Abstract
B cell receptor (BCR) signaling is a central pathway promoting the survival and proliferation of normal and malignant B cells. Chronic lymphocytic leukemia (CLL) arises from mature B cells, expressing functional BCRs, mainly of IgM and IgD isotypes. Importantly, 30% of CLL patients express quasi-identical BCRs, so-called “stereotyped” receptors, indicating the existence of common antigenic determinants, which may drive disease initiation and favor its progression. Although the antigenic specificity of IgM and IgD receptors is identical, there are distinct isotype-specific responses after IgM and IgD triggering. Here, we discuss the most important steps of normal B cell development, and highlight the importance of BCR signaling for CLL pathogenesis, with a focus on differences between IgM and IgD isotype signaling. We also highlight the main characteristics of CLL patient subsets, based on BCR stereotypy, and describe subset-specific BCR function and antigen binding characteristics. Finally, we outline the key biologic and clinical responses to kinase inhibitor therapy, targeting the BCR-associated Bruton tyrosine kinase (BTK), phosphoinositide-3-kinase (PI3K), and spleen tyrosine kinase (SYK) in patients with CLL.
The B cell receptor (BCR) during B cell development
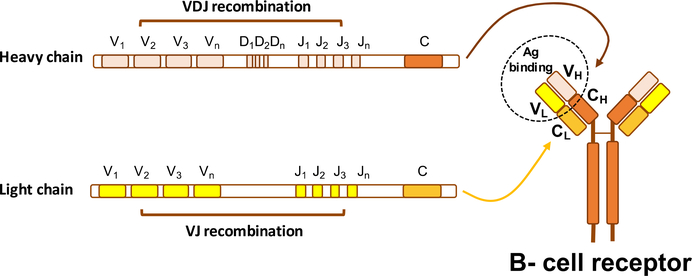
B lymphocytes develop from hematopoietic stem cells through a continuum of developmental stages that originate within the primary lymphoid tissues (i.e. fetal liver and fetal/adult marrow), with later stages of maturation occurring in secondary lymphoid organs, including the lymph nodes and the spleen (Figure 1).1 One of the first essential steps towards maturation of a normal B cell is the successful rearrangement of immunoglobulin (Ig) heavy chain (IGH) gene segments (V, D and J segments), during a process named VDJ recombination (Figure 2), which occurs in progenitor (pro)-B cells, and leads to precursor (pre)-B-cell development.2 During this process, a highly diverse repertoire of antigen-binding HCDR3 regions3 is generated, a key determinant for the antigen specificity of the developing BCR. Pre-B cells express an immature BCR, termed pre-B cell receptor (pre-BCR), which is composed of fully rearranged heavy chains and “surrogate” light chains. During this stage of differentiation, the rearrangement of the immunoglobulin light (IGL) chain V and J gene segments takes place, allowing for the expression of a complete BCR on the surface of immature B cells, expressing heavy chains of the M isotype (i.e. IgM). The complete BCR molecule includes two heavy chains and two light chains, which associate with two Igα/Igβ subunits (i.e. CD79a and CD79b), which are necessary for signal transduction and indispensable for B cell survival.4 VDJ recombination is an error-prone process, which generates a high number of BCRs (up to 3*1011), including some with reactivity towards self-antigens. These autoreactive B cells normally are negatively selected and undergo apoptosis, while cells expressing non self-reactive BCRs may proceed further in development, and acquire expression of surface IgDs (i.e. immunoglobulins carrying heavy chains of the D isotype), with the same specificity as IgM, resulting in mature B cells expressing both, IgM and IgD.5 After antigen encounter in the periphery, B-cell activation and differentiation in secondary lymphoid tissues (i.e. lymph nodes, spleen) occurs in specialized structures, named germinal centers (GC), where B cell clonal expansion and somatic hypermutation (SHM) of the variable regions of both heavy and light chain genes takes place (Figure 1). While most of the somatic mutations introduced by SHM reduce the affinity of the BCR for the stimulating antigen and result in cellular apoptosis, in a minority of cases antigen affinity increases, and such B cells are positively selected for further differentiation into memory B cells or antibody-secreting plasma cells.5 Affinity selection occurs after direct recognition of antigens exposed on the surface of follicular dendritic cells (FDC),6 a cellular component of GCs, and positive selection of BCRs with the highest affinity for foreign antigens devoids the IgM+ memory B cell pool from autoreactive B cells, which would otherwise increase the risk for autoimmunity.7 In addition to the SHM process, B cells can diversify their receptors during a process named class switch recombination (CSR), which also occurs within the GCs, allowing the generation of BCRs that carry heavy chains of different isotypes than IgM and IgD. The immunoglobulin heavy chains constant regions μ (IgM) and δ (IgD), are substituted by either γ, ε, or α heavy chains, generating IgG, IgE and IgA isotypes, which are characteristically involved in responses to viruses and bacteria (IgG), parasites (IgE), and mucosal microbes (IgA) (for a more complete review of IgG, IgE and IgA isotype functions please refer to 8).
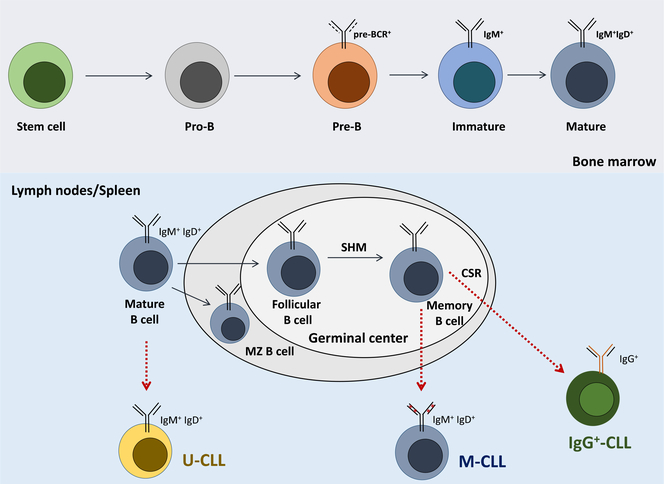
Figure 1. B cell receptor maturation during B cell development and antigen responses.
B cells undergo a series of maturation steps in the bone marrow, that lead to the generation of mature B cells, which express IgM and IgD isotype receptors on their surface. B cells then continue their maturation in secondary lymphoid organs, including the lymph nodes and the spleen, where, after antigen encounter, the BCRs are further diversified through somatic hypermutaion (SHM) and class switch recombination (CSR). CLL arises from distinct precursors, with U-CLL deriving from naïve B cells expressing unmutated immunoglobulins, while M-CLL deriving from memory B cells which have undergone SHM. A smaller, IgG-expressing subset, can arise from memory B cells which have undergone CSR.
Figure 2. The VDJ recombination process.
Recombination of VDJ genetic regions of the heavy chain and VJ regions of the light chain at the pro- and pre-B stage allows generation of the variable regions of the heavy and light chain of the mature BCR. The high variability of the BCRs is in part due to the large number of V, D, and J gene regions of both Ig chains (e.g. the heavy chain includes 51V, 27D, and 6J genes). The complete BCR is composed by two variable heavy (VH) and two variable light (VL) chains, responsible for antigen binding, as well as two constant heavy (CH) and two constant light (CL) chains, involved in effector functions.
IgM and IgD isotypes: structural and functional diversity in normal B cells
Mature B cells express 20,000–150,000 IgM molecules and 250,000–300,000 IgD molecules.9 Early studies in IgM10 or IgD11, 12 knockout mice demonstrated highly interchangeable functions of the two receptors in B cell development, affinity maturation and CSR. Several later studies, however, supported the existence of structural and functional diversity in IgM and IgD isotypes in normal B cells. The main structural difference between IgD and IgM is the IgD-specific “hinge” region, an extended peptide sequence located between the Fab (i.e. antigen-binding region) and Fc portion (i.e. tail region of the BCR that interacts with the cell membrane). This hinge region, present in IgD and absent in IgM, favors flexibility of the Fab region of IgD, permitting binding to polyvalent antigens.13 In line with this finding, IgD but not IgM receptors have been associated with prolonged signaling activation in normal B cells.14 The distinct properties of IgM and IgD receptors may also be related to their spatial organization on the plasma membrane and distinct interactions with positive and negative signaling regulators. IgMs are indeed largely monomeric, whereas IgD can frequently be found in large clusters (also named “protein islands”),15, 16 which may at least in part explain the differential threshold for activation of the two isotypes. IgD, but not IgM, was also recently described to be located in close proximity to the chemokine receptor CXCR4, facilitating transduction of CXCR4 activation signals to downstream effectors.17 This nanoscale organization of BCRs is determined by the actin cytoskeleton,9 which regulates the size and lifetime of receptor aggregations18 (for a more complete review on cytoskeletal regulation of BCR aggregation please refer to 19).
