Abstract
Introduction
Pistacia atlantica subsp. kurdica is an important food source and a well-known medicinal plant in the Zagros Mountains of Iran. The present study aimed to investigate the effect of P. atlantica extract and essential oil in streptozotocin-induced diabetic mice.
Materials and Methods
Different doses of hydroalcoholic extract and essential oil of P. atlantica subsp. kurdica (50, 100, and 200 mg/kg) were given to streptozotocin-induced diabetic mice in separate groups for three weeks. At the end of treatment, blood samples were collected; then, oxidative stress markers, TNF-α, and lipid profile were determined in its serum samples.
Results
Our findings showed that the administration of P. atlantica extract for three consecutive weeks significantly improved the lipid profile, oxidative stress, and inflammation process by reducing lipid peroxidation and increasing total antioxidant capacity.
Conclusion
This study showed that P. atlantica subsp. kurdica has antioxidant and blood lipid-lowering effects that can be used as a supplement to improve diabetes complications.
Keywords: Pistacia atlantica subsp. kurdica, phytochemical, diabetes, lipid profile, oxidative stress
Introduction
Diabetes mellitus, as a global main public health concern, is increasing due to urbanization, obesity, and aging.1 It is anticipated that the number of diabetic patients will reach 366 million in 2030.2 Increased oxidative stress and lipid profile alterations are important contributors to the initiation and progression of diabetes as well as its complications.3,4 In addition to insulin therapy, diabetes mellitus could be controlled by a combination of fasting, diet control, and plant therapeutics.5
It has been estimated that around 80% of the people in some Asian/African regions rely on traditional medicine for their health care.6 Commonly the pharmaceutically efficient forms of these plants are their extractions.7 In addition, the chemical factors of extracts/essential oils qualify their usage in food industries. Recently, there has been increasing attention on traditional herbs with antioxidant, anti-inflammatory and antidiabetic properties.8,9 The efficiency of traditional herbs needs to be confirmed in diabetes; therefore, the World Health Organization (WHO) recommends the assessment of traditional plant treatments for diabetes mellitus.5,10
Pistacia atlantica subsp. kurdica is from the Anacardiaceae family and is found in the Zagros Mountains of Iran (west and northwest of Iran with a mild climate). Oleoresin derived from P. atlantica is applied to produce chewing gum. The fruit of P. atlantica is consumed by inhabitants as food and the unripe fruit is served in pickles. Its husk and kernel oil is consumed as frying oil by local people. In addition to food consumption, P. atlantica has been employed in traditional medicine in the treatment of various diseases including stomach disorders and throat infections.11 It has been shown that P. atlantica and its chemical factors induce some pharmacological actions including anticancer, antioxidant, anti-inflammatory, and antimicrobial activities.12–15 Therefore, this project was initiated to evaluate the impact of long-term treatment with hydroalcoholic extract (HE) and essential oil (EO) of P. atlantica subsp. kurdica on glucose, lipid profile, TNF-α and oxidative stress biomarkers in streptozotocin (STZ)-induced diabetic mice.
Materials and Methods
Chemical substances were bought from Merck, Darmstadt, Germany unless otherwise mentioned. 2,4,6-tripyridyl-s-triazine (TPTZ) and STZ were provided from Sigma-Aldrich Company (St Louis, MO, USA). The ripe P. atlantica subsp. kurdica fruits were gathered in May 2016 from the Awraman region in Marivan, Kurdistan in the west of Iran. A voucher specimen (MPH-11854) is deposited in the Herbarium of the Research Institute of Forests and Range-land Research by Hossein Maroufi, Sanandaj, Iran.
Preparation of the Extract and Essential Oil
The extract and essential oil of P. atlantica subsp. kurdica were prepared according to our previous study.15
Animal Experiments
Male albino mice (25–30 g) were bought from the animal house of Hamadan University of Medical Science. The animals were kept under a temperature of 22–25°C, humidity of 50–55%, and 12 h light/dark cycle. Animals were given the standard diet with water ad libitum.
