Abstract
Background
Adult, community-acquired pneumonia (CAP) guidelines from the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) include indications for urinary antigen tests (UATs) for Streptococcus pneumoniae (SP) and Legionella pneumophila (LP). These recommendations were based on expert opinions and have not been rigorously evaluated.
Methods
We used data from a multicenter, prospective, surveillance study of adults hospitalized with CAP to evaluate the sensitivity and specificity of the IDSA/ATS UAT indications for identifying patients who test positive. SP and LP UATs were completed on all included patients. Separate analyses were completed for SP and LP, using 2-by-2 contingency tables, comparing the IDSA/ATS indications (UAT recommended vs not recommended) and UAT results (positive vs negative). Additionally, logistic regression was used to evaluate the association of each individual criterion in the IDSA/ATS indications with positive UAT results.
Results
Among 1941 patients, UATs were positive for SP in 81 (4.2%) and for LP in 32 (1.6%). IDSA/ATS indications had 61% sensitivity (95% confidence interval [CI] 49–71%) and 39% specificity (95% CI 37–41%) for SP, and 63% sensitivity (95% CI 44–79%) and 35% specificity (95% CI 33–37%) for LP. No clinical characteristics were strongly associated with positive SP UATs, while features associated with positive LP UATs were hyponatremia, fever, diarrhea, and recent travel.
Conclusions
Recommended indications for SP and LP urinary antigen testing in the IDSA/ATS CAP guidelines have poor sensitivity and specificity for identifying patients with positive tests; future CAP guidelines should consider other strategies for determining which patients should undergo urinary antigen testing.
Keywords: pneumonia, urine antigen test, Legionella, pneumococcal
The recommended criteria from the Infectious Diseases Society of America and American Thoracic Society community-acquired pneumonia guidelines regarding which patients should undergo pneumococcal and Legionella urinary antigen tests have poor sensitivity and specificity for detection of these pathogens.
(See the Editorial Commentary by Murdoch on pages 2034–5.)
Pneumonia is the leading cause of hospitalization and death due to infection in the United States [1–3]. In 2007, the Infectious Disease Society of America and the American Thoracic Society (IDSA/ATS) jointly published management guidelines for community-acquired pneumonia (CAP) in adults, which included recommendations regarding which patients should undergo urinary antigen testing for Streptococcus pneumoniae (SP) or Legionella pneumophila (LP) [4]. These guidelines recommend SP and LP urinary antigen tests (UAT) for hospitalized adults with any of several clinical characteristics (risk factors) associated with S. pneumoniae or Legionella infections. These recommendations were based on expert opinions and have not been extensively evaluated since publication. Prior studies examining risk factors for positive SP and LP UATs have been retrospective and limited by indication biases, with UATs being ordered at the discretion of the treating clinician or local practice [5–16].
The objective of this study was to use a large, prospective cohort of adult CAP patients with universal urinary antigen testing to evaluate the accuracy of the risk factor–based approach described in the 2007 IDSA/ATS CAP guidelines for identifying which patients to test with UATs. Additionally, we evaluated the association of clinical characteristics not included in the current guidelines with SP and LP UAT results to identify additional risk factors that may improve future guidelines.
METHODS
We conducted a cross-sectional study using data prospectively collected as part of the multicenter Etiology of Pneumonia in the Community (EPIC) Study [17]. The EPIC study was sponsored by the Centers for Disease Control and Prevention and was approved by the Institutional Review Boards of each participating institution and by the Centers for Disease Control and Prevention. Written informed consent was obtained from each participant or from their authorized representative.
Setting and Participants
Adults with CAP were enrolled in the EPIC study from 1 January 2010 until 30 June 2012 at 5 hospitals, including 3 in Chicago, Illinois, and 2 in Nashville, Tennessee. Eligibility criteria for the EPIC study have been previously described [17] and are listed in Supplementary Table 1. Trained research personnel enrolled patients at least 18 hours a day, 7 days a week, and collected urine for SP and LP UATs. For the current analysis, we included patients enrolled in the EPIC study who had UATs performed for both LP and SP.
Data Collection and Study Definitions
Demographic and clinical data were collected by patient and caregiver interviews and chart reviews using standardized data collection instruments [17]. Data were collected to ascertain each indication for SP and LP urinary antigen testing as recommended in the 2007 IDSA/ATS CAP guidelines. These guidelines recommend obtaining a SP UAT for patients meeting any of the 7 pneumococcal criteria outlined in Table 1. Similarly, the guidelines recommend LP urinary antigen testing for patients meeting any of the 5 Legionella criteria in Table 1. Definitions used in this study for each of the variables in Table 1 are detailed in Supplementary Table 2.
Table 1.
Indications for Urinary Antigen Test for Streptococcus pneumoniae and Legionella pneumophila
| Indication | SP UAT | LP UAT |
|---|---|---|
| ICU admission | x | x |
| Failure of outpatient antibiotic therapy | x | x |
| Leukopenia | x | |
| Active alcohol abuse | x | x |
| Chronic liver disease | x | |
| Asplenia | x | |
| Recent travel | x | |
| Pleural effusion | x | x |
Indications shown are recommended in the 2007 Infectious Diseases Society of America and American Thoracic Society Community-acquired Pneumonia Guidelines [4].
