Abstract
The plasma membrane forms the physical barrier between the cytoplasm and extracellular space, allowing for biochemical reactions necessary for life to occur. Plasma membrane damage needs to be rapidly repaired to avoid cell death. This relies upon the coordinated action of the machinery that polarizes the repair response to the site of injury, resulting in resealing of the damaged membrane and subsequent remodeling to return the injured plasma membrane to its pre-injury state. As lipids comprise the bulk of the plasma membrane, the acts of injury, resealing, and remodeling all directly impinge upon the plasma membrane lipids. In addition to their structural role in shaping the physical properties of the plasma membrane, lipids also play an important signaling role in maintaining plasma membrane integrity. While much attention has been paid to the involvement of proteins in the membrane repair pathway, the role of lipids in facilitating plasma membrane repair remains poorly studied. Here we will discuss the current knowledge of how lipids facilitate plasma membrane repair by regulating membrane structure and signaling to coordinate the repair response, and will briefly note how lipid involvement extends beyond plasma membrane repair to the tissue repair response.
Keywords: Lipids, plasma membrane, membrane injury, tissue repair
1. Introduction
The plasma membrane separates the extracellular environment from the cell interior, where biochemical reactions necessary for life occur. The plasma membrane is semi-permeable, allowing the cell to communicate with and utilize resources from its surrounding environment. However, all cells are susceptible to plasma membrane damage, which results in the mixture of the intracellular fluid and extracellular milieu and can result in death if the damage is not rapidly repaired. Plasma membrane repair relies on the coordinated activity of repair machinery, which carries out vesicle fusion to the membrane, membrane shedding, and polymerization of F-actin at the site of repair (Horn & Jaiswal, 2018). Repair of injured cells is tied closely to tissue repair and regeneration, as shown by studies demonstrating release of inflammatory mediators, including small molecules, peptides and proteins that signal to cells in the inflammatory and regenerative systems to initiate a tissue level reparative response.
The most abundant component of the cell’s plasma membrane is the lipids. Lipids are a class of biomolecules, which are generally insoluble in water, and may refer to fatty acids, sterols, mono-, di-, and triglycerides, as well as phospholipids, among others. While lipids are often ascribed a metabolic role as energy storage molecules, they also play important structural and signaling roles in the cell. Acute and chronic release of lipids and free fatty acids following cell and tissue injury has been widely recognized to be involved in the process of tuning the inflammatory and subsequent tissue repair response. Being the most abundant component of the plasma membrane, lipids are also essential player in the process of plasma membrane repair; however, much of the research committed to identifying the mechanisms of plasma membrane repair has focused on the proteins associated with plasma membrane repair (Cooper & McNeil, 2015).
Lipids contribute to cellular physiology at both an individual and population level. Individual lipids can serve as signaling molecules on their own or through binding proteins, and chemical changes to a single lipid can initiate change in local membrane composition. At the population level, the composition of lipids in a membrane can result in formation of signaling platforms that can change the properties of an entire membrane, enabling the cell to finely tune tension, shape, and rigidity. At each of these levels the structural and signaling aspects of lipids are critical for the cell to mount an efficient response to plasma membrane injury. In this review, we will focus on the role of lipids during plasma membrane repair by discussing their functions as both structural and signaling molecules. We will highlight how lipids respond to injury and facilitate repair both at the level of individual molecules and at the bulk level by collectively altering the plasma membrane form and function.
2. Plasma membrane lipid composition, organization, and response to injury
The plasma membrane has a unique lipid composition that helps distinguish its structural and functional properties from the other internal membrane-bound compartments. Plasma membrane lipids can be grouped into three classes – glycerophospholipids, sphingolipids, and sterols. Each of these lipids contributes their own qualities that affect the structural and signaling characteristics of the plasma membrane (Nicolson, 2014). In mammalian cells, lipids formed upon the phosphate and glycerol (e.g. diacylglycerol - DAG) backbone are called glycerophospholipids (referred to as phospholipids hereafter) and make up the majority of the plasma membrane. These phospholipids are derived from glycerol-3-phosphate, itself a product of cellular metabolism that is enzymatically modified into phosphatidic acid (PA). From PA, cells generate DAG, or cytidine diphosphate-DAG (CDP-DAG), which serve as inputs into the phospholipid biosynthetic pathways (Figure 1A, B). Changing the head group attached to the DAG backbone creates different phospholipid species, which include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), phosphatidylglycerol (PG), and PA (Oropeza, 2017) (Figure 1B). Among these lipids, PC is the most abundant in the plasma membrane and is formed by adding choline to the DAG backbone. It can also be produced by methylation of existing PE, while PE can be produced by the decarboxylation of PS. Further, PE and PC head groups can be cleaved and replaced with serine to produce PS (Oropeza, 2017). The variety of possible phospholipid interconversions, such as these, allows the cell to rapidly change the lipid composition of the plasma membrane. Thus, each of these lipids contributes significantly to the organization, structure, and function of the plasma membrane as a whole.
Figure 1: Lipid biosynthesis and signaling interactions.
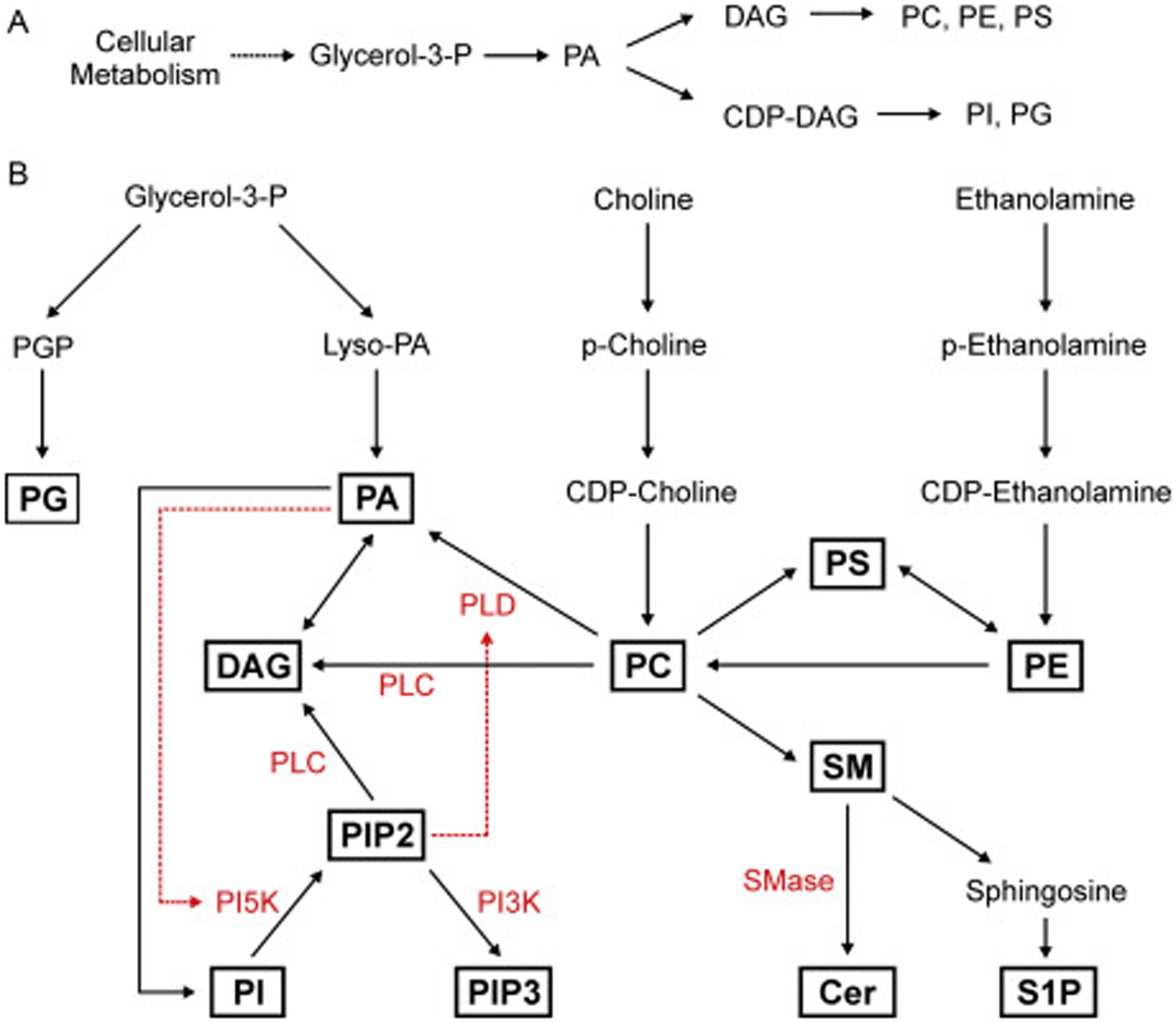
(A) Plasma membrane phospholipids are generated from biosynthetic pathways, which rely on formation of the molecule diacylglycerol (DAG) or cytidine diphosphate diacylglycerol (CDP-DAG) from phosphatidic acid (PA). PA is itself generated primarily from glycerol-3-phosphate, which is a product of glycolysis. Phospholipid biosynthetic pathways require the addition of different head groups to the DAG backbone. (B) Phospholipid biosynthesis depends on multiple inputs from metabolism (glycerol-3-phosphate) and extracellular import (choline and ethanolamine). Glycerol-3-phosphate is used to generate PA as well as PG through the intermediate phosphatidylglycerol phosphate (PGP). PA is then used to create other phospholipids including PI, PC, PS, and PE, through the use of the DAG backbone. Phospholipids and sphingolipids are connected by the head group choline, found on both PC and sphingomyelin (SM). SM utilizes the ceramide (Cer) backbone and can be used to generate the signaling lipid sphingosine-1-phosphate (S1P). Plasma membrane lipids are routinely interconverted as a means to maintain lipid homeostasis and for lipid-mediated signaling. Enzymes that generate lipid signaling after plasma membrane injury (in red) include the phospholipases C and D (PLC and PLD), phosphatidyloinositol kinases (PI5K and PI3K), and sphingomyelinase (SMase). Each of these provides another mechanism for generating specific membrane lipids acutely in parallel to biosynthetic inputs.
Plasma membrane lipids containing the alkanoamine sphingosine are called sphingolipids. The primary plasma membrane sphingolipid in mammalian cells is sphingomyelin, which utilizes a ceramide backbone (Merrill Jr, 2008). Sphingomyelin also confers different properties to the plasma membrane than the phospholipids, not least due to its preferential association with sterols (Ramstedt & Slotte, 2006). This preferential association between different lipids results in an important feature of the plasma membrane – its transverse and lateral heterogeneity. Many of the sphingolipids also contain a glycosidically bound carbohydrate moiety causing formation of the glycosphingolipids. Here the sugar (oligosaccharide) moiety faces the extracellular space interacting with other cells and extracellular ligands required for processes such as signaling, cell adhesion or intercellular interactions.