Induction of Experimental Diabetes
The induction of diabetes was done through intra-peritoneal administration of STZ in 0.1 M citrate buffer (pH 4.5) at a dose of 60 mg/kg to mice following the estimation of baseline fasting blood sugar concentration. At 72 h following STZ administration, animals with fasting blood glucose levels over 200 mg/dL were considered to be diabetic.
Experimental Design
Animals were divided into 10 groups and each one comprised 6 mice. The groups were 1) normal untreated mice; 2) diabetic untreated mice; 3) normal mice treated with 200 mg/kg of HE; 4, 5, and 6) diabetic mice treated with 50, 100, 200 mg/kg of HE, respectively; 7) normal mice treated with 200 mg/kg of EO; 8, 9, and 10) diabetic mice treated with 50, 100, 200 mg/kg of EO, respectively. Three weeks following intervention, animals were anesthetized with intraperitoneal administration of 40 mg/kg ketamine and 10 mg/kg xylazine mixture, after overnight fasting. Blood specimens were gathered from the heart and centrifuged at 1500 g for 10 min for preparing serum.
Determination of Glucose and Lipid Profile
Serum levels of glucose, triglyceride (TG), total cholesterol (TC), HDL-C and LDL-C were measured using commercial kits (Pars Azmoon, Tehran, Iran). In addition, the Atherogenic Index (AI) and Coronary Risk Index (CRI) were estimated by the following formulas:
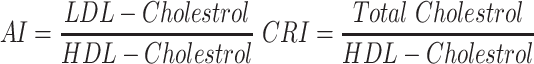 |
Lipid Peroxidation (LPO) Assay
LPO was determined in serum samples by the use of thiobarbituric acid reactive substances (TBARS) method as described by Navaei-Nigjeh et al.16 Briefly, a reaction mixture was provided containing 0.2% TBA in 0.05 M H2SO4. The specimens were read by a microplate reader (Synergy HTX, BioTek Instruments Inc., Winooski, VT, USA) set at 532 nm against malondialdehyde (MDA) as the standard provided at several concentrations (1, 2, 4 and 8 µM). Finally, results were expressed as nmol/mL.
Ferric Reducing/Antioxidant Power Assay
Total antioxidant capacity (TAC), called FRAP, in serum samples was measured according to the reduction of Fe3+ to Fe2+. In this experiment, the complex between Fe2+ and TPTZ, as an indicator, results in a blue color with an absorbance maximum at 593 nm as previously described Navaei-Nigjeh et al.16 Results were expressed as µmol/mL.
TNFα Assay
The serum level of TNF-α was measured using the ELISA kit (Crystal day Biotech Co., Shanghai, China). The optical density of the produced color was assessed at 450 nm. Data were expressed as ng/L.
Statistical Analyses
Data were indicated as means ± SEM and compared by ANOVA. Tukey–Kramer was used as a post hoc test. A P-value of less than 0.05 was statistically significant.
Results
HE/EO Effects on Glucose and Lipid Profile
As shown in Table 1, administration of STZ significantly increased serum levels of glucose (P<0.001), TG (P<0.001), TC (P<0.001), VLDL (P<0.001), and LDL-C (P<0.001); and decreased HDL-C serum level in comparison to the control group. No significant changes were observed in glucose level and HDL-C in diabetic mice during HE and/or EO therapy when compared with the STZ group. HE treatment at the doses of 50, 100, and 200 mg/kg inhibited the rise of TG and TC in diabetic mice. In addition, LDL-C level was remarkably decreased in diabetic mice treated with HE (200 mg/kg). No significant changes were observed in TC and LDL-C levels during EO therapy when compared with the STZ group. Only a significant decrease was observed in TG levels in diabetic mice treated with EO (200 mg/kg) in comparison to the STZ group.
Table 1.
