Abstract
Background:
Prostate cancer (PC) is the second-most common cause of cancer.68Ga-prostate-specific membrane antigen (PSMA)-11 positron-emission tomography/computed tomography (PET/CT) scan help in accurate staging of PC owing to its high PSMA avidity and specificity. The aim of this prospective observational study was to determine the incremental value of Ga-68 PSMA-11 PET/CT over multiparametric magnetic resonance imaging (mpMRI) in the locoregional staging of intermediate- and high-risk PC using histopathology from radical prostatectomy specimens as a gold standard.
Materials and Methods:
This was a prospective study, including 35 patients with biopsy-proven prostate carcinoma. All the patients underwent whole-body Ga-68 PSMA-11 PET/CT scans along with mpMRI including a dedicated pelvic imaging protocol within a time window of ± 10 days. The reference standard was based on histopathological results, postprostatectomy.
Results:
All 35 patients showed Ga-68 PSMA-11-avid disease, of which 29 underwent radical prostatectomy, one underwent radiation therapy, and five did not undergo surgery owing to metastases. A total of 52 PC lesions were detected in 29 patients on histopathology. Of 52 lesions, 29 lesions were identified in prostate parenchyma and 23 were extraprostatic lesions on histopathology. Ga-68 PSMA-11 PET/CT detected a total of 45 lesions, of which 29 lesions were located within the prostate parenchyma and 16 were representative of extraprostatic lesions. mpMRI detected a total of 36 lesions, of which 29 lesions were located within the prostate parenchyma and seven were representative of extraprostatic lesions. The overall sensitivity of 68Ga-PSMA PET/CT and mpMRI in the detection of lesions was 86.2% and 68.6%, respectively. However, the overall specificity was 94.7% and 89.1% for 68Ga-PSMA and mpMRI, respectively.
Conclusion:
Ga-68 PSMA-11 PET/CT provided superior locoregional preoperative staging of PC as compared to mpMRI in intermediate- and high-risk PC patients.
Keywords: Ga-68 prostate-specific membrane antigen-11 positron-emission tomography/computed tomography, prostate cancer, risk stratification
Introduction
Prostate cancer (PC) is a global disease and is the second-most common cause of cancer. It is also one among the leading causes of cancer death in men of all races. PC burden is growing worldwide with 1.7 million new cases and 499,000 new deaths by 2030.[1] Although PC incidence rates are low in Asian and North African countries that is 1 to 9/100,000 people, developing countries such as India show an increasing trend.[2]
Most crucial part of patient management in PC is precise local staging of disease.[3] In addition, treatment choices (such as radical prostatectomy, radiotherapy or palliative systemic treatment and the extent of pelvic lymph node dissection during surgery or planning of the radiation field, and also the consideration of multimodal therapy) are also greatly influenced by staging. Currently, in a recently diagnosed PC, bone scan, computed tomography (CT), and magnetic resonance imaging (MRI) are the most commonly used modalities in staging.[4]
Favorable results were obtained in localizing PC by multiparametric MRI (mpMRI) which combines information obtained with T2-weighted, diffusion-weighted imaging (DWI), and dynamic contrast-enhanced sequences.[5] However, mpMRI is also associated with some pitfalls such as lack of standardization and limited tumor detection rates in certain areas such as transitional zones.[6]
With the advancement in molecular technology and imaging, molecular imaging with positron-emission tomography (PET) is evolving as a favorable diagnostic approach.[7] Prostate-specific membrane antigen (PSMA) is a transmembrane protein which is expressed mainly in PC cells with low or no expression in the normal prostate gland.[8] In the recent era, Ga-68 labeled PSMA ligands have been shown to have high specificity and sensitivity for the detection of recurrent PC and metastatic disease.[9]
The purpose of our study was to evaluate the incremental value of Ga-68 PSMA-11 PET/CT over mpMRI in locoregional staging of intermediate and high-risk PC, using histopathological correlation as the reference standard.
