dose) SBP and DBP. If blood pressure measurements were available at more than one time point within the acceptable window, we used the means of blood pressures taken in the 3‐ to 12‐week range.
Secondary outcomes
Withdrawals due to adverse effects.
Change in pulse pressure (mmHg).
Change in heart rate (beats/minute).
Search methods for identification of studies
Electronic searches
For this update, the Cochrane Hypertension Information Specialist searched the following databases without language, publication year, or publication status restrictions:
Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (to 6 August 2021);
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 7) via the Cochrane Register of Studies;
MEDLINE Ovid (1946 to 5 August 2021);
Embase Ovid (1974 to 5 August 2021);
ProQuest Dissertations & Theses Global (to 6 August 2021);
Web of Science Clarivate (to 7 August 2021);
Food Science and Technology Abstracts Clarivate (to 7 August 2021);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (to 6 August 2021);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (to 6 August 2021).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. Where appropriate, these were combined with subject strategy adaptations of the Highly Sensitive Search Strategy designed by Cochrane for identifying RCTs (as described in the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2019). We present the search strategies for these databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE and Embase for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials
The first review author (PP) checked the reference lists of relevant papers and reviews identified, and searched local and regional databases using English and/or Thai language without any other restrictions as follows:
Google Scholar (searched 8 September 2020);
Thai Journals Online (ThaiJO) (searched 9 September 2020);
the digital collection of full‐text academic works by faculty members, researchers, and graduate students of Chulalongkorn University, Chulalongkorn University Intellectual Repository (CUIR) (searched 9 September 2020);
ThaiLIS Digital Collection (TDC) (searched 9 September 2020);
E‐Theses (CMU e‐Theses) (searched 9 September 2020);
OpenGrey (OpenSIGLE) (www.opengrey.eu) (searched 10 September 2020);
Chinese BioMedical Literature Database (CBM) (searched 11 September 2020);
Chinese Medical Current Contents (CMCC) (searched 11 September 2020);
Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS) (searched 11 September 2020);
China Proceedings of Conference Full‐text Database (CNKI) and Full‐text Database of Academic Conferences in China (English version) (searched 13 September 2020);
China Medical Academic Conference (CMAC) (searched 14 September 2020);
Index to Chinese Periodical Literature (searched 14 September 2020);
Thai Clinical Trials Registry (TCTR) (searched 3 October 2020).
We present the search strategies for these resourcesin Appendix 2.
Data collection and analysis
Selection of studies
We considered predefined eligibility criteria, Ngamjarus 2009, throughout the process of study selection.
For this update, the Cochrane Hypertension Information Specialist searched the databases listed in Electronic searches, merged all of the search results, screened obviously irrelevant records, and sent us the remaining potentially relevant records. We imported these records into Covidence, the number of search results of each databases, included and excluded studies re‐checked with Mendeley Desktop software. Two review authors (PP, FB) independently screened the titles and abstracts of these records. Any disagreements were resolved by discussion, or the report/study(s) was preserved for the next step, full‐text assessment. We retrieved the full‐text reports for all potentially relevant records left after the screening process, and PP and FB independently assessed the full texts using Covidence software (Covidence). Any disagreements were resolved through discussion or by consulting a third review author (CN or CS) if necessary. The primary reasons for the exclusion of studies are provided in Characteristics of excluded studies.
Regarding the results obtained from searching the resources listed in Searching other resources, two review authors (PP, CN) independently screened the titles and abstracts and evaluated the full texts of all studies deemed potentially relevant. Any disagreements were resolved by consensus, or with the involvement of a third review author (CS) where necessary.
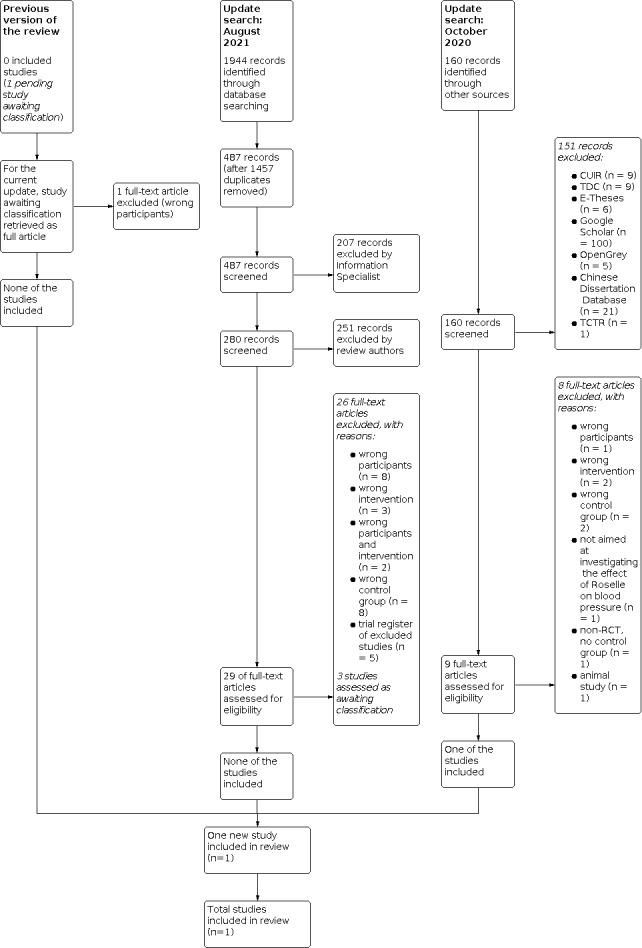
The study selection process is illustrated in a PRISMA flow diagram (Figure 1).
1.
study flow diagram.
Data extraction and management
Using templates for data extraction forms available from Cochrane Review Groups, we designed a data extraction form specifically to match the needs of our review. Prior to the data extraction process, we performed a pilot test of our data extraction form, to arrive at a standardised data extraction form. The data extraction form included PICO components, general information (study date, setting, duration of follow‐up period, country, sample size, funding), baseline characteristics (age, gender, SBP, DBP), inclusion and exclusion criteria, and risk of bias table.
Two review authors (CN, PP) independently extracted the data from the included studies. Any disagreements were resolved by discussion or by consultation with a third review author (CS) if needed. We contacted the authors of the original study(s) to provide additional information or clarification where required. We entered all relevant data into Review Manager 5 (Review Manager 2020). One review author (CN) double‐checked the data presented in the data extraction form against the data entered into the Review Manager 5 file. The main characteristics of the included studies are presented in the Characteristics of included studies section.
Assessment of risk of bias in included studies
In accordance with our protocol (Ngamjarus 2009), two review authors (PP, CN) independently assessed the risk of bias for each included study based on the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The risk of bias tool addresses the following six domains.
Selection bias (sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessment)
Attrition bias (incomplete outcome data)
Reporting bias (selective outcome reporting)
Other bias, if any
We assessed each domain as low, high, or unclear risk of bias. We provided the supporting information from the included study together with our comment/judgement in the risk of bias tables.
We recorded the results of the risk of bias assessment in the data extraction form. Any disagreements were resolved by discussion or by consultation with a third review author (CS) if necessary. The risk of bias assessment is presented in the risk of bias tables (Characteristics of included studies), risk of bias graph (Figure 2), and risk of bias summary (Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For the one dichotomous outcome (withdrawals due to adverse effects), we planned to present the treatment effects as risk ratio (RR) with 95% confidence intervals (CIs).
For continuous outcomes (change in SBP, DBP, pulse pressure, heart rate), we expressed the treatment effect as mean difference (MD) of change with corresponding 95% CIs.
We did not plan to use the standardised mean difference (SMD), as there were no issues related to the use of different scales for our outcomes of interest.
Unit of analysis issues
In the published protocol (Ngamjarus 2009), we planned to consider the following components.
Studies with multiple treatment groups
In the case of studies with multiple treatment groups, we planned to combine all relevant intervention groups into a single group, and all relevant control groups into a single control group. We would follow the methods outlined in Section 6.2.9, Higgins 2021a, and Section 23.3.2, Higgins 2021b, of the Cochrane Handbook for Systematic Reviews of Interventions.
Repeated observations on participants
We intended to consider a single time point and analyse only data at this time point for trials in which it was presented following the recommendations in Section 6.2.4 of the Cochrane Handbook (Li 2021).
Dealing with missing data
All of the required information was obtained from the included study, therefore for this update we contacted the principal investigator of one trial to obtain the publication status of their study. We noted levels of attrition (dropout rate) for missing participants.
Assessment of heterogeneity
We did not investigate heterogeneity across studies because there was only one included study.
If there are sufficient studies in future updates of this review, we will quantify heterogeneity of treatment effect amongst the included studies using a visual examination of the forest plot, the Chi2 test (P value less than 0.10), and the I2 statistic. We defined an I2 greater than 50% as indicative of substantial heterogeneity.
Assessment of reporting biases
We originally planned in our protocol to use a funnel plot and Egger’s test and Egger’s plot to investigate publication bias (Ngamjarus 2009), employing the Stata program (Stata 2017), if at least 10 trials were included in a meta‐analysis.
Examining publication bias was not possible as there was only one included study.