HE/EO Effects on Glucose Level and Lipid Profile in the Serum of Diabetic Mice
| Group | Glucose | TG (mg/dL) | TC (mg/dL) | HDL-C (mg/dL) | LDL-C (mg/dL) | VLDL (mg/dL) | Atherogenic Index (AI) | Coronary Risk Index (CRI) |
|---|---|---|---|---|---|---|---|---|
| Control | 129±10 | 64.1±7.3 | 92.8±6.4 | 41±5.2 | 35±4.7 | 12.8±1.4 | 0.8±0.1 | 1.5±0.2 |
| STZ | 363±36aaa | 125.1±16.7aaa | 134.4±5.9aaa | 25±3.1a | 58.7±6.4a | 25.0±3.3aaa | 2.3±0.3aa | 5.1±0.7aaa |
| HE (200 mg/kg) | 144±11 | 80.1±14.1 | 95.6±7.6 | 37±6.4 | 28.8±8.1 | 16.1±2.8 | 0.7±0.2 | 2.2±0.4 |
| STZ+HE (50 mg/kg) | 338±35 | 78.3±9.1bbb | 93.6±4.8bbb | 26±4.4 | 52.3±7.9 | 15.6±1.8bbb | 2.1±0.4 | 3.1±0.5bb |
| STZ+HE (100 mg/kg) | 398±23 | 67.9±9.8bbb | 103.5±6.8bb | 28±3.6 | 48.1±9.1 | 13.5±1.9bbb | 1.7±0.3 | 2.4±0.4bbb |
| STZ+HE (200 mg/kg) | 392±38 | 76.7±6.0bbb | 86.2±12.8bbb | 28±5.5 | 39.8±2.8b | 15.3±1.2bbb | 1.4±0.3 | 2.8±0.5bbb |
| EO (200 mg/kg) | 219±17 | 97.6±15.3 | 106.8±13.2 | 44±6.1 | 43.1±3.2 | 19.5±5.1 | 0.9±0.1 | 2.2±0.4 |
| STZ+EO (50 mg/kg) | 392±18 | 101.7±9.2 | 130.8±10.8 | 26±4.8 | 59.2±11.2 | 20.3±1.8 | 2.3±0.5 | 3.9±0.7 |
| STZ+EO (100 mg/kg) | 370±25 | 108.6±9.4 | 121.6±17.6 | 24±4.1 | 64.8±8.7 | 21.7±1.8 | 2.7±0.5 | 4.6±0.8 |
| STZ+EO (200 mg/kg) | 355±31 | 88.6±11bbb | 122±18.1 | 21±3.6 | 61.9±12.3 | 17.7±2.2bbb | 2.9±0.6 | 4.3±0.7 |
Notes: Statistical analysis used one-way ANOVA with Tukey’s test. Values are expressed as means± SEM, n=6 for each group. Significantly different from the control group; aP<0.05, aaP<0.01, and aaaP<0.001. Significantly different from the diabetic group b(P<0.05), bb(P<0.01) and bbb(P<0.001).
Abbreviations: HE, hydroalcoholic extract; EO, essential oil; TG, triglycerides; TC, total cholesterol; HDL-C, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein; AI, atherogenic index; CRI, coronary risk index.
HE/EO Effects on TNF-α Levels
TNF-α levels significantly increased in diabetic in comparison to normal mice (P<0.05). Following HE therapy, TNF-α concentration significantly decreased in the dose of 200 mg/kg in comparison to diabetic mice (P<0.01). No significant alteration was found in the TNF-α level in diabetic mice during EO therapy when compared with the STZ group (Table 2).
Table 2.