Abbreviations: ICU, intensive care unit; LP, Legionella pneumophila; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
In addition to recommendations in the IDSA/ATS CAP guidelines for UAT indications, we also evaluated other clinical characteristics that were identified in prior studies as potentially predictive of pneumococcal and Legionella CAP (Supplementary Table 3) [7, 11–16, 18–21]. These variables included age ≥65 years, fever, nausea, diarrhea, confusion, headache, hyponatremia (serum sodium < 130 mmol/L), severe CAP, and empiric use of broad-spectrum antibiotics. We evaluated 2 definitions for severe CAP: (1) ≥3 IDSA/ATS minor criteria present at the time of hospital arrival [4]; and (2) Pneumonia Severity Index (PSI) risk class IV or V [22]. Empiric use of broad-spectrum antibiotics was defined as administration of at least 1 of the following antibiotics on the date of admission or the next calendar day: an antipseudomonal beta-lactam, aminoglycoside, carbapenem, vancomycin, linezolid, aztreonam, or daptomycin.
To simulate the clinical environment in which UATs are typically used, we limited the predictor variables in our analysis to the clinical data that are routinely available within the first few hours after a CAP diagnosis. Hence, variables such as viral detection by polymerase chain reaction and procalcitonin concentration were not evaluated in the main analysis. In supplemental analyses, results for SP and LP UATs were described in the context of viral testing and procalcitonin results. Methods for viral testing and procalcitonin measurement have been previously described [17, 23].
Urinary Antigen Tests
Biological samples, including urine, were systematically collected for pathogen testing as soon as possible after the initial hospital presentation [17]. UATs for SP and LP were performed at study sites using BinaxNow (Alere), according to manufacturer recommendations [24, 25]. The SP UAT targets C-polysaccharide, which is common to all pneumococcal serotypes. The LP UAT identifies a soluble antigen present only in Legionella pneumophila serogroup 1, which causes approximately 80–90% of known legionellosis in North America [26]. Urine antigen testing was performed systematically on patients enrolled in the EPIC study, regardless of whether they met IDSA/ATS testing indications. Results for SP and LP UATs were reported as either positive or negative.
Data Analysis
We classified each patient as meeting or not meeting IDSA/ATS UAT testing indications for SP and, separately, for LP. Patients with any of the pneumococcal criteria in Table 1 were classified as meeting SP UAT testing indications. Patients with any of the Legionella criteria in Table 1 were classified as meeting LP UAT testing indications. First, we compared the prevalence of each of the IDSA/ATS indications for SP and LP urinary antigen testing between patients who had positive and negative UAT results. Simple logistic regression was used to calculate the odds ratio (OR) for each indication (odds of the indication being present in patients with a positive UAT compared to those with a negative UAT). Then, separate 2-by-2 contingency tables were developed for SP and LP, with the exposure variable being whether testing indications were met (UAT recommended vs not recommended) and the outcome variable being the UAT result (positive vs negative). Using these contingency tables, sensitivity and specificity for the recommended testing indications were calculated.
Using simple logistic regression, we also assessed the association of potential predictors of SP and LP pneumonia, as identified in a literature review (Supplementary Table 3), with actual SP and LP UAT results. Next, we constructed separate multivariable logistic regression models to identify the factors independently associated with a positive SP or LP UAT result. To prevent model overfitting, we limited the number of predictor variables to approximately 10 degrees of freedom per outcome (positive UAT) [27]. We included 8 predictor variables in the SP model (sex, age ≥65 years, failure of outpatient antibiotics, fever [temperature > 38°C], hyponatremia, intensive care unit admission, PSI risk class ≥IV, and use of empiric broad-spectrum antibiotics), and 4 predictor variables in the LP model (recent travel, fever, diarrhea, and hyponatremia). The selection of these variables was based on the strength of the association between characteristics and positive tests, as described in the prior literature (Supplementary Table 3). Calculations were performed using Stata 14 (StataCorp LP, College Station, TX). We considered 2-sided P values <.05 as significant.
RESULTS
Study Population
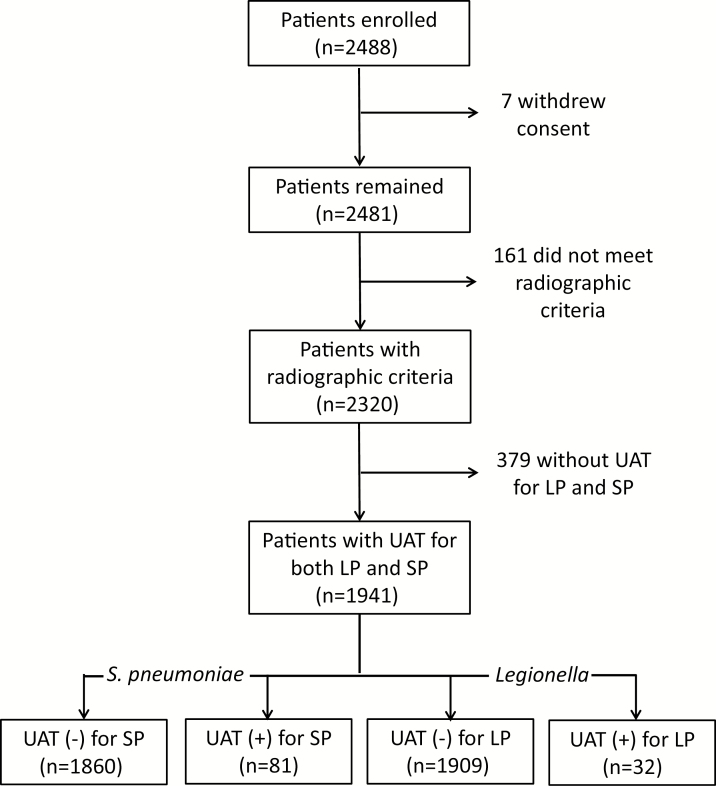
The EPIC study included 2320 adults hospitalized with radiographically-confirmed CAP; 379 (16%) patients were excluded from the current analysis due to missing UAT results, resulting in a final sample size of 1941 (84% of the original EPIC population; Figure 1). The median age of included patients was 57 years; 54.4% were White; and asthma and chronic obstructive pulmonary disease were the most common comorbidities (Table 2). Compared with the patients excluded due to missing UATs, patients included in this analysis were less likely to have a history of chronic kidney disease (13.8% versus 25.9%, respectively) or dialysis dependency (1.2% versus 17.9%, respectively), likely due to anuric patients being unable to provide urine specimens for the study. Other baseline characteristics were similar between the included patients and those excluded due to missing UAT results (Supplementary Table 4).