In the context of self-antigen stimulation, IgM, but not IgD, can be down-regulated following prolonged antigenic stimulation,20 demonstrating that IgM is the principal isotype that can become “anergic”, a state in which B cells become unresponsive to antigen. More recent work demonstrated that IgD is less sensitive than IgM to endogenous antigens, possibly maintaining the quiescence of B cells in the context of autoantibody stimulation, and limiting autoreactivity of cells carrying anergized IgMs.21,22 BCR-desensitization associated to anergy is frequently associated to constitutive increase in basal intracellular Ca2+ levels, together with an overall reduced responsiveness in terms of phospho-protein activation following BCR stimulation.23,24 A summary of the structural and functional differences of IgM and IgD isotype receptors is provided in Table 1.
Table 1.
IgM and IgD isotype expression and function in normal B cells and CLL cells.
| BCR isotype | Expression in normal B cells | Function in normal B cells | Expression in CLL cells | Function in CLL cells |
|---|---|---|---|---|
| IgM | Expressed at the immature B-cell stage after productive VDJ recombination 5; secreted or membrane-bound | Lower threshold for B-cell activation13; down-modulated in vivo following chronic antigen exposure 20 | Higher levels on U-CLL, as compared to M-CLL 27, 38, 39 | Survival and proliferation49, 59, 60, cell cycle entry49, long-lived signaling activation 49; cross-talk with CXCR444, TLR signaling 45 and IL-4 46; higher IgM responsiveness in U-CLL 27, 38, 39 |
| IgD | Co-expressed with IgM in mature B-cells5; secreted or membrane-bound; contains a hinge region necessary to polyvalent antigen binding 13 | Higher threshold for B-cell activation13; lower association with the CD19 co-receptor 16 | Co-expressed with IgM27, 38, 39 | Short-lived signaling and cytoskeletal activation, rapid internalization 48, 49 |
BCR signaling in Chronic Lymphocytic Leukemia (CLL)
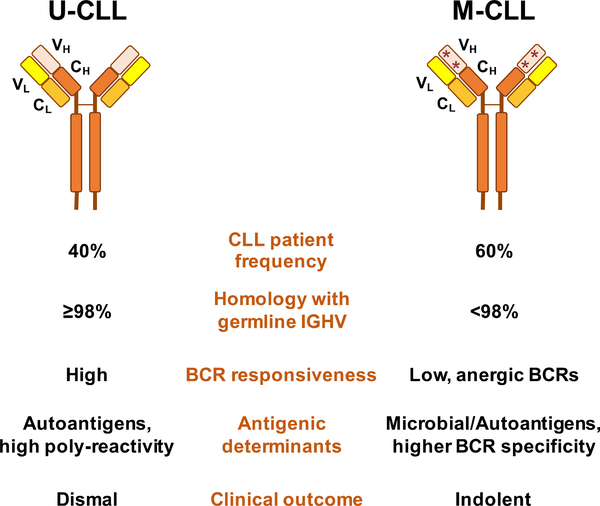
Several lines of evidence support the hypothesis that CLL is a BCR-dependent malignancy. First, the mutational status of IGHV genes shows significant variability among patients with CLL, which in turn correlates with the clinical outcome. Specifically, unmutated CLL (U-CLL), defined as cases in which the CLL BCRs have 98% or more identity with the germline IGHV sequence, is typically associated with a more aggressive clinically behavior,25–27 whereas mutated CLL (M-CLL) cases carrying BCRs with less than 98% IGHV identity, characteristically present with more indolent disease (Figure 3). Second, around 30% of CLL patients express a largely skewed immunoglobulin repertoire, with virtually identical BCRs, the so-called “stereotyped” receptors.28–33 Third, the BCR signaling pathway is the central pathway activated in the lymph node microenvironment of CLL patients, the primary site of CLL cell proliferation in so-called proliferation centers or pseudofollicles.34 While most CLL cells express BCRs of both, IgM and IgD isotypes (Table 1), a smaller proportion of cases, around 5–10%, express isotype-switched IgGs.35, 36 The cell of origin for U and M-CLL is also distinct,37 with U-CLL deriving from pre-germinal center CD5+ naïve B cells, while M-CLL originating from post-germinal center CD5+CD27+ memory B cells (Figure 1), further distinguishing these subgroups of patients.
Figure 3. Characteristics of U-CLL and M-CLL patient subsets.
CLL patients can be categorized into two main subsets (U-CLL, M-CLL), characterized by a different degree of somatic hypermutations, BCR responsiveness, antigenic determinants and clinical outcome.
Responsiveness of CLL cells to IgM stimulation differs substantially among samples from different patients; CLL cells from U-CLL patients typically have higher responsiveness to IgM, which promotes CLL cell survival and proliferation, and a more clinical aggressive phenotype than M-CLL cases.25–27, 38, 39 M-CLL show characteristic features of anergized B cells resulting from prolonged antigen engagement, including reduced surface IgM levels, baseline activation of Ca2+ signaling, and constitutive ERK phosphorylation. 39–41 These features result in diminished IgM responsiveness, which can be restored by IgD ligation, or can spontaneously recover in vitro in the absence of stimulatory ligands.39, 41 In contrast, U-CLL cells express higher sIgM levels,27, 39 and also tend to express higher levels of the ZAP70 signaling adaptor42, 43 which may further facilitate increased signaling responsiveness. IgM stimulation results in marked down-regulation of the chemokine receptor CXCR4;44 vice versa, IgM signaling is modulated by TLR45 and IL-4 receptor activation,46, 47 demonstrating cross-talk between the BCR and other signaling pathways in CLL cells. The functional importance of the IgD isotype in CLL remains less defined. IgD signaling can be induced in all CLL samples, without significant differences between U-CLL and M-CLL, 27, 38, 39 and, in contrast to normal B cells,14 IgD responses appear to be more short-lived,48 and unable to induce c-MYC protein expression or cell-cycle entry,49 most likely because of rapid and more pronounced IgD internalization following stimulation.48 IgM and IgD isotype features in CLL cells are summarized in Table 1. The antigen-driven pathogenicity of the CLL-BCRs has been suggested by a number of studies characterizing several self-antigens for the CLL-BCRs, in particular for U-CLL, including proteins exposed on the cell surface during apoptosis (e.g. myosin heavy chain IIA),50–52 lipoproteins,53 cytoskeletal proteins (e.g. vimentin),36 and microbial proteins (e.g. LPS).53 Largely autoreactive BCRs derived from pairing of virus-specific heavy-chains with a restricted number of light chains have also been recently described as pathogenic in the Eμ-TCL1 mouse model of CLL,54 confirming the importance of autoantigenic interactions in leukemia development also in vivo in mice. M-CLL derived BCR are less poly-reactive than U-CLL, and possess higher specificity and affinity for antigens, such as fungal antigens, with some cases carrying BCRs displaying rheumatoid factor activity.55, 56,57
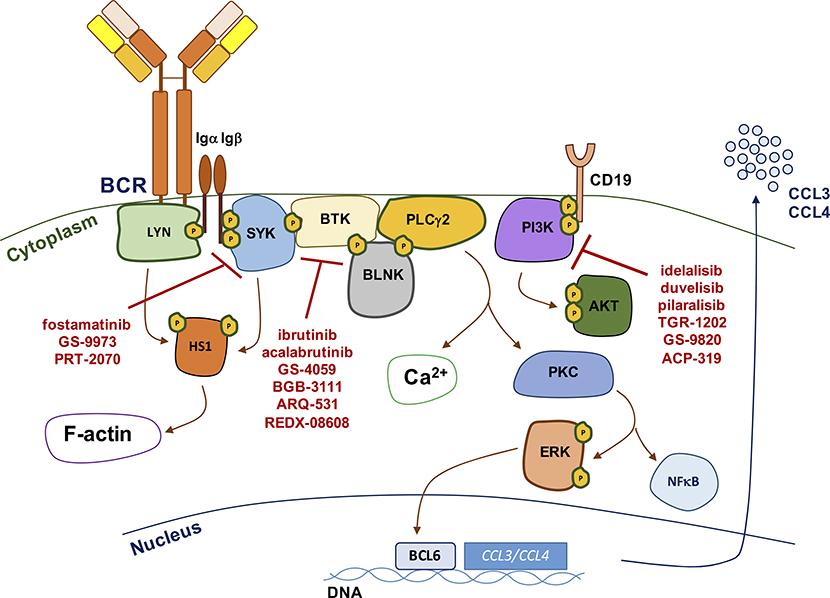
At a functional level, CLL-BCR engagement activates a complex cascade of intracellular signaling molecules, including upstream kinases LYN, SYK, BTK and PI3K, which transduce signals to calcium signaling modulators (e.g. PLCγ2), cytoskeletal activators (e.g. HS1 protein),58 and to downstream effectors, including AKT and ERK kinases, the NF-κB pathway, nuclear transcription factors, but also anti-apoptotic proteins of the BCL2 family, such as MCL1 59, 60. Nuclear transcription results in numerous outcomes, including production and secretion of CCL3 and CCL4 chemokines,61, 62 two important chemoattractants for lymphocytes and monocytes/macrophages (Figure 4). IgM stimulation induces more prolonged signaling resulting in secretion of higher levels of CCL3 and CCL4; such effect cannot be appreciated following IgD signaling, which is largely restricted to early cytoskeletal activation (e.g. HS1 phosphorylation).48 BCR signaling duration and intensity is tightly regulated by several mechanisms including receptor endocytosis, positive (e.g. CD19) and negative (e.g. CD5, CD22) co-receptor signaling, and activation of phosphatases, which fine-tune the functional outcome of the response.63 CLL-BCRs can also signal in the absence of external antigen, in an autonomous fashion, by interactions between individual BCRs through recognition of conserved epitopes within specific regions of the immunoglobulin heavy and light chains.64, 65 The autonomous signaling activity of the CLL-BCRs has also been defined indispensable for leukemia development and progression in the Eμ-TCL1 mouse model of CLL,66 increasing the complexity of BCR activation mechanisms involved in disease pathogenesis.