Data synthesis
Due to insufficient data, we were unable to carry out a meta‐analysis.
If more data are available in future updates of this review, we will apply the random‐effects model to combine the treatment effect amongst the studies where there is an absence of considerable heterogeneity (defined as an I2 greater than 80%). Where meta‐analysis is inappropriate due to the presence of considerable heterogeneity, we will undertake a narrative synthesis.
Subgroup analysis and investigation of heterogeneity
We did not conduct an investigation of heterogeneity as there was only one included study.
In future updates of this review where substantial heterogeneity is identified, we will investigate possible causes for it by undertaking the following subgroup analyses as prespecified in our protocol (Ngamjarus 2009).
Type of Roselle
Underlying disease of the participants
Sensitivity analysis
We did not conduct sensitivity analysis as there was only one included study.
In future updates of this review if sufficient data are available, we will undertake the following sensitivity analyses in order to assess the robustness of the effect estimate;
Removing study(s) at high risk of bias in any of the risk of bias domains
Removing study(s) that contribute to high levels of heterogeneity
Undertaking fixed‐effect versus random‐effects model
Summary of findings and assessment of the certainty of the evidence
For this update, two review authors (PP, CN) independently assessed the certainty of the evidence for the main outcomes across the included studies using the GRADE approach (Schünemann 2021), employing GRADEpro GDT software (GRADEpro GDT). Any discrepancies were resolved by discussion. The GRADE approach considers the following five factors: study limitations, inconsistency, imprecision, indirectness, and publication bias. The overall certainty of the evidence for each outcome is classified as high, moderate, low, or very low. We presented the main findings of the review in a summary of findings table, and justified our decisions to downgrade the certainty of the evidence in footnotes.
Results
Description of studies
For details, see Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
For this update, the Information Specialist performed the searches of the core databases in August 2021. The search of the electronic databases identified 1944 records. After removal of 1457 duplicates, 487 records were screened by the Information Specialist, of which 207 were excluded. Two review authors (PP and FB or CN) performed title and abstract screening of the remaining 280 records using Covidence (Covidence), excluding 251 records. We retrieved the full‐text articles for the remaining 29 records, of which 26 were excluded, for the following reasons: wrong participants (8 articles) (Abubakar 2019; Asgary 2016; Hajifaraji 2018; Jalalyazdi 2019; Kafeshani 2017; Najarzade 2016; NCT03804801; Pelliccia 2017), wrong intervention (3 articles) (Boix‐Castejon 2017; Boix‐Castejon 2021; Marhuenda 2021), wrong participants and intervention (2 articles) (Boix‐Castejon 2018; Herranz‐Lopez 2019), wrong control group (8 articles) (Bourqui 2020; Elkafrawy 2020; Nwachukwu 2015; Nwachukwu 2015a; Nwachukwu 2016; Nwachukwu 2017; Xu 2021; Yusni 2020), and trial register of excluded studies (5 articles) (IRCT201310089662N8; IRCT2014041510826N8; NCT01682291; NCT02637570; NCT03507023). See Characteristics of excluded studies.
We identified three potentially eligible studies that we assessed as studies awaiting classification (see Characteristics of studies awaiting classification), as follows.
NCT04339283 randomly assigned participants to Roselle or a water group. We contacted the primary trial author for the publication status and found that it is under consideration for publication.
Al‐Anbaki 2021 randomly assigned one study site as a control group (health awareness lecture on hypertension management), and took into account clustering in sample size calculation, therefore we considered this study as cluster‐randomised trial. However, the study provided no information on adjusting for clustering in the analyses. We planned to contact the primary trial author for more information (e.g. the intracluster correlation coefficient). In the case that the desired value cannot be obtained, we plan to account for clustering according to the methods described in Chapter 23 of the Cochrane Handbook (Higgins 2021b).
Izadi 2021 randomly assigned participants to Roselle or placebo. Blood pressure measurements at baseline and after study were likely reported as change in blood pressure. We planned to contact the primary trial author for baseline blood pressure and other relevant information.
Our searches of other sources obtained 160 additional records between 8 September 2020 and 3 October 2020. We identified two reference lists of relevant papers; one record from tracking of the trial registration number; three records from ThaiJO; 12 records from CUIR; nine records from TDC; six records from E‐Theses; 100 records from Google Scholar (first 10 pages); five records from OpenGrey; 21 records from Chinese Dissertation Database; one record from TCTR; and no records from CMB, CMCC, TCMLARS, CMAC, and Index to Chinese Periodical Literature.
Two review authors (PP, CN) screened the titles and abstracts of the 160 records, of which 151 were excluded. We retrieved the remaining nine full‐text articles, of which eight were excluded, as follows: wrong participants (1 article) (Salman 2017), wrong intervention (2 articles) (Aroonsiriwatana 2010; Wiruttanapornkul 2012), wrong control group (2 articles) (Intarit 2012; Thanasatirakul 2015), aim of study was not to investigate the effect of Roselle on blood pressure (1 article) (Hadi 2017), not an RCT (1 article) (Tangkomsaengtong 2020), and an animal study (1 article) (Bunbupha 2018). See Characteristics of excluded studies.
We included one RCT in the review with a total of 60 participants (Sarbini 2019). See Characteristics of included studies. The study selection process was shown in Figure 1.
Included studies
See Characteristics of included studies.
For this update, one RCT with a two‐arm, parallel‐group design fulfilled the inclusion criteria (Sarbini 2019). The follow‐up duration was eight weeks. The study was conducted in Yogyakarta, Indonesia between 2018 to 2019. The study reported the outcomes of interest at a single time point, and completely reported the number of participants, mean and standard deviation (SD) of changes in SBP and DBP. There were no issues related to multiple treatment groups, repeated observations on participants, or missing data.
Study setting and participants
All participants were outpatients at public health centres in Yogyakarta. A total of 60 participants (both males and females) were randomly assigned to either Roselle or placebo. Most participants were elderly (50 years and older), and all had type 2 diabetes mellitus (T2DM) (average duration of 7 years or more), fasting blood sugar level of 151.11 mg/dL, fasting insulin level of 12.19 μIU/mL, and homeostasis model assessment‐insulin resistance (HOMA‐IR) of 2.38. The average body mass index (BMI) was above 25 kg/m2.
The average age of participants was 54.08 years (SD 5.89) and 56.14 years (SD 5.11) in the Roselle and placebo groups, respectively. At baseline, the average SBP (mmHg) of participants was 188 (SD 143.54) and 146.80 (SD 20), and the average DBP (mmHg) of participants was 79.14 (SD 8.24) and 81.47 (SD 9.71) in the Roselle and placebo groups, respectively.
Intervention
Participants in the intervention group received two capsules a day after meals (morning or evening) for eight weeks; each capsule contains 50 mg of pure extract Roselle. Therefore, the total pure extract Roselle dosage was 5600 mg. for each participants.
Control
Participants in the control group received placebo capsules (500 mg lactose) and consumed the same amount of capsules under the same direction as the intervention group.
Outcomes
Sarbini 2019 reported the primary outcome of this review, that is changes in SBP and DBP. Blood pressure (mmHg) was measured using a stethoscope and mercury sphygmomanometer (Omron brand); the average of three times blood pressure value was reported: at baseline, at the end of study visit, and changes. Sarbini 2019 reported change‐from‐baseline outcomes calculated by end measurement minus baseline measurement. There was no information of the secondary outcomes of this review, that is withdrawals due to adverse effects, change in pulse pressure, and change in heart rate.
Additional outcomes reported in Sarbini 2019 were physical activities, food intakes, nutritional status, blood glucose level, insulin resistance, and compliance of capsule intake of each participant, assessed using participant‐recorded diary data (see Characteristics of included studies).
Excluded studies
See Characteristics of excluded studies.
No studies met our inclusion criteria in the previous version of this review. However, one study was assessed as awaiting classification (McKay 2008), which was in the process of publishing the full article. For this update, we retrieved the full text for this study (McKay 2010), and two review authors (PP, CN) independently assessed eligibility of the study for inclusion in the review. We excluded this study because both the baseline SBP and DBP of participants did not meet our inclusion criteria (wrong participants) (see Characteristics of excluded studies).
For this update, we excluded a total of 27 studies, 19 identified from searching the electronic databases and 8 identified from searching other resources. The reasons for exclusion are provided in Results of the search and Characteristics of excluded studies.
Risk of bias in included studies
Review authors’ judgements for each risk of bias domain for the included study are presented in Characteristics of included studies; summaries of risk of bias are provided in Figure 2 and Figure 3 (Sarbini 2019).
Allocation
Selection bias (sequence generation and allocation concealment)
Sarbini 2019 used a permuted block design with a computer random number generator. The randomisation was performed by randomisation team which acted as the central randomisation office. We therefore assessed Sarbini 2019 as having low risk of bias for sequence generation and allocation concealment.
Blinding
Performance bias (blinding of participants and personnel)
The capsules in both the intervention and control groups were identical in appearance (shape, size, colour), taste, and capsule container bottles, therefore the participants were unlikely to be aware of which one they received.