Figure 1.
Patient enrollment. Abbreviations: LP, Legionella pneumophila; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
Table 2.
Characteristics of Patients Who Tested Positive and Negative for Streptococcus pneumoniae and Legionella pneumophila by Urinary Antigen Test
| Characteristic | Overall Sample (n = 1941) | SP UAT Positive (n = 81) |
SP UAT Negative (n = 1860) | LP UAT Positive (n = 32) |
LP UAT Negative (n = 1909) |
|---|---|---|---|---|---|
| Median age, years (IQR) | 57 (46–71) | 60 (50–70) | 57 (46–71) | 58.5 (49–63) | 57 (46–71) |
| Male sex | 958 (49.4) | 35 (43.2) | 923 (49.6) | 26 (81.3) | 932 (48.8) |
| Race | |||||
| White | 1055 (54.4) | 46 (56.8) | 1009 (54.3) | 18 (56.3) | 1037 (54.3) |
| Black | 748 (38.5) | 30 (37.0) | 718 (38.6) | 14 (43.8) | 734 (38.5) |
| Asian | 41 (2.1) | 1 (1.2) | 40 (2.2) | 0 | 41 (2.2) |
| Other | 97 (5.0) | 4 (4.9) | 93 (5.0) | 0 | 97 (5.1) |
| Hispanic ethnicity | 203 (10.5) | 11 (13.6) | 192 (10.3) | 3 (9.4) | 200 (10.5) |
| Current smoking | 504 (26.0) | 29 (35.8) | 475 (25.5) | 10 (31.3) | 494 (25.9) |
| Heavy alcohol use | 96 (5.0) | 4 (4.9) | 92 (5.0) | 2 (6.3) | 94 (4.9) |
| City of enrollment | |||||
| Chicago | 1310 (67.5) | 47 (58.0) | 1263 (67.9) | 19 (59.4) | 1291 (67.6) |
| Nashville | 631 (32.5) | 34 (42.0) | 597 (32.1) | 13 (40.6) | 618 (32.4) |
| Comorbidities | |||||
| Chronic kidney disease | 268 (13.8) | 17 (21.0) | 251 (13.5) | 5 (15.6) | 263 (13.8) |
| Dialysis | 23 (1.2) | 1 (1.2) | 22 (1.2) | 0 | 23 (1.2) |
| Asthma | 502 (25.9) | 19 (23.5) | 483 (26.0) | 5 (15.6) | 497 (26.0) |
| Chronic obstructive pulmonary disease | 451 (23.2) | 25 (30.9) | 426 (22.9) | 2 (6.3) | 449 (23.5) |
| Heart failure | 361 (18.6) | 18 (22.2) | 343 (18.4) | 4 (12.5) | 357 (18.7) |
| Liver disease | 108 (5.6) | 3 (3.7) | 105 (5.7) | 1 (3.1) | 107 (5.6) |
| Sickle cell disease | 29 (1.5) | 0 | 29 (1.6) | 0 | 29 (1.5) |
| Immunosuppressed | 326 (16.8) | 11 (13.6) | 315 (16.9) | 7 (21.9) | 319 (16.7) |
| Human immunodeficiency virus infection | 61 (3.1) | 2 (2.5) | 59 (3.2) | 2 (6.3) | 59 (3.1) |
| Cancer | 398 (20.5) | 13 (16.1) | 385 (20.7) | 2 (6.3) | 396 (20.7) |
| Leukemia, lymphoma, or Hodgkin disease | 62 (3.2) | 1 (1.2) | 61 (3.3) | 0 | 62 (3.3) |
All values shown are n (%), unless otherwise indicated.