Figure 4. The BCR signaling pathway and its targeted inhibition.
Schematic representation of the main activation events in the BCR signaling pathway. BCR signaling activation is initiated by upstream kinases including SYK, BTK and PI3K, which can be targeted by novel small molecule kinase inhibitors, including the SYK inhibitors fostamatinib, GS-9973, and PRT-2070, the BTK kinase inhibitors ibrutinib, acalabrutinib, GS-4059, BGB-3111, ARQ-531 and REDX-08608 and the PI3K inhibitors idelalisib, duvelisib, pilaralisib, TGR-1202, GS-9820, and ACP-319.
CLL- BCR stereotyped subsets
Dissecting the functional and molecular features of subsets of CLL patients with unique “stereotyped” BCR has provided valuable insight into the role of BCR signaling in CLL. 28–33 BCR “stereotypy” refers to highly restricted and sometimes identical variable heavy complementarity determining region 3 (VH-CDR3) sequences among different CLL patients, a characteristic that can be detected in approximately 30–35% of CLL cases, almost two thirds being U-CLL. 28–33 In the most recent analysis of a series of 21,123 IGHV sequences from CLL patients, 67 23 “ “major” (i.e. most populated) subsets were identified and represented 12% of all CLLs. {Agathangelidis, 2012 #20844;Agathangelidis, 2016 #20975}HCDR3 stereotypy between geographically distant and unrelated patients implies that CLL ontogeny is not stochastic, but rather related to common antigenic determinants. Stereotypy extends to shared somatic mutations, similar genetic and epigenetic profile of the leukemic clones, similar antigen-binding properties and functional responses through the BCR and other immune receptors, and also to similar clinical outcomes (Table 2).
Table 2.
Biological and molecular features of the most common stereotyped subsets
| Stereotyped subset | Subset #1 | Subset #2 | Subset #4 | Subset #8 |
|---|---|---|---|---|
| Frequency | ˜2,4% 28, 67 | ˜2.8% 28, 67 | ˜1% 28, 67 | ˜0.5 % 28 |
| IGHV/IGVL gene identity | Clan I IGHV genes/IGHD6–19 /IGHJ4/IGKV1[D]-39 28, 29, 32 | IGHV3–21/ IGHJ6/ IGLV3–21 28, 73 | γ-switched IGHV4–34/ IGHJ6/ IGKV2–30 28, 29 |
γ-switched IGHV4–39/ IGHD6–13/ IGHJ5/ IGKV1[D]-39 28, 29, 83 |
| IGHV mutational status | Unmutated 28, 29 | Mutated (60%) Unmutated (40%) 28, 29, 74 | Mutated 28, 29, 33 | Unmutated 28, 29, 83 |
| BCR signaling properties | functional BCR signaling 36, 68 | functional BCR signaling 36 | anergic BCRs 82 | functional BCR signaling 36 |
| Predicted antigens | Vimentin; Calreticulin; MEACs; healthy Hep-2; apoptotic RAMOS; apoptotic Jurkat; oxidation markers; dsDNA; Insulin/LPS 36,50, 52, 89, 90 |
cofilin-1; stomach chief cells; pancreatic exocrine glands 53 |
intact anti-I/i motif; viable human memory B cells 33, 52,99 |
MEACs; healthy Hep-2; apoptotic RAMOS; apoptotic Jurkat oxidation markers; extractable nuclear antigens; dsDNA microbial antigens/TLR ligands, calreticulin 36,50, 52, 90 |
| Genetic lesions in treatment-naïve CLL | deletion of 11q, 17p, NOTCH1, NFKBIE mutations 68, 70, 71, 76 | deletion of 13q, SF3B1 mutations 69, 70,75, 76 | deletion of 13q 69, 70,75 | trisomy 12, NOTCH1 mutations 69,70, 84 |
| Clinical course/ Risk of transformation | Aggressive (median TTFT of 1.6 yrs)30 | Aggressive (median TTFT of 1.9 yrs)30 | Indolent (median TTFT of 11 yrs)30 | Aggressive (median TTFT of 1.5 yrs),30 increased risk of Richter’s transformation84 |
Two paradigmatic stereotyped subsets associated with poor clinical outcome are subset #1 and #2. Subset #1 exhibits an aberrant and distinctive gene-expression profile signature with several differentially expressed transcripts involved in the regulation of apoptosis, cell proliferation, oxidative processes and BCR signaling.68 Additionally, subset #1 is enriched for NOTCH169, 70 and NFKBIE (i.e. gene encoding for IκBε, a negative NF-κB regulator) aberrations.71 In CLL cells from subset #1, BCR cross-linking by anti-IgM results in significantly higher rates of cell proliferation when compared to non-subset #1 cases using the same IGHV genes but heterogeneous HCDR3s.68 However, Bergh et al showed that triggering subset #1 leukemic cells with one of their putative antigens, namely oxidized low-density lipoprotein (oxLDL),53 induced BCR clustering and internalization but did not result in intracellular signal transduction. 72 In a proportion of these cases, TLR9 stimulation could bypass BCR silencing, inducing cell cycle entry, and suggesting that interaction with oxLDL alone is not sufficient to drive cellular proliferation of subset #1 cells.72 Stereotyped subset #2 cases express either mutated (60%) or unmutated (40%) BCRs encoded by the IGHV3–21/IGVL3–21 gene pair, but is uniformly aggressive independently of SHM status.28, 29, 73–75 Similarities among subset #2 cases include a distinctive pattern of SHM for M-CLL cases, and a remarkable high frequency of SF3B1 mutations.33, 69, 70, 76 Importantly, IGHV3–21 expressing CLL cells show the highest levels of signaling responsiveness when compared to non-IGHV3–21 CLL of both U-CLL and M-CLL.27 Interestingly, IGLV3–21 was recently shown to have independent poor prognostic significance, irrespective of its association with stereotyped subset #2,77 and to be associated with high levels of MYC target gene expression and low CXCR4 surface expression, implying an ongoing, and possibly cell-autonomous signaling activity, in line with the intramolecular recognition properties of IGLV3–21 described by Minici et al, 65 as discussed in more detail at the end of this section.
Other exemplary subsets for their internal biological and clinical homogeneity are stereotyped subsets #4 and subset #8, both expressing γ-switched BCRs (i.e. IgG isotypes). On the one side, subset #4 is defined by the expression of mutated IGHV4–34/IGKV2–30 BCRs, with long and positively charged VH CDR3s,28, 29 reminiscent of pathogenic anti-DNA antibodies,78 and SHM patterns suggestive of edited autoreactive antibodies.33, 79 The ongoing SHM results in intraclonal diversification of the BCR and implies ongoing interaction with antigen(s).80, 81 Subset #4 patients are relatively young at diagnosis and experience an indolent clinical course.30 Subset #4 CLL cells show biochemical and functional features of anergy, including constitutive ERK1/2 activation and lack of responsiveness after BCR cross-linking in terms of MAPK signaling activation and intracellular Ca2+ release.82 Interestingly, anergy could be reversed in this subset by ligation of TLR1/2 and subsequent activation of the miR-17∼92 cluster, a regulator of MAPK expression. Stimulation through TLR1/2 resulted in a distinct gene and miRNA expression profiles that were clearly distinct from those of CLL cells from non–subset #4 CLL cases, suggesting a subset-specific regulation of the anergic state.82 The indolent behavior of other stereotyped subsets, such as subset #148 (stereotyped BCRs carrying mutated IGHV2–5) suggests that anergy and reduced BCR signaling responsiveness may also characterize these subsets,30 albeit biochemical characterization of anergic features is yet to be performed.