Sterols, or steroid alcohols, are a third type of plasma membrane lipid, with cholesterol being the predominant form found in mammalian cells. Unlike phospholipids and sphingolipids, which are structurally analogous, cholesterol is composed of a steroid backbone that results in a planar and more rigid molecule. While being a mostly non-polar molecule, owing to its steroid backbone and hydrocarbon chain, cholesterol does possess a hydroxyl group opposite the hydrocarbon chain. This structural arrangement is important for the organization of cholesterol in biological membranes as it results in the hydroxyl group associating with the neighboring lipid head groups and water, while the majority of the cholesterol molecule resides within the hydrophobic core of the membrane. In mammalian cells the majority of cellular cholesterol is found in the plasma membrane, where it can make up to 50% of lipid content (Van Meer, Voelker, & Feigenson, 2008). Cholesterol interacts with both phospholipids and sphingolipids, and its interactions with these lipids play a crucial role in determining the overall physical properties of the plasma membrane. For example, when inserted into a region abundant in phospholipids, cholesterol has a rigidifying effect; however, the opposite can be true with sphingolipids. In this case, cholesterol actually increases the fluidity among these lipids, which prevents them from forming a gel-like phase that is incompatible with the overall fluid nature of the plasma membrane (Krause & Regen, 2014). While the structural importance of plasma membrane cholesterol is well appreciated, it can also play a role in signaling. This is due to its protein-binding ability, resulting in protein translocation to the cholesterol-rich regions of plasma membrane (Sheng et al., 2012; Van Meer et al., 2008).
The plasma membrane is not a homogenous mixture of the different lipids described above (Figure 2A). Instead, there exists distinct inter- and intra-leaflet heterogeneity of lipids. This allows local and functional diversity between the two leaflets as well as various parts of the single contiguous plasma membrane (Figure 2B). Phospholipids in particular show inter-leaflet heterogeneity. While PC exists in both the inner and outer leaflet of the plasma membrane, the charged phospholipids – PE, PI, and PS – are almost exclusively maintained within the inner leaflet (Nicolson, 2014; van Meer, 1989). Similar inter-leaflet heterogeneity exists among sphingolipids, with the glycosphingolipids maintained exclusively in the outer leaflet. With an abundance of uncharged (zwitterionic) lipids and smaller amounts of neutral and anionic glycosphingolipids in the outer leaflet, and the negatively charged phosphatidylserine, phosphatidic acid and phosphatidylinositol within the inner leaflet, there exists a charge differential between the two plasma membrane leaflets (Steck & Lange, 2018; Zachowski, 1993). There also exists lateral heterogeneity of lipid composition within each leaflet, which is demonstrated by the formation of lipid microdomains, such as the sphingomyelin and cholesterol-rich domains that exist interspersed among the phospholipids throughout the plasma membrane (Cebecauer et al., 2018; Sezgin, Levental, Mayor, & Eggeling, 2017) (Figure 2B). These examples of organizational heterogeneity, along with the differences among the lipids that comprise the plasma membrane confer a variety of structural and signaling properties to the plasma membrane and allow the plasma membrane to mount and sustain localized signaling despite being fully interconnected and fluid. In the subsequent sections, we will discuss how this is achieved and utilized by the cell.
Figure 2: Plasma membrane structure and dynamics.
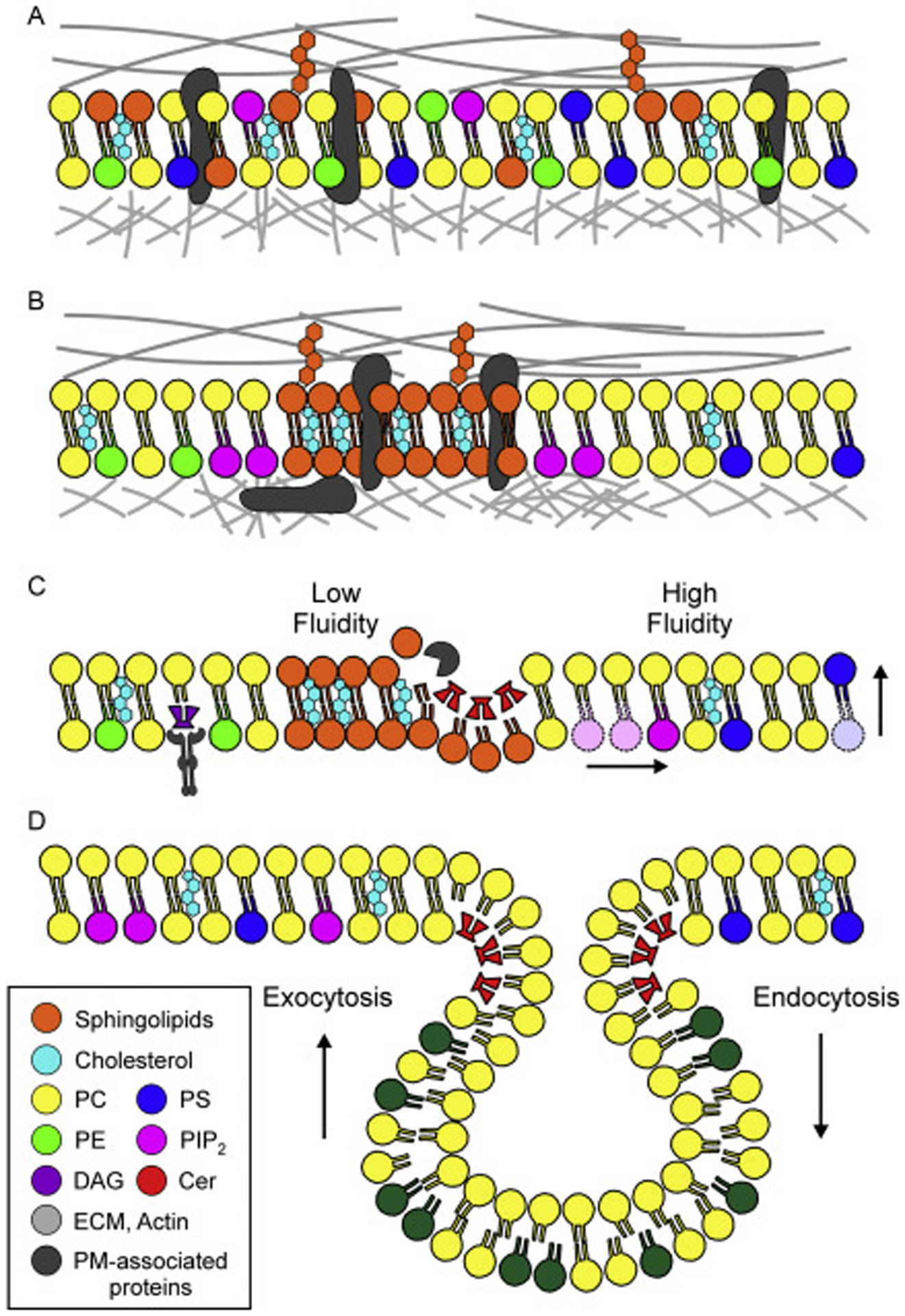
(A) The plasma membrane is a fluid-mosaic of lipids, including glycerophospholipids, sphingolipids, and cholesterol, as well as plasma membrane-associated proteins. Plasma membrane lipids and proteins interact with the extracellular matrix (ECM) and the cortical actin network, both of which provide sources of tension that support the structure of the membrane. (B) The plasma membrane is not a homogenous mixture of proteins and lipids (as in A). Instead, distinct inter- and intra-leaflet heterogeneity exists. Charged phospholipids such as PIP2, PS, and PE are almost exclusively found on the inner leaflet, while the glycosphingolipids are only found on the outer leaflet. Within a single leaflet, lateral heterogeneity is found in the form of lipid microdomains, such as the cholesterol and sphingolipid-rich domains that serve as signaling platforms to accumulate plasma membrane-associated proteins. The organization of lipids within the membrane also affects the structure of underlying cortical cytoskeleton. For example, PIP2 interacts with actin-associated proteins, resulting in a local strengthening of the plasma membrane-cytoskeleton interface. (C) Plasma membrane is dynamic and individual lipids have the capacity to move laterally within a leaflet (shown in pink) and between the leaflets (shown in blue). The fluidity of the membrane is determined in part by its composition, with cholesterol and sphingolipid-rich regions being less fluid than those areas comprised primarily of phospholipids. Furthermore, individual lipids may be modified by proteins, which generate new lipid species that can change membrane structural properties (red, Cer) or be used for signaling (purple, DAG). (D) The lipid make-up of the plasma membrane constantly changes. To regulate the composition of plasma membrane lipids, cells utilize vesicle trafficking, which can add lipids (by exocytosis) or remove lipids (by endocytosis) from the plasma membrane. This form of membrane remodeling maintains lipid homeostasis and plasma membrane functionality.
The plasma membrane is a dynamic barrier that separates the cell interior from the extracellular space (Figure 2C). Not only is the composition and organization of the plasma membrane in constant flux, the membrane itself also must interact with forces being applied to it from all directions. In some cases, these forces are benign, such as membrane protrusion driven by cortical actin polymerization (Pontes, Monzo, & Gauthier, 2017). However, excessive force applied to the membrane can result in physical damage, such as the shear force applied on the endothelial cells by blood flow, on muscle cells during contraction, on lung alveolar cells during breathing, or by a blunt force trauma to any cell (Cong, Hubmayr, Li, & Zhao, 2017; Demonbreun & McNally, 2016; McNeil & Steinhardt, 2003). While small injuries to the plasma membrane (on the nanometer scale) can be spontaneously resealed due to the line tension imposed by plasma membrane lipids alone (McNeil & Terasaki, 2001), larger membrane injuries (on the micron scale) require a series of coordinated mechanisms to undergo repair. Early observations of cells undergoing plasma membrane repair identified that membrane bound vesicles are involved in the process of membrane resealing (Bi, Alderton, & Steinhardt, 1995). It has since become evident that in addition to resealing, remodeling of the plasma membrane through vesicle fusion (exocytosis), vesicle internalization (endocytosis), and shedding (ectocytosis) also contribute to the repair process (reviewed in (Horn & Jaiswal, 2018)). Each of these pathways that facilitate repair through membrane remodeling also alter the local composition and distribution of plasma membrane lipids in healthy cells (van Meer, 1989) (Figure 2D). Constitutive fusion of biosynthetic vesicles is a major mechanism for delivery of new lipids and proteins, which helps to build and maintain the plasma membrane. This homeostatic process of vesicle fusion that maintains the plasma membrane at rest also enables plasma membrane repair through regulated fusion of vesicles triggered by calcium influx following plasma membrane injury (Horn & Jaiswal, 2018; McNeil & Steinhardt, 2003).