HE/EO Effects on TNF-α Level and Oxidative Stress Biomarkers in the Serum of Diabetic Mice
| Groups | TNF-α (ng/L) | TAC (µmol/mL) | LPO (nmol/mL) |
|---|---|---|---|
| Control | 5.8±1.1 | 1.4±0.15 | 1.8±0.36 |
| STZ | 8.1±0.91aa | 0.8±0.07aa | 2.1±0.49aaa |
| HE (200 mg/kg) | 6.1±0.82 | 1.25±0.17 | 4.9±0.94 |
| STZ+HE (50 mg/kg) | 6.8±0.49 | 0.94±0.08 | 4.4±0.50 |
| STZ+HE (100 mg/kg) | 7.2±0.65 | 1.07±0.05 | 3.8±0.36b |
| STZ+HE (200 mg/kg) | 6.2±0.37b | 1.3±0.13bb | 2.9±0.31bbb |
| EO (200 mg/kg) | 6.4±1.2 | 1.3±0.08 | 2.5±0.53 |
| STZ+EO (50 mg/kg) | 7.8±0.97 | 0.9±0.21 | 3.1±0.94 |
| STZ+EO (100 mg/kg) | 7.4±0.38 | 1.1±0.25 | 2.9±0.54 |
| STZ+EO (200 mg/kg) | 6.9±0.41 | 1.2±0.09 | 3.6±0.63 |
Notes: Analysis used one-way ANOVA with Tukey’s test. Values are expressed as means±SEM, n=6 for each group. Significantly different from the control group; aaP<0.01 and aaaP<0.001. Significantly different from the diabetic group bP<0.05, bbP<0.01, and bbbP<0.001.
Abbreviations: HE, hydroalcoholic extract; EO, essential oil; STZ, streptozotocin; TNF-α, tumor necrosis factor-α; TAC, total antioxidant capacity; LPO, lipid peroxidation.
HE/EO Effects on Oxidative Stress Biomarkers
As indicated in Table 2, in the diabetic mice group, serum TAC significantly decreased (P<0.01), but returned to normal after usage of HE (200 mg/kg; P<0.05), 50 and 100 mg/kg of EO (P<0.001 and P<0.001, respectively). No significant alteration was seen following administration of HE (50 and 100 mg/kg) and EO (200 mg/kg). There was a remarkable increase in the level of LPO in diabetic mice when compared to control (P<0.001). Following HE therapy, LPO decreased at the dosage of 200 mg/kg in comparison to the diabetic group (P<0.01). EO suppressed increment of LPO at the dosages of 50 (P<0.05) and 100 (P<0.01) mg/kg.
Discussion
In the present project, the administration of STZ for three consecutive weeks gave rise to a remarkable rise in glucose, TC, TG, LDL, and VLDL serum concentration in the mice. In contrast, the HDL level in serum samples significantly decreased in diabetic mice. Hyperglycemia in STZ-induced diabetes mice could be caused by destroying β-cells in Langerhans islets, which has an adverse impact on serum lipid concentration.16,17 Secondary elevation of free fatty acid in the blood owing to their mobilization from adipocytes can lead to the clearance of HDL and might be one of the probable mechanisms of hyperlipidemia in diabetes.17,18 Therefore, STZ-induced lipid disturbances may be due in part to extra action of lipolytic hormones on adipocytes.19 In addition, hypertriglyceridemia in diabetic mice could result from an increment in hepatic VLDL overproduction and impaired metabolism of TG-rich particles. It can cause the deposition of fat in ectopic sites such as skeletal muscle (lipotoxicity).20 Lipoprotein lipase (LPL) dysfunction may be another contributor to hypertriglyceridemia. Observed elevation in TC of diabetic mice may be due to a decrease in HDL-C. These changes may be relevant in explaining the increased predisposition of diabetes to atherosclerosis and coronary heart disease. HE administration for three consecutive weeks resulted in a remarkable decrease in serum concentration of TG, TC, LDL, and VLDL in the diabetic mice. Serum glucose levels had no significant alteration in both normal and STZ diabetic mice after HE and/or EO treatment. It seems that HE/EO changed the serum lipid profile without decreasing blood glucose levels. Phytochemical studies on different subspecies of P. atlantica extract indicated the presence of flavonoids and phenolic compounds as major chemical components.11,21-23 These compounds may be associated with the therapeutic effects of P. atlantica. Previously, Hashemnia et al have reported that N-hexane extract of P. atlantica seed produced a potent antihyperglycemic effect in STZ diabetic mice. Unfortunately, the subspecies of P. atlantica in this research was not specified; P. atlantica comprises three subspecies in Iran, kurdica, mutica, and cabulica.24 This discrepancy may be explained at least in part by the difference in subspecies and extract of P. atlantica in the study. Our findings showed that HE may attenuate hypercholesterolemia via modifying the lipoprotein state. On the other hand, enhancement of LDL receptors and/or the lecithin–cholesterol acyltransferase (LCAT) activity may participate in the regulation of lipid profile. LCAT has a critical role in consolidating free cholesterol into HDL and transferring back to VLDL or IDL, which is returned through the liver cells.25 In addition, a decrease of TG in serum diabetic mice is possibly due to the inhibition of lipogenesis in adipose tissue.