Subset #8 (IGHV4–39/IGKV1(D)-39) patients, at the opposite side of the clinical spectrum, are characterized by a high risk for developing Richter’s transformation, and by presence of distinct genetic aberrations (i.e. high frequency of trisomy 12 and NOTCH1 mutations).28, 30, 69, 70, 76, 83, 84 CLL cells from subset #8 patients display robust BCR pathway activation upon antigen binding, even when compared to cells from stereotyped subsets #1 and #2, along with an extremely promiscuous binding to both microbial and auto-antigens (e.g. Sm, dsDNA, CpG, LPS).36 Chu and colleagues demonstrated that several CLL BCRs mostly of the U-CLL subtype, including subset #8 and subset #6 (unmutated IGHV1–69/IGKV3–20 stereotyped BCRs), bind to apoptotic cells with exposed non-muscle myosin heavy chain IIA (MYHIIA)51 and such binding significantly correlates with poor patient survival.50 U-CLL-like stereotyped Ig sequences, mostly utilizing the IGHV1–69 gene have also been identified in naïve B cells from healthy donors85, 86, demonstrating that an early selection of restricted BCR Igs with properties that resemble natural antibodies generated to fight common pathogens or to clear apoptotic debris,87, 88 may occur even in normal individuals. The identification of such Ig sequences in the normal B cell repertoire suggests that certain B cell subsets carrying discrete BCR Igs escape immune tolerance possibly due to low autoreactivity. Auto-antigenic stimulation may also take place in the lymph node microenvironment, where calreticulin can be found on the surface of macrophages (i.e. nurselike cells)89, 90, which in turn may trigger BCR signaling, especially in CLL cells from subsets #1 and #8.90 Despite these subset-specific characteristics and binding activities, several studies using mimetic epitopes have revealed a largely shared epitopic reactivity of CLL-BCRs, demonstrating that common antigenic structures can be recognized even amongst unrelated CLL clonotypes.91–94 BCR-BCR interactions driving autonomous signaling have instead been recently shown to have subset-specific epitopes, binding kinetics and affinity.65 Stronger affinities and longer binding half-lives associate with indolent cases (e.g. subset #4) and weaker, short-lived contacts with progressive ones (e.g. subset #2), again linking the quality of the BCR signal to the distinct clinical outcomes.65 Of note, BCR homotypic interactions are not an intrinsic property of the germline clonotypic BCR-Ig, but are acquired through specific Ig affinity maturation. In particular, a subset #2 unifying SHM of the residue corresponding to the splice site between the variable and the constant Ig lambda domain, leads to subset #2 BCR self-recognition. In subset #4, CSR to IgG introduces the binding epitope, thus providing also a structural explanation for the exclusive usage of IgG isotypes by the cases assigned to this stereotyped subset.29, 33, 65, 95,96 Of note, light-chain mediated binding to the bacterial protein L and to the actin-binding protein cofilin was described for subset #2 recombinant Igs, independent of the subset #2 heavy chain, suggesting that binding to these antigens cannot account for the non-stochastic pairing of subset #2 heavy and lambda light chain.97 In the structural analysis by Minici et al. the subset #2 BCR homotypic interactions are also largely mediated through the subset #2 IgL. Importantly, however, the subset #2 heavy chain with the characteristically short VH-CDR3{Agathangelidis, 2012 #13724;Agathangelidis, 2012 #13724}28, 29 facilitates the spatial proximity between the two BCRs, while establishing one direct hydrogen bond with the epitope on the light chain,65 implying that BCR homotypic interactions may indeed account for the biased pairing of subset #2 heavy and lambda light Ig chain. Regarding subset #4 antigen binding activity, recent BCR specificity studies using a variety of antigenic targets revealed the importance of the autoantigen-mediated selection. In particular, unlike most CLL Igs that bind apoptotic cells, subset #4 BCR Igs recognize elements on viable human memory B cells and this binding necessitates the distinctive immunogenetic characteristics of subset #4, such as the specific SHM and the CSR to IgG. 52, 98, 99 All these evidences demonstrate that both antigenic and BCR-autonomous interactions influence the non-stochastic pairing of the heavy and light chains of stereotyped BCRs, suggesting a fine regulation of subset-specific Ig features.
BCR signaling inhibitors for CLL treatment
The management of patients has fundamentally changed since the introduction of small molecule inhibitors targeting BCR signaling-related kinases SYK,100 BTK,101 and PI3K 102(Figure 4). Durable responses, even in heavily pretreated patients, and/or patients carrying unfavorable cytogenetic risk features [i.e. del(17p), del(11q)], are common and led to the FDA and EMA approval of the BTK inhibitor ibrutinib101, 103 and the PI3Kδ inhibitor idelalisib102, 104, the latter typically used in combination with the anti-CD20 monoclonal antibody rituximab. A common mechanism of action of these drugs involves the rapid redistribution of CLL cells from the lymphatic tissues into the peripheral blood, which correlates with rapid resolution of lymphadenopathy within the first weeks of treatment,105 together with abrogated leukemia proliferation and accelerated CLL cell death.106 BTK and PI3K kinases participate not only in CLL survival- and proliferation-related BCR signaling, but also in signaling of receptors related to cell migration, adhesion and tissue homing, including chemokine receptor and adhesion molecule signaling. Accordingly, preclinical studies using BTK and PI3Kδ inhibitors demonstrated inhibition of integrin and chemokine receptor signaling,104, 107–111 along with BCR signaling blockade.
Ibrutinib disrupts pro-survival signals from nurselike cells (NLC),107 CD40 ligation, TLR9, BAFF, fibronectin, IL-6, IL-4, TNFα,110 chemotaxis towards CXCL12 and CXCL13,107, 108 integrin-mediated adhesion,108 and CCL3 and CCL4 chemokine production, in vitro and in CLL patients receiving ibrutinib therapy.107 CLL cells carrying unmutated IGHV genes generally show higher dependence on BTK and BCR signaling for survival, which presumably explains the higher sensitivity of CLL cells with unmutated IGHV to ibrutinib treatment in vitro112 and in vivo in patients receiving ibrutinib therapy.101, 113 Ibrutinib as single agent,101, 114, 115 or in combination with rituximab,116, 117 fludarabine, cyclophosphamide and rituximab (FCR),116 or bendamustine and rituximab (BR),118 induces durable remissions in previously treated 101, 119–121 or untreated patients.103,119, 122 Ibrutinib was also shown to promote graft-versus-leukemia (GvL) effects in CLL patients following hematopoietic cell transplantation (HCT),123 and improve expansion of CD19-directed CAR T cells. 124 This outcome may be related to the effects of ibrutinib on T cells, including increased T cell receptor (TCR) repertoire diversity,125 promotion of Th1 instead of Th2 CD4+ T cell responses,126 and downregulation of the immunosuppressive molecules PD-1 and CTLA-4.127 Five-year follow up of phase II studies of single-agent ibrutinib therapy recently reported high rates of progression free survival (92%128 to not reached113) in treatment naïve patients and 44%128 to 64.8% in R/R CLL,113 reemphasizing the remarkable efficacy of this agent. Despite this efficacy and tolerability, resistance to ibrutinib has been described, and is commonly associated with point mutations at the ibrutinib-binding site within BTK (C481S), or with activating mutations of the BCR signaling molecule PLCγ2,129, 130 but also with clonal evolution 131, 132 and emergence of mutations in BCR-independent proteins, such as EP300 and MLL2, which are implicated in chromatin and histone regulation.131 In vitro, NFKBIE mutations have also been associated with reduced responses to ibrutinib treatment.71 An interesting mode to circumvent BTK (C481S) mutations has been proposed, and involves miRNA-mediated targeting of BTK total protein, which can be achieved through HDAC inhibition.133 Inhibitors of non-BCR related pathways, including nuclear export 134 and the para-caspase MALT1135 together with novel small molecule inhibitors with comparable blocking activities against wild-type and C418S-mutant BTK, namely ARQ531136 and REDX08608,137 are also currently tested in preclinical settings, with encouraging results. In addition to ibrutinib, novel small molecule BTK kinase inhibitors with higher selectivity towards BTK kinase, and less cross-reactivity with other Tec kinase family members, are currently under clinical development, including acalabrutinib,138,139 GS-4059,140 and BGB-3111.141 Whether these agents will provide greater responses and/or less side effects than ibrutinib remains to be evaluated.
The PI3Kδ inhibitor idelalisib has been tested as single agent,142 in combination with rituximab102, 143 or with rituximab and/or bendamustine.144 Similar to patients receiving ibrutinib, idelalisib induces early lymphocytosis followed by lymphocyte count normalization. Also similar to ibrutinib, idelalisib effectively antagonizes CLL-survival signals coming from the microenvironment, 104, 110 reduces CLL-cell chemotaxis,109 and CCL3 and CCL4 release by CLL cells in vitro and in vivo in patients receiving idelalisib therapy.109 Additional PI3K inhibitors have been tested in preclinical and early clinical studies, including duvelisib, also called IPI-145, a PI3K γ/δ inhibitor,145, 146 the pan-PI3K inhibitor pilaralisib, also called SAR245408,147 the PI3K β,δ inhibitor GS-9820, 148 and the PI3Kδ inhibitors ACP-319149 and TGR-1202. 150, 151
SYK kinase inhibition has also been explored, with promising results obtained in relapsed/refractory CLL patients after treatment with fostamatinib.100, 152 The drug was then further developed for the treatment of rheumatoid arthritis, and other novel SYK inhibitors are currently tested, including GS-9973153,154 and PRT-2070.