The role of lipids during plasma membrane repair goes far beyond a passive role in vesicle-mediated delivery of membrane lipids. As structural components of the plasma membrane, lipids are responsible for contributing to membrane tension, rigidity, and overall shape. After an injury, the biophysical properties of the plasma membrane, and the individual lipids themselves, are altered, eliciting changes to membrane rigidity and fluidity. These structural changes to lipids are both necessary for repair and potentially pathogenic if left uncorrected (Sreetama et al., 2018). In addition to their structural role, and often as an extension of it, lipids also provide a signaling function that facilitates plasma membrane repair. Injury to the plasma membrane also changes the biochemical environment within the cell. Influx of calcium from the extracellular space, as well as locally increased oxidation, both trigger lipid signaling that is required for repair. Given their integral role in forming the structural backbone of the membrane, lipids are ideally placed to act as damage sensors, initiating early signaling that sets the stage for subsequent repair machinery. Further, the complexity of signaling afforded by lipids allows the cell to coordinate a highly organized response to injury across space and time (Figure 1B). These observations suggest that lipids are not bystanders during the repair process, but are instead actively involved in organizing the playing field on which repair machinery operates.
3. Structural role for lipids during plasma membrane repair
The physical properties of the plasma membrane are governed in large part by the effect of lipid interactions at the population level. For example, lipid composition, distribution, and inter-lipid interactions actively control the rigidity and tension acting upon the plasma membrane, which in turn regulates cellular functions such as vesicle fusion, cell motility, and membrane resealing (Diz-Muñoz, Fletcher, & Weiner, 2013; Gauthier, Fardin, Roca-Cusachs, & Sheetz, 2011; Togo, Krasieva, & Steinhardt, 2000). The tension forces acting on the plasma membrane are generally applied by three sources: the difference in hydrostatic pressure between the cell interior and extracellular space, the cortical cytoskeletal network, and the extracellular matrix to which the cell is attached (Gauthier, Masters, & Sheetz, 2012; Kozlov & Chernomordik, 2015) (Figure 2B). The plasma membrane itself forms the barrier responsible for the hydrostatic pressure experienced by the cell, while the cortical actin cytoskeleton, focal adhesions, and extracellular matrix regulate the structural tension (Burridge & Guilluy, 2016; Gauthier et al., 2012). As these features change dynamically, tension forces experienced by the plasma membrane are not static and the cell’s ability to respond to them appropriately allows for essential functions such as change in morphology, movement, adhering to new substrates, cell division, and membrane fusion. These functions also require modulating the membrane area, such as through the processes of exocytosis and endocytosis or by changing the existing membrane architecture (Kozlov & Chernomordik, 2015; Nassoy & Lamaze, 2012).
While tension acts at the level of an entire membrane, the physical properties of the individual lipids and lipid domains dynamically guide the local changes required for adapting to the change in tension. Lateral movement, rotation, and flipping of lipids between the leaflets of the plasma membrane are the physical changes that work together to allow the fluid membrane to adapt to the changes in membrane tension (Nicolson, 2014) (Figure 2C). These same processes also work to dynamically control membrane fluidity by regulating the distribution of phospholipids, sphingolipids, and cholesterol in the membrane. Due to the differences in the three-dimensional conformations of membrane lipids, a change in their distribution changes the lipid packing density in a given lipid domain. For example, replacement of a cylindrical lipid (e.g. PC), with a conical shaped, charged phospholipid (e.g. PS) in the inner leaflet, causes the plasma membrane to attain an overall outward curvature. In contrast to PC, sphingomyelin forms a taller and narrower cylinder, which along with the configuration of its acyl chains, allowing this lipid to pack more tightly than phospholipids (Cebecauer et al., 2018). The tight packing, along with the preference of cholesterol to intercalate the acyl chains of sphingomyelin creates the liquid-ordered membrane domains, such as the so-called “lipid rafts” (Van Meer et al., 2008) (Figure 2C). These domains decrease the local fluidity in the membrane relative to the regions comprised primarily of phospholipids. This is especially important for membrane signaling functions as the liquid-ordered domains often serve to aggregate membrane-associated proteins (Cebecauer et al., 2018). Lipids also interact with plasma membrane localized proteins to regulate membrane tension and fluidity. For example, the cytoskeletal proteins interact with membrane lipids to supply the cortical tension that regulates the global shape of the plasma membrane and produces cell movement (Cebecauer et al., 2018; Sezgin et al., 2017). Membrane proteins also help locally shape and provide rigidity to the plasma membrane by interacting with specific lipids or lipid domains (Cebecauer et al., 2018). All of the above mechanisms for regulating the physical properties of the membrane play important roles in determining how a cell responds to plasma membrane injury and undergoes successful repair.
3.1. Plasma membrane tension and dynamics are interrelated after injury
In response to an injury, a sudden change in hydrostatic pressure and local disassembly of the cortical cytoskeleton causes a rapid drop in membrane tension (Jaiswal et al., 2014; Miyake, McNeil, Suzuki, Tsunoda, & Sugai, 2001; Togo et al., 2000). Further, the addition of new membrane by increased calcium-triggered exocytosis also decreases membrane tension and is required for plasma membrane repair (Togo et al., 2000). However, to successfully repair the cell also needs to restore the barrier function of the resealed membrane. This process is itself facilitated by mechanisms that regulate membrane tension, in particular the re-establishment of the actin cortex. While initial loss of cortical actin aids in repair by allowing physical access for vesicles to fuse with the plasma membrane, the accumulation of F-actin at the injury site in the minutes following injury, is also required to provide structural support to the repairing/repaired membrane and prevent additional injury due to membrane fragility (Demonbreun et al., 2016; Horn et al., 2017; Jaiswal et al., 2014; McDade, Archambeau, & Michele, 2014; Miyake et al., 2001). Quantification of membrane tension after injury to fibroblasts demonstrated that a minimum tension force was reached by approximately 30 seconds post-injury, but tension was fully restored by 80 seconds post-injury (Togo et al., 2000). These observations align well with the observed kinetics of membrane resealing, which suggests that plasma membrane wounds can be resealed within 30 seconds; however, restoration of membrane tension shortly afterward is required for successful membrane remodeling and completion of the repair process.
While reassembly of the cortical cytoskeleton in the minutes following injury is known to restore membrane tension (described in Section 3.3), membrane remodeling also contributes to increasing tension. In order to balance any decrease in membrane tension, such as that caused by exocytosis, cells routinely utilize endocytosis, which reduces the membrane area and results in increased tension (Dai & Sheetz, 1995). Endocytosis also occurs in response to plasma membrane injury and has been described as a mechanism for membrane resealing (Idone et al., 2008). However, in addition to this role, restoring membrane tension is another role that has been proposed for endocytosis after the membrane is resealed following a pore forming toxin injury (Skalman, Holst, Larsson, & Lundmark, 2018). This study showed that endocytosis occurs after membrane resealing and is mediated by the CLIC/GEEC pathway effector GRAF1, which is implicated in repair of muscle fiber plasma membrane injury (Lenhart et al., 2015). Accumulation of GRAF1 at the repair site occurs 2 minutes after injury, supporting its potential role in membrane remodeling following resealing. Such a role of CLIC/GEEC-mediated endocytosis in membrane remodeling is also supported by the observation that CLIC/GEEC is immediately activated in response to decreased membrane tension and has the capacity to turn over large amounts of plasma membrane (Thottacherry et al., 2018). The observation, that endocytosis may not be required for resealing through the removal of pore-forming toxins but instead acts as a mechanosensor to buffer membrane tension, may also explain how endocytosis participates in the repair of mechanical damage, where no membrane-spanning pores need to be removed.
Endocytosis can also occur at sites of membrane invagination known as caveolae, and caveolae internalization has been implicated in membrane repair (Corrotte et al., 2013). In response to increased stress on the plasma membrane, caveolae have been proposed to act as mechanosensors, buffering membrane tension in response to mechanical stress (Cheng et al., 2015; Sinha et al., 2011). These membrane invaginations require the presence of caveolin proteins, loss of which can result in poor membrane repair and muscular dystrophy (Gazzerro, Sotgia, Bruno, Lisanti, & Minetti, 2010; Minetti et al., 1998). However, whether caveolae facilitate repair by buffering membrane tension remains unclear because, unlike the capacity of CLIC/GEEC endocytosis, caveolae make up a small portion (as small as 0.03%) of the membrane area and are not found ubiquitously in all cells (Gauthier et al., 2012; Sinha et al., 2011). It remains possible that caveolar endocytosis does help partially balance membrane tension, but another possibility is that they serve as mechanosensitive platforms during membrane repair. For example, caveolae are the site for assembling membrane repair proteins such as EHD2 and MG53 (Cai et al., 2009; Daumke et al., 2007; Marg et al., 2012). Thus, caveolae may serve a signaling function during membrane repair (see Section 4).
3.2. Regulation of plasma membrane lipid mobility and oxidation is required for repair
Similar to the changes in tension described above, the fluidity of the plasma membrane is also dynamic after injury. Plasma membrane damage increases the fluidity of individual lipids, allowing them more freedom to migrate laterally, rotate, or even flip – appearing in the opposite leaflet of the membrane. This phenomenon may be explained by the kinetic energy imparted on the membrane lipids by mechanical disruption, which can result in lipid mixing without relying on membrane tension (Petersen, Chung, Nayebosadri, & Hansen, 2016). Indeed, mechanical injury of healthy cells transiently increases the mobile fraction of plasma membrane lipids by as much as 9% (Sreetama et al., 2018). However, uninjured muscle cells from Limb Girdle Muscular Dystrophy 2B (LGMD2B) patients show increased membrane fluidity, which is associated with their poor membrane repair ability (Sreetama et al., 2018). The poor repair of these patient cells can be mirrored in healthy cells by increasing their membrane lipid mobility through the removal of cholesterol or by addition of a glucocorticoid (prednisone) (Heier et al., 2013; Sreetama et al., 2018). Thus, while increase in lipid mobility following plasma membrane injury is associated with successful repair, excessive lipid mobility leading to membrane instability is detrimental to the repair process. This Goldilocks principle applies to several aspects of lipid involvement in the repair process. In the case of lipid mobility, this principle is exemplified by the observation that decreasing the excessive lipid mobility in LGMD2B patient cells using a membrane permeant modified glucocorticoid improves repair (Sreetama et al., 2018). Similar benefits have been attributed to membrane stabilizing copolymers such as poloxamer 188, that improved repair after physiological mechanical injury (Plataki, Lee, Rasmussen, & Hubmayr, 2011), and injury to dystrophic cells (Houang et al., 2015; Yasuda et al., 2005). While membrane stabilization at the time of injury appears to improve membrane repair, a chronically rigid plasma membrane may inhibit the beneficial effects of transient lipid mobility after injury. Copolymers such as poloxamer 188 avoid this potential issue by only stabilizing the plasma membrane once lipid packing density is sufficiently reduced, such as after an injury. This is due to their lack of integration into the membrane under normal lipid packing conditions. Once membrane tension is restored, the poloxamer compound is forced out of the outer membrane leaflet (Houang, Sham, Bates, & Metzger, 2018). These examples illustrate the far-reaching consequence of lipid movement on structural stability of the plasma membrane and its ability to successfully repair.