In addition, Sarbini 2019 declared that the researchers, participants, and laboratory analysts were unaware of the interventions provided. We therefore assessed Sarbini 2019 as having low risk of bias for blinding of participants and personnel.
Detection bias (blinding outcome assessment)
As mentioned above, the researchers, participants, and laboratory analysts were unaware of the interventions provided. It is unlikely that the blinding could have been broken. We therefore assessed Sarbini 2019 as having low risk of bias for blinding of outcome assessment.
Incomplete outcome data
Eight participants were lost to follow‐up; the overall dropout rate was 13.4%. There was an imbalance of dropouts across groups, with 20% dropout in the intervention group versus 6.6% in the control group. No reason for dropout was mentioned for one withdrawal in the intervention group. We therefore assessed Sarbini 2019 as having high risk of bias for incomplete outcome data.
Selective reporting
The study protocol for Sarbini 2019 was not available. All of the outcomes specified in the Methods section of the study have been reported; however, adverse effects was not an outcome of interest for this study. In order to investigate the effectiveness of Roselle as a treatment for hypertension, it is important to consider adverse effects. We therefore assessed Sarbini 2019 as having high risk of bias for selective outcome reporting.
Other potential sources of bias
An other potential source of bias was baseline imbalance between groups (Sarbini 2019). At baseline, the average SBP of participants in the Roselle group (grade II hypertension) was higher than the control group (grade I hypertension); the classification of hypertension based on the 2020 International Society of Hypertension Global Hypertension Practice Guidelines (Unger 2020). We therefore assessed Sarbini 2019 as having high risk of other bias.
Effects of interventions
See: Table 1
See Table 1.
Comparison: Roselle extract versus placebo
Primary outcome
Change in blood pressure at eight weeks
One RCT investigated the effect of consuming Roselle capsules versus placebo (lactose), and reported baseline measurement, end measurement, and change‐from‐baseline outcomes, calculated by end measurement minus baseline measurement (Sarbini 2019). Three blood pressure measurements were taken using a stethoscope and mercury sphygmomanometer (Omron brand) before and after the interventions; the authors of the original study calculated the mean blood pressure.
For SBP, the effect size calculated was not statistically significant. The mean difference of change in SBP was 1.65, 95% confidence interval (CI) −7.89 to 11.19 mmHg, 52 participants, very low‐certainty evidence, Analysis 1.1. We noted a large amount of variation, coefficient of variation > 300% for both groups. This result should be interpreted with caution (Table 1).
1.1. Analysis.

Comparison 1: Roselle extract versus placebo, Outcome 1: Change in systolic blood pressure (mmHg) at week 8
Roselle group: baseline SBP mean 188 (SD 143.54) mmHg, end SBP mean 139.5 (SD 18.27) mmHg, 24 participants.
Control group: baseline SBP mean 146.8 (SD 20.0) mmHg, end SBP mean 141.11 (SD 20.63) mmHg, 28 participants.
For DBP, the effect size calculated was not statistically significant. The mean difference of change in DBP was 4.60, 95% CI −1.38 to 10.58 mmHg, 52 participants, very low‐certainty evidence, Analysis 1.2. We noted a large amount of variation, coefficient of variation > 270% for both groups. This result should be interpreted with caution (Table 1).
1.2. Analysis.

Comparison 1: Roselle extract versus placebo, Outcome 2: Change in diastolic blood pressure (mmHg) at week 8
Roselle group: baseline SBP mean 79.14 (SD 8.24) mmHg, end SBP mean 83.87 (SD 10.68) mmHg, 24 participants.
Control group: baseline SBP mean 81.47 (SD 9.71) mmHg, end SBP mean 81.61 (SD 8.29) mmHg, 28 participants.
Secondary outcomes
Withdrawals due to adverse effects
This outcome was not reported in the included study.
Change in pulse pressure (mmHg)
This outcome was not reported in the included study.
Change in heart rate (beats/min)
This outcome was not reported in the included study.
Subgroup analysis
We performed no subgroup analyses due to an insufficient number of included studies.
Sensitivity analysis
We were unable to test the robustness of the overall treatment effect as only one study was included in the review.
Publication and small‐study bias
We were not able to draw funnel plots due to an insufficient number of included studies.
Discussion
Summary of main results
This first update of the review included one RCT that examined the effect of Roselle extract capsules versus placebo in 52 adults with T2DM. Currently, the available evidence is insufficient to determine the use of Roselle extract (total dose of 5600 mg) for controlling or lowering blood pressure in hypertensive patients with T2DM compared to placebo (lactose) at eight weeks (very low‐certainty evidence).
There was no reporting of the review secondary outcomes: withdrawals due to adverse effects, change in pulse pressure, and change in heart rate.
For a future update of this review, we will determine whether one study assessed as awaiting classification that is currently under consideration for publication meets the inclusion criteria of the review.
Overall completeness and applicability of evidence
We included only one RCT in the review, which involved a small number of participants, most of whom were elderly (at least 50 years old), and all of whom had T2DM. The applicability of the evidence in this review is therefore limited due to the lack of variation in the characteristics of participants, the lack of variation in the use of Roselle (forms, dosages, timing of Roselle; short‐ or long‐term use), and the lack of data for the secondary outcomes of the review. In addition, the evidence in this review came from a single study with few participants.
Quality of the evidence
We assessed the one included study to have low risk of bias for sequence generation, allocation concealment, blinding of participants and personnel, and blinding outcome assessment. Conversely, we judged the study to have high risk of bias for incomplete outcome data, selective outcome reporting, and imbalance in baseline characteristics.
The true effect is likely to be substantially different from the estimate of effect of the primary outcomes, as the certainty of the evidence based on the GRADE approach was very low for mean difference of change in SBP and DBP.
Of five factors that can reduce the certainty of the evidence, we downgraded three levels for limitations in study design as mentioned above. Data were insufficient to undertake an assessment of inconsistency and publication bias. Additionally, we downgraded two levels for imprecision due to (i) the small numbers of participants, as the total number of participants was fewer than 400, and (ii) wide confidence intervals; consideration of minimally important difference at 5 mmHg. Finally, we downgraded one level for indirectness, as the evidence was specific to participants aged 50 years and older.
Potential biases in the review process
In order to minimise potential bias, we conducted this review in accordance with a predefined protocol and the Cochrane Handbook for Systematic Reviews of Interventions.
Because the original content is out of date and the protocol was published in 2009, we revised the Background and methodologies (Assessment of heterogeneity, Assessment of reporting biases, Data synthesis, Subgroup analysis and investigation of heterogeneity, Sensitivity analysis), and performed a GRADE assessment of the certainty of the evidence. We reported all changes in the Differences between protocol and review section.
We attempted to minimise the risk of publication bias by performing a comprehensive search to identify all relevant trials in all languages, without date or publication status restrictions. The Cochrane Hypertension Information Specialist and Assistant Managing Editor undertook the systematic search process for the electronic databases. We also searched trial registers for unpublished or ongoing trials. We attempted to obtain additional relevant data by closely checking the reference lists of relevant articles identified, searching for grey literature, as well as searching the local and regional Chinese and Thai databases.
We applied rigorous processes for study selection, data extraction, and risk of bias and certainty of evidence assessment. We organised all search results using Mendeley Desktop, re‐checked them with Covidence, and standardised our data extraction form by performing a pilot test.
However, the performance of the initial screening of the records identified from the search of the electronic databases by the Information Specialist could have introduced selection bias.
Agreements and disagreements with other studies or reviews
To our knowledge, there are no systematic reviews of RCTs that have addressed the research question posed in this review.
There are six potentially relevant non‐Cochrane systematic reviews (Adeola 2019; Najafpour 2020; Serban 2015; Wahabi 2010; Walton 2016; Zhang 2020). These reviews investigated the effect of Roselle on blood pressure, glycaemic status (fasting plasma glucose), or metabolic syndrome. We tracked all included studies of these six reviews and found that some of them were not RCTs, and all were outside the scope of our study.
In addition, we found one comprehensive review that assessed the effectiveness of Roselle as a treatment for hypertension and hyperlipidaemia in humans and animals by searching the evidence up to November 2012 (Hopkins 2013). With regard to human studies, five RCTs were included in Hopkins 2013, all of which we excluded in our review.
Authors' conclusions
Implications for practice.
The available evidence regarding the effectiveness and safety of Roselle for hypertensive adults compared to placebo or no treatment is very uncertain and did not permit the drawing of any conclusions.
Implications for research.
There is a need for well‐conducted randomised controlled trials (RCTs) investigating the effectiveness of Roselle compared to placebo on controlling or lowering blood pressure in hypertensive patients, that evaluate both short‐ and long‐term administration of treatment. Future studies should provide information regarding preparation of Roselle, and report findings according to the CONSORT Statement. In addition, future RCTs need to be well‐planned for investigating and reporting adverse events of Roselle. The current evidence is limited to adults with an average age of 55.2 years; there is a need for future RCTs to investigate adults with an age range of 18 to 60 years.