The remarkable clinical effectiveness of BCR signaling inhibitors underscores the importance of B cell receptor signaling and of BCR-associated kinases in the proliferation and homing of CLL cells, in particular at the level of the lymph node microenvironment, making this class of agent the treatment of choice for CLL patients with a wide variety of clinical presentations, biological characteristics and response to prior therapies.
Conclusions
A large number of studies has highlighted the importance of BCR signaling in CLL pathogenesis. The complexity of BCR signaling in CLL subsets is further increased by the existence of isotype-specific functions for IgM and IgD, and of stereotype-specific antigen binding and signaling properties. The therapeutic landscape has remarkably changed since the introduction of small molecule inhibitors targeting BCR-associated kinases, which abrogate CLL cell proliferation and induce durable remissions, even in high-risk and refractory CLL patients. Nonetheless, resistances to these novel agents can emerge, primarily in high-risk patients, and can be challenging in patients receiving long-term therapy with these drugs. It is therefore essential to identify and target additional pathways, which contribute to CLL survival and proliferation in the presence of continuous therapy with these agents, and especially in cases with resistance mutations in BCR signaling molecules. Studies to characterize the mutational landscape driving resistance to BCR signaling inhibitors,129, 131, 132 and randomized clinical trials utilizing these drugs in combination with newer agents (e.g. the BCL2 inhibitor venetoclax) are underway and will allow a better refinement of individualized treatment strategies for CLL patients.
Acknowledgments
The work was supported by a Leukemia & Lymphoma Society Scholar Award in Clinical Research (J.A.B.), and MD Anderson’s Moon Shot Program in CLL. This research is also supported in part by the MD Anderson Cancer Center Support Grant CA016672 and Associazione Italiana per la Ricerca sul Cancro AIRC Investigator grants #20246 (to P.G.), and Research Program AIRC 5 per mille #21198 (to P.G.).
Disclosures
J. A. B. received research funding from Pharmacyclics, Gilead, and Portola and is a consultant for Janssen. P.G. received commercial research grants from AbbVie, Janssen, Gilead and Novartis. E. ten Hacken and M. Gounari have no competing financial interests.
Abbreviations used in this article
- BCR
B-cell receptor
- CLL
Chronic Lymphocytic Leukemia
- Ig
Immunoglobulin
- U-CLL
unmutated-CLL
- M-CLL
mutated-CLL
References
- 1.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008. September 1; 112(5): 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghia P, ten Boekel E, Sanz E, de la Hera A, Rolink A, Melchers F. Ordering of human bone marrow B lymphocyte precursors by single-cell polymerase chain reaction analyses of the rearrangement status of the immunoglobulin H and L chain gene loci. J Exp Med 1996. December 1; 184(6): 2217–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 2000. July; 13(1): 37–45. [DOI] [PubMed] [Google Scholar]
- 4.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell 2004. June 11; 117(6): 787–800. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol 2005. March; 5(3): 230–242. [DOI] [PubMed] [Google Scholar]
- 6.Kranich J, Krautler NJ. How Follicular Dendritic Cells Shape the B-Cell Antigenome. Front Immunol 2016; 7: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol 2008. December; 20(6): 632–638. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol 2012. July; 12(7): 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattila PK, Feest C, Depoil D, Treanor B, Montaner B, Otipoby KL, et al. The actin and tetraspanin networks organize receptor nanoclusters to regulate B cell receptor-mediated signaling. Immunity 2013. March 21; 38(3): 461–474. [DOI] [PubMed] [Google Scholar]
- 10.Lutz C, Ledermann B, Kosco-Vilbois MH, Ochsenbein AF, Zinkernagel RM, Kohler G, et al. IgD can largely substitute for loss of IgM function in B cells. Nature 1998. June 25; 393(6687): 797–801. [DOI] [PubMed] [Google Scholar]
- 11.Roes J, Rajewsky K. Immunoglobulin D (IgD)-deficient mice reveal an auxiliary receptor function for IgD in antigen-mediated recruitment of B cells. J Exp Med 1993. January 1; 177(1): 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitschke L, Kosco MH, Kohler G, Lamers MC. Immunoglobulin D-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc Natl Acad Sci U S A 1993. March 1; 90(5): 1887–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ubelhart R, Hug E, Bach MP, Wossning T, Duhren-von Minden M, Horn AH, et al. Responsiveness of B cells is regulated by the hinge region of IgD. Nat Immunol 2015. May; 16(5): 534–543. [DOI] [PubMed] [Google Scholar]
- 14.Kim KM, Reth M. The B cell antigen receptor of class IgD induces a stronger and more prolonged protein tyrosine phosphorylation than that of class IgM. J Exp Med 1995. March 1; 181(3): 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maity PC, Yang J, Klaesener K, Reth M. The nanoscale organization of the B lymphocyte membrane. Biochim Biophys Acta 2015. April; 1853(4): 830–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klasener K, Maity PC, Hobeika E, Yang J, Reth M. B cell activation involves nanoscale receptor reorganizations and inside-out signaling by Syk. Elife 2014; 3: e02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker M, Hobeika E, Jumaa H, Reth M, Maity PC. CXCR4 signaling and function require the expression of the IgD-class B-cell antigen receptor. Proc Natl Acad Sci U S A 2017. May 16; 114(20): 5231–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treanor B, Depoil D, Bruckbauer A, Batista FD. Dynamic cortical actin remodeling by ERM proteins controls BCR microcluster organization and integrity. J Exp Med 2011. May 9; 208(5): 1055–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattila PK, Batista FD, Treanor B. Dynamics of the actin cytoskeleton mediates receptor cross talk: An emerging concept in tuning receptor signaling. J Cell Biol 2016. February 1; 212(3): 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 1988. August 25; 334(6184): 676–682. [DOI] [PubMed] [Google Scholar]
- 21.Noviski M, Mueller JL, Satterthwaite A, Garrett-Sinha LA, Brombacher F, Zikherman J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. Elife 2018. March 9; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabouri Z, Perotti S, Spierings E, Humburg P, Yabas M, Bergmann H, et al. IgD attenuates the IgM-induced anergy response in transitional and mature B cells. Nat Commun 2016. November 10; 7: 13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub BC, Jun JE, Bishop AC, Shokat KM, Thomas ML, Goodnow CC. Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J Exp Med 2000. April 17; 191(8): 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity 1997. April; 6(4): 419–428. [DOI] [PubMed] [Google Scholar]
- 25.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999. September 15; 94(6): 1848–1854. [PubMed] [Google Scholar]
- 26.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999. September 15; 94(6): 1840–1847. [PubMed] [Google Scholar]
- 27.D’Avola A, Drennan S, Tracy I, Henderson I, Chiecchio L, Larrayoz M, et al. Surface IgM expression and function are associated with clinical behavior, genetic abnormalities, and DNA methylation in CLL. Blood 2016. August 11; 128(6): 816–826. [DOI] [PubMed] [Google Scholar]
- 28.Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood 2012. May 10; 119(19): 4467–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatopoulos K, Belessi C, Moreno C, Boudjograh M, Guida G, Smilevska T, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: Pathogenetic implications and clinical correlations. Blood 2007. January 1; 109(1): 259–270. [DOI] [PubMed] [Google Scholar]
- 30.Baliakas P, Hadzidimitriou A, Sutton LA, Minga E, Agathangelidis A, Nichelatti M, et al. Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: a retrospective multicentre study. The lancet haematology 2014; 1(2): e74–e84. [DOI] [PubMed] [Google Scholar]
- 31.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors [In Process Citation]. J Clin Invest 1998; 102(8): 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messmer BT, Albesiano E, Efremov DG, Ghiotto F, Allen SL, Kolitz J, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med 2004. August 16; 200(4): 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray F, Darzentas N, Hadzidimitriou A, Tobin G, Boudjogra M, Scielzo C, et al. Stereotyped patterns of somatic hypermutation in subsets of patients with chronic lymphocytic leukemia: implications for the role of antigen selection in leukemogenesis. Blood 2008. February 1; 111(3): 1524–1533. [DOI] [PubMed] [Google Scholar]
- 34.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011. January 13; 117(2): 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palacios F, Moreno P, Morande P, Abreu C, Correa A, Porro V, et al. High expression of AID and active class switch recombination might account for a more aggressive disease in unmutated CLL patients: link with an activated microenvironment in CLL disease. Blood 2010. June 3; 115(22): 4488–4496. [DOI] [PubMed] [Google Scholar]