Transient change in lipid mobility can have many effects on plasma membrane function related to both structure and signaling, and deciphering which of these are beneficial for repair requires further studies. One of the roles of lipid mobility may be to allow for the movement of individual lipids to form microdomains near the site of injury, which has been shown to be important for repair (Vaughan et al., 2014), perhaps by facilitating lipid-mediated signaling. For example, shear force on the plasma membrane, such as that experienced during a mechanical injury, results in lipid mixing, which increases the mobility of signaling lipids and proteins residing in stable lipid microdomains (Petersen et al., 2016). This may allow these lipids and proteins to interact with new partners that were unavailable due to spatial segregation prior to injury. Furthermore, exposure of the plasma membrane hydrophobic core as a result of reduced lipid packing provides the opportunity for injury-triggered lipid signaling through the binding of cholesterol (see Section 4).
Endogenous mechanisms of repair in healthy cells appear to mimic the beneficial effect provided by poloxamer 188 by allowing for transient increases in lipid mobility while restricting fluidity increase to a relatively small spatial or temporal window. One such mechanism for this may be mediated by the protein MG53. While intracellular MG53 is a redox-sensitive protein capable of facilitating vesicle aggregation, it is proposed to have multiple functions during membrane repair. It also works extracellularly to improve repair of injured muscle and other cells (Gushchina et al., 2017; Weisleder et al., 2012). Repair of muscle fibers lacking the dysferlin protein, which results in reduced membrane stability, is improved by the presence of extracellular (oxidized) MG53 protein, suggesting that this protein can act on the outer leaflet of the plasma membrane to improve plasma membrane in diseased cells. This raises the intriguing possibility that MG53 may affect plasma membrane structural properties. As MG53 interacts with the plasma membrane in a cholesterol-dependent manner ((Zhu et al., 2012); see Section 4), it’s extracellular role may involve a mode of action similar to poloxamer 188, where it inserts within membranes in a disordered lipid environment. This mechanism is particularly intriguing in light of the redox-sensitive nature of MG53. Local oxidation at the site of membrane injury activates MG53 oligomerization (Cai et al., 2009), which may locally increase membrane rigidity. This is notable because it is hypothesized that a beneficial role of decreasing membrane fluidity is preventing the spread of harmful lipid hydroperoxides, which likely form in the oxidative environment near the site of injury (Braughler & Hall, 1992; Hall, Wang, Miller, Cebak, & Hill, 2018). Transient and local lipid peroxidation may affect membrane stabilization as peroxidation of polyunsaturated fatty acids changes head group separation and acyl chain interdigitation, increasing their molecular volume and causing formation of distinct cholesterol domains in the membrane, which increases the membrane rigidity (Jacob & Mason, 2005; Mason, Walter, & Mason, 1997). Thus, local lipid peroxidation may provide transient membrane stabilization, while mechanisms such as redox-dependent MG53 binding may limit the spread of lipid peroxides. Limiting the spread of lipid hydroperoxides may also explain the beneficial effect of vitamin E treatment on plasma membrane repair (Howard, McNeil, & McNeil, 2011; Labazi et al., 2015). Compared with cytosolic antioxidants, which can be detrimental to repair (Spaeth et al., 2012), vitamin E is membrane-localized and could therefore allow for the local buildup of oxidized lipids at the site of injury while preventing the global spread of lipid oxidation. Another endogenous mechanism of acutely decreasing membrane lipid mobility after injury involves the lipid species ceramide, the formation of which causes endocytosis in response to membrane injury through the activity of extracellular acid sphingomyelinase (Tam et al., 2010). This process, facilitated by the dysferlin-mediated release of acid sphingomyelinase (Defour et al., 2014) creates microdomains of ceramide from sphingomyelin. However, ceramide formation also changes membrane dynamics by forming rigid microdomains and potentially by displacing cholesterol from lipid rafts (London, 2004).
3.3. Lipid-protein interactions shape the repairing membrane
The discussion above illustrates several roles for lipids in changing biophysical properties of the injured plasma membrane during repair. Additional structural changes the plasma membrane must adopt for repair include molding the shape of the membrane to limit wound expansion as well as pulling in the membrane edges to close the wound. In addition to working on their own, lipids also interact with proteins to coordinate these processes. Annexins are a class of calcium-sensitive proteins that rapidly accumulate at the site of a membrane injury (Potez et al., 2011). Their localization and function in response to injury is controlled by the patterning of anionic lipids such as PS, which is present at the wound edge and is a known binding partner of annexins (Gerke, Creutz, & Moss, 2005). Annexins can physically manipulate the injured plasma membrane by stabilizing, folding, and contracting in order to facilitate repair (Gerke et al., 2005; Jaiswal & Nylandsted, 2015). With their calcium and lipid affinities spanning a wide spectrum, annexins accumulate slightly differently from each other at the site of injury and perform different functions to facilitate repair of the wounded plasma membrane (see Section 4.1). For example, Annexin 5 accumulates first to form a lattice structure, which provides a force opposing the tension at the wound edge (Bouter et al., 2011). This can limit the expansion of the wound area itself. Following this, accumulation of Annexin 4 and Annexin 6 results in folding and inward contraction of the injured membrane, respectively (Boye et al., 2017). In contrast, Annexin 1, one of the earliest responders to plasma membrane injury (Leikina et al., 2015; McNeil, Rescher, Gerke, & McNeil, 2006), does not appear to be essential for plasma membrane repair (Leikina et al., 2015; McNeil et al., 2006). Along with the annexins, which act quickly to physically manipulate the membrane itself, reassembly of the cytoskeleton plays a crucial role in restoring membrane tension in the later stages of repair. This cytoskeletal assembly is regulated by Rho GTPase activity, which triggers F-actin accumulation at the site of injury. Spatial arrangement of lipids is also known to regulate Rho family GTPase activity (see Section 4.3).
4. Signaling role for lipids during plasma membrane repair
For plasma membrane repair to occur successfully, the cell must possess a means to sense that injury has occurred, coordinate the change in activity and localization of repair machinery, and ultimately close the wounded area. To achieve these tasks, cells employ signaling networks, which respond to the changing microenvironment after injury and activate the diverse plasma membrane repair mechanisms with precise control in time and space. The structural role of lipids described above illustrates how their ability to modify the biophysical properties of the plasma membrane is beneficial for resealing. Lipids also react to the changing biochemical environment to become signaling molecules that determine the spatiotemporal dynamics of protein activation (Eyster, 2007) (Figure 1B). This is in part achieved through the activity of lipid modifying enzymes, such as kinases, phosphatases, and phospholipases. Influx of calcium, increase in oxidation, and change in tension on the plasma membrane due to injury all trigger changes in lipid structure and behavior to initiate signaling. Therefore, injury-triggered protein-lipid interactions that result in modified lipids generates signaling that allows for changes in activity and localization of plasma membrane repair machinery. Below we describe the biochemical signaling role of lipids in facilitating plasma membrane repair.
Lipids act as signaling molecules through their ability to directly bind or otherwise alter the activity or localization of target proteins (Figure 2B, C). Lipids may serve as ligands for specific proteins, or act as a scaffold to bring cytosolic proteins to the plasma membrane. Direct lipid modification of proteins, such as GTPases, provides another method to regulate protein localization and activity (Casey, 1995). While a common thread in lipid signaling is the modification of a target protein, either directly or indirectly, the numerous mechanisms lipids use to achieve this outcome allows for a great diversity of signaling. For example, the dynamic arrangement of lipids in the plasma membrane as discussed above, and the electrostatic or chemical changes in lipids due to enzymatic activity of lipid modifying enzymes rapidly affect lipids themselves as well as the target proteins in the plasma membrane (Figure 2B, C). Collectively, these changes enable lipids to initiate/regulate local signaling allowing precise spatial and temporal control over downstream plasma membrane repair pathways. The goal of signaling during plasma membrane repair is to generate a polarized response such that the repair machinery can be spatially and temporally localized and activated at the repair site. Plasma membrane lipids help with successful repair by being part of the affected entity that also works to sense membrane injury, providing spatial and temporal cues to trigger signaling for downstream repair pathways, and ultimately being the benefactor of the successful wound repair response.
4.1. Sensing and containing the membrane damage
One signaling function of lipids is the recruitment of peripheral membrane proteins to the plasma membrane. As described above, damage to the plasma membrane transiently increases the mobility of individual lipids. This allows for the movement and patterning of lipids into signaling domains, changing the spatial arrangement of proteins that selectively interact with a particular lipid species. In this way, lipids play an important role in polarizing the cellular response to an injury.
The signaling lipid PS is interesting in this regard due to its localization to the wound edge after injury. Accumulation of PS at the site of membrane injury allows it to act as a damage sensor, marking a key site for the recruitment of repair proteins such as annexins (Boye et al., 2017). Unlike Annexin 5 that forms a lattice structure at the wound edge (Bouter et al., 2011), Annexins A4 and A6 work together to mediate constriction of the wounded edges of the plasma membrane, aiding in the closure of the wound (Boye et al., 2017). Presence of PS at the free membrane wound edge helps directs the annexin proteins to this site in a calcium-dependent manner, where they perform vital functions required for stabilization and shaping of the repairing membrane (see Section 3.3). In a similar manner, PS signaling may be involved in the early recruitment of other membrane repair proteins such as dysferlin (McDade et al., 2014; Middel et al., 2016) and MG53 (Cai et al., 2009). In addition to allowing for the lateral translocation of membrane lipids, injury-triggered increase in membrane fluidity also results in reduced lipid packing. This causes the exposure of the membrane hydrophobic core and allows proteins to interact with cholesterol. The MG53 binding protein PTRF (cavin-1) binds cholesterol in response to membrane injury (Zhu et al., 2012). PTRF presumably contributes to the translocation of MG53 to the injury-site, where MG53 is also able to bind PS and become activated in an oxidation-dependent manner (Cai et al., 2009).