Materials and Methods
Study population
This prospective, non-randomized study was conducted in Fortis Memorial Research Institute, Gurgaon in the Department of Nuclear Medicine between October 2016and October 2018. A total of 35 patients with biopsy-proven prostate carcinoma with Gleason score (GS) ≥6, who were planned for radical prostatectomy underwent Ga-68 PSMA-11 PET/CT in the Department of Nuclear Medicine and PET/CT for staging and initial treatment planning. Patients with low-risk PC with GS <6, Digital rectal examination (DRE)/Magnetic Resonance imaging (MRI) showing bladder neck or sphincter involvement, MRI/bone scan/imaging showing evidence of gross metastatic disease, patients who had undergone transurethral resection of the prostate or prostatic surgery and those who had received prior hormonal therapy or chemotherapy for PC were not included in the study. The institutional review board approved this study, and all patients provided signed informed consent before enrollment.
Radiopharmaceutical preparation
Good Manufacturing Practice grade Ga-68 PSMA-11 was prepared in-house as per the following protocol. To the Ga-68 eluted from Ge-68/Ga-68 generator with 0.05 HCL, 10 μg PSMA-11 (ABX, Radeberg, Germany) was added in 0.25M sodium acetate, and then the reaction mixture was heated for 10 min at 95°C. For purification, C-18 cartridge was preconditioned with 5 ml of 70% ethanol, purged with air and water. The labeling mixture was passed through the cartridge and the final product was passed through 0.22 μm filter before injecting into the patients. Samples with radiopharmaceutical purity of >95% were used for patient administration.
Image acquisition
PET/CT was performed on an integrated Phillips True flight select time of flight PET scanner with 40 slices/s multidetector computerized tomography. Intravenous contrast was administered. 1 h after intravenous injection of a mean activity of 1.76 MBq/kg (2–5 mCi) Ga-68 PSMA-11. Patients were asked to empty the bladder before the scanning procedure. A CT scanning (120 kV, 250 mAs) was performed first followed with the PET (acquisition time was 3 min/bed position (axial field of view 21.8 cm, matrix size 256 × 256). PET data were corrected for attenuation and reconstructed using an iterative ordered-subsets expectation-maximization algorithm (three iterations, 21 subsets, and 4 mm gaussian filter). A delayed sequence of the pelvis was also acquired after 1 h following 20 mg of intravenous injection of furosemide. All mpMRIs sequences (T2-weighted, T1-weighted, 3D VISTA SPIR, BTFE, DWI, and m-Dixon) were performed by outpatient radiology on Philips Ingenia 3T MR scanner.
Image analysis
mpMRI scans and whole-body Ga-68 PSMA-11 PET/CT scans were analyzed by two experienced radiologists and two experienced nuclear medicine physicians independently using interactive computer display and fusion software (Philips). For Ga-68 PSMA-11 PET/CT, any focal Ga-68-PSMA uptake in sites other than physiological sites of uptake and higher than background was considered as a lesion. Corresponding CT images were used for the localization of the lesion. Uptake sites were interpreted on the basis of shape, location, and intensity. Each lesion was assessed on transverse, coronal, sagittal section, and its Ga-68-PSMA uptake was expressed as the maximal standardized uptake value (SUV) corrected for the administered dose and patient body weight. Tumor sizes and SUVmax for areas of radiotracer uptake in enlarged nodes and organs with extranodal disease was measured for each scan. The lesions were quantified by drawing region of interest on the hypermetabolic lesions and tumor volume was derived by thresholding method. The summation of the volume of the lesions in an individual patient was further used for the data analysis.
Statistical methods
Descriptive statistics were analyzed with SPSS version 21.0 software. Continuous variables were presented as mean ± standard deviation. P < 0.05 was considered statistically significant. Detection rates with Ga-68 PSMA-11 PET/CT and mpMRI were compared using the Chi-square test.
