Abstract
Background & aims:
Dietary diversity is widely advocated in national and international recommendations although whether the beneficial effects on survival or longevity still apply in the final phase of the lifespan remains understudied. We aimed to prospectively examine the association of dietary diversity, food items with all-cause mortality among the oldest old (80+) and determine whether dietary diversity recommendations were appropriate for this population.
Methods:
The study included 28,790 participants aged 80+ (9957 octogenarians, 9925 nonagenarians, and 8908 centenarians). A baseline dietary diversity score (DDS) was constructed based on nine food items of a food frequency questionnaire. Cox models with penalized splines evaluated non-linear associations of DDS as continuous variable with mortality to identify cut-offs of DDS.
Results:
We documented 23,503 deaths during 96,739 person-years of follow-up. Each one unit increase in DDS was associated with a 9% lower risk of mortality (adjusted hazard ratio (HR): 0.91; 95% confidential interval (CI): 0.90–0.92). Compared to participants whose DDS less than 2 scores, those with a DDS of 2, 3, 4, 5, and higher than 6 scores had a lower mortality risk, the HRs were 0.86 (0.82–0.89), 0.78 (0.75–0.81), 0.69 (0.66–0.72), 0.65 (0.62–0.68), and 0.56 (0.53–0.58) respectively, and a significant trend emerged (p<0.001). Protein-rich food items were associated with prominent beneficial effects on mortality including meat (HR and 95% CI for high vs low frequency: 0.70 (0.68–0.72)), fish and sea food (HR, 0.74 (0.72–0.77)), egg (HR, 0.75 (0.73–0.77)), and bean (HR, 0.80 (0.78–0.82)).
Conclusions:
Even after the age of 80, the DDS tool may offer a simple and straightforward mean of identifying and screening individuals at high risk for mortality. Recommendation of dietary diversity, especially consumption of protein-rich food, may be advocated to reduce mortality risk and promote longevity in the oldest old.
Keywords: Dietary diversity, protein-rich food, mortality, cohort study, oldest old, elderly
Introduction
Most previous studies related to development of chronic diseases and under-nutrition, all-cause and cause-specific mortality have investigated the role of single food items, macro- and micro-nutrient intake and bioactive components [1–4]. Although a high correlation and interaction among individual foods and nutrients in typical human diets is recognized, less studies to date have focused on the appraisal of the total diet or broad dietary patterns for human health [5–7].
One widely used approach to evaluation of total diet quality is assessment of dietary diversity, a rapid, user-friendly and cost-effective tool [8, 9]. This tool is able to provide an overall assessment of dietary behaviors, to evaluate the effect of several food items simultaneously on health [8], bypass methodological problems of interactions, and facilitate a more comprehensive approach to disease control and prevention [9]. We previously showed a significant association between the dietary diversity score (DDS), constructed from information on nine food, and cognitive impairment among Chinese elderly and oldest old (80+) [10]. A number of national and international dietary guidelines have advocated improving the diversity of the diet to meet daily nutrient requirements and reduce the risk of various chronic diseases [11–14], while evidence from observational studies to date that support benefits of higher dietary diversity for health outcome or survival is limited among the elderly, especially among the oldest old.
It is generally acknowledged that multiple factors, such as biological, environmental, and psychosocial factors, act together to promote human longevity [15]. The impact of dietary behavior is considered one of the key modifiable determinants of longevity [16]. A beneficial effect of dietary diversity for reducing mortality risk has been established for middle aged and younger elderly individuals [17]. Whether these effects of dietary diversity still apply in the final phase of the lifespan remains understudied. Consequently, there is currently little consensus regarding dietary behavior and longevity in the oldest old. Much of the evidence on the role of dietary diversity in mortality for this age group is limited by short follow-up periods, small sample sizes, survivor biases for healthier individuals, scarcity of longitudinal studies, and the focus on populations of developed countries [18, 19]. Due to these limitations, the generalizability to the population of Chinese oldest old is unclear.
As a result of a decline in social or economic status and physiological function with aging, the oldest old are most likely to suffer from nutritional deficiencies [20–22]. A deeper understanding of the link of diet diversity to survival could facilitate establishment of evidence-based recommendations for the oldest old to promote longevity and improve their life quality [23]. To examine whether recommendations regarding dietary diversity are appropriate for this population, in the current study, we prospectively examined the associations of the DDS with all-cause mortality in the Chinese Longitudinal Healthy Longevity Study (CLHLS) of 28,790 oldest old, with adjustment for age, sex, residence, occupation, educational background, income, marital status, lifestyle, oral health status, prevalence of diseases, cognitive impairment, and disability in activities of daily living (ADL). Furthermore, the associations between each food item composed of DDS and all-cause mortality were examined to identify key food groups that could be targeted to help achieve and maintain better survival.