While the local accumulation of proteins such as annexins acts to stabilize the structurally unstable membrane after injury, remodeling of the membrane itself can achieve a similar outcome. For example, membrane shedding is a known mechanism of plasma membrane repair, which functions to limit the lateral expansion of the wound area (Andrews, Almeida, & Corrotte, 2014; Horn & Jaiswal, 2018). Formation of these outward budding vesicles at the plasma membrane is associated with an increase in cytosolic calcium and oxidation, as well as the disruption of the actin cytoskeleton–plasma membrane interface (Pollet, Conrard, Cloos, & Tyteca, 2018), and each of these occurs locally in the immediate aftermath of membrane injury (Andrews et al., 2014; Horn & Jaiswal, 2018). Mechanistically, the process of membrane shedding is mediated by the endosomal sorting complexes required for transport (ESCRT) proteins (Jimenez et al., 2014; Scheffer et al., 2014). ESCRT assembly for membrane shedding is activated by the calcium-dependent ALG-2 protein, which then recruits its interacting partner ALIX leading to subsequent recruitment of the remaining complex members (Scheffer et al., 2014). While this mechanism outlines how ESCRT proteins assemble, the lipid signaling that determines the appropriate spatial localization relative to the membrane injury in order to limit wound expansion has not been elucidated. Of potential interest in this regard is the unconventional phospholipid lysobisphosphatidic acid (LBPA), which is found on endolysosomes. This lipid binds ALIX and is capable of budding vesicles away from the cytosol (Matsuo et al., 2004). Presence of LBPA at the plasma membrane through vesicle fusion could allow for ALIX recruitment and ESCRT assembly (Bissig et al., 2013); however, the presence of LBPA at membrane wound sites has not been directly observed. Following injury, lysosomes are known to fuse with the damaged membrane and may deposit LBPA at the site of injury, which would in turn facilitate ALIX-mediated vesicle shedding. Lysosome fusion is required for the process of repair (Reddy, Caler, & Andrews, 2001). Delivery of membrane (i.e. lipids) is one of the roles proposed for lysosome-mediated repair (Andrews et al., 2014; McNeil, 2002). Another role identified for lysosomal fusion in membrane repair is the secretion of the lipid modifying enzyme acid sphingomyelinase (ASM) (Defour et al., 2014; Tam et al., 2010). ASM activity at the plasma membrane has been shown to facilitate microvesicle shedding (Bianco et al., 2009). Furthermore, ASM activity at the injury site would locally produce ceramide from sphingomyelin. Structurally, the conical ceramide molecule results in negative membrane curvature associated with rapid nucleation of ESCRT complex proteins (Lee, Kai, Carlson, Groves, & Hurley, 2015). Ceramide microdomains formed by the activity of extracellular acid sphingomyelinase near the site of injury could appear on the extracellular leaflet (resulting in endocytosis) or the inner leaflet either by selective flipping across the membrane bilayer (Pollet et al., 2018) or through diffusion of sphingomyelinase through the wounded area to act on inner leaflet sphingomyelin found proximal to the wound edge. In either case, the outward budding of the membrane produced by these ceramide-rich microdomains is thought to assist in microvesicle shedding.
Another role of plasma membrane lipids in wound detection is that of mechanosensing provided by lipid microdomains, such as caveolae, by way of sensing injury-triggered alteration in membrane stress (see Section 3.1). While it is unclear whether caveolae can provide structural assistance to the damaged membrane in the context of a large membrane injury (as opposed to sub-injury threshold stress, (Sinha et al., 2011)), caveolae may still provide a signaling function during the repair process. This signaling function may occur due to the activity of the repair protein EHD2, which accumulates at the site of membrane injury and is thought to play a role in shaping membrane dynamics (Daumke et al., 2007; Demonbreun et al., 2016; Marg et al., 2012). EHD2 localization at caveolae results from its affinity for phosphatidylinositol 4,5-bisphosphate (PIP2), which is enriched around the caveolae boundary (Parton & Del Pozo, 2013; Simone, Caplan, & Naslavsky, 2013). PIP2 also accumulates at the site of membrane injury (Demonbreun et al., 2016; Vaughan et al., 2014). The precise role of EHD2 in membrane repair is not known, although it is known as a membrane shaping protein that may be involved in membrane fusion (Campelo, Fabrikant, McMahon, & Kozlov, 2010). Interestingly, as a result of caveolae flattening, EHD2 dissociates from the plasma membrane and translocates to the nucleus where it alters gene transcription (Torrino et al., 2018). This suggests the possibility that caveolae could act as mechanosensors that facilitate adaptation to membrane injury through gene transcription, although this remains to be explored.
4.2. Initiating and coordinating downstream repair pathways
Translocation of existing lipid species to the site of injury provides a mechanism to recruit and activate protein machinery. This is especially important in sensing membrane damage. However, lipid signaling also helps to coordinate downstream repair pathways that result in the membrane trafficking and cytoskeletal reorganization required for repair. This is achieved in part through the activity of lipid modifying enzymes, such as phospholipases, which are activated by the changing biochemical environment after injury. These enzymes initiate signaling through the generation of new lipid species, providing an added spatial, as well as a temporal component to lipid signaling, helping to more precisely coordinate the repair response.
Calcium entry after plasma membrane injury is required for repair. Calcium can activate proteins directly, and ultimately is the initiator of many downstream repair pathways. Calcium also initiates lipid signaling after plasma membrane injury by activating phospholipases, such as phospholipase C (PLC). While PLC is able to cleave PC, the classic substrate of PLC is PIP2, which upon cleavage generates the membrane bound DAG and the cytosolic inositol trisphosphate (IP3) – both of which are increased after injury (Lamb, Harper, McKinney, Rzigalinski, & Ellis, 1997; Vaughan et al., 2014). Similar to PS, DAG directly binds proteins to provide its signaling function; however, because it is generated de novo after injury, an additional temporal component to signaling is achieved. Additionally, in contrast to PS, which recruits proteins directly involved in repair, DAG appears to recruit signaling proteins such as protein kinase C (PKC) (Vaughan et al., 2014; Zuzek, Fan, Spaeth, & Bittner, 2013). PKC, in turn, influences a wide variety of downstream pathways known to facilitate repair including vesicle fusion, Rho GTPase patterning, as well as repair potentiation (Togo, 2004; Vaughan et al., 2014; Zuzek et al., 2013). Thus, DAG acts as a scaffold that initiates and coordinates downstream signaling pathways within a tight spatial and temporal window rather than as a marker of membrane injury for fast-acting structural proteins. Along with DAG, IP3 is also generated by the activity of PLC. It is unclear what role, if any, that IP3 may have in repair, but its role in calcium signaling and the fact that injured cells secrete IP3 for hours post-injury (Lamb et al., 1997) suggest a possible signaling role in repair that may extend beyond the process of membrane resealing, which needs further investigation.
Similar to PLC, phospholipase D (PLD) activity is also increased after injury and is required for repair (Arun et al., 2013). While PLD is also activated by calcium, recent findings have provided insight into the role of mechanical stress on initiating lipid signaling regulated by PLD (Petersen et al., 2016). This study showed that shear force on the membrane, as experienced by cells during mechanical injury, facilitated increased PLD activity through the process of lipid mixing. Prior to increase in shear force, PLD associates with lipid rafts, physically segregated from its activator PIP2 and its substrate PC (Petersen et al., 2016). However, shear force on the membrane increases the kinetic energy required for lipid mixing (i.e. increased lipid fluidity described in Section 3) and allows for the membrane associated PLD to diffuse outward from lipid rafts and interact with PIP2 in order to metabolize PC. Importantly, sequestration of cholesterol alone increased PLD activity, supporting the idea that transient increase in lipid fluidity after membrane injury may be required for PLD-mediated signaling. The product of PC metabolism by PLD is the signaling lipid PA (Cazzolli, Shemon, Fang, & Hughes, 2006). While no defined roles for PA after membrane injury are known, PA has been observed to rapidly appear at the wound edge (Vaughan et al., 2014), which fits with the fast (650 μs) timescale of PA generation by PLD (Petersen et al., 2016). Further, PA is implicated in processes critical to the success of membrane repair such as vesicle fusion with the plasma membrane and GTPase signaling (Cazzolli et al., 2006; Zhang & Du, 2009). In addition to PA, PG is another lipid product of PLD activity relevant to membrane repair, and its synthesis has been implicated in the process of membrane repair and repair of epithelial wounds (Arun et al., 2013; Piazza & Marmer, 2007).
4.3. Closing the wound and membrane remodeling
Wound closure is mediated in part by the interactions of the cortical actin cytoskeleton with the plasma membrane. While initial depolymerization of the local F-actin network is thought to assist with vesicle fusion and membrane shedding, delayed accumulation of F-actin may facilitate repair either by working in coordination with myosin to pull the wounded membrane edges toward each other or by providing a barrier and stabilizing function for the newly formed membrane. F-actin accumulation is also responsible for providing support to the newly resealed membrane, restoring tension, and preventing subsequent injury. F-actin reorganization during repair is coordinated by the activity of lipids through their regulation of signaling proteins (reviewed in (Horn & Jaiswal, 2018)), as well as through direct interaction with actin-binding proteins such as F-actin bundling by the Annexin A2-S100 A11 complex (Jaiswal et al., 2014).
Small GTPases, such as Rho family members, associate with biological membranes via lipid modifications (ten Klooster & Hordijk, 2007); however, selectivity for the plasma membrane is regulated through the polybasic domain comprised of a cluster of positively charged amino acids (Do Heo et al., 2006; Maxwell, Zhou, & Hancock, 2018). This allows small GTPases, such as Rac1 and Cdc42 to preferentially bind the negatively charged PIP2 and PIP3 (Johnson, Erickson, & Cerione, 2012; Maxwell et al., 2018; Remorino et al., 2017). Thus, electrostatic interaction with signaling lipids helps to facilitate GTPase localization, which is a critical feature that determines the efficiency and magnitude of the downstream response (Das et al., 2015; Yogurtcu & Johnson, 2018). These lipids also exist at the boundary of lipid-ordered domains, such as lipid rafts, indicating that GTPases are targeted to these regions where protein accumulation at the membrane is common (Moissoglu et al., 2014), increasing their relative signaling capacity. Rac1, a Rho family GTPase required for repair (Verboon & Parkhurst, 2015), forms nanoclusters at sites enriched in PA and PIP3, whose roles in regulating Rac1 appear to be non-overlapping (Maxwell et al., 2018). While PA mediates targeting to the membrane, Rac1 activity depends on PIP3, suggesting multiple roles for signaling lipids in GTPase activity after repair.