Oxidative stress plays a leading part in the progress of diabetes complications such as dyslipidemia and inflammatory process. One of the critical biomarkers of oxidative damages is lipid peroxidation, which is the most explored area of research when it comes to reactive oxygen species (ROS).6 In addition, it has been reported that TNFα could affect glucose hemostasis and adipose tissue dysfunction.25 TNFα could suppress tyrosine phosphorylation inside the insulin receptors and may inhibit glucose transporter gene expression.26 In the current study a remarkable rise in LPO biomarker and decrease in TAC of serum were observed, as supported by previous studies, such as Pourkhalili et al.1 In addition, TNFα was remarkably increased in diabetic mice. Stimulation of oxidative stress signaling pathways and TNFα seems to take part in the stimulation of some redox-sensitive transcription factors such as nuclear factor kappa B (NF-kB).27 On the other hand, it has been shown that NF-kB stimulation in hepatocytes has a contributory role in progressing insulin resistance and the occurrence of diabetes mellitus.28,29
HE administration for three consecutive weeks significantly improved oxidative damage and inflammatory processes through decreasing lipid peroxidation and increasing TAC, as well as modulation of TNFα in the serum of diabetic mice. However, the effects of EO were less than HE in diabetic animals. It seems that phenols and flavonoids, the major constituents of HE, have more potential to remove free radicals and decrease the inflammatory reactions than monoterpene hydrocarbons in EO.
Conclusion
In conclusion, our survey indicated that continued oral intake of HE for three weeks might provide beneficial effects on the hyperlipidemia, oxidative stress, and inflammatory response in diabetic mice. The finding might support its usage by the Iranian population in the management and treatment of diabetes. This suggests that P. atlantica subsp. kurdica intake may prevent or be helpful in decreasing dyslipidemia related to diabetes. Finally, the exact mechanisms and the active constituents of P. atlantica subsp. kurdica involved are still to be determined via additional studies.
Acknowledgments
The authors gratefully acknowledge the Research Vice-chancellor of Kermanshah University of Medical Sciences due to the assignment of the relevant grant (Grant Number: 96166). This research is a prerequisite for the MSc of Seyran Hosseini, in the School of Nutritional Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran. In this study all experiments were selected and kept in during study based on Care and Use of Laboratory Animals Guideline that was approved by the Ethical Board of the Research Council of Kermanshah University of Medical Sciences (Ethical Number: KUMS.REC.1395.624). The abstract of this manuscript has been presented at the 3 International & 15 Iranian Nutrition Congress, December 19–21, 2018, Tehran, Iran.
Funding Statement
This study was funded by the Research Council of Kermanshah University of Medical Sciences (Grant Number: 96166).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work and declare that the content has not been published elsewhere.
Disclosure
The authors have no conflicts of interest to disclose.