Results
Thirty-five patients with biopsy-proven PC and GS ≥6 underwent preoperative staging with simultaneous Ga-68 PSMA-11 PET/CT and multiparametric MRI of the prostate within a time window of 30 days before radical prostatectomy. Demographic and pathologic characteristics are listed in Table 1. The mean age of our population was 63.92 years (range = 49–75 years). Of the 35 patients, 29 underwent radical prostatectomy, and one underwent radiation therapy, five patients did not undergo surgery owing to skeletal metastases in four, and locally advanced disease in one patient.
Table 1.
Demographic characteristics of patients (n=35)
| Characteristics | Number of patients |
|---|---|
| Age (mean) | 63.92 |
| Serum PSA levels (median) | 12.4 |
| Gleason’s score | |
| 6 | 9 |
| 7-8 | 18 |
| 9-10 | 8 |
| Radical prostatectomy | 29 |
| Radical radiation therapy | 1 |
| Distant metastatic disease | 5 |
PSA: Prostate-specific antigen
Lesion-based analysis
A total of 52 lesions were evaluated in 29 patients. The lesions were grouped into two groups; Prostate parenchymal lesions and extraprostatic lesions. The extraprostatic lesions were further divided into periprostatic extension, lymph node, seminal vesicle involvement, and bladder neck invasion. On comparison with final surgical histopathology, 29 lesions were identified in prostate parenchyma and 22 were extraprostatic lesions as detailed in Table 2.
Table 2.
Frequencies of lesions detected in different modalities
| Categories | HPE | Ga-68 PSMA-11 PET/CT | MRI |
|---|---|---|---|
| Prostate parenchymal lesions | 29 | 29 | 29 |
| Extraprostatic lesions | 23 | 16 | 7 |
| Lymph nodes | 7 | 5 | 1 |
| Bladder neck | 2 | 1 | 1 |
| Seminal vesicles | 6 | 4 | 2 |
| PPE | 8 | 6 | 3 |
PPE: Periprostatatic extension, HPE: Histopathological examination, PSMA: Prostate-specific membrane antigen, PET: Positron-emission tomography, CT: Computed tomography, MRI: Magnetic resonance imaging
Ga-68 PSMA-11 PET/CT detected a total of 45 lesions, of which 29 lesions were located within the prostate parenchyma, and 16 were representative of extraprostatic lesions. Whereas mpMRI detected a total of 36 lesions, of which 29 lesions were located within the prostate parenchyma and 7 were representative of extraprostatic lesions. The overall sensitivity of Ga-68 PSMA-11 PET/CT and mpMRI in the detection of lesions was 86.2% and 68.6%, respectively. The sensitivity of Ga-68 PSMA-11 PET/CT and mpMRI for lesions within prostate parenchyma was 100% for both modalities. Whereas, the sensitivity of Ga-68 PSMA-11 PET/CT and mpMRI was 68.1% and 27.2%, respectively, for extraprostatic lesions.
Further, the delineation of extraprostatic lesions in Ga-68 PSMA-11 PET/CT and mpMRI are shown in Table 2. Of 16 extraprostatic lesions detected on Ga-68 PSMA-11, four were seminal vesicle involvement, five were representative of locoregional lymph node involvement, six were peri prostatic extension, and one was bladder neck invasion. Of seven extraprostatic lesions detected on mpMRI, two were seminal vesicle involvement, one was representative of locoregional lymph node involvement, three were peri prostatic extension, one was bladder neck invasion. The sensitivity of Ga-68 PSMA-11 PET/CT in the detection of extraprostatic lesions, i.e. lymph nodes, periprostatic lesions, lesions in the seminal vesicles, and the bladder neck was 71.4%, 75%, 60%, and 50%, respectively. The sensitivity of mpMRI in the detection of lymph nodes, periprostatic lesions, lesions in the seminal vesicles, and bladder neck is 14.2%, 37.5%, 20%, and 50%, respectively. There were no false-positive ratings with either modality for detection of lymph nodes, periprostatic lesions, and bladder neck but both modalities detected one lesion in the seminal vesicles which were falsely positive as compared to the histopathology, and hence, the specificity of each was 95.8% in case of seminal vesicle detection.