The spatial arrangement of lipids at the plasma membrane is not only important for GTPase recruitment, but also for their activity. GTPases are molecular switches that require the cycling of nucleotides to remain active. This process is more efficient when GTPases and their regulatory proteins (which are themselves regulated by lipids) are clustered (Ligeti, Dagher, Hernandez, Koleske, & Settleman, 2004). The nanoclusters appear to form specifically at the boundary of ordered raft domains and disordered domains where signaling lipids such as PIP3 and PIP2 are found. Shear stress on the plasma membrane also results in the dissociation of the negative regulator RhoGDI and its binding partner Rho (Shao et al., 2018). This dissociation has the effect of allowing Rho GTPase translocation to the membrane where it can interact with signaling lipids. Intriguingly, PA preferentially binds the Rho family member Rac1, resulting in nanocluster formation (Maxwell et al., 2018).
In addition to regulating the patterning and activity of Rho family GTPases, lipids also have a more direct role in regulating F-actin association with the plasma membrane. The membrane phosphoinositides, and PIP2 in particular, play an important role in regulating the interaction of F-actin with the plasma membrane (Kapus & Janmey, 2013; Saarikangas, Zhao, & Lappalainen, 2010). In general, PIP2 is a positive regulator of F-actin polymerization and the presence of PIP2 increases the stability of the actin cytoskeleton–plasma membrane interface. This occurs primarily through the direct interaction of PIP2 with actin-binding proteins, and change in PIP2 distribution has been shown to precede actin build-up at the plasma membrane (Nebl, Oh, & Luna, 2000; Senju & Lappalainen, 2019; Tran, Masedunskas, Weigert, & Ten Hagen, 2015). PIP2 dynamics after plasma membrane injury support a role for PIP2 in actin assembly during repair as its accumulation near the site of injury is generally delayed. Myofiber injury in zebrafish was found to cause rapid loss of PIP2, followed by restoration to pre-injury levels by 30 seconds post-injury (Middel et al., 2016). Similarly, peak PIP2 accumulation at the injury site occurred 45 seconds post-injury in Xenopus oocytes (Vaughan et al., 2014). PIP2 accumulation was observed as early as 4 seconds in mouse myofibers; however, it continued to accumulate even 1 minute post-injury suggesting a role in the later stages of repair (Demonbreun et al., 2016). These observations on PIP2 kinetics line up remarkably well with the accumulation of F-actin at the injury site, which begins around 30 seconds after injury and extends for several minutes (Godin, Vergen, Prakash, Pagano, & Hubmayr, 2011; Horn et al., 2017). Regulation of actin binding proteins by PIP2 occurs in part through electrostatic interactions (Senju et al., 2017), suggesting that clusters of PIP2 molecules may be necessary to achieve build-up of F-actin itself. Interestingly, several methods for PIP2 micro-domain formation may allow for this to occur after plasma membrane injury. The primary method for PIP2 formation in cells is by the activity of PI(4)P-5 kinase (PI5K) (Kolay, Basu, & Raghu, 2016). PI5K activity is itself driven by regulators of membrane repair including Rho GTPases (Gilmore & Burridge, 1996) and PLD (Roach et al., 2012). PLD-mediated activation of PI5K relies on the formation of PA, which itself is able to determine the spatial localization of PI5K as well as cause its activation (Roach et al., 2012). Intriguingly, PIP2 is needed for PLD activity suggesting the possibility that a feed-forward loop leads to increasing PIP2 concentrations as repair progresses ultimately facilitating the necessary build-up of F-actin (Figure 1B).
The lipid-mediated cytoskeletal rearrangement described above provides the cell with a mechanism to close the wounded site and add structural support to the newly resealed membrane. This phase represents restoration of the barrier function of the plasma membrane; however, cells must still undergo a membrane remodeling phase due to the presence of cytoskeletal as well as other repair proteins and lipids that accumulate during the repair process. Without adequate remodeling, the plasma membrane protein and lipid composition would change dramatically, particularly after repeat injuries, and no longer function as in its pre-injury state. This remodeling is likely provided by processes described previously: membrane endocytosis (Section 3.1) and membrane shedding (Section 4.1). These remodeling events actively promote plasma membrane repair; however, they also act as extensions of the repair response and may continue long after successful resealing in order to restore the plasma membrane to its pre-injury state.
5. Lipids as a mediator of tissue repair
Failure of injured cells to repair results in cell death and activates a tissue repair response. Despite the many different types of tissue, there is a common repair program involved in tissue repair. This involves a series of distinct, but mutually dependent stages including inflammation, regeneration, and remodeling of the tissue (Gurtner, Werner, Barrandon, & Longaker, 2008). Use of lipidomics during epidermal wound repair identified that several of the plasma membrane lipids discussed above are enriched during wound repair. They include glycerophosphocholines, glycerophosphoglycerols, glycerophosphoinositols, as well as triacylglycerols (Taverna, Nanney, Pollins, Sindona, & Caprioli, 2011). By direct imaging of skeletal myofibers during the course of repair from a focal injury, exposure of PS on the injured myofiber surface was found to attract macrophages to the injury site. This helps to remodel the newly repaired plasma membrane (Middel et al., 2016), but may also serve to potentiate tissue inflammation.
Inflammatory exudates provide insights into the regulation of the first stage of wound repair – inflammation. They consist of a variety of lipid mediators derived from the omega-3 essential fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and include lipoxins, resolvins and protectins. These mediators promote wound resolution by acting upon the immune and endothelial cells and help to control the duration and nature of the immune and phagocytic cell infiltration at the injury site (Serhan, 2014). Alteration in this response inhibits the subsequent stages, tissue regeneration and remodeling, leading to increased tissue scarring. The second stage of tissue repair, regeneration, makes use of signaling by different lipids, one of which is sphingolipid. As discussed above, sphingolipids in the plasma membrane are an important regulator of plasma membrane repair. During regeneration, sphingolipids such as sphingosine 1-phosphate (S1P) are known for their growth promoting effect on tissue resident stem cells (Calise et al., 2012; Nojima, Freeman, Gulbins, & Lentsch, 2015). Another broad group of lipid carriers that are recognized for their role in activating stem cells are extracellular vesicles (EVs), which are released locally at the site of injury or from a distant site and through their lipid and other cargoes regulate regeneration of injured tissues by way of stem cell activation (Riazifar, Pone, Lötvall, & Zhao, 2017). EVs are known to be generated in response to plasma membrane injury of single cells, and this is required for successful repair (Jimenez et al., 2014; Scheffer et al., 2014). Given their ability to traverse away from the site of synthesis (Verweij et al., 2019), the EVs generated during plasma membrane injury may activate both local and distant tissue regeneration responses needed for repair. This method of extracellular communication may allow for the proper execution of inflammatory and regenerative responses needed for appropriate tissue remodeling required to restore organ function. Failure or delay in these processes, as in chronic inflammatory conditions and conditions of regenerative deficit would lead to aberrant tissue remodeling resulting in fibrotic or adipogenic replacement of the lost tissue.
6. Conclusion
While often considered to be a passive resident of the plasma membrane, there is ample evidence to support a more active role of lipids in the process of plasma membrane repair as well as tissue repair. In this review, we have discussed how lipids working at the individual as well as at the population level facilitate the proper orchestration of the repair response. These roles of lipids in plasma membrane repair include both a structural role and a signaling role. Rather than these roles being separated from each other, they overlap significantly such that one can easily be an extension of the other. The structural role of lipids may extend into the signaling role, which can then impact back on the structural characteristics of the repairing membrane by changing the composition or distribution of individual lipids. Thus, the signaling role of lipids affects membrane structure and how the wound is eventually resealed and remodeled, returning the plasma membrane to homeostasis. We have briefly discussed how such an integral role of lipids in plasma membrane repair also extends into tissue-level repair and restoration of organ function. This role of lipids extends further by way of regulating the response of proteins during the repair process as well as long-term gene expression-based adaptations required for the recovery of injured cells and tissues. Thus, it is clear that there is a need to acknowledge the role of lipids as an active participant in the processes of both cell and tissue repair.
Acknowledgements
J.K.J. and A.H. acknowledge NIH for financial support - NIAMS (R01AR055686) and NICHD (U54HD090257) and thank our lab members for useful discussions and inputs during the course of writing and editing this work.