References
- 1.Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, et al. Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes. 2011;2(11):204–210. doi: 10.4239/wjd.v2.i11.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Li P, Zhang T, Wang S, Copeland L. Trypsin and chymotrypsin are necessary for in vitro enzymatic digestion of rice starch. RSC Adv. 2017;7(7):3660–3666. doi: 10.1039/C6RA24816K [DOI] [Google Scholar]
- 3.Pickering RJ, Rosado CJ, Sharma A, Buksh S, Tate M, de Haan JB. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin Transl Immunol. 2018;7(4):1–20. doi: 10.1002/cti2.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozder A. Lipid profile abnormalities seen in T2DM patients in primary healthcare in Turkey: a cross-sectional study. Lipids Health Dis. 2014;13(1):183. doi: 10.1186/1476-511X-13-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96(4):660–666. doi: 10.1079/BJN20061849 [DOI] [PubMed] [Google Scholar]
- 6.Nili-Ahmadabadi A, Pourkhalili N, Fouladdel S, et al. On the biochemical and molecular mechanisms by which malathion induces dysfunction in pancreatic islets in vivo and in vitro. Pestic Biochem Physiol. 2013;106(1):51–60. doi: 10.1016/j.pestbp.2013.04.003 [DOI] [Google Scholar]
- 7.Han DG, Cho SS, Kwak JH, Yoon IS. Medicinal plants and phytochemicals for diabetes mellitus: pharmacokinetic characteristics and herb-drug interactions. J Pharm Investig. 2019;49(7):603–612. doi: 10.1007/s40005-019-00440-4 [DOI] [Google Scholar]
- 8.Abdali E, Javadi S, Akhgari M, Hosseini S, Dastan D. Chemical composition and biological properties of Satureja avromanica Maroofi. J Food Sci Technol. 2017;54(3):727–734. doi: 10.1007/s13197-017-2512-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghahremani-majd H, Dashti F, Dastan D, Mumivand H, Hadian J, Esna-Ashari M. Antioxidant and antimicrobial activities of Iranian mooseer (Allium hirtifolium Boiss) populations. Hortic Environ Biotechnol. 2012;53(2):116–122. doi: 10.1007/s13580-012-0131-2 [DOI] [Google Scholar]
- 10.Rahimifard M, Navaei-Nigjeh M, Mahroui N, Mirzaei S, Siahpoosh Z. Improvement in the function of isolated rat pancreatic islets through reduction of oxidative stress using traditional Iranian medicine. Cell J. 2014;16(2):147–163. [PMC free article] [PubMed] [Google Scholar]
- 11.Hatamnia AA, Abbaspour N, Darvishzadeh R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014;145:306–311. doi: 10.1016/j.foodchem.2013.08.031 [DOI] [PubMed] [Google Scholar]
- 12.Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology. Sci World J. 2013;15:1–32. doi: 10.1155/2013/219815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidarian E, Jafari-Dehkordi E, Valipour P, Ghatreh-Samani K, Ashrafi-Eshkaftaki L. Nephroprotective and anti-inflammatory effects of Pistacia atlantica leaf hydroethanolic extract against gentamicin-induced nephrotoxicity in rats. J Diet Suppl. 2017;14:1–14. [DOI] [PubMed] [Google Scholar]
- 14.Rigane G, Ghazghazi H, Aouadhi C, Ben Salem R, Nasr Z. Phenolic content, antioxidant capacity and antimicrobial activity of leaf extracts from Pistacia atlantica. Nat Prod Res. 2017;31(6):696–699. doi: 10.1080/14786419.2016.1212035 [DOI] [PubMed] [Google Scholar]
- 15.Nachvak SM, Hosseini S, Nili-Ahmadabadi A, Dastan D, Rezaei M. Chemical composition and antioxidant activity of Pistacia atlantica subsp. Kurdica from Awraman. J Rep Pharm Sci. 2018;7(3):222–230. [Google Scholar]