Abbreviations: IQR, interquartile range; LP, Legionella pneumophila; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
Streptococcus pneumoniae Results
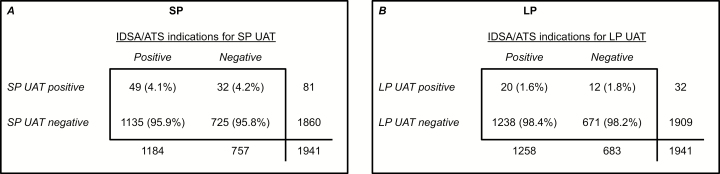
Among the 1941 included patients, 1184 (61.0%) patients had ≥1 IDSA/ATS indication for SP urinary antigen testing; 81 (4.2%) patients had a positive SP UAT. The percentage of patients with a positive SP UAT was very similar among those with ≥1 IDSA/ATS indication (4.1%) and those without any IDSA/ATS indication (4.2%; P = .92; Figure 2). The presence of ≥1 IDSA/ATS indication had 61% sensitivity (95% confidence interval [CI] 49–71%) and 39% specificity (95% CI 37–41) for identifying patients with a positive SP UAT. No individual indication within the IDSA/ATS guidelines was more common among patients who tested positive by SP UATs than patients who tested negative (Table 3).
Figure 2.
The 2-by-2 contingency tables for (A) Streptococcus pneumoniae (SP) and (B) Legionella pneumophila (LP). In the tables, columns represent the Infectious Diseases Society of America and American Thoracic Society (IDSA/ATS) indications for obtaining a SP or LP urinary antigen test (UAT). Patients with ≥1 testing indication from the IDSA/ATS guidelines were classified as positive for meeting the indications for testing. Rows represent SP and LP UAT results. Percentages, listed in parentheses, represent column percentages. Abbreviations: IDSA/ATS, Infectious Diseases Society of America and American Thoracic Society; LP, Legionella pneumophila; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
Table 3.
Prevalence of Each Indication Recommended in the IDSA/ATS Community-acquired Pneumonia Guidelines for Obtaining Streptococcus pneumoniae and Legionella pneumophila Urinary Antigen Test (UAT), by UAT Result
| IDSA/ATS Indication | SP UAT Positive (n = 81) | SP UAT Negative (n = 1860) | SP OR (95% CI) |
LP UAT Positive (n = 32) |
LP UAT Negative (n = 1909) | LP OR (95% CI) |
|---|---|---|---|---|---|---|
| ICU admission | 24 (29.6) | 396 (21.3) | 1.56 (0.95–2.54) | 5 (15.6) | 415 (21.7) | 0.67 (0.26–1.74) |
| Failure of outpatient antibiotic therapy | 12 (14.8) | 386 (20.8) | 0.66 (0.36–1.24) | 4 (12.5) | 394 (20.6) | 0.55 (0.19–1.58) |
| Leukopenia | 3 (3.7) | 71 (3.8) | 0.97 (0.30–3.15) | n/a | n/a | n/a |
| Active alcohol abuse | 4 (4.9) | 92 (5.0) | 1.00 (0.36–2.79) | n/a | n/a | n/a |
| Chronic liver disease | 3 (3.7) | 105 (5.7) | 0.64 (0.20–2.07) | 1 (3.1) | 107 (5.6) | 0.54 (0.07–4.02) |
| Asplenia | 0 | 28 (1.5) | n/c | n/a | n/a | n/a |
| Recent travel | n/a | n/a | n/a | 10 (31.3) | 330 (17.3) | 2.17 (1.02–4.64) |
| Pleural effusion | 25 (30.9) | 564 (30.3) | 1.03 (0.63–1.66) | 9 (28.1) | 580 (30.4) | 0.90 (0.41–1.95) |
| ≥1 IDSA/ATS indication | 49 (60.5) | 1135 (61.0) | 0.98 (0.62–1.54) | 20 (62.5) | 1238 (64.9) | 0.90 (0.44–1.86) |
All values shown are n (%), unless otherwise indicated. The designation of n/a (not applicable) indicates characteristics that were not included in the IDSA/ATS CAP guidelines for the specific pathogens. The designation of n/c (not calculated) indicates no patients who tested positive for S. pneumoniae had asplenia.
Abbreviations: CAP, community-acquired pneumonia; CI, confidence interval; ICU, intensive care unit; IDSA/ATS, Infectious Diseases Society of America and American Thoracic Society; IQR, interquartile range; LP, Legionella pneumophila; OR, odds ratio; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
A univariate evaluation of additional variables that were selected from the literature as potential predictors of SP pneumonia showed that hyponatremia (OR 2.15, 95% CI 1.16–3.98) and a PSI risk class ≥IV (OR 1.62, 95% CI 1.03–2.53) were associated with a positive SP UAT (Table 4). However, in the pre-specified multivariable model, no variables were significantly associated with a positive SP UAT (Table 5).
Table 4.