- 36.Gounari M, Ntoufa S, Apollonio B, Papakonstantinou N, Ponzoni M, Chu CC, et al. Excessive antigen reactivity may underlie the clinical aggressiveness of chronic lymphocytic leukemia stereotyped subset 8. Blood 2015. April 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seifert M, Sellmann L, Bloehdorn J, Wein F, Stilgenbauer S, Durig J, et al. Cellular origin and pathophysiology of chronic lymphocytic leukemia. J Exp Med 2012. November 19; 209(12): 2183–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood 2003. February 1; 101(3): 1087–1093. [DOI] [PubMed] [Google Scholar]
- 39.Mockridge CI, Potter KN, Wheatley I, Neville LA, Packham G, Stevenson FK. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood 2007. May 15; 109(10): 4424–4431. [DOI] [PubMed] [Google Scholar]
- 40.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood 2008. July 1; 112(1): 188–195. [DOI] [PubMed] [Google Scholar]
- 41.Apollonio B, Scielzo C, Bertilaccio MT, Ten Hacken E, Scarfo L, Ranghetti P, et al. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood 2013. May 9; 121(19): 3879–3888, S3871–3878. [DOI] [PubMed] [Google Scholar]
- 42.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003. May 1; 348(18): 1764–1775. [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 2002. December 15; 100(13): 4609–4614. [DOI] [PubMed] [Google Scholar]
- 44.Vlad A, Deglesne PA, Letestu R, Saint-Georges S, Chevallier N, Baran-Marszak F, et al. Down-regulation of CXCR4 and CD62L in chronic lymphocytic leukemia cells is triggered by B-cell receptor ligation and associated with progressive disease. Cancer Res 2009. August 15; 69(16): 6387–6395. [DOI] [PubMed] [Google Scholar]
- 45.Chatzouli M, Ntoufa S, Papakonstantinou N, Chartomatsidou E, Anagnostopoulos A, Kollia P, et al. Heterogeneous Functional Effects of Concomitant B Cell Receptor and TLR Stimulation in Chronic Lymphocytic Leukemia with Mutated versus Unmutated Ig Genes. J Immunol 2014. May 15; 192(10): 4518–4524. [DOI] [PubMed] [Google Scholar]
- 46.Aguilar-Hernandez MM, Blunt MD, Dobson R, Yeomans A, Thirdborough S, Larrayoz M, et al. IL-4 enhances expression and function of surface IgM in CLL cells. Blood 2016. June 16; 127(24): 3015–3025. [DOI] [PubMed] [Google Scholar]
- 47.Guo B, Zhang L, Chiorazzi N, Rothstein TL. IL-4 rescues surface IgM expression in chronic lymphocytic leukemia. Blood 2016. July 28; 128(4): 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ten Hacken E, Sivina M, Kim E, O’Brien S, Wierda WG, Ferrajoli A, et al. Functional Differences between IgM and IgD Signaling in Chronic Lymphocytic Leukemia. J Immunol 2016. August 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krysov S, Dias S, Paterson A, Mockridge CI, Potter KN, Smith KA, et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood 2012. January 5; 119(1): 170–179. [DOI] [PubMed] [Google Scholar]
- 50.Chu CC, Catera R, Zhang L, Didier S, Agagnina BM, Damle RN, et al. Many chronic lymphocytic leukemia antibodies recognize apoptotic cells with exposed nonmuscle myosin heavy chain IIA: implications for patient outcome and cell of origin. Blood 2010. May 13; 115(19): 3907–3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu CC, Catera R, Hatzi K, Yan XJ, Zhang L, Wang XB, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood 2008. December 15; 112(13): 5122–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Catera R, Silverman GJ, Hatzi K, Seiler T, Didier S, Zhang L, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol Med 2008. Nov-Dec; 14(11–12): 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanemo Myhrinder A, Hellqvist E, Sidorova E, Soderberg A, Baxendale H, Dahle C, et al. A new perspective: molecular motifs on oxidized LDL, apoptotic cells, and bacteria are targets for chronic lymphocytic leukemia antibodies. Blood 2008. April 1; 111(7): 3838–3848. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez de Oya N, De Giovanni M, Fioravanti J, Ubelhart R, Di Lucia P, Fiocchi A, et al. Pathogen-specific B-cell receptors drive chronic lymphocytic leukemia by light-chain-dependent cross-reaction with autoantigens. EMBO Mol Med 2017. November; 9(11): 1482–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoogeboom R, van Kessel KP, Hochstenbach F, Wormhoudt TA, Reinten RJ, Wagner K, et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J Exp Med 2013. January 14; 210(1): 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoogeboom R, Wormhoudt TA, Schipperus MR, Langerak AW, Dunn-Walters DK, Guikema JE, et al. A novel chronic lymphocytic leukemia subset expressing mutated IGHV3–7-encoded rheumatoid factor B-cell receptors that are functionally proficient. Leukemia 2013. March; 27(3): 738–740. [DOI] [PubMed] [Google Scholar]
- 57.Kostareli E, Gounari M, Janus A, Murray F, Brochet X, Giudicelli V, et al. Antigen receptor stereotypy across B-cell lymphoproliferations: the case of IGHV4–59/IGKV3–20 receptors with rheumatoid factor activity. Leukemia 2012. May; 26(5): 1127–1131. [DOI] [PubMed] [Google Scholar]
- 58.ten Hacken E, Scielzo C, Bertilaccio MT, Scarfo L, Apollonio B, Barbaglio F, et al. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood 2013. March 21; 121(12): 2264–2273. [DOI] [PubMed] [Google Scholar]
- 59.Petlickovski A, Laurenti L, Li X, Marietti S, Chiusolo P, Sica S, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood 2005. June 15; 105(12): 4820–4827. [DOI] [PubMed] [Google Scholar]
- 60.Paterson A, Mockridge CI, Adams JE, Krysov S, Potter KN, Duncombe AS, et al. Mechanisms and clinical significance of BIM phosphorylation in chronic lymphocytic leukemia. Blood 2012. February 16; 119(7): 1726–1736. [DOI] [PubMed] [Google Scholar]
- 61.Burger JA, Quiroga MP, Hartmann E, Burkle A, Wierda WG, Keating MJ, et al. High-level expression of the T-cell chemokines CCL3 and CCL4 by chronic lymphocytic leukemia B cells in nurselike cell cocultures and after BCR stimulation. Blood 2009. March 26; 113(13): 3050–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sivina M, Hartmann E, Kipps TJ, Rassenti L, Krupnik D, Lerner S, et al. CCL3 (MIP-1{alpha}) plasma levels and the risk for disease progression in chronic lymphocytic leukemia (CLL). Blood 2011. November 29; 117(5): 1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood 2011. October 20; 118(16): 4313–4320. [DOI] [PubMed] [Google Scholar]
- 64.Duhren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012. September 13; 489(7415): 309–312. [DOI] [PubMed] [Google Scholar]
- 65.Minici C, Gounari M, Ubelhart R, Scarfo L, Duhren-von Minden M, Schneider D, et al. Distinct homotypic B-cell receptor interactions shape the outcome of chronic lymphocytic leukaemia. Nat Commun 2017. June 9; 8: 15746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iacovelli S, Hug E, Bennardo S, Duehren-von Minden M, Gobessi S, Rinaldi A, et al. Two types of BCR interactions are positively selected during leukemia development in the Emu-TCL1 transgenic mouse model of CLL. Blood 2015. March 5; 125(10): 1578–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agathangelidis A, Hadzidimitriou A, Minga E, Sutton L, Polychronidou E, Shanafelt T, et al. Reappraising Immunoglobulin Repertoire Restrictions in Chronic Lymphocytic Leukemia: Focus on Major Stereotyped Subsets and Closely Related Satellites. Blood 2016; 128: 4376. [Google Scholar]
- 68.Del Giudice I, Chiaretti S, Santangelo S, Tavolaro S, Peragine N, Marinelli M, et al. Stereotyped subset #1 chronic lymphocytic leukemia: a direct link between B-cell receptor structure, function, and patients’ prognosis. American journal of hematology 2014. January; 89(1): 74–82. [DOI] [PubMed] [Google Scholar]
- 69.Sutton LA, Young E, Baliakas P, Hadzidimitriou A, Moysiadis T, Plevova K, et al. Different spectra of recurrent gene mutations in subsets of chronic lymphocytic leukemia harboring stereotyped B-cell receptors. Haematologica 2016. August; 101(8): 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossi D, Spina V, Bomben R, Rasi S, Dal-Bo M, Bruscaggin A, et al. Association between molecular lesions and specific B-cell receptor subsets in chronic lymphocytic leukemia. Blood 2013. June 13; 121(24): 4902–4905. [DOI] [PubMed] [Google Scholar]
- 71.Mansouri L, Sutton LA, Ljungstrom V, Bondza S, Arngarden L, Bhoi S, et al. Functional loss of IkappaBepsilon leads to NF-kappaB deregulation in aggressive chronic lymphocytic leukemia. J Exp Med 2015. June 01; 212(6): 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergh AC, Evaldsson C, Pedersen LB, Geisler C, Stamatopoulos K, Rosenquist R, et al. Silenced B-cell receptor response to autoantigen in a poor-prognostic subset of chronic lymphocytic leukemia. Haematologica 2014. November; 99(11): 1722–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tobin G, Thunberg U, Johnson A, Eriksson I, Soderberg O, Karlsson K, et al. Chronic lymphocytic leukemias utilizing the VH3–21 gene display highly restricted Vlambda2–14 gene use and homologous CDR3s: implicating recognition of a common antigen epitope. Blood 2003. June 15; 101(12): 4952–4957. [DOI] [PubMed] [Google Scholar]