Ga-68 PSMA-11 PET/CT and mpMRI showed discordant results in a total of lesions: three periprostatic extension, two seminal vesicle lesions, and four lymph nodal lesions. As compared to histopathology, PET/CT could not detect two lymph nodal lesions, two peri-prostatic extension, and two seminal vesicle lesions but could detect four additional lesions showing nodal involvement, two seminal vesicle lesions and three in peri-prostatic region than mpMRI, but there was no lesion which was detected on mpMRI and missed on PSMA PET/CT [Figures 1 and 2]. mpMRI could not detect five peri-prostatic extensions, six lymph nodal involvement and three lesions in the seminal vesicles. Both the modalities could not detect one lesion in the bladder neck; however, both detected one false-positive lesion in the seminal vesicle.
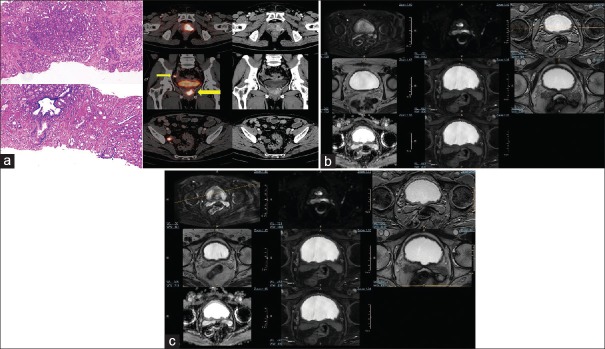
Figure 1.
(a) A 68-year-old male, serum prostate-specific antigen 76.8 ng/ml, transrectal ultrasound-guided biopsy: adenocarcinoma with Gleason score-4 + 3. Ga-68 prostate-specific membrane antigen-11 positron-emission tomography/computed tomography image shows an enhancing lesion noted in the left lobe of the prostate gland involving the posterior and anterior peripheral zones in the apical, mid glandular, and basal regions and the periurethral central zone with extension into the right lobe. There is evidence of contiguous extension of the lesion into the left seminal vesicle (thin arrow) with Ga-68 prostate-specific membrane antigen-avid metastatic right internal iliac lymph node (thick arrow). (b) Corresponding axial multiparametric magnetic resonance imaging (T2-weighted, T1-weighted, 3D VISTA SPIR, BTFE, DWI, and m-Dixon) images could not categorically delineate the left seminal vesicle. (c) Corresponding axial multiparametric magnetic resonance imaging (T2-weighted, T1-weighted, 3D VISTA SPIR, BTFE, DWI, and m-Dixon) images could not categorically delineate the right internal iliac lymph node
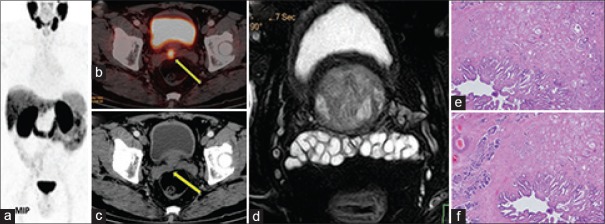
Figure 2.
A 60-year-old male, serum prostate-specific antigen 27.8 ng/ml, transrectal ultrasound-guided biopsy: adenocarcinoma with Gleason score-4 + 3. Ga-68 prostate-specific membrane antigen-11 positron-emission tomography/computed tomography image showed Ga-68 prostate-specific membrane antigen-11-avid lesion in the right posterior peripheral zone of the prostate gland (a) and contiguous right seminal vesicle involvement (b), with no enhancing lesion in computed tomography image (c). Magnetic resonance imaging did not show any lesion (d). Histopathological sections showed the involvement of seminal vesicle (e and f)
The mean SUVmax of the lesions on Ga-68 PSMA-11 PET/CT scan in patients with the prostate confined disease, lymph nodal disease, and skeletal metastases was found to be 17.23 ± 10.01, 16.07 ± 12.94, and 34.16 ± 7.57, respectively. The metastatic lesions showed more SUVmax values.