Prevalence of Potential Predictors for Positive Urinary Antigen Tests (UATs) Identified in the Literature but Not Included in the IDSA/ATS, by Result of Streptococcus pneumoniae UAT and Legionella pneumophila UAT
| Characteristic | SP UAT Positive (n = 81) |
SP UAT Negative (n = 1860) |
SP OR (95% CI) |
LP UAT Positive (n = 32) | LP UAT Negative (n = 1909) |
LP OR (95% CI) |
|---|---|---|---|---|---|---|
| Age ≥65 | 33 (40.7) | 647 (34.8) | 1.29 (0.82–2.03) | 5 (15.6) | 675 (35.4) | 0.34 (0.13–0.88) |
| Fever (>38°C) | 28 (34.6) | 476 (25.6) | 1.54 (0.96–2.46) | 18 (56.3) | 486 (25.5) | 3.76 (1.86–7.63) |
| Nausea | 29 (35.8) | 649 (34.9) | 1.04 (0.65–1.66) | 13 (40.6) | 665 (34.8) | 1.28 (0.63–2.61) |
| Diarrhea | 18 (22.2) | 382 (20.5) | 1.11 (0.65–1.89) | 14 (43.8) | 386 (20.2) | 3.07 (1.51–6.23) |
| Confusion | 17 (21.0) | 374 (20.1) | 1.06 (0.61–1.82) | 7 (21.9) | 384 (20.1) | 1.1 (0.47–2.59) |
| Headache | 40 (49.4) | 858 (46.1) | 1.14 (0.73–1.78) | 23 (71.9) | 875 (45.8) | 3.02 (1.39–6.56) |
| Hyponatremia Na ≤130 mE/L |
13 (16.1) | 152 (8.2) | 2.15 (1.16–3.98) | 13 (40.6) | 152 (8.0) | 7.91 (3.83–16.3) |
| Pneumonia Severity Index risk class ≥IV | 37 (45.7) | 636 (34.2) | 1.62 (1.03–2.53) | 6 (18.8) | 667 (34.9) | 0.43 (0.18–1.05) |
| ≥3 ATS minor criteria | 7 (8.6) | 129 (6.9) | 1.27 (0.57–2.81) | 2 (6.3) | 134 (7.0) | 0.88 (0.21–3.74) |
| Empiric broad-spectrum antibiotics | 31 (38.3) | 583 (31.3) | 1.36 (0.86–2.15) | 9 (28.1) | 605 (31.7) | 0.84 (0.39–1.83) |
All values shown are n (%), unless otherwise indicated.
Abbreviations: ATS, American Thoracic Society; CI, confidence interval; LP, Legionella pneumophila; OR, odds ratio; SP, Streptococcus pneumoniae; UAT, urinary antigen test.
Table 5.
Multivariable Models for Predicting Positive Streptococcus pneumoniae and Legionella pneumophila Urinary Antigen Tests
| Multivariable OR (95% CI) | |
|---|---|
| Streptococcus pneumoniae (n = 81) | |
| Male sex | 0.69 (0.43–1.09) |
| Age ≥65 | 1.04 (0.61–1.77) |
| Failure of outpatient antibiotics | 0.67 (0.36–1.26) |
| Fever (>38°C) | 1.50 (0.93–2.42) |
| Hyponatremia | 1.81 (0.96–3.41) |
| ICU admission | 1.29 (0.75–2.24) |
| Pneumonia Severity Index risk class ≥IV | 1.46 (0.84–2.55) |
| Empiric broad spectrum antibiotics | 1.16 (0.70–1.94) |
| Legionella pneumophila (n = 32) | |
| Recent travel | 2.18 (0.99–4.76) |
| Fever (>38°C) | 3.21 (1.56–6.60) |
| Hyponatremia | 7.44 (3.5–15.67) |
| Diarrhea | 2.88 (1.39–5.95) |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Legionella pneumophila Results
Among the 1941 included patients, 1258 (64.8%) had ≥1 IDSA/ATS indication for LP urinary antigen testing and 32 (1.6%) had a positive LP UAT. The percentage of patients with a positive LP UAT was very similar among those with ≥1 IDSA/ATS indication (1.6%) and those without any IDSA/ATS indications (1.8%; P = .78; Figure 2). The presence of ≥1 IDSA/ATS indication for LP urinary antigen testing had 63% sensitivity (95% CI 44–79%) and 35% specificity (95% CI 33–37%) for identifying patients with a positive LP UAT. The only IDSA/ATS indication more common in patients with a positive UAT than a negative UAT was recent travel (OR 2.17, 95% CI:1.02–4.64) (Table 3).
In univariate analyses, several clinical characteristics selected from the literature that are not included in the IDSA/ATS indications for LP testing were found to be associated with a positive LP UAT result, including age ≥65 years, fever, diarrhea, headache, and hyponatremia (Table 4). In the multivariable model for LP, hyponatremia, fever, and diarrhea were significantly associated with a positive LP UAT, with recent travel nearly reaching significance (Table 5). The sensitivity and specificity of having ≥1 of these features (hyponatremia, fever, diarrhea, or recent travel) for a positive LP UAT was 88% (95% CI 71–97%) and 47% (95% CI 45–49%), respectively.
Urinary Antigen Test Results in the Context of Results for Viral Testing and Procalcitonin
SP and LP UAT results stratified by viral detection and procalcitonin levels are presented in Supplementary Tables 5 and 6. Positive SP and LP UAT results were rare in patients with respiratory viral detection, and more common in patients with high procalcitonin compared with low procalcitonin.
DISCUSSION
Using prospective, active-surveillance data with systematic urinary antigen testing of adults hospitalized with CAP, we found that positive SP and LP UAT results were infrequent and that the current IDSA/ATS CAP guideline recommendations for which patients should undergo these tests were not associated with positive test results. Targeted urinary antigen testing based on these criteria would have resulted in a large volume of testing with rare positive results in the EPIC study population. Additionally, we evaluated the association of other clinical variables not in the current IDSA/ATS indications for urinary antigen testing to explore whether a targeted testing approach based on other variables could potentially lead to a more efficient testing strategy. Our results suggest no clinical characteristic is strongly associated with a positive SP UAT. However, hyponatremia, recent travel, temperature > 38°C, and diarrhea were all associated with a positive LP UAT; further evaluating these criteria as indications for LP urine antigen testing may help inform future CAP guidelines.