References
- Andrews NW, Almeida PE, & Corrotte M (2014). Damage control: cellular mechanisms of plasma membrane repair. Trends in cell biology, 24, 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun SN, Xie D, Howard AC, Zhong Q, Zhong X, McNeil PL, & Bollag WB (2013). Cell wounding activates phospholipase D in primary mouse keratinocytes. Journal of lipid research, 54, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G-Q, Alderton JM, & Steinhardt RA (1995). Calcium-regulated exocytosis is required for cell membrane resealing. The Journal of cell biology, 131, 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, … Clementi E. (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO journal, 28, 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig C, Lenoir M, Velluz M-C, Kufareva I, Abagyan R, Overduin M, & Gruenberg J (2013). Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Developmental cell, 25, 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouter A, Gounou C, Bérat R, Tan S, Gallois B, Granier T, … Brisson AR. (2011). Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nature communications, 2, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye TL, Maeda K, Pezeshkian W, Sønder SL, Haeger SC, Gerke V, … Nylandsted J. (2017). Annexin A4 and A6 induce membrane curvature and constriction during cell membrane repair. Nature communications, 8, 1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braughler JM, & Hall E (1992). Involvement of lipid peroxidation in CNS injury. Journal of neurotrauma, 9, S1–7. [PubMed] [Google Scholar]
- Burridge K, & Guilluy C (2016). Focal adhesions, stress fibers and mechanical tension. Experimental cell research, 343, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, Hwang M, … Zhao X. (2009). MG53 nucleates assembly of cell membrane repair machinery. Nature cell biology, 11, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, & Bruni P (2012). Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1823, 439–450. [DOI] [PubMed] [Google Scholar]
- Campelo F, Fabrikant G, McMahon HT, & Kozlov MM (2010). Modeling membrane shaping by proteins: Focus on EHD2 and N‐BAR domains. FEBS letters, 584, 1830–1839. [DOI] [PubMed] [Google Scholar]
- Casey PJ (1995). Protein lipidation in cell signaling. Science, 268, 221–225. [DOI] [PubMed] [Google Scholar]
- Cazzolli R, Shemon AN, Fang MQ, & Hughes WE (2006). Phospholipid signalling through phospholipase D and phosphatidic acid. IUBMB life, 58, 457–461. [DOI] [PubMed] [Google Scholar]
- Cebecauer M, Amaro M, Jurkiewicz P, Sarmento M. J. o., Šachl R, Cwiklik L, & Hof M (2018). Membrane lipid nanodomains. Chemical reviews, 118, 11259–11297. [DOI] [PubMed] [Google Scholar]
- Cheng JP, Mendoza-Topaz C, Howard G, Chadwick J, Shvets E, Cowburn AS, … Nichols BJ (2015). Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J Cell Biol, 211, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong X, Hubmayr RD, Li C, & Zhao X (2017). Plasma membrane wounding and repair in pulmonary diseases. American Journal of Physiology-Lung Cellular and Molecular Physiology, 312, L371–L391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ST, & McNeil PL (2015). Membrane repair: mechanisms and pathophysiology. Physiological reviews, 95, 1205–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrotte M, Almeida PE, Tam C, Castro-Gomes T, Fernandes MC, Millis BA, … Maugel TK. (2013). Caveolae internalization repairs wounded cells and muscle fibers. Elife, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, & Sheetz M (1995). Regulation of endocytosis, exocytosis, and shape by membrane tension. Paper presented at the Cold Spring Harbor symposia on quantitative biology. [DOI] [PubMed] [Google Scholar]
- Das S, Yin T, Yang Q, Zhang J, Wu YI, & Yu J (2015). Single-molecule tracking of small GTPase Rac1 uncovers spatial regulation of membrane translocation and mechanism for polarized signaling. Proceedings of the National Academy of Sciences, 112, E267–E276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJG, & McMahon HT (2007). Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature, 449, 923. [DOI] [PubMed] [Google Scholar]
- Defour A, Van der Meulen JH, Bhat R, Bigot A, Bashir R, Nagaraju K, & Jaiswal JK (2014). Dysferlin regulates cell membrane repair by facilitating injury-triggered acid sphingomyelinase secretion. Cell death & disease, 5, e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, & McNally EM (2016). Plasma membrane repair in health and disease Current topics in membranes (Vol. 77, pp. 67–96): Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demonbreun AR, Quattrocelli M, Barefield DY, Allen MV, Swanson KE, & McNally EM (2016). An actin-dependent annexin complex mediates plasma membrane repair in muscle. J Cell Biol, 213, 705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A, Fletcher DA, & Weiner OD (2013). Use the force: membrane tension as an organizer of cell shape and motility. Trends in cell biology, 23, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Heo W, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, & Meyer T (2006). PI (3, 4, 5) P3 and PI (4, 5) P2 lipids target proteins with polybasic clusters to the plasma membrane. Science, 314, 1458–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster KM (2007). The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Advances in physiology education, 31, 5–16. [DOI] [PubMed] [Google Scholar]
- Gauthier NC, Fardin MA, Roca-Cusachs P, & Sheetz MP (2011). Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proceedings of the National Academy of Sciences, 108, 14467–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier NC, Masters TA, & Sheetz MP (2012). Mechanical feedback between membrane tension and dynamics. Trends in cell biology, 22, 527–535. [DOI] [PubMed] [Google Scholar]
- Gazzerro E, Sotgia F, Bruno C, Lisanti MP, & Minetti C (2010). Caveolinopathies: from the biology of caveolin-3 to human diseases. European journal of human genetics, 18, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, & Moss SE (2005). Annexins: linking Ca 2+ signalling to membrane dynamics. Nature reviews Molecular cell biology, 6, 449. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, & Burridge K (1996). Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4–5-bisphosphate. Nature, 381, 531. [DOI] [PubMed] [Google Scholar]
- Godin LM, Vergen J, Prakash Y, Pagano RE, & Hubmayr RD (2011). Spatiotemporal dynamics of actin remodeling and endomembrane trafficking in alveolar epithelial type I cell wound healing. American Journal of Physiology-Lung Cellular and Molecular Physiology, 300, L615–L623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, & Longaker MT (2008). Wound repair and regeneration. Nature, 453, 314. [DOI] [PubMed] [Google Scholar]
- Gushchina LV, Bhattacharya S, McElhanon KE, Choi JH, Manring H, Beck EX, … Weisleder N. (2017). Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Molecular Therapy, 25, 2360–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Wang JA, Miller DM, Cebak JE, & Hill RL (2018). Newer pharmacological approaches for antioxidant neuroprotection in traumatic brain injury. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, … Scheffer L. (2013). VBP15, a novel anti‐inflammatory and membrane‐stabilizer, improves muscular dystrophy without side effects. EMBO molecular medicine, 5, 1569–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, & Jaiswal JK (2018). Cellular mechanisms and signals that coordinate plasma membrane repair. Cellular and Molecular Life Sciences, 75, 3751–3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Van der Meulen JH, Defour A, Hogarth M, Sreetama SC, Reed A, … Jaiswal JK. (2017). Mitochondrial redox signaling enables repair of injured skeletal muscle cells. Sci Signal, 10. doi: 10.1126/scisignal.aaj1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houang EM, Haman KJ, Filareto A, Perlingeiro RC, Bates FS, Lowe DA, & Metzger JM (2015). Membrane-stabilizing copolymers confer marked protection to dystrophic skeletal muscle in vivo. Molecular Therapy-Methods & Clinical Development, 2, 15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houang EM, Sham YY, Bates FS, & Metzger JM (2018). Muscle membrane integrity in Duchenne muscular dystrophy: recent advances in copolymer-based muscle membrane stabilizers. Skeletal muscle, 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AC, McNeil AK, & McNeil PL (2011). Promotion of plasma membrane repair by vitamin E. Nature communications, 2, 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idone V, Tam C, Goss JW, Toomre D, Pypaert M, & Andrews NW (2008). Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. The Journal of cell biology, 180, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob RF, & Mason RP (2005). Lipid peroxidation induces cholesterol domain formation in model membranes. Journal of Biological Chemistry, 280, 39380–39387. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Lauritzen SP, Scheffer L, Sakaguchi M, Bunkenborg J, Simon SM, … Nylandsted J (2014). S100A11 is required for efficient plasma membrane repair and survival of invasive cancer cells. Nature communications, 5, 3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, & Nylandsted J (2015). S100 and annexin proteins identify cell membrane damage as the Achilles heel of metastatic cancer cells. Cell cycle, 14, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, & Perez F (2014). ESCRT machinery is required for plasma membrane repair. Science, 343, 1247136. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Erickson JW, & Cerione RA (2012). C-terminal di-arginine motif of Cdc42 protein is essential for binding to phosphatidylinositol 4, 5-bisphosphate-containing membranes and inducing cellular transformation. Journal of Biological Chemistry, 287, 5764–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A, & Janmey P (2013). Plasma membrane—Cortical cytoskeleton interactions: A cell biology approach with biophysical considerations. Comprehensive Physiology, 3, 1231–1281. [DOI] [PubMed] [Google Scholar]
- Kolay S, Basu U, & Raghu P (2016). Control of diverse subcellular processes by a single multi-functional lipid phosphatidylinositol 4, 5-bisphosphate [PI (4, 5) P2]. Biochemical Journal, 473, 1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, & Chernomordik LV (2015). Membrane tension and membrane fusion. Current opinion in structural biology, 33, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause MR, & Regen SL (2014). The structural role of cholesterol in cell membranes: from condensed bilayers to lipid rafts. Accounts of chemical research, 47, 3512–3521. [DOI] [PubMed] [Google Scholar]
- Labazi M, McNeil AK, Kurtz T, Lee TC, Pegg RB, Angeli JPF, … McNeil PL (2015). The antioxidant requirement for plasma membrane repair in skeletal muscle. Free Radical Biology and Medicine, 84, 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RG, Harper CC, McKinney JS, Rzigalinski BA, & Ellis EF (1997). Alterations in Phosphatidylcholine Metabolism of Stretch‐Injured Cultured Rat Astrocytes. Journal of neurochemistry, 68, 1904–1910. [DOI] [PubMed] [Google Scholar]
- Lee I-H, Kai H, Carlson L-A, Groves JT, & Hurley JH (2015). Negative membrane curvature catalyzes nucleation of endosomal sorting complex required for transport (ESCRT)-III assembly. Proceedings of the National Academy of Sciences, 112, 15892–15897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E, Defour A, Melikov K, Van der Meulen JH, Nagaraju K, Bhuvanendran S, … Jaiswal JK (2015). Annexin A1 deficiency does not affect myofiber repair but delays regeneration of injured muscles. Scientific reports, 5, 18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart KC, O’Neill TJ, Cheng Z, Dee R, Demonbreun AR, Li J, … Taylor JM (2015). GRAF1 deficiency blunts sarcolemmal injury repair and exacerbates cardiac and skeletal muscle pathology in dystrophin-deficient mice. Skeletal muscle, 5, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeti E, Dagher M-C, Hernandez SE, Koleske AJ, & Settleman J (2004). Phospholipids can switch the GTPase substrate preference of a GTPase-activating protein. Journal of Biological Chemistry, 279, 5055–5058. [DOI] [PubMed] [Google Scholar]
- London E (2004). Ceramide selectively displaces cholesterol from ordered lipid domains (rafts) implications for lipid raft structure and function. Journal of Biological Chemistry, 279, 9997–10004. [DOI] [PubMed] [Google Scholar]
- Marg A, Schoewel V, Timmel T, Schulze A, Shah C, Daumke O, & Spuler S (2012). Sarcolemmal repair is a slow process and includes EHD2. Traffic, 13, 1286–1294. [DOI] [PubMed] [Google Scholar]