Correlation between Ga-68 prostate-specific membrane antigen uptake, serumprostate-specific antigen and Gleason score
The SUVmax was calculated in different categories of Gleason's score. It was observed that the higher the value of Gleason's score, the higher would be the SUVmax of prostatic lesions. The bivariate correlation analysis indicated a statistically significant correlation between Ga-68-PSMA uptake (SUVmax) and Gleason's score. The spearman's correlation coefficient was 0.561 (P = 0.000), which indicates moderate correlation. However, no correlation (rs= 0.101) was observed between serum prostate-specific antigen (PSA) level and Ga-68-PSMA uptake (SUVmax).
Discussion
Major consideration in the management of PC is initial diagnosis and risk stratification into low, intermediate, and high-grade groups. Patients are risk-stratified based on serum PSA level, tumor grade and clinical stage to select optimal therapy and to predict the prognosis. However, these parameters have limited accuracy in the staging of disease leading to under or over the treatment of PC. The crucial part in the management of PC is precise local staging which predicts prognosis and also provides an approach for selection of treatment.[10] In patients with high-risk disease (GS >7, PSA >20 ng/mL, clinical-stage T2c–3a), there are high chances of lymph node and bone metastases. The detection of radiologically occult lymph node metastases can significantly influence patient management. The most commonly used modalities in staging are bone scan, CT and MRI, but have their limitations. Although mpMRI is a major breakthrough, it has several limitations such as claustrophobia, confinement to the pelvic region and radiologist expertise.[9]
Ga-68 PSMA-11 PET/CT is a non-invasive diagnostic imaging modality for imaging patients with PC. Small-molecule radiotracers-targeting PSMA has led to a change in the care of patients with PC. Several studies have been published showing the superiority of PSMA-based PET over choline in patients with biochemical recurrence with low PSA levels. Subsequently, Ga-68 PSMA-11 PET/CT has emerged as an imaging modality of choice in PC staging.[11,12]
This study is a prospective study to investigate the role of the Ga-68 PSMA-11 PET/CT scan in locoregional staging of primary PC. 35 biopsy-proven intermediate- and high-risk PC patients with Gleason's score of ≥6 were recruited. All the patients underwent Ga-68 PSMA-11 PET/CT and showed evidence of Ga-68 PSMA-11-avid disease. All patients also underwent mpMRI. On the analysis, the Ga-68 PSMA-11 PET/CT scan was found to demonstrate a higher tumor detection rate in patients per lesion basis for the preoperative loco-regional staging of PC with 68Ga-PSMA-11 PET/CT than with mpMRI.
The current study emphasizes the usefulness of Ga-68 PSMA-11 PET/CT for lymph nodal staging in intermediate to high-risk PC patients, preoperatively with moderate sensitivity (71.4%) and high specificity (100%). There were 7/29 patients histologically proven to have regional lymph nodal involvement. Of seven, Ga-68 PSMA-11 PET/CT could detect lymph nodal involvement in five patients and could not detect in two patients. However, in these two patients, the size of lymph nodes was <0.5 cm, which is a limiting factor for PET resolution and also histopathology showed microscopic metastases. Van Leeuwen et al.[13] in their study on the role of Ga-68 PSMA-11 PET/CT for LN staging found sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 64%, 95%, 88%, and 82%, respectively. Maurer et al.,[14] in a retrospective review, also showed a sensitivity of 65.9% and specificity of 98.9% for Ga-68 PSMA-11 PET/CT for the detection of lymph node involvement in high-risk PC. Similar to these studies, our study exhibited a sensitivity, specificity, PPV, and NPV of 71.4%, 100%, 100%, and 91.7%, respectively, for lymph node staging in intermediate and high-risk PC. mpMRI findings showed a sensitivity, specificity, PPV and NPV of 14.2%, 100%, 100%, and 76%. mpMRI missed to detect six lymph nodal lesions and hence had a low sensitivity. With these results, it can be suggested that Ga-68 PSMA-11 PET/CT is superior in lymph node detection in the primary staging of intermediate- to high-risk PC and can replace the other current imaging modalities for lymph nodal staging.