- 16.Navaei-Nigjeh M, Rahimifard M, Pourkhalili N, et al. Multi-organ protective effects of cerium oxide nanoparticle/selenium in diabetic rats: evidence for more efficiency of nanocerium in comparison to metal form of cerium. Asian J Anim Vet Adv. 2012;7(7):605–612. doi: 10.3923/ajava.2012.605.612 [DOI] [Google Scholar]
- 17.Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.) Sm., in STZ-induced diabetic rats. Chem Biol Interact. 2009;182(1):67–72. doi: 10.1016/j.cbi.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 18.Geethan P, Prince P. Antihyperlipidemic effect of D‐pinitol on streptozotocin‐induced diabetic wistar rats. J Biochem Mol Toxicol. 2008;22(4):220–224. doi: 10.1002/jbt.20218 [DOI] [PubMed] [Google Scholar]
- 19.Eddouks M, Lemhadri A, Michel J-B. Hypolipidemic activity of aqueous extract of Capparis spinosa L. in normal and diabetic rats. J Ethnopharmacol. 2005;98(3):345–350. doi: 10.1016/j.jep.2005.01.053 [DOI] [PubMed] [Google Scholar]
- 20.Pushparaj P, Low H, Manikandan J, Tan B, Tan C. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111(2):430–434. doi: 10.1016/j.jep.2006.11.028 [DOI] [PubMed] [Google Scholar]
- 21.Satheesh MA, Pari L. Effect of pterostilbene on lipids and lipid profiles in streptozotocin–nicotinamide induced type 2 diabetes mellitus. J Appl Biomed. 2008;6(1):31–37. doi: 10.32725/jab.2008.005 [DOI] [Google Scholar]
- 22.Belyagoubi-Benhammou N, Belyagoubi L, El Zerey-Belaskri A, Atik Bekkara F. In vitro antioxidant properties of flavonoid fractions from Pistacia atlantica Desf. subsp. atlanticafruit using five techniques. J Mech Eng Sci. 2014;6(4):1118–1125. [Google Scholar]
- 23.Ben Ahmed Z, Yousfi M, Viaene J, et al. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. leaves. Pharm Biol. 2017;55(1):1185–1194. doi: 10.1080/13880209.2017.1291690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashemnia M, Nikousefat Z, Yazdani-Rostam M. Antidiabetic effect of Pistacia atlantica and Amygdalus scoparia in streptozotocin-induced diabetic mice. Comp Clin Path. 2015;24(6):1301–1306. doi: 10.1007/s00580-015-2068-1 [DOI] [Google Scholar]
- 25.Nili-Ahmadabadi A, Akbari Z, Ahmadimoghaddam D, Larki-Harchegani A. The role of ghrelin and tumor necrosis factor alpha in diazinon-induced dyslipidemia: insights into energy balance regulation. Pestic Biochem Physiol. 2019;157:138–142. doi: 10.1016/j.pestbp.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 26.Pakzad M, Fouladdel S, Nili-Ahmadabadi A, et al. Sublethal exposures of diazinon alters glucose homeostasis in Wistar rats: biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic Biochem Physiol. 2013;105(1):57–61. doi: 10.1016/j.pestbp.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 27.Kajbaf F, Mojtahedzadeh M, Abdollahi M. Mechanisms underlying stress-induced hyperglycemia in critically ill patients. Future Med. 2007;4(1):97–106. [Google Scholar]
- 28.Mostafalou S, Eghbal MA, Nili-Ahmadabadi A, Baeeri M, Abdollahi M. Biochemical evidence on the potential role of organophosphates in hepatic glucose metabolism toward insulin resistance through inflammatory signaling and free radical pathways. Toxicol Ind Health. 2012;28(9):840–851. doi: 10.1177/0748233711425073 [DOI] [PubMed] [Google Scholar]
- 29.Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11(2):183–190. doi: 10.1038/nm1166 [DOI] [PMC free article] [PubMed] [Google Scholar]