If the IDSA/ATS recommendations were used in our study population to determine which patients to test with SP UATs, 61% of patients would have undergone testing, which would have identified 49 (60%) of the 81 patients with a positive test, yielding a number-needed-to-test (NNT) of 25 tests per positive test result. Assuming a cost of $17 dollars per test [28], the cost per each positive result would be $425. If all patients underwent SP urinary antigen testing without considering the IDSA/ATS recommendations, the NNT would have been 24, and the cost per positive test would have been $408.
Most prior work suggests pneumococcal pneumonia cannot be accurately identified based on clinical features, because its presentation overlaps substantially with pneumonia of other etiologies [13]. Our study supports the notion that pneumococcal pneumonia cannot be prospectively identified based on clinical features alone.
Given that empirical coverage of pneumococcus is standard for adult CAP [4], the clinical utility of pneumococcal urinary antigen testing has been questioned [29, 30]. A potential role for SP UATs is to use positive results as an indication to deescalate empiric antibiotics to narrower-spectrum agents (eg, penicillin). However, the prior literature suggests clinicians rarely narrow antibiotics started empirically for CAP, even when pathogens are detected [31, 32].
The prevalence of pneumococcal urinary antigen detection in our study (4.2%) was lower than reported in some prior CAP studies [13–15, 21]. Reasons for lower pneumococcal prevalence in the current study may be multifactorial, including patient enrollment after the introduction of widespread pneumococcal conjugate vaccine use; inclusion of patients with all-cause CAP, including those with no pathogen detected; and systematic urinary antigen testing in the study population that did not rely on a clinician’s decision to obtain the test.
If patients in our study underwent LP urinary antigen testing based on the IDSA/ATS recommendations, 63% would have undergone testing, which would have identified 20 (62.5%) of the 32 patients with a positive result, yielding a NNT of 50. Assuming each LP UAT costs $17 [28], the cost per positive test result would have been $850. If all patients in our study were tested with LP UATs without regard to the IDSA/ATS recommendations, the NNT would have been 59 and the cost per positive test results would have been $1003. Alternatively, if patients in our study underwent LP UAT based on the presence of hyponatremia, fever, diarrhea, or recent travel (the 4 variables in our multivariable model for LP), then 53.7% would have undergone testing, which would have identified 28 (87.5%) of the 32 patients with positive a UAT, yielding a NNT of 37, and a cost per positive test of $629.
Our study adds to the growing literature on clinical features of Legionella pneumonia. Historically, the clinical presentation of Legionella pneumonia was largely studied using retrospective study designs that were dependent on Legionella testing obtained during routine care [5–12, 33–36]. These studies were limited by selection biases, with the selective inclusion of patients specifically tested for Legionella by a treating clinician. Selective testing for Legionella in patients with high pneumonia severities may have led to overestimating the association between Legionella infection and severe pneumonia in prior studies [7, 35]. A strength of our study was systematic Legionella urinary antigen testing of all study patients. Using this strategy, we observed no association between Legionella pneumonia and either intensive care unit admission or other markers of severe pneumonia. Our results do support previous reports of Legionella pneumonia being associated with hyponatremia, recent travel, and gastrointestinal symptoms [7, 11, 12]
The incidence of Legionella cases appears to be increasing [37], and a positive LP UAT may have important clinical and public health implications. If empiric beta-lactam monotherapy is used for CAP, as is common in Europe [38], a positive LP UAT would prompt clinicians to change antibiotics to cover Legionella. While additional data are needed on the comparative effectiveness of fluoroquinolones and macrolides for the treatment of Legionella, some recent data suggest an advantage for fluoroquinolones [39]; hence, a positive LP UAT may also be used by clinicians to change from beta-lactam plus macrolide therapy to fluoroquinolone therapy. Furthermore, identifying Legionella cases can lead to outbreak investigations and source remediation [40].
Limitations of our study should be noted. First, the study included modest numbers of patients who tested positive for SP or LP by urinary antigen testing, which prohibited multivariable models with more predictor variables and reduced the precision of our estimates. Second, urine was not collected from 379 pneumonia patients enrolled in the parent EPIC study. As expected, the primary difference between patients who did and did not have urine samples collected was a higher prevalence of chronic dialysis in those who did not have urine collected. In addition to anuria, other factors leading to no urine collection included incontinence and patient refusal. This highlights some inherent limitations to using urine tests to evaluate for pathogens. Third, false-positive UATs are possible, due to prior infections with persistent antigen detection or, in the case of SP, recent vaccination [41–44]. Fourth, the EPIC study excluded some patients with high-risk features for S. pneumoniae and Legionella infections, including severe immunosuppression. Fifth, participation in EPIC required written informed consent, which resulted in the differential non-enrollment of patients who lacked the capacity to consent, leading to a differential exclusion of patients with high illness severities [17]. Sixth, this study was limited geographically to Chicago and Nashville, 2 urban areas in the United States [17]. Lastly, our analysis did not assess the clinical impact of urinary antigen test results, nor did it account for considerations beyond the yield of a positive result that may inform the decision to obtain a diagnostic test. For example, in patients empirically treated with broad-spectrum antibiotics, an etiologic diagnosis is particularly important for antibiotic stewardship. Similarly, a Legionella UAT may be useful to evaluate for potential outbreaks associated with contaminated water sources [40]. Urinary antigen testing may be useful in these patients, despite a low probability of a positive result.