According to the current guidelines, mpMRI because of its higher soft-tissue contrast and multi-parametric information is considered as the imaging modality of choice to detect seminal vesicle involvement and periprostatic extension.[15] In our study, four patients were found to have Ga-68 PSMA-11 uptake in seminal vesicles, of which three were proven to have seminal vesicle involvement in histopathological examination. The false-positive result in one of the patients could be due to increased bladder activity masquerading as uptake in seminal vesicles. In this study, we observed that by administering furosemide injection (dose-20 mg) intravenously 2 h before the acquisition of the delayed image, the sensitivity for detection of seminal vesicle involvement can be increased, as the bladder activity reduces in the delayed image which enhances seminal vesicle uptake. The sensitivity and specificity of Ga-68 PSMA-11 PET/CT for the identification of seminal vesicle involvement were 60.0% and 95.8%, respectively. mpMRI findings, when compared with histopathology, could identify seminal vesicle involvement in only two patients (including one false positive). The sensitivity and specificity of mpMRI in the detection of the lesion in seminal vesicles were 20% and 95.8%, respectively. Both 68Ga-PSMA-11 PET/CT and mpMRI showed concordant false-positive findings.
There were 8/29 patients in whom periprostatic extension was observed in the histopathological specimen. Ga-68 PSMA-11 PET/CT showed focal uptake in the periprostatic region in 6/29 patients and was histologically proven to have the periprostatic extension. The sensitivity and specificity for Ga-68 PSMA-11 PET/CT in periprostatic extension were 75% and 100%, respectively, whereas it was 37.5% and 100%, respectively for mpMRI. With these findings, it was observed that Ga-68 PSMA-11 PET/CT had a similar specificity to mpMRI but higher sensitivity. The reason for false-negative for periprostatic extension in MRI could be due to variability in radiologist's expertise in reporting.
In the current study, two patients had bladder neck involvement in histopathology. One of two of these patients had Ga-68 PSMA-11 uptake in the bladder neck area giving a sensitivity and specificity of 50.0% and 100%, respectively. Concordant results were found between Ga-68 PSMA-11 PET/CT and mpMRI for the detection of bladder neck involvement.
mpMRI provides a fine anatomical structural detail, therefore a higher accuracy for the assessment of the delineation between the tumor and surrounding structures. However, regional lymph nodes and extrapelvic metastases were poorly detected with mpMRI. The advantage of Ga-68 PSMA-11 PET/CT is that it can detect lymph nodes of diameter 2-3 mm and other distant metastases as whole-body scan is done due to its ability to detect uptake of radiotracer molecule. This ability to detect metastases to lymph nodes is a deciding factor in the management of patients with PC when curative local treatment is considered.[16]
In our study, we found a mean SUVmax of 17.23 ± 10.01 in the primarily prostate confined disease. However, when the prostatic lesions were associated with lymph nodal involvement and skeletal metastases, the mean SUVmax was 16.07 ± 12.94 and 34.16 ± 7.57, respectively. This increase in uptake of the radiotracer was statistically significant. The increase in radiotracer uptake could be explained by increased PSMA expression in patients with metastases. This suggests that the tumors showing high PSMA expression are more aggressive and needs aggressive treatment. This is one of the most valuable aspects of Ga-68 PSMA-11 PET/CT compared to other conventional regional imaging to correctly define the staging and to select the appropriate treatment.