What's new
| Date | Event | Description |
|---|---|---|
| 5 November 2021 | Amended | One additional review author, Fonthip Buttramee (FB), contributed to the update. The review contact person and the order of the review authors have changed. The Background has been updated, and the methodologies have been revised in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. We assessed one study as awaiting classification in the previous version of the review; however, it was excluded in this update. For this update, the searches were updated to 7 August 2021 for the main databases and to 3 October 2020 for the local and regional databases. We added one new included study and assessed one study as awaiting classification. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 1, 2010
Acknowledgements
The review authors would like to acknowledge Cochrane Thailand for providing office space. We would also like to acknowledge all staff of Cochrane Hypertension, especially Information Specialist and Assistant Managing Editor Douglas Salzwedel, for the systematic literature search as well as the initial screening of the records obtained from the search of the electronic databases.
Appendices
Appendix 1. Search strategies
(1.1) Database: Ovid MEDLINE(R) ALL <1946 to August 04, 2021> Search Date: 5 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 hibiscus/ 2 (hibiscus$ or rosella or roselle or ambary or anthocyanin$ or burao or chemparathampoo or erragogu or esculetin or gogu or karkad$ or kenaf or sorrel or red tea or sabdarifa or sabdariffa or sour tea or tellagogu or zobo).mp. 3 or/1‐2 4 hypertension/ 5 essential hypertension/ 6 (antihypertens$ or hypertens$ or prehypertens$).tw,kf. 7 exp blood pressure/ 8 (blood pressure or bloodpressure).tw,kf. 9 ((arterial or diastolic or systolic) adj pressure).tw,kf. 10 or/4‐9 11 randomized controlled trial.pt. 12 controlled clinical trial.pt. 13 randomized.ab. 14 placebo.ab. 15 dt.fs. 16 randomly.ab. 17 trial.ab. 18 groups.ab. 19 or/11‐18 20 animals/ not (humans/ and animals/) 21 19 not 20 22 3 and 10 and 21
(1.2) Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies
Search Date: 6 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 hibiscus* AND INSEGMENT #2 (ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR red tea OR rosella OR roselle OR sabdarifa OR sabdariffa OR sorrel OR sour tea OR tellagogu OR zobo) AND INSEGMENT #3 (#1 OR #2) #4 RCT:DE AND INSEGMENT #5 Review:ODE AND INSEGMENT #6 (#4 OR #5) #7 #3 AND #6
(1.3) Database: Cochrane Central Register of Controlled Trials (Issue 7, 2021) via Cochrane Register of Studies
Search Date: 5 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 hibiscus* AND CENTRAL:TARGET #2 (ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR red tea OR rosella OR roselle OR sabdarifa OR sabdariffa OR sorrel OR sour tea OR tellagogu OR zobo) AND CENTRAL:TARGET #3 (#1 OR #2) #4 MESH DESCRIPTOR Hypertension AND CENTRAL:TARGET #5 MESH DESCRIPTOR Essential Hypertension AND CENTRAL:TARGET #6 (antihypertens* OR hypertens* OR prehypertens*) AND CENTRAL:TARGET #7 MESH DESCRIPTOR Blood Pressure EXPLODE ALL AND CENTRAL:TARGET #8 (blood pressur* OR bloodpressur*) AND CENTRAL:TARGET #9 (arterial OR diastolic or systolic) NEXT pressur* AND CENTRAL:TARGET #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 #10 AND #3
(1.4) Database: Embase <1974 to 2021 August 04> Search Date: 5 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 hibiscus/ 2 hibiscus sabdariffa extract/ 3 (hibiscus* or rosella or roselle or ambary or anthocyanin$ or burao or chemparathampoo or erragogu or esculetin or gogu or karkad$ or kenaf or sorrel or red tea or sabdarifa or sabdariffa or sour tea or tellagogu or zobo).mp. 4 or/1‐3 5 exp hypertension/ 6 (antihypertens$ or hypertens$ or prehypertens$).tw. 7 exp blood pressure/ 8 blood pressure.mp. 9 bloodpressure.tw. 10 ((arterial or diastolic or systolic) adj pressure).tw. 11 or/5‐10 12 randomized controlled trial/ 13 crossover procedure/ 14 double‐blind procedure/ 15 (randomi?ed or randomly).tw. 16 (crossover$ or cross‐over$).tw. 17 placebo.ab. 18 doubl$ blind$.tw. 19 assign$.ab. 20 allocat$.ab. 21 or/12‐20 22 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 23 21 not 22 24 4 and 11 and 23
(1.5) Database: ProQuest Dissertations & Theses Global Search Date: 6 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ noft((hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR red tea OR sabdarifa OR sabdariffa OR sour tea OR tellagogu OR zobo)) AND noft((antihypertens* OR hypertens* prehypertens* OR blood pressur*)) AND noft((allocat* OR assign* OR controlled OR group* OR placebo* OR randomi* OR randomly OR trial OR doubl* blind* OR singl* blind* OR blinded))
(1.6) Database: Clarivate Web of Science (Indexes=SCI‐EXPANDED, CPCI‐S, ESCI‐Timespan=All years) Search Date: 7 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #4 #1 AND #2 AND #3 #3 TS=(allocat* OR assign* OR controlled OR doubl* blind* OR group* OR placebo* OR randomi* OR randomly OR singl* blind* OR trial) #2 TS=(antihypertens* OR hypertens* OR prehypertens* OR "blood pressur*") #1 TI=(hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo) OR AB=(hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo)
(1.7) Database: Clarivate Food Science and Technology Abstracts (1969‐2020) Search Date: 7 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #4 #1 AND #2 AND #3 #3 TS=(allocat* OR assign* OR controlled OR doubl* blind* OR group* OR placebo* OR randomi* OR randomly OR singl* blind* OR trial) #2 TS=(antihypertens* OR hypertens* OR prehypertens* OR "blood pressur*") #1 TI=(hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo) OR AB=(hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo)
(1.8) Database: ClinicalTrials.gov Search Date: 6 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition or disease: Hypertension Other terms: randomized Intervention/treatment: (hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo) Study type: Interventional Studies (Clinical Trials)
(1.9) Database: International Clinical Trials Registry Platform (WHO ICTRP) Search Date: 6 August 2021 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Condition: Hypertension
Title: (hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo)
OR
Intervention: (hibiscus* OR rosella OR roselle OR ambary OR anthocyanin* OR burao OR chemparathampoo OR erragogu OR esculetin OR gogu OR karkad* OR kenaf OR sorrel OR "red tea" OR sabdarifa OR sabdariffa OR "sour tea" OR tellagogu OR zobo)
Appendix 2. Searching other resources
(2.1) to (2.5); using Thai language SeeFigure 4
4.

Figure 4 Searching other resources (2.1) to (2.5); using Thai language
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
(2.6) Database: OpenGrey (http://www.opengrey.eu/) Search Date: 10 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
OpenGrey (http://www.opengrey.eu/) using
keyword in Medicine discipline; "Hibiscus" (4)
"Roselle" in Medicine discipline; "Hibiscus" (1)
(2.7) Database: Chinese BioMedical Literature Database (CBM; http://allie.dbcls.jp/pubmed_all/CBM;Chinese+Biomedical+Literature+Database.html) Search Date: 11 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
There were 939 records for this database, we searched using "Hibiscus" or "Roselle" (0)
(2.8) Database: Chinese Medical Current Contents (CMCC; https://benthamscience.com/journals/current‐chinese‐medical‐science) Search Date: 11 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
the Advanced search in abstract, title, keywords, and specified Subject as "Hypertension" using
"Hibiscus" or "Roselle" (0)
(2.9) Database: Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS; http://lists.healthnet.org/archive/cgi‐bin/namazu.cgi?idxname=afro‐nets) Search Date: 11 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
the Advanced search in title; "Hibiscus" or "Roselle" in title (0)
(2.10) Database: Chinese Dissertation Database Search Date: 13 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
China Proceedings of Conference Full‐text Database (CNKI; https://epub.cnki.net/KNS/brief/result.aspx?dbPrefix=CPFD) using "roselle" found 2 records, 2 excluded or "hibiscus" (21)
Full‐text Database of Academic Conferences in China (English version) (Wanfang data; http://c.wanfangdata.com.cn/conference) using using "roselle" or or "hibiscus" in Medicine&Health (0)
(2.11) Database: China Medical Academic Conference (CMAC; http://www.chinacmac.com/en/) Search Date: 14 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
in Medicine&Health; "roselle" OR "hibiscus" (0)
(2.12) Database: Index to Chinese Periodical Literature (https://www.library.ucsb.edu/node/7793) Search Date: 14 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
"roselle" OR "hibiscus" (0)
(2.13) Database: Thai Clinical Trials Registry (TCTR) (https://www.clinicaltrials.in.th/) Search Date: 3 October 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
"roselle" (0)
Data and analyses
Comparison 1. Roselle extract versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in systolic blood pressure (mmHg) at week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Change in diastolic blood pressure (mmHg) at week 8 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sarbini 2019.