In conclusion, clinical indications for pneumococcal and Legionella urinary antigen testing recommended in the 2007 IDSA/ATS CAP guidelines had poor sensitivity and specificity for identifying patients with positive UATs. Even when considering clinical characteristics outside the 2007 IDSA/ATS indications, we could not find any characteristics strongly associated with positive pneumococcal UATs. While 4 clinical characteristics (hyponatremia, diarrhea, fever, and recent travel) were associated with a positive Legionella UAT, positive Legionella tests were quite rare (1.6%); using a risk factor–based approach to Legionella testing, even using these 4 characteristics would have resulted in dozens of negative tests for each positive test. A consideration for future CAP guidelines may be to abandon the risk factor–based approach for testing indications in favor of other approaches, such as evaluating which patient types and clinical scenarios would benefit most from microbiological diagnoses.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. This work was supported by a cooperative agreement with the Centers for Disease Control and Prevention (number U18 IP000299).
Potential conflicts of interest. C. G. G. was supported in part by the National Institute on Aging (grant number R01AG043471) and has served as a consultant for Pfizer Inc and Merck. E. J. A. reports grants and non-financial support from MedImmune and grants from NovaVax and Regeneron, and has served as a consultant for Abbvie. W. H. S. was supported in part by the National Institute of General Medical Sciences (grant number K23GM110469) and has served as a consultant or scientific advisor for Cempra Pharmaceuticals, Ferring Pharmaceuticals, BioTest AG, Abbott Point of Care, Gilead Pharmaceuticals, and Pfizer. S. B. received support from the Office of Academic Affiliation, Department of Veterans Affairs (VA), and VA National Quality Scholars Program, and used the facilities of VA Tennessee Valley Healthcare System, Nashville, Tennessee. K. M. E. has served on a Data and Safety Monitoring Board for Novartis and her institution has received research support from Novartis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2015. NCHS Data Brief 2016; 267:1–8. [PubMed] [Google Scholar]
- 2. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in U.S. Hospitals, 2011: statistical brief #162. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US), 2006–2013. PMID: 24228292. [PubMed] [Google Scholar]
- 3. Sato R, Gomez Rey G, Nelson S, Pinsky B. Community-acquired pneumonia episode costs by age and risk in commercially insured US adults aged ≥50 years. Appl Health Econ Health Policy 2013; 11:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cunha BA. Clinical features of legionnaires’ disease. Semin Respir Infect 1998; 13:116–27. [PubMed] [Google Scholar]
- 6. Gupta SK, Imperiale TF, Sarosi GA. Evaluation of the Winthrop-University Hospital criteria to identify Legionella pneumonia. Chest 2001; 120:1064–71. [DOI] [PubMed] [Google Scholar]
- 7. Fiumefreddo R, Zaborsky R, Haeuptle J, et al. Clinical predictors for Legionella in patients presenting with community-acquired pneumonia to the emergency department. BMC Pulm Med 2009; 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haeuptle J, Zaborsky R, Fiumefreddo R, et al. Prognostic value of procalcitonin in Legionella pneumonia. Eur J Clin Microbiol Infect Dis 2009; 28:55–60. [DOI] [PubMed] [Google Scholar]
- 9. Hollenbeck B, Dupont I, Mermel LA. How often is a work-up for Legionella pursued in patients with pneumonia? a retrospective study. BMC Infect Dis 2011; 11:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Engel MF, van Manen L, Hoepelman AI, Thijsen S, Oosterheert JJ. Diagnostic, therapeutic and economic consequences of a positive urinary antigen test for Legionella spp. in patients admitted with community-acquired pneumonia: a 7-year retrospective evaluation. J Clin Pathol 2013; 66:797–802. [DOI] [PubMed] [Google Scholar]
- 11. Haubitz S, Hitz F, Graedel L, et al. Ruling out Legionella in community-acquired pneumonia. Am J Med 2014; 127:1010.e11–9. [DOI] [PubMed] [Google Scholar]
- 12. Roed T, Schønheyder HC, Nielsen H. Predictors of positive or negative Legionella urinary antigen test in community-acquired pneumonia. Infect Dis (Lond) 2015; 47:484–90. [DOI] [PubMed] [Google Scholar]
- 13. Huijts SM, Boersma WG, Grobbee DE, et al. ; CAP Diagnostics investigators. Predicting pneumococcal community-acquired pneumonia in the emergency department: evaluation of clinical parameters. Clin Microbiol Infect 2014; 20:1316–22. [DOI] [PubMed] [Google Scholar]
- 14. Molinos L, Zalacain R, Menéndez R, et al. Sensitivity, specificity, and positivity predictors of the pneumococcal urinary antigen test in community-acquired pneumonia. Ann Am Thorac Soc 2015; 12:1482–9. [DOI] [PubMed] [Google Scholar]
- 15. West DM, McCauley LM, Sorensen JS, Jephson AR, Dean NC. Pneumococcal urinary antigen test use in diagnosis and treatment of pneumonia in seven Utah hospitals. ERJ Open Res 2016; 2. doi: 10.1183/23120541.00011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Gu L, Qu JX, Liu YM, Cao B; For CAPCn. Evaluating the utility of Binax NOW Streptococcus pneumoniae urinary antigen test in adults with community acquired pneumonia in China. Clin Respir J 2018; 12:425–32. [DOI] [PubMed] [Google Scholar]