The patients were categorized into two groups according to their Gleason's score, i.e. <6, 7–8 and 9–10. The mean SUVmax of these groups was 11.8 ± 3.69, 15.62 ± 8.24 and 33.8 ± 7.6, respectively. The difference in mean SUVmax of these groups was statistically significant (P < 0.05). We analyzed the correlation between SUVmax and Gleason's score and also between SUVmax and S.PSA values (Spearman's rank test). A strong positive correlation was observed with Gleason' score (<6, 7–8, and 9–10) and Ga-68 PSMA-11 uptake for prostate confined and extraprostatic disease; however, no correlation was observed with serum PSA levels and Ga-68 PSMA-11 uptake (SUVmax) of the prostate gland.
Considering the advantages and disadvantages of each modality, and the results of this study, we can emphasize that the sensitivity of Ga-68 PSMA-11 PET/CT is significantly higher than that of MRI in the detection of extraprostatic lesions and can provide superior locoregional preoperative staging in the patients. However, since the study group is quite small, to determine which of these two modalities is better or more accurate, its better to leave room for future research. Furthermore, there are studies such as Eiber et al.[17] who emphasized that Ga-68 PSMA-11 PET/CT increases the diagnostic accuracy when used with mpMRI for the staging of PC. Thus, further prospective randomized controlled trials are required to justify the role of Ga-68-PSMA-11 PET/MRI in the locoregional staging of PC.
Conclusion
The study concludes that in patients of carcinoma prostate, Ga-68 PSMA-11 PET/CT can adequately identify the intraglandular tumor and correlates well with histopathological analysis. In comparison to mpMRI, the Ga-68 PSMA-11 PET/CT scan has superior sensitivity in the detection of lymph nodal metastases, periprostatic extension, and seminal vesicle involvement and can provide superior locoregional preoperative staging in intermediate- and high-risk PC patients.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Perin NN. Global variation in cancer incidence and mortality. Curr Sci. 2001;81:465–74. [Google Scholar]
- 3.Soylu FN, Eggener S, Oto A. Local staging of prostate cancer with MRI. Diagn Interv Radiol. 2012;18:365–73. doi: 10.4261/1305-3825.DIR.4970-11.2. [DOI] [PubMed] [Google Scholar]
- 4.Makarov DV, Loeb S, Ulmert D, Drevin L, Lambe M, Stattin P. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst. 2013;105:1306–13. doi: 10.1093/jnci/djt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 6.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: A multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 7.Wibmer AG, Burger IA, Sala E, Hricak H, Weber WA, Vargas HA. Molecular Imaging of Prostate Cancer. Radiographics. 2016;36:142–59. doi: 10.1148/rg.2016150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5. [PubMed] [Google Scholar]
- 9.Mottaghy FM, Heinzel A, Verburg FA. Molecular imaging using PSMA PET/CT versus multiparametric MRI for initial staging of prostate cancer: Comparing apples with oranges? Eur J Nucl Med Mol Imaging. 2016;43:1397–9. doi: 10.1007/s00259-016-3389-2. [DOI] [PubMed] [Google Scholar]
- 10.Whitmore WF., Jr Expectant management of clinically localized prostatic cancer. Semin Oncol. 1994;21:560–8. [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68) Ga-labelled PSMA ligand and (18) F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–15. doi: 10.1111/bju.13540. [DOI] [PubMed] [Google Scholar]
- 14.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68) gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Grivas N, Hinnen K, de Jong J, Heemsbergen W, Moonen L, Witteveen T, et al. Seminal vesicle invasion on multi-parametric magnetic resonance imaging: Correlation with histopathology. Eur J Radiol. 2018;98:107–12. doi: 10.1016/j.ejrad.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier W, Rius M, et al. Intra-individual comparison of (68) Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:1400–6. doi: 10.1007/s00259-016-3346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–36. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]