- 74.Baliakas P, Agathangelidis A, Hadzidimitriou A, Sutton LA, Minga E, Tsanousa A, et al. Not all IGHV3–21 chronic lymphocytic leukemias are equal: prognostic considerations. Blood 2015. January 29; 125(5): 856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marincevic M, Cahill N, Gunnarsson R, Isaksson A, Mansouri M, Goransson H, et al. High-density screening reveals a different spectrum of genomic aberrations in chronic lymphocytic leukemia patients with ‘stereotyped’ IGHV3–21 and IGHV4–34 B-cell receptors. Haematologica 2010. September; 95(9): 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Strefford JC, Sutton LA, Baliakas P, Agathangelidis A, Malcikova J, Plevova K, et al. Distinct patterns of novel gene mutations in poor-prognostic stereotyped subsets of chronic lymphocytic leukemia: the case of SF3B1 and subset #2. Leukemia 2013. November; 27(11): 2196–2199. [DOI] [PubMed] [Google Scholar]
- 77.Stamatopoulos B, Smith T, Crompot E, Pieters K, Clifford R, Mraz M, et al. The light chain IgLV3–21 defines a new poor prognostic subgroup in Chronic Lymphocytic Leukemia: results of a multicenter study. Clin Cancer Res 2018. June 26. [DOI] [PubMed] [Google Scholar]
- 78.Jang YJ, Stollar BD. Anti-DNA antibodies: aspects of structure and pathogenicity. Cell Mol Life Sci 2003. February; 60(2): 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hadzidimitriou A, Darzentas N, Murray F, Smilevska T, Arvaniti E, Tresoldi C, et al. Evidence for the significant role of immunoglobulin light chains in antigen recognition and selection in chronic lymphocytic leukemia. Blood 2009. January 8; 113(2): 403–411. [DOI] [PubMed] [Google Scholar]
- 80.Kostareli E, Sutton LA, Hadzidimitriou A, Darzentas N, Kouvatsi A, Tsaftaris A, et al. Intraclonal diversification of immunoglobulin light chains in a subset of chronic lymphocytic leukemia alludes to antigen-driven clonal evolution. Leukemia 2010. July; 24(7): 1317–1324. [DOI] [PubMed] [Google Scholar]
- 81.Sutton LA, Kostareli E, Hadzidimitriou A, Darzentas N, Tsaftaris A, Anagnostopoulos A, et al. Extensive intraclonal diversification in a subgroup of chronic lymphocytic leukemia patients with stereotyped IGHV4–34 receptors: implications for ongoing interactions with antigen. Blood 2009. November 12; 114(20): 4460–4468. [DOI] [PubMed] [Google Scholar]
- 82.Ntoufa S, Papakonstantinou N, Apollonio B, Gounari M, Galigalidou C, Fonte E, et al. B Cell Anergy Modulated by TLR1/2 and the miR-17 approximately 92 Cluster Underlies the Indolent Clinical Course of Chronic Lymphocytic Leukemia Stereotyped Subset #4. J Immunol 2016. May 15; 196(10): 4410–4417. [DOI] [PubMed] [Google Scholar]
- 83.Ghiotto F, Fais F, Valetto A, Albesiano E, Hashimoto S, Dono M, et al. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J Clin Invest 2004. April; 113(7): 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossi D, Spina V, Cerri M, Rasi S, Deambrogi C, De Paoli L, et al. Stereotyped B-cell receptor is an independent risk factor of chronic lymphocytic leukemia transformation to Richter syndrome. Clin Cancer Res 2009. July 1; 15(13): 4415–4422. [DOI] [PubMed] [Google Scholar]
- 85.Forconi F, Potter KN, Wheatley I, Darzentas N, Sozzi E, Stamatopoulos K, et al. The normal IGHV1–69-derived B-cell repertoire contains stereotypic patterns characteristic of unmutated CLL. Blood 2010. January 7; 115(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 86.Vergani S, Bagnara D, Mazzarello AN, Ferrer G, Yancopoulos S, Stamatopoulos K, et al. CLL Stereotyped IGHV-D-J Rearrangements Can Be Detected Throughout Normal B-Cell Developmental Stages in Aged People When Using Ultra-Deep, Next Generation Sequencing Techniques. Blood 2016; (128): 2028.26825708 [Google Scholar]
- 87.Milner EC, Anolik J, Cappione A, Sanz I. Human innate B cells: a link between host defense and autoimmunity? Springer Semin Immunopathol 2005. March; 26(4): 433–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol 2008. January 15; 180(2): 800–808. [DOI] [PubMed] [Google Scholar]
- 89.Binder M, Lechenne B, Ummanni R, Scharf C, Balabanov S, Trusch M, et al. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS One 2010; 5(12): e15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ten Hacken E, Gounari M, Back JW, Shimanovskaya E, Scarfo L, Kim E, et al. Calreticulin as a novel B cell receptor antigen in chronic lymphocytic leukemia. Haematologica 2017. July 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Binder M, Muller F, Jackst A, Lechenne B, Pantic M, Bacher U, et al. B-cell receptor epitope recognition correlates with the clinical course of chronic lymphocytic leukemia. Cancer 2011. May 1; 117(9): 1891–1900. [DOI] [PubMed] [Google Scholar]
- 92.Mimmi S, Vecchio E, Iaccino E, Rossi M, Lupia A, Albano F, et al. Evidence of shared epitopic reactivity among independent B-cell clones in chronic lymphocytic leukemia patients. Leukemia 2016. December; 30(12): 2419–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seiler T, Woelfle M, Yancopoulos S, Catera R, Li W, Hatzi K, et al. Characterization of structurally defined epitopes recognized by monoclonal antibodies produced by chronic lymphocytic leukemia B cells. Blood 2009. October 22; 114(17): 3615–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marcatili P, Ghiotto F, Tenca C, Chailyan A, Mazzarello AN, Yan XJ, et al. Igs expressed by chronic lymphocytic leukemia B cells show limited binding-site structure variability. J Immunol 2013. June 1; 190(11): 5771–5778. [DOI] [PubMed] [Google Scholar]
- 95.Hadzidimitriou A, Agathangelidis A, Darzentas N, Murray F, Delfau-Larue MH, Pedersen LB, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood 2011. September 15; 118(11): 3088–3095. [DOI] [PubMed] [Google Scholar]
- 96.Vardi A, Agathangelidis A, Sutton LA, Chatzouli M, Scarfo L, Mansouri L, et al. IgG-switched CLL has a distinct immunogenetic signature from the common MD variant: ontogenetic implications. Clin Cancer Res 2014. January 15; 20(2): 323–330. [DOI] [PubMed] [Google Scholar]
- 97.Ghia EM, Widhopf GF 2nd, Rassenti LZ, Kipps TJ. Analyses of recombinant stereotypic IGHV3–21-encoded antibodies expressed in chronic lymphocytic leukemia. J Immunol 2011. June 1; 186(11): 6338–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Y, Catera R, Gao C, Yan XJ, Allen SL, Kolitz JE, et al. CLL Stereotyped Subset #4 Igs Acquire Binding to Viable B Lymphocyte Surfaces by Somatic Mutations, Isotype Class Swtiching, and with the Prerequisite of IG Self-Association. Blood 2017; 130: 58. [Google Scholar]
- 99.Catera R, Liu Y, Gao C, Yan XJ, Magli A, Allen SL, et al. Binding of CLL subset 4 B-cell receptor immunoglobulins to viable human memory B lymphocytes requires a distinctive IGKV somatic mutation. Mol Med 2017. January 12; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010. April 1; 115(13): 2578–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013. July 4; 369(1): 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med 2014. March 13; 370(11): 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med 2015. December 17; 373(25): 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, et al. CAL-101, a p110{delta} selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011. January 13; 117(2): 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wodarz D, Garg N, Komarova NL, Benjamini O, Keating MJ, Wierda WG, et al. Kinetics of CLL cells in tissues and blood during therapy with the BTK inhibitor ibrutinib. Blood 2014. June 26; 123(26): 4132–4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burger JA, Li KW, Keating MJ, Sivina M, Amer AM, Garg N, et al. Leukemia cell proliferation and death in chronic lymphocytic leukemia patients on therapy with the BTK inhibitor ibrutinib. JCI Insight 2017. January 26; 2(2): e89904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood 2012. February 2; 119(5): 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Rooij MF, Kuil A, Geest CR, Eldering E, Chang BY, Buggy JJ, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood 2012. March 15; 119(11): 2590–2594. [DOI] [PubMed] [Google Scholar]