| Study characteristics | ||
| Methods |
Study design: individually randomised controlled trial; parallel‐group design
Study dates: 2018 to 2019
Setting: 5 public health centres in Yogyakarta, Indonesia Length of intervention: 8 weeks Duration of follow‐up periods: weekly evaluation of outcomes Country: Indonesia Preparation methods of Roselle: no information |
|
| Participants |
Sample size: randomised 60 (intervention 30, control 30); at the end of study 52 (intervention 24, control 28)
Baseline characteristics
Age (years):
Gender (M/F)
Baseline SBP (mmHg):
Baseline DBP (mmHg):
Inclusion criteria: T2DM patients with fasting blood glucose levels ≥ 126 mg/dL or random blood glucose level ≥ 200 mg/dL, willing to be sample by signing informed consent, able to communicate well, T2DM patients without kidney complication and heart disease, T2DM patients ranged between 35 and 65 years, and consuming the same drugs with the type, dose/amount, and frequency until the study was completed. Exclusion criteria:
|
|
| Interventions |
Intervention: Pure Roselle extract of 50 mg, twice a day for 8 weeks; total dose of 5600 mg (The extract dose added in capsules was 10% of the weight of 500 mg; each capsule contains 50 mg of pure extract Roselle) Comparison: Placebo capsules (500 mg lactose) Notes: Both study groups consumed 2 capsules a day after meals (morning and evening) for 8 weeks. Each participant was given a diary containing a record of compliance with capsule consumption, the remaining capsules, reasons for not consuming capsules, and a record of complaints during capsule consumption in which they were to write every day. During the study, samples were not permitted to take supplements or other herbal medicines. |
|
| Outcomes | The following outcomes were reported both at baseline and after the interventions, and change‐from‐baseline (calculated by end measurement minus baseline measurement);
|
|
| Notes |
Ethical considerations: Universitas Gajah Mada Faculty of Medicine, Public Health and Nursing Research Ethics Commission to obtain Ethical Clearance with Ref: KE FK/00995/EC/2018. Funding: Education fund management agency of the Republic of Indonesia’s Finance Minister, Gadjah Mada University, and Universitas Muhammadiyah Surakarta. Trial authors contacted for additional data: No |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The study design used double‐blinded & placebo‐controlled randomized clinical trial with intervention (placebo and Rosella) for 8 weeks.” “This study used experimental design with a double‐blinded & placebo‐controlled randomized clinical trial (RCT) design conducted in 2018‐2019 with total participants of 60 T2DM outpatients.” “Samples were randomly divided based on permuted block randomization by computer using 4 random numbers consisting of 2 (two) groups, the group that received Rosella capsules and the group with no Rosella prescription but received placebo capsules (500 mg lactose). The samples were selected by convenience sampling technique from affordable population by dividing it into 2 groups (control and Rosella groups) and then drawing for each group division. Randomization was performed by PT Liza Herbal International and the randomization team.” Comment: the random sequence was computer generated |
| Allocation concealment (selection bias) | Low risk | “Samples were randomly divided based on permuted block randomization by computer using 4 random numbers consisting of 2 (two) groups, the group that received Rosella capsules and the group with no Rosella prescription but received placebo capsules (500 mg lactose). The samples were selected by convenience sampling technique from affordable population by dividing it into 2 groups (control and Rosella groups) and then drawing for each group division. Randomization was performed by PT Liza Herbal International and the randomization team.” Comment: "PT Liza Herbal International and the randomization team" acted as the central randomisation office, therefore the allocation sequence was concealed to staff |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Researchers, samples and laboratory analysts of the study outcomes were completely unaware of the interventions provided. Rosella and placebo capsules were made in an identical shape, size, color, taste, and bottle package.” Comment: unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Researchers, samples and laboratory analysts of the study outcomes were completely unaware of the interventions provided. Rosella and placebo capsules were made in an identical shape, size, color, taste, and bottle package.” Comment: unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “In the intervention, 60 samples fulfilled the inclusion and exclusion criteria included in the clinical trial, 30 samples for control group and 30 samples for Rosella group. Before the intervention was given, 3 samples withdrew after taking blood for the baseline, 2 samples from the control group and 1 sample from intervention group so there was total of 57 samples received the intervention. The withdrawal reasons were due to family members’ permission issues, abundant medicine consumption and out of town for a long time. At the time of data analysis, only there were 52 participants could be analyzed because percentage of compliance with the intervention capsules below 80% (n = 3) and changing in the type and dose of hypoglycemic drugs consumed before the study was completed (n = 1)” Comment:
|
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is not available. All of the study’s outcomes specified in the methods have been reported; however, adverse effects was not an outcome of interest for this study. |
| Other bias | High risk |
Comment: imbalance in participant characteristics at baseline
*2020 International Society of Hypertension Global Hypertension Practice Guidelines (Unger 2020) |
DBP: diastolic blood pressure SBP: systolic blood pressure SD: standard deviation T2DM: type 2 diabetes mellitus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abubakar 2019 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Aroonsiriwatana 2010 | Intervention was a combination of Roselle and Stevia. |
| Asgary 2016 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Boix‐Castejon 2017 | Intervention was MetabolAids dietary ingredient (Lippia citriodora extract + Hibiscus sabdariffa extract). |
| Boix‐Castejon 2018 | Intervention was a combination of Hibiscus sabdariffa and Lippia citriodora; participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Boix‐Castejon 2021 | Intervention was a combination of Hibiscus sabdariffa and Lippia citriodora. |
| Bourqui 2020 | No inactive substance (5‐arm study: Roselle (tablet or brew), Combretum micranthum ‐ kinkeliba (tablet or brew), captopril) |
| Bunbupha 2018 | Animal study; rat |
| Elkafrawy 2020 | No inactive substance (3‐arm study: Roselle (low or high dose), captopril) |
| Hadi 2017 | Study aimed to investigate the effect on oxidative stress reduction and serum muscle damage factors in soccer players. No information on SBP and DBP, as they were not the outcomes of interest for this study. |
| Hajifaraji 2018 | Study aimed to investigate the effect on polygenic dyslipidaemia. No information for SBP and DBP, as they were not the outcomes of interest for this study. |
| Herranz‐Lopez 2019 | Intervention was a combination of polyphenolic extracts from Lippia citriodora L. and Hibiscus sabdariffa L.; participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Intarit 2012 | Control group received simvastatin. |
| IRCT201310089662N8 | Trial registration information of Asgary 2016 |
| IRCT2014041510826N8 | Trial registration information of Najarzade 2016 |
| Jalalyazdi 2019 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Kafeshani 2017 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Marhuenda 2021 | Intervention was a combination of Hibiscus sabdariffa and Lippia citriodora. |
| McKay 2008 | Trial registration information of McKay 2010 |
| McKay 2010 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Najarzade 2016 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline; Persian language, Google translation. |
| NCT01682291 | Trial registration information of Pelliccia 2017 |
| NCT02637570 | Trial registration information of Hadi 2017 |
| NCT03507023 | Trial registration information of Herranz‐Lopez 2019 |
| NCT03804801 | Inclusion criteria: SBP 120 to 139 mmHg, DBP 80 to 89 mmHg (information presented in ClinicalTrials.gov) |
| Nwachukwu 2015 | Comparison group was black currant infusion. |
| Nwachukwu 2015a | Comparison group was black currant infusion. |
| Nwachukwu 2016 | Comparison group was black currant infusion. |
| Nwachukwu 2017 | Comparison group was black currant infusion. |
| Pelliccia 2017 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Salman 2017 | Participants had SBP < 140 mmHg and DBP < 90 mmHg at baseline. |
| Tangkomsaengtong 2020 | Not a randomised controlled trial, no control group |
| Thanasatirakul 2015 | Comparison group was Caesalpinia sappan L. and Garcinia cowa Roxb. (Evidence of potential pharmacological properties of Caesalpinia sappan L: improve blood circulation, treatment of blood pressure, Nirmal 2015) |
| Wiruttanapornkul 2012 | Intervention was Roselle and Stevia. |
| Xu 2021 | Intervention was anthocyanin purified from bilberry (Vaccinium myrtillus) and black currant (Ribes nigrum). |
| Yusni 2020 | Comparison group was antihypertensive and antidiabetic drugs. |
DBP: diastolic blood pressure SBP: systolic blood pressure
Characteristics of studies awaiting classification [ordered by study ID]
Al‐Anbaki 2021.
| Methods | Cluster‐randomised controlled trial (a multicentric comparative pilot intervention) |
| Participants | All participants with uncontrolled hypertension registered in the participating health centres were encouraged to join the pilot intervention if the following criteria were met. Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: Bags of HS calyxes (10 g per day) and health awareness lecture on hypertension management (recommending reduction of sodium intake, physical activity, etc.) Control: Health awareness lecture on hypertension management (recommending reduction of sodium intake, physical activity, etc.) |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes | Location: 4 health centres located in 3 regions in the centre and north of Iraq Funding: Antenna Foundation funded this programme. |
Izadi 2021.
| Methods | RCT |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: 1 capsule of sour tea powder (450 mg capsule containing at least 250 mg of anthocyanin) for 8 weeks Control: 1 placebo capsule (pure microcrystalline cellulose) for 8 weeks |
| Outcomes |
Primary outcomes:
Secondary outcomes: Anthropometric indices (means of body weight, BMI, and waist circumferences) |
| Notes | Location: Khorshid Hospital in Isfahan, Iran Funding: Isfahan University of Medical Sciences Iranian Registry of Clinical Trials: IRCT20140208016529N3 |
NCT04339283.
| Methods | RCT (open‐label) |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: Standardised Hibiscus sabdariffa tea: 300 mL of freshly prepared standardised Hibiscus sabdariffa tea (containing 102.49 mg/L of total monomeric anthocyanin) is administered daily to participants for 28 days. Control: No intervention: water 300 mL of distilled water is administered to participants daily for 28 days. |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Notes |
Source: clinicaltrials.gov/ct2/show/NCT04339283 Locations: Nigeria, Department of Clinical Pharmacy Laboratory, University of Ibadan Ibadan, Oyo, Nigeria, 200284 Sponsors and Collaborators: University of Ibadan Principal Investigator: Segun J Showande, PhD, University of Ibadan ClinicalTrials.gov Identifier: NCT04339283 Other Study ID Numbers: Hibiscus‐tea Study First Posted: 9 April 2020 Last Update Posted: 9 April 2020 Last Verified: April 2020 Trial authors contacted for additional data: Yes (Date: 2 October 2020) Reply received: 2 October 2020. This study is currently under consideration for publication. |
BMI: body mass index DBP: diastolic blood pressure HS: Hibiscus sabdariffa SBP: systolic blood pressure
Differences between protocol and review
For this update, we made the following changes to the protocol.