- 17. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farr BM, Kaiser DL, Harrison BD, Connolly CK. Prediction of microbial aetiology at admission to hospital for pneumonia from the presenting clinical features. British Thoracic Society Pneumonia Research Subcommittee. Thorax 1989; 44:1031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roig J, Aguilar X, Ruiz J, et al. Comparative study of Legionella pneumophila and other nosocomial-acquired pneumonias. Chest 1991; 99:344–50. [DOI] [PubMed] [Google Scholar]
- 20. Sopena N, Sabrià-Leal M, Pedro-Botet ML, et al. Comparative study of the clinical presentation of Legionella pneumonia and other community-acquired pneumonias. Chest 1998; 113:1195–200. [DOI] [PubMed] [Google Scholar]
- 21. Choi MJ, Song JY, Cheong HJ, et al. Clinical usefulness of pneumococcal urinary antigen test, stratified by disease severity and serotypes. J Infect Chemother 2015; 21:672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 23. Self WH, Balk RA, Grijalva CG, et al. Procalcitonin as a marker of etiology in adults hospitalized with community-acquired pneumonia. Clin Infect Dis 2017; 65:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alere. BinaxNOW(R) Streptococcus pneumoniae Antigen Card [package insert]. Scarborough, ME: Alere, 2017. [Google Scholar]
- 25.Alere. BinaxNOW(R) Legionella Antigen Card [package insert]. Scarborough, ME: Alere, 2017. [Google Scholar]
- 26. Shimada T, Noguchi Y, Jackson JL, et al. Systematic review and metaanalysis: urinary antigen tests for Legionellosis. Chest 2009; 136:1576–85. [DOI] [PubMed] [Google Scholar]
- 27. Harrell FE. Regression modeling strategies. New York, New York: Springer, 2001. [Google Scholar]
- 28. Centers for Medicare and Medicaid. Clinical diagnostic laboratory fee schedule 2017 Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Accessed 20 May 2018.
- 29. Falguera M, Ruiz-González A, Schoenenberger JA, et al. Prospective, randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax 2010; 65:101–6. [DOI] [PubMed] [Google Scholar]
- 30. Mandell L. Prospective randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax 2010; 65:93–4. [DOI] [PubMed] [Google Scholar]
- 31. Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003; 123:1142–50. [DOI] [PubMed] [Google Scholar]
- 32. Blasi F, Garau J, Medina J, Ávila M, McBride K, Ostermann H; REACH study group. Current management of patients hospitalized with community-acquired pneumonia across Europe: outcomes from REACH. Respir Res 2013; 14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsai TF, Finn DR, Plikaytis BD, McCauley W, Martin SM, Fraser DW. Legionnaires’ disease: clinical features of the epidemic in Philadelphia. Ann Intern Med 1979; 90:509–17. [DOI] [PubMed] [Google Scholar]
- 34. Wingfield T, Rowell S, Peel A, Puli D, Guleri A, Sharma R. Legionella pneumonia cases over a five-year period: a descriptive, retrospective study of outcomes in a UK district hospital. Clin Med (Lond) 2013; 13:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ueda A, Oki M, Yanagi H, Ozawa H, Takagi A. Clinical characteristics of Legionella pneumonia diagnosed with Legionella urinary antigen test. Tokai J Exp Clin Med 2016; 41:8–13. [PubMed] [Google Scholar]
- 36. Henry C, Boethel C, Copeland LA, Ghamande S, Arroliga AC, White HD. Clinical utility of testing for Legionella pneumonia in central Texas. Ann Am Thorac Soc 2017; 14:65–9. [DOI] [PubMed] [Google Scholar]
- 37. Dooling KL, Toews KA, Hicks LA, et al. Active bacterial core surveillance for legionellosis - United States, 2011-2013. MMWR Morb Mortal Wkly Rep 2015; 64:1190–3. [DOI] [PubMed] [Google Scholar]
- 38. Torres A, Blasi F, Peetermans WE, Viegi G, Welte T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis 2014; 33:1065–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burdet C, Lepeule R, Duval X, et al. Quinolones versus macrolides in the treatment of legionellosis: a systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:2354–60. [DOI] [PubMed] [Google Scholar]
- 40. Garrison LE, Kunz JM, Cooley LA, et al. Vital signs: Deficiencies in environmental control identified in outbreaks of Legionnaires’ Disease-North America, 2000–2014. Am J Transplant 2016; 16:3049–58. [DOI] [PubMed] [Google Scholar]
- 41. Centers for Disease Control and Prevention. Legionella (Legionnaires’ Disease and Pontiac Fever): surveillance and reporting Available at: https://www.cdc.gov/legionella/surv-reporting.html. Accessed 18 December 2017.
- 42. Grijalva CG, Wunderink RG, Zhu Y, et al. In-hospital pneumococcal polysaccharide vaccination is associated with detection of pneumococcal vaccine serotypes in adults hospitalized for community-acquired pneumonia. Open Forum Infect Dis 2015; 2:ofv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murdoch DR, Laing RT, Cook JM. The NOW S. pneumoniae urinary antigen test positivity rate 6 weeks after pneumonia onset and among patients with COPD. Clin Infect Dis 2003; 37:153–4. [DOI] [PubMed] [Google Scholar]
- 44. Sordé R, Falcó V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med 2011; 171:166–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.