- 109.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3’-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 2011. September 29; 118(13): 3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton’s tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011. June 9; 117(23): 6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fiorcari S, Brown WS, McIntyre BW, Estrov Z, Maffei R, O’Brien S, et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS One 2013; 8(12): e83830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guo A, Lu P, Galanina N, Nabhan C, Smith SM, Coleman M, et al. Heightened BTK-dependent cell proliferation in unmutated chronic lymphocytic leukemia confers increased sensitivity to ibrutinib. Oncotarget 2016. January 26; 7(4): 4598–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ahn IE, Farooqui MZH, Tian X, Valdez J, Sun C, Soto S, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 2018. May 24; 131(21): 2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014. July 17; 371(3): 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Coutre SE, Furman RR, Flinn IW, Burger JA, Blum K, Sharman J, et al. Extended Treatment with Single-Agent Ibrutinib at the 420 mg Dose Leads to Durable Responses in Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Clin Cancer Res 2017. January 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brown JR, Barrientos JC, Barr PM, Flinn IW, Burger JA, Tran A, et al. The Bruton tyrosine kinase inhibitor ibrutinib with chemoimmunotherapy in patients with chronic lymphocytic leukemia. Blood 2015. May 7; 125(19): 2915–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jain P, Keating MJ, Wierda W, Campbell M, Thompson PA, Ferrajoli A, et al. Long term follow up of treatment with ibrutinib and rituximab (IR) in patients with high-risk Chronic Lymphocytic Leukemia (CLL). Clin Cancer Res 2016. October 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol 2016. February; 17(2): 200–211. [DOI] [PubMed] [Google Scholar]
- 119.Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol 2015. February; 16(2): 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 2014. September; 15(10): 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015. April 16; 125(16): 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014. January; 15(1): 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ryan CE, Sahaf B, Logan AC, O’Brien S, Byrd JC, Hillmen P, et al. Ibrutinib efficacy and tolerability in patients with relapsed chronic lymphocytic leukemia following allogeneic HCT. Blood 2016. November 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fraietta JA, Beckwith KA, Patel PR, Ruella M, Zheng Z, Barrett DM, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016. March 03; 127(9): 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yin Q, Sivina M, Robins H, Yusko E, Vignali M, O’Brien S, et al. Ibrutinib Therapy Increases T Cell Repertoire Diversity in Patients with Chronic Lymphocytic Leukemia. J Immunol 2017. January 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013. October 10; 122(15): 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long M, Beckwith K, Do P, Mundy BL, Gordon A, Lehman AM, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017. August 01; 127(8): 3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.O’Brien S, Furman RR, Coutre S, Flinn IW, Burger JA, Blum K, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018. April 26; 131(17): 1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med 2014. June 12; 370(24): 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahn IE, Underbayev C, Albitar A, Herman SE, Tian X, Maric I, et al. Clonal evolution leading to ibrutinib resistance in chronic lymphocytic leukemia. Blood 2017. January 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burger JA, Landau DA, Taylor-Weiner A, Bozic I, Zhang H, Sarosiek K, et al. Clonal evolution in patients with chronic lymphocytic leukaemia developing resistance to BTK inhibition. Nat Commun 2016. May 20; 7: 11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Landau DA, Sun C, Rosebrock D, Herman SEM, Fein J, Sivina M, et al. The evolutionary landscape of chronic lymphocytic leukemia treated with ibrutinib targeted therapy. Nat Commun 2017. December 19; 8(1): 2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bottoni A, Rizzotto L, Lai TH, Liu C, Smith LL, Mantel R, et al. Targeting BTK through microRNA in chronic lymphocytic leukemia. Blood 2016. October 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hing ZA, Mantel R, Beckwith KA, Guinn D, Williams E, Smith LL, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood 2015. May 14; 125(20): 3128–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saba NS, Wong DH, Tanios G, Iyer JR, Lobelle-Rich P, Dadashian EL, et al. MALT1 Inhibition Is Efficacious in Both Naive and Ibrutinib-Resistant Chronic Lymphocytic Leukemia. Cancer Res 2017. December 15; 77(24): 7038–7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Reiff SD, Mantel R, Smith LL, Greene JT, Muhowski EM, Fabian CA, et al. The BTK Inhibitor ARQ 531 Targets Ibrutinib Resistant CLL and Richter’s Transformation. Cancer Discov 2018. August 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guisot NES, Best SA, Wright V, Thomason A, Woyach JA, Mantel R, et al. REDX08608, a Novel, Potent and Selective, Reversible BTK Inhibitor with Efficacy and Equivalent Potency Against Wild-Type and Mutant C481 S BTK. Blood 2016; (128): 4399. [Google Scholar]
- 138.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med 2016. January 28; 374(4): 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Awan FT, Schuh A, Brown JR, Furman R, Pagel JM, Hillmen P, et al. Acalabrutinib Monotherapy in Patients with Ibrutinib Intolerance: Results from the Phase 1/2 ACE-CL-001 Clinical Study. Blood 2016; (128): 638. [Google Scholar]
- 140.Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B, et al. A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood 2016. January 28; 127(4): 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tam CS, Opat S, Cull G, Trotman J, Gottlieb D, Simpson D, et al. Twice Daily Dosing with the Highly Specific BTK Inhibitor, Bgb-3111, Achieves Complete and Continuous BTK Occupancy in Lymph Nodes, and Is Associated with Durable Responses in Patients (pts) with Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL). Blood 2016; (128): 642. [Google Scholar]
- 142.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3 kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood 2014. March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.O’Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naive older patients with chronic lymphocytic leukemia. Blood 2015. December 17; 126(25): 2686–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barrientos JC, Furman RR, Leonard J, Flinn I, Rai K, De vos S, et al. Update on a phase I study of the selective PI3Kd inhibitor idelalisib (GS-1101) in combination with rituximab and/or bendamustine in patients with relapsed or refractory CLL. Journal of Clinical Oncology 2013; 31 (Suppl): abstract 7017. [Google Scholar]
- 145.Balakrishnan K, Peluso M, Fu M, Rosin NY, Burger JA, Wierda WG, et al. The Phosphoinositide-3-Kinase (PI3K)-Delta and Gamma Inhibitor, IPI-145, Overcomes Signals from the PI3K/AKT/S6 Pathway and Promotes Apoptosis in CLL. Leukemia 2015. April 28; 29(9): 1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dong S, Guinn D, Dubovsky JA, Zhong Y, Lehman A, Kutok J, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood 2014. December 4; 124(24): 3583–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Brown JR, Davids MS, Rodon J, Abrisqueta P, Kasar SN, Lager J, et al. Phase I trial of the pan-PI3K inhibitor pilaralisib (SAR245408/XL147) in patients with chronic lymphocytic leukemia (CLL) or relapsed/refractory lymphoma. Clin Cancer Res 2015. July 15; 21(14): 3160–3169. [DOI] [PubMed] [Google Scholar]
- 148.Kater AP, Tonino SH, Spiering M, Chamuleau MED, Liu R, Adewoye AH, et al. Final results of a phase 1b study of the safety and efficacy of the PI3Kdelta inhibitor acalisib (GS-9820) in relapsed/refractory lymphoid malignancies. Blood Cancer J 2018. February 12; 8(2): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Niemann CU, Mora-Jensen HI, Dadashian EL, Krantz F, Covey T, Chen SS, et al. Combined BTK and PI3Kdelta Inhibition with Acalabrutinib and ACP-319 Improves Survival and Tumor Control in CLL Mouse Model. Clin Cancer Res 2017. October 1; 23(19): 5814–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maharaj KK, Powers JJ, Pabon-Saldana M, Fonseca R, Achille A, Deng S, et al. Modulation of T Cell Compartment in a Preclinical CLL Murine Model By a Selective PI3K Delta Inhibitor, TGR-1202. Blood 2016; (128): 3236. [Google Scholar]
- 151.Davids MS, Kim HT, Nicotra A, Savell A, Francoeur K, Hellman J, et al. TGR-1202 in Combination with Ibrutinib in Patients with Relapsed or Refractory CLL or MCL: Preliminary Results of a Multicenter Phase I/Ib Study. Blood 2016; (128): 641. [Google Scholar]
- 152.Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, et al. B cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel Syk inhibitor, R406. Blood 2009. July 30; 114(5): 1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sharman J, Hawkins M, Kolibaba K, Boxer M, Klein L, Wu M, et al. An open-label phase 2 trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in chronic lymphocytic leukemia. Blood 2015. April 9; 125(15): 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sharsssman JP, Shustov AR, Smith M, Hagenstad CT, Kolibaba KS, Abella-Dominicis E, et al. Updated Results on the Clinical Activity of Entospletinib (GS-9973), a Selective Syk Inhibitor, in Patients with CLL Previously Treated with an Inhibitor of the B-Cell Receptor Signaling Pathway. Blood 2016; (128): 3225.27127303 [Google Scholar]