The third review author, Fonthip Buttramee (FB), has been added.
The affiliation of the first and second review authors has changed.
The review contact person and the order of review authors have changed.
The Background section has been revised.
-
The Methods have been revised to align with the Cochrane Handbook for Systematic Reviews of Interventions Versions 5 and 6, as follows.
We made minor changes to the measures of treatment effect and data synthesis. For example, we changed the term "weighted mean difference (WMD)" to "mean difference (MD)", and defined substantial heterogeneity and considerable heterogeneity as an I2 greater than 50% and 80%, respectively.
We added the GRADE assessment of the certainty of the evidence and included a summary of findings table.
The phrasing of the secondary outcomes has been revised, as follows: "change of pulse pressure" to "change in pulse pressure"; and "change of heart rate" to "change in heart rate".
Contributions of authors
P Pattanittum (PP): performed previous work that was the foundation of the current review (provide a methodological perspective, screened search results in the previous version of the review). For this update, PP revised the Background, Methods, undertook the tasks in Searching other resources, obtained unpublished studies, organised all search results, screened search results, extracted data, assessed risk of bias and certainty of evidence, wrote to the authors of the original study for additional information, entered data into Review Manager 5, analysed data, interpreted data, provided a methodological perspective, took the lead in writing the review.
C Ngamjarus (CN): performed previous work that was the foundation of the current review (developed the protocol, performed searches, and screened search results in the previous version of the review). For this update, CN screened search results obtained from Searching other resources, extracted data, assessed risk of bias and certainty of evidence, re‐checked the data in Review Manager 5, provided both general advice on the review and a methodological perspective.
F Buttramee (FB): for this update, FB screened search results obtained from Electronic searches.
C Somboonporn (CS): performed previous work that was the foundation of the current review (provided a clinical perspective on the previous version of the review). For this update, CS provided both general advice on the review and a clinical perspective.
All review authors provided critical feedback and helped shape the review.
Sources of support
Internal sources
-
Department of Epidemiology and Biostatistics, Faculty of Public Health, Khon Kaen University, Thailand
salary support
-
Department of Radiology (Division of Nuclear Medicine), Faculty of Medicine, Khon Kaen University, Thailand
salary support
External sources
-
No sources of support provided, Other
No sources of support provided
Declarations of interest
P Pattanittum: none known.
C Ngamjarus: none known.
F Buttramee: none known.
C Somboonporn: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Sarbini 2019 {published data only}
- Sarbini DWI, Huriyati EMY, Sadewa H, Wahyuningsih MSH. The effect of rosella (Hibiscus sabdariffa linn) on insulin resistance in patients with type 2 diabetes mellitus: a randomized clinical trial. International Journal of Pharmaceutical Research 2019;11:547-57. [Google Scholar]
References to studies excluded from this review
Abubakar 2019 {published data only}
- Abubakar SM, Ukeyima MT, Spencer JPE, Lovegrove JA. Acute effects of hibiscus sabdariffa calyces on postprandial blood pressure, vascular function, blood lipids, biomarkers of insulin resistance and inflammation in humans. Nutrients 2019;11(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aroonsiriwatana 2010 {published data only}
- Aroonsiriwatana S. The Effects of Roselle (Hibiscus Sabdariffa) and Stevia (Stevia Rebaudiana) on Hypertension in Patients with Type 2 Diabetes at King Chulalongkorn Memorial Hospital [Masters thesis]. Bangkok (TH): Chulalongkorn University, 2010. [Google Scholar]
Asgary 2016 {published data only}
- Asgary S, Soltani R, Zolghadr M, Keshvari M, Sarrafzadegan N. Evaluation of the effects of roselle (Hibiscus sabdariffa L.) on oxidative stress and serum levels of lipids, insulin and hs-CRP in adult patients with metabolic syndrome: a double-blind placebo-controlled clinical trial. Journal of Complementary & Integrative Medicine 2016;13(2):175-80. [DOI] [PubMed] [Google Scholar]
Boix‐Castejon 2017 {published data only}
- Boix-Castejon M, Herranz-Lopez M, Caturla N, Roche E, Barrajon-Catalan E, Micol V. Hibiscus and lemon verbena polyphenols: assessment for weight management in overweight volunteers. Appetite control and satiety. Free Radical Biology and Medicine 2017;108(Suppl 1):S96. [Google Scholar]
Boix‐Castejon 2018 {published data only}
- Boix-Castejon M, Herranz-Lopez M, Perez Gago A, Olivares-Vicente M, Caturla N, Roche E, et al. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: a randomized controlled trial. Food & Function 2018;9(6):3173-84. [DOI] [PubMed] [Google Scholar]
Boix‐Castejon 2021 {published data only}
- Boix-Castejon M, Herranz-Lopez M, Olivares-Vicente M, Campoy P, Caturla N, Jones J, et al. Effect of metabolaid (R) on pre- and stage 1 hypertensive patients: a randomized controlled trial. Journal of Functional Foods 2021;84:1-8. [Google Scholar]
Bourqui 2020 {published data only}
- Bourqui A, Niang EAB, Graz B, Diop EA, Dahaba M, Thiaw I, et al. Hypertension treatment with Combretum micranthum or Hibiscus sabdariffa, as decoction or tablet: a randomized clinical trial. Journal of Human Hypertension 2020;34:1-9. [DOI] [PubMed] [Google Scholar]
Bunbupha 2018 {published data only}
- Bunbupha S, Prachaney P, Berkban T, Kukongviriyapan U, Pakdeechote P. Hibiscus sabdariffa L. extract alleviates vascular endothelial dysfunction by enhancing nitric oxide bioavailability in high-fructose diet induced insulin resistance rats. Srinagarind Medical Journal 2018;33(4):294-300. [Google Scholar]
Elkafrawy 2020 {published data only}
- Elkafrawy N, Younes K, Naguib A, Badr H, Kamal Zewain S, Kamel M, et al. Antihypertensive efficacy and safety of a standardized herbal medicinal product of Hibiscus sabdariffa and Olea europaea extracts (NW Roselle): a phase-II, randomized, double-blind, captopril-controlled clinical trial. Phytotherapy Research: PTR 2020;34(12):3379-87. [DOI] [PubMed] [Google Scholar]
Hadi 2017 {published data only}
- Hadi A, Pourmasoumi M, Kafeshani M, Karimian J, Maracy MR, Entezari MH. The effect of green tea and sour tea (Hibiscus sabdariffa L.) supplementation on oxidative stress and muscle damage in athletes. Journal of Dietary Supplements 2017;14(3):346-57. [DOI] [PubMed] [Google Scholar]
Hajifaraji 2018 {published data only}
- Hajifaraji M, Matlabi M, Ahmadzadeh-Sani F, Mehrabi Y, Rezaee MS, Hajimehdipour H, et al. Effects of aqueous extracts of dried calyx of sour tea (Hibiscus sabdariffa L.) on polygenic dyslipidemia: a randomized clinical trial. Avicenna Journal of Phytomedicine 2018;8(1):24-32. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Herranz‐Lopez 2019 {published data only}
- Herranz-Lopez M, Olivares-Vicente M, Boix-Castejon M, Caturla N, Roche E, Micol V. Differential effects of a combination of Hibiscus sabdariffa and Lippia citriodora polyphenols in overweight/obese subjects: a randomized controlled trial. Scientific Reports 2019;9(1):2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Intarit 2012 {published data only}
- Intarit P, Kuman Pava K, Itharat A, Chinsoi P. Comparative study on the efficacy and side effects of hibiscus sabdariffa linn. extract versus simvastatin in reducing blood lipids levels on hyperlipidemias patient (clinical trial phase II). Thammasat Medical Journal 2012;12(3):506-17. [Google Scholar]
IRCT201310089662N8 {published data only}
- IRCT201310089662N8. Evaluation of the effects of sour tea on lipid profile, insulin resistance, oxidative stress, and inflammatory markers in adult patients with metabolic syndrome. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201310089662N8 (first received 4 December 2013).