- Mason RP, Walter MF, & Mason PE (1997). Effect of oxidative stress on membrane structure: small-angle X-ray diffraction analysis. Free Radical Biology and Medicine, 23, 419–425. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Fauré J, … Sadoul R (2004). Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science, 303, 531–534. [DOI] [PubMed] [Google Scholar]
- Maxwell KN, Zhou Y, & Hancock JF (2018). Rac1 nanoscale organization on the plasma membrane is driven by lipid binding specificity encoded in the membrane anchor. Molecular and cellular biology, 38, e00186–00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade JR, Archambeau A, & Michele DE (2014). Rapid actin-cytoskeleton–dependent recruitment of plasma membrane–derived dysferlin at wounds is critical for muscle membrane repair. The FASEB Journal, 28, 3660–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil AK, Rescher U, Gerke V, & McNeil PL (2006). Requirement for annexin A1 in plasma membrane repair. Journal of Biological Chemistry, 281, 35202–35207. [DOI] [PubMed] [Google Scholar]
- McNeil PL (2002). Repairing a torn cell surface: make way, lysosomes to the rescue. Journal of cell science, 115, 873–879. [DOI] [PubMed] [Google Scholar]
- McNeil PL, & Steinhardt RA (2003). Plasma membrane disruption: repair, prevention, adaptation. Annual review of cell and developmental biology, 19, 697–731. [DOI] [PubMed] [Google Scholar]
- McNeil PL, & Terasaki M (2001). Coping with the inevitable: how cells repair a torn surface membrane. Nature cell biology, 3, E124. [DOI] [PubMed] [Google Scholar]
- Merrill AH Jr (2008). Sphingolipids Biochemistry of lipids, lipoproteins and membranes (pp. 363–397): Elsevier. [Google Scholar]
- Middel V, Zhou L, Takamiya M, Beil T, Shahid M, Roostalu U, … Nienhaus GU (2016). Dysferlin-mediated phosphatidylserine sorting engages macrophages in sarcolemma repair. Nature communications, 7, 12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, … Donati MA (1998). Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nature genetics, 18, 365. [DOI] [PubMed] [Google Scholar]
- Miyake K, McNeil PL, Suzuki K, Tsunoda R, & Sugai N (2001). An actin barrier to resealing. Journal of cell science, 114, 3487–3494. [DOI] [PubMed] [Google Scholar]
- Moissoglu K, Kiessling V, Wan C, Hoffman BD, Norambuena A, Tamm LK, & Schwartz MA (2014). Regulation of Rac1 translocation and activation by membrane domains and their boundaries. J Cell Sci, 127, 2565–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassoy P, & Lamaze C (2012). Stressing caveolae new role in cell mechanics. Trends in cell biology, 22, 381–389. [DOI] [PubMed] [Google Scholar]
- Nebl T, Oh SW, & Luna EJ (2000). Membrane cytoskeleton: PIP2 pulls the strings. Current Biology, 10, R351–R354. [DOI] [PubMed] [Google Scholar]
- Nicolson GL (2014). The Fluid—Mosaic Model of Membrane Structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1838, 1451–1466. [DOI] [PubMed] [Google Scholar]
- Nojima H, Freeman CM, Gulbins E, & Lentsch AB (2015). Sphingolipids in liver injury, repair and regeneration. Biological chemistry, 396, 633–643. [DOI] [PubMed] [Google Scholar]
- Oropeza NPF (2017). Development of Biomimetic Membrane Assemblies on Microspheres for High-Throughput and Multiplexable Studies. The University of New Mexico. [Google Scholar]
- Parton RG, & Del Pozo MA (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nature reviews Molecular cell biology, 14, 98. [DOI] [PubMed] [Google Scholar]
- Petersen EN, Chung H-W, Nayebosadri A, & Hansen SB (2016). Kinetic disruption of lipid rafts is a mechanosensor for phospholipase D. Nature communications, 7, 13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza GJ, & Marmer WN (2007). Conversion of phosphatidylcholine to phosphatidylglycerol with phospholipase D and glycerol. Journal of the American Oil Chemists’ Society, 84, 645–651. [Google Scholar]
- Plataki M, Lee YD, Rasmussen DL, & Hubmayr RD (2011). Poloxamer 188 facilitates the repair of alveolus resident cells in ventilator-injured lungs. American journal of respiratory and critical care medicine, 184, 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet H, Conrard L, Cloos A-S, & Tyteca D (2018). Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules, 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes B, Monzo P, & Gauthier NC (2017). Membrane tension: A challenging but universal physical parameter in cell biology. Paper presented at the Seminars in cell & developmental biology. [DOI] [PubMed] [Google Scholar]
- Potez S, Luginbühl M, Monastyrskaya K, Hostettler A, Draeger A, & Babiychuk EB (2011). Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. Journal of Biological Chemistry, 286, 17982–17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramstedt B, & Slotte JP (2006). Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1758, 1945–1956. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, & Andrews NW (2001). Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell, 106, 157–169. [DOI] [PubMed] [Google Scholar]
- Remorino A, De Beco S, Cayrac F, Di Federico F, Cornilleau G, Gautreau A, … Coppey M (2017). Gradients of Rac1 nanoclusters support spatial patterns of Rac1 signaling. Cell reports, 21, 1922–1935. [DOI] [PubMed] [Google Scholar]
- Riazifar M, Pone EJ, Lötvall J, & Zhao W (2017). Stem cell extracellular vesicles: extended messages of regeneration. Annual review of pharmacology and toxicology, 57, 125–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach AN, Wang Z, Wu P, Zhang F, Chan RB, Yonekubo Y, … Du G (2012). Phosphatidic acid regulation of PIPKI is critical for actin cytoskeletal reorganization. Journal of lipid research, 53, 2598–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, & Lappalainen P (2010). Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiological reviews, 90, 259–289. [DOI] [PubMed] [Google Scholar]
- Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, & Jaiswal JK (2014). Mechanism of Ca 2+-triggered ESCRT assembly and regulation of cell membrane repair. Nature communications, 5, 5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju Y, Kalimeri M, Koskela EV, Somerharju P, Zhao H, Vattulainen I, & Lappalainen P (2017). Mechanistic principles underlying regulation of the actin cytoskeleton by phosphoinositides. Proceedings of the National Academy of Sciences, 114, E8977–E8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju Y, & Lappalainen P (2019). Regulation of actin dynamics by PI (4, 5) P2 in cell migration and endocytosis. Current opinion in cell biology, 56, 7–13. [DOI] [PubMed] [Google Scholar]
- Serhan CN (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature, 510, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, & Eggeling C (2017). The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature reviews Molecular cell biology, 18, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Liao X, Xie F, Deng S, Liu X, Ristaniemi T, & Liu B (2018). FRET biosensor allows spatiotemporal observation of shear stress-induced polar RhoGDIα activation. Communications biology, 1, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng R, Chen Y, Gee HY, Stec E, Melowic HR, Blatner NR, … Fujiwara TK (2012). Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nature communications, 3, 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone LC, Caplan S, & Naslavsky N (2013). Role of phosphatidylinositol 4, 5-bisphosphate in regulating EHD2 plasma membrane localization. PloS one, 8, e74519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, … Johannes L (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell, 144, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalman LN, Holst MR, Larsson E, & Lundmark R (2018). Plasma membrane damage caused by listeriolysin O is not repaired through endocytosis of the membrane pore. Biology open, 7, bio035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth C, Fan J, Spaeth E, Robison T, Wilcott R, & Bittner G (2012). Neurite transection produces cytosolic oxidation, which enhances plasmalemmal repair. Journal of neuroscience research, 90, 945–954. [DOI] [PubMed] [Google Scholar]
- Sreetama SC, Chandra G, Van der Meulen JH, Ahmad MM, Suzuki P, Bhuvanendran S, … Jaiswal JK (2018). Membrane stabilization by modified steroid offers a potential therapy for muscular dystrophy due to dysferlin deficit. Molecular Therapy, 26, 2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck TL, & Lange Y (2018). Transverse distribution of plasma membrane bilayer cholesterol: Picking sides. Traffic, 19, 750–760. [DOI] [PubMed] [Google Scholar]
- Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, … Andrews NW (2010). Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. The Journal of cell biology, 189, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna D, Nanney LB, Pollins AC, Sindona G, & Caprioli R (2011). Multiplexed molecular descriptors of pressure ulcers defined by imaging mass spectrometry. Wound Repair and Regeneration, 19, 734–744. [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, & Hordijk PL (2007). Targeting and localized signalling by small GTPases. Biology of the Cell, 99, 1–12. [DOI] [PubMed] [Google Scholar]
- Thottacherry JJ, Kosmalska AJ, Kumar A, Vishen AS, Elosegui-Artola A, Pradhan S, … Chaudhary N (2018). Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nature communications, 9, 4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T (2004). Long-term Potentiation of Wound-induced Exocytosis and Plasma Membrane Repair Is Dependant on cAMP-response Element-mediated Transcription via a Protein Kinase C-and p38 MAPK-dependent Pathway. Journal of Biological Chemistry, 279, 44996–45003. [DOI] [PubMed] [Google Scholar]
- Togo T, Krasieva TB, & Steinhardt RA (2000). A decrease in membrane tension precedes successful cell-membrane repair. Molecular biology of the cell, 11, 4339–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrino S, Shen W-W, Blouin CM, Mani SK, de Lesegno CV, Bost P, … Chambon V (2018). EHD2 is a mechanotransducer connecting caveolae dynamics with gene transcription. J Cell Biol, 217, 4092–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DT, Masedunskas A, Weigert R, & Ten Hagen KG (2015). Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nature communications, 6, 10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G (1989). Lipid traffic in animal cells. Annual review of cell biology, 5, 247–275. [DOI] [PubMed] [Google Scholar]
- Van Meer G, Voelker DR, & Feigenson GW (2008). Membrane lipids: where they are and how they behave. Nature reviews Molecular cell biology, 9, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan EM, You J-S, Yu H-YE, Lasek A, Vitale N, Hornberger TA, & Bement WM (2014). Lipid domain–dependent regulation of single-cell wound repair. Molecular biology of the cell, 25, 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verboon JM, & Parkhurst SM (2015). Rho family GTPases bring a familiar ring to cell wound repair. Small GTPases, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, … Zimmermann P (2019). Live tracking of inter-organ communication by endogenous exosomes in vivo. Developmental cell, 48, 573–589. e574. [DOI] [PubMed] [Google Scholar]
- Weisleder N, Takizawa N, Lin P, Wang X, Cao C, Zhang Y, … Chen P-J (2012). Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Science translational medicine, 4, 139ra185–139ra185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, & Metzger JM (2005). Dystrophic heart failure blocked by membrane sealant poloxamer. Nature, 436, 1025. [DOI] [PubMed] [Google Scholar]
- Yogurtcu ON, & Johnson ME (2018). Cytosolic proteins can exploit membrane localization to trigger functional assembly. PLoS computational biology, 14, e1006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski A (1993). Phospholipids in animal eukaryotic membranes: transverse asymmetry and movement. Biochemical Journal, 294, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, & Du G (2009). Phosphatidic acid signaling regulation of Ras superfamily of small guanosine triphosphatases. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1791, 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Lin P. h., De G, Choi K. h., Takeshima H, Weisleder N, & Ma J (2012). PTRF Anchors MG53 to Cell Injury Site for Initiation of Membrane Repair. Biophysical Journal, 102, 366a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuzek A, Fan JD, Spaeth CS, & Bittner GD (2013). Sealing of transected neurites of rat B104 cells requires a diacylglycerol PKC-dependent pathway and a PKA-dependent pathway. Cellular and molecular neurobiology, 33, 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