IRCT2014041510826N8 {published data only}
- IRCT2014041510826N8. Effect of Hibiscus subdariffa on albuminuria, total antioxidant capacity, hypertension and hs-CRP in patient with diabetic nephropathy. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014041510826N8 (first received 4 December 2013).
Jalalyazdi 2019 {published data only}
- Jalalyazdi M, Ramezani J, Izadi-Moud A, Madani-Sani F, Shahlaei S, Ghiasi SS. Effect of hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. Journal of Advanced Pharmaceutical Technology & Research 2019;10(3):107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kafeshani 2017 {published data only}
- Kafeshani M, Entezari MH, Karimian J, Pourmasoumi M, Maracy MR, Amini MR, et al. A comparative study of the effect of green tea and sour tea on blood pressure and lipid profile in healthy adult men. Arya Atherosclerosis 2017;13(3):109-16. [PMC free article] [PubMed] [Google Scholar]
Marhuenda 2021 {published data only}
- Marhuenda J, Pérez-Piñero S, Arcusa R, Victoria-Montesinos D, Cánovas F, Sánchez-Macarro M, et al. A randomized, double-blind, placebo-controlled trial to determine the effectiveness of a polyphenolic extract (Hibiscus sabdariffa and Lippia citriodora) for reducing blood pressure in prehypertensive and type 1 hypertensive subjects. Molecules (Basel, Switzerland) 2021;26(6):1-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2008 {published data only}
McKay 2010 {published data only}
- McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. Journal of Nutrition 2010;140(2):298-303. [PMID: ] [DOI] [PubMed] [Google Scholar]
Najarzade 2016 {published data only}
- Najarzade A, Hemayati R, Reza JZ, Fallahzade H, Mozaffari-Khosravi H, Taghizadeh M, et al. Effects of hibiscus subdariffa on albuminuria and hypertension in patients with diabetic nephropathy. Toloo-e-behdasht 2016;14(6):Pe107-18. [Google Scholar]
NCT01682291 {published data only}
- NCT01682291. Prehypertension and dietary supplements - The PYRAMIDS study [Pre-hypertension treatment with a combination of dietary supplements and life-style modifications]. clinicaltrials.gov/show/NCT01682291 (first received 10 September 2012).
NCT02637570 {published data only}
- NCT02637570. Effect of hibiscus tea and green tea supplements on athletic performance, blood pressure, muscle damage indices and oxidative stress. clinicaltrials.gov/show/NCT02637570 (first received 22 December 2015).
NCT03507023 {published data only}
- NCT03507023. Effect of hibiscus and lippia extract on blood pressure [Effect of the combination of polyphenols derived from the hibiscus sabdariffa and lippia citriodora on slightly hypertensive or type 1 hypertensive volunteers]. clinicaltrials.gov/show/NCT03507023 (first received 24 April 2018).
NCT03804801 {published data only}
- NCT03804801. Effect of hibiscus sabdariffa on blood pressure in a university population [Effect of hibiscus sabdariffa on blood pressure in a university population]. clinicaltrials.gov/show/NCT03804801 (first received 15 January 2019).
Nwachukwu 2015 {published data only}
- Nwachukwu DC, Aneke EI, Obika LF, Nwachukwu NZ. Effects of aqueous extract of Hibiscus sabdariffa on the renin-angiotensin-aldosterone system of Nigerians with mild to moderate essential hypertension: a comparative study with lisinopril. Indian Journal of Pharmacology 2015;47(5):540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nwachukwu 2015a {published data only}
- Nwachukwu DC, Aneke E, Nwachukwu NZ, Obika LF, Nwagha UI, Eze AA. Effect of Hibiscus sabdariffa on blood pressure and electrolyte profile of mild to moderate hypertensive Nigerians: a comparative study with hydrochlorothiazide. Nigerian Journal of Clinical Practice 2015;18(6):762-70. [DOI] [PubMed] [Google Scholar]
Nwachukwu 2016 {published data only}
- Nwachukwu DC, Aneke EI, Nwachukwu NZ, Azubike N, Obika LFO. Thirst perception in mild to moderate hypertensive Nigerians treated with aqueous extract of hibiscus sabdariffa l. African Journal of Pharmacy and Pharmacology 2016;10(18):403-10. [Google Scholar]
Nwachukwu 2017 {published data only}
- Nwachukwu DC, Aneke EI, Nwachukwu NZ, Azubike N, Obika LF. Does consumption of an aqueous extract of hibiscus sabdariffa affect renal function in subjects with mild to moderate hypertension? Journal of Physiological Sciences: JPS 2017;67(1):227-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pelliccia 2017 {published data only}
- Pelliccia F, Pasceri V, Marazzi G, Arrivi A, Cacciotti L, Pannarale G, et al. Randomised, double-blind, placebo-controlled, assessment of the efficacy and safety of dietary supplements in prehypertension. Journal of Human Hypertension 2017;31(10):647-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Salman 2017 {published data only}
- Salman A, Abdullah I, Saib I, Abdulaziz B, Yahya K, Awad M, et al. Acute effect of drinking cold hibiscus beverage on blood pressure in adult females: a randomized controlled trial. International Journal of Academic Scientific Research 2017;5(1):26-34. [Google Scholar]
Tangkomsaengtong 2020 {published data only}
- Tangkomsaengtong C, Arunakul I-O, Itharat A. Preliminary study on effect and safety of roselle extract tablets for treating patient with stage 1 hypertension. Thai Journal of Pharmacy Practice 2020;12(3):913-22. [Google Scholar]
Thanasatirakul 2015 {published data only}
- Thanasatirakul P. The effects of roselle flower tea (hibiscus sabdariffa extract) on home blood pressure in patients with mild hypertension: a randomized double blinded placebo controlled trial [Masters thesis]. Bangkok (TH): Chulalongkorn University, 2015. [Google Scholar]
Wiruttanapornkul 2012 {published data only}
- Wiruttanapornkul K. The effect of roselle and stevia on global cardiovascular risk factor in type 2 diabetes mellitus and hypertensive patients at King Chulalongkorn Memorial Hospital. Master of Science Program in Medicine (Master's thesis) 2012.
Xu 2021 {published data only}
- Xu Z, Xie J, Zhang H, Pang J, Li Q, Wang X, et al. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia - a randomized controlled trial. European Journal of Clinical Nutrition 2021;75(2):345-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yusni 2020 {published data only}
- Yusni Y, Meutia F. Action mechanism of rosella (Hibiscus sabdariffa L.) used to treat metabolic syndrome in elderly women. Evidence-Based Complementary and Alternative Medicine: eCAM 2020;2020:5351318. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Al‐Anbaki 2021 {published data only}
- Al-Anbaki M, Cavin AL, Nogueira RC, Taslimi J, Ali H, Najem M, et al. Hibiscus sabdariffa, a treatment for uncontrolled hypertension. Pilot comparative intervention. Plants (Basel, Switzerland) 2021;10(5):1-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Izadi 2021 {published data only}
- Izadi F, Farrokhzad A, Tamizifar B, Tarrahi MJ, Entezari MH. Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled clinical trial. Phytotherapy Research: PTR 2021;35(1):477-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT04339283 {published data only}
- NCT04339283. Effect of standardized hibiscus sabdariffa tea in seemingly healthy human volunteers [Standardized hibiscus sabdariffa linn tea, potential nutraceutical candidate for the prevention of hypertension, diabetes, and hypercholesterolemia - a pilot study]. clinicaltrials.gov/show/NCT04339283 (first received 9 April 2020).
Additional references
Abegaz 2017
- Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine 2017;96(4):e5641. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adeola 2019
- Adeola O, De Souza Batista CM, Fungwe TV. The effectiveness of hibiscus sabdariffa for improving metabolic syndrome: a systematic review. EC Nutrition 2019;10:887-92. [Google Scholar]
Ali 2005
- Ali BH, Al Wabel N, Blunden G. Phytochemical, pharmacological and toxicological aspects of hibiscus sabdariffa l.: a review. Phytotherapy Research 2005;19(5):369-75. [DOI: 10.1002/ptr.1628] [DOI] [PubMed] [Google Scholar]
Amin 2020
- Amin AR, Kassab RB, Moneim AEA, Amin HK. Comparison among garlic, berberine, resveratrol, hibiscus sabdariffa, genus zizyphus, hesperidin, red beetroot, catha edulis, portulaca oleracea, and mulberry leaves in the treatment of hypertension and type 2 DM: a comprehensive review. Natural Product Communications 2020;15(4):1-24. [Google Scholar]
Chen 2018
- Chen YJ, Li LJ, Tang WL, Song JY, Qiu R, Li Q, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 11. Art. No: CD008170. [DOI: 10.1002/14651858.CD008170.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 8 October 2021. Melbourne, Australia: Veritas Health Innovation, August 2021. Available at covidence.org.
Da‐Costa‐Rocha 2014
- Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L. – a phytochemical and pharmacological review. Food Chemistry 2014;165:424-43. [DOI: 10.1016/j.foodchem.2014.05.002] [DOI] [PubMed] [Google Scholar]
Ernst 2005
- Ernst E. Complementary/alternative medicine for hypertension: a mini-review. Wiener Medizinische Wochenschrift 2005;155(17-18):386-91. [DOI: 10.1007/s10354-005-0205-1] [DOI] [PubMed] [Google Scholar]
Garjon 2020
- Garjon J, Saiz LC, Azparren A, Gaminde I, Ariz MJ, Erviti J. First-line combination therapy versus first-line monotherapy for primary hypertension. Cochrane Database of Systematic Reviews 2020, Issue 2. Art. No: CD010316. [DOI: 10.1002/14651858.CD010316.pub3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2018
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392(10159):1923-94. [DOI] [PMC free article] [PubMed]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 15 October 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), May 2018. Available at gradepro.org.
Haji 1999
- Haji Faraji M, Haji Tarkhani A. The effect of sour tea (Hibiscus sabdariffa) on essential hypertension. Journal of Ethnopharmacology 1999;65(3):231-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Herrera‐Arellano 2004
- Herrera-Arellano A, Flores-Romero S, Chavez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardized extract from hibiscus sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine 2004;11(5):375-82. [0944-7113] [DOI] [PubMed] [Google Scholar]
Herrera‐Arellano 2007
- Herrera-Arellano A, Miranda-Sanchez J, Avila-Castro P, Herrera-Alvarez S, Jiminez-Ferrer JE, Zamilpa A, et al. Clinical effects produced by a standardized herbal medicinal product of Hibiscus sabdariffa on patients with hypertension. A randomized, double-blind, lisinopril-controlled clinical trial. Planta Medica 2007;73(1):6-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021a
- Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021b
- Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hopkins 2013
- Hopkins AL, Lamm MG, Funk JL, Ritenbaugh C. Hibiscus sabdariffa L. in the treatment of hypertension and hyperlipidemia: a comprehensive review of animal and human studies. Fitoterapia 2013;85:84-94. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Igwe 2019
- Igwe EO, Charlton KE, Probst YC. Usual dietary anthocyanin intake, sources and their association with blood pressure in a representative sample of Australian adults. Journal of Human Nutrition and Dietetics: The Official Journal of the British Dietetic Association 2019;32(5):578-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jabeur 2017
- Jabeur I, Pereira E, Barros L, Calhelha RC, Sokovic M, Oliveira MBPP, et al. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Research International (Ottawa, Ont.) 2017;100(Pt 1):717-23. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laurent 2017
- Laurent S. Antihypertensive drugs. Pharmacological Research 2017;124:116-25. [PMID: ] [DOI] [PubMed] [Google Scholar]
Li 2021
- Li TJ, Higgins JPT, Deeks JJ. Chapter 5: Collecting data. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Mendeley Desktop [Computer program]
- Mendeley Desktop. Version 1.19.8. Glyph & Cog, LLC, 2020.
Mojiminiyi 2007
- Mojiminiyi FBO, Dikko M, Muhammad BY, Ojobor PD, Ajagbonna OP, Okolo RU, et al. Antihypertensive effect of an aqueous extract of the calyx of Hibiscus Sabdariffa. Fitoterapia 2007;78(4):292-7. [0367-326X] [DOI] [PubMed] [Google Scholar]
Mozaffari‐Khosravi 2009
- Mozaffari-Khosravi H, Jalali-Khanabadi B-A, Afkhami-Ardekani M, Fatehi F, Noori-Shadkam M. The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. Journal of Human Hypertension 2009;23(1):48-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Najafpour 2020
- Najafpour Boushehri S, Karimbeiki R, Ghasempour S, Ghalishourani SS, Pourmasoumi M, Hadi A, et al. The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: a systematic review and meta-analysis of randomized clinical trials. Phytotherapy Research: PTR 2020;34(2):329-39. [PMID: ] [DOI] [PubMed] [Google Scholar]
NCD‐RisC 2017
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389(10064):37-55. [DOI] [PMC free article] [PubMed]
Ngamjarus 2009
- Ngamjarus C, Pattanittum P, Somboonporn C. Roselle for hypertension in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007894. [DOI: 10.1002/14651858.CD007894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngamjarus 2010
- Ngamjarus C, Pattanittum P, Somboonporn C. Roselle for hypertension in adults. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD007894. [DOI: 10.1002/14651858.CD007894.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management. NICE Guideline (NG136). London (UK): National Institute for Health and Care Excellence, 2019. [Google Scholar]
Nielsen 2017
- Nielsen JA, Shrestha AD, Neupane D, Kallestrup P. Non-adherence to anti-hypertensive medication in low- and middle-income countries: a systematic review and meta-analysis of 92443 subjects. Journal of Human Hypertension 2017;31(1):14-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Nirmal 2015
- Nirmal NP, Rajput MS, Prasad RG, Ahmad M. Brazilin from Caesalpinia sappan heartwood and its pharmacological activities: a review. Asian Pacific Journal of Tropical Medicine 2015;8(6):421-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Odigie 2003
- Odigie IP, Ettarh RR, Adigun SA. Chronic administration of aqueous extract of Hibiscus Sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2K-1C hypertensive rats. Journal of Ethnopharmacology 2003;86(2-3):181-5. [0378-8741] [DOI] [PubMed] [Google Scholar]
Onyenekwe 1999
- Onyenekwe PC, Ajani EO, Ameh DA, Gamaniel KS. Antihypertensive effect of roselle (hibiscus sabdariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in wistar rats. Cell Biochemistry and Function 1999;17(3):199-206. [0263-6484] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Riaz 2018
- Riaz G, Chopra R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomedicine & Pharmacotherapy/Biomedecine & Pharmacotherapie 2018;102:575-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Serban 2015
- Serban C, Sahebkar A, Ursoniu S, Andrica F, Banach M. Effect of sour tea (hibiscus sabdariffa l.) on arterial hypertension: a systematic review and meta-analysis of randomized controlled trials. Journal of Hypertension 2015;33(6):1119-27. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stata 2017 [Computer program]
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Thavorn 2006
- Thavorn K, Chaiyakunapruk N, Thamalikitkul V. A systematic review of clinical efficacy of hibiscus sabdariffa. Thai Pharmaceutical and Health Science Journal 2006;1:219-25. [Google Scholar]
Thiagarajah 2019
- Thiagarajah K, Ong MK, Teh LK, Lye HS. Plants infused water as preferred healthy drinks. In: Grumezescu AM, Holban AM, editors(s). Bottled and Packaged Water. Vol. 4: The Science of Beverages. Woodhead Publishing, 2019:367-402. [Google Scholar]
Unger 2020
- Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Journal of Hypertension 2020;38(6):982-1004. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vendrame 2019
- Vendrame S, Klimis-Zacas D. Potential factors influencing the effects of anthocyanins on blood pressure regulation in humans: a review. Nutrients 2019;11(6):1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wahabi 2010
- Wahabi HA, Alansary LA, Al-Sabban AH, Glasziuo P. The effectiveness of Hibiscus sabdariffa in the treatment of hypertension: a systematic review. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 2010;17(2):83-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walton 2016
- Walton RJ, Whitten DL, Hawrelak JA. The efficacy of hibiscus sabdariffa (rosella) in essential hypertension: a systematic review of clinical trials. Australian Journal of Herbal Medicine 2016;28(2):48-51. [Google Scholar]
WHO 2003
- World Health Organization. The magnitude of the problem of poor adherence. In: Sabate E, editors(s). Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization, 2003:7-10. [Google Scholar]
WHO 2016
- World Health Organization. Global NCD target: reduce high blood pressure. Overview 2016;31(4):190-215.
WHO 2020
- World Health Organization. Improving Hypertension Control in 3 Million People: Country Experiences of Programme Development and Implementation. Geneva: World Health Organization, 2020. [Google Scholar]
Williams 2018
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Journal of Hypertension 2018;36(10):1953-2041. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wright 2007
Wright 2018
- Wright JM, Musini VM, Gill R. First-line drugs for hypertension. Cochrane Database of Systematic Reviews 2018, Issue 4. Art. No: CD001841. [DOI: 10.1002/14651858.CD001841.pub3] [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2018
- Wu HY, Yang KM, Chiang PY. Roselle anthocyanins: antioxidant properties and stability to heat and pH. Molecules (Basel, Switzerland) 2018;23(6):3-13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2020
- Zhang B, Yue R, Wang Y, Wang L, Chin J, Huang X, et al. Effect of hibiscus sabdariffa (roselle) supplementation in regulating blood lipids among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis. Phytotherapy Research: PTR 2020;34(5):1083-95. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ngamjarus 2009b
- Ngamjarus C, Pattanittum P, Somboonporn C. Roselle for hypertension in adults. Cochrane Database of Systematic Reviews 2009, Issue 7. Art. No: CD007894. [DOI: 10.1002/14651858.CD007894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngamjarus 2010b
- Ngamjarus C, Pattanittum P, Somboonporn C. Roselle for hypertension in adults. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD007894. [DOI: 10.1002/14651858.CD007894.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