Materials and Methods
Study Setting
Participants, derived from CLHLS, were enrolled in 1998, 2000, 2002, 2005, 2008–09, 2011–12, and 2014 and have been followed up ever since. The CLHLS randomly selected 806 cities and counties in 23 provinces of China using multi-stage stratified sampling, and the study areas covering 85% Chinese population. All centenarians who voluntarily agreed to participate in the study were attempted to be interviewed. For each centenarian, one nearby nonagenarian (90–99), octogenarian (80–89) and younger elderly (65–79) of predefined sex were interviewed. To obtain comparable numbers of women and men for each age group, the predefined sex were randomly determined by the randomly assigned centenarians’ code numbers [24, 25]. A more detailed description and assessment of the CLHLS data quality have been presented in previous publications [24, 25]. Among 43,487 CLHLS participants recruited from 1998 to 2014, 28,790 (66.2%) oldest old met the inclusion criteria: aged 80 and older, successfully followed up once or more, available dietary information; excluded participants included 9131 (21.0%) participants aged 79 and younger, 5019 (11.5%) participants who only had the baseline information and were lost-to-follow-up at the first follow-up survey, and 547 (1.3%) participants with unavailable dietary information. The participant flow chart can be seen in Appendix Figure 1. The design of CLHLS was approved by the Biomedical Ethics Committee of Peking University (IRB00001052–13074), and a consent form was signed by all participants or their representatives.
Assessment of DDS
In a face-to-face interview, DDS was assessed by trained personnel at baseline based on a food frequency questionnaire [26] related to the nine major food groups: meat, fish and seafood, eggs, beans, fruits, salty vegetables, tea, garlic, and fresh vegetables, which were recorded as “often or almost every day” or “occasionally” or “rarely or never”. One DDS unit was defined as “often or almost every day” consumption of any food group without considering a minimum intake; the maximum possible DDS score was 9 points representing highest level of dietary diversity. Cereals and oil were not included in the construction of the DDS since almost all Chinese consume these two food groups daily [10].
Ascertainment of deaths
In the follow-up surveys conducted between the baseline and 2014, information on deaths and the date of death were obtained from close family member of participants or village doctors [24]. Person years were measured from baseline until the date of death, loss to follow-up, or September 1, 2014, whichever occurred first. Lost-to follow-up in this study referred to participants who could not be contacted after at least three reasonable efforts. The participants who were lost-to follow-up in the second or later follow-up survey were included in the main analyses.
Statistical analysis
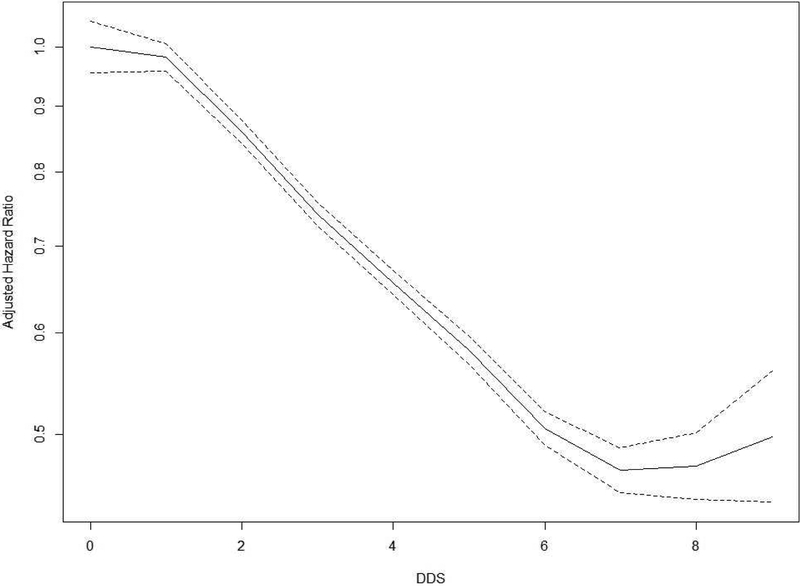
On the whole, the missing data for any individual covariates at baseline amounted to less than 1.0%, to correct missing values on the individual covariates, multiple imputation methods were performed. The assumption of proportional hazard was satisfied testing with Kaplan-Meier curves and scaled Schoenfeld residuals on functions of time when DDS was treated as a categorical or continuous variable. Cox models with penalized splines evaluated non-linear associations of DDS with disability [27]; two DDS score cut-offs were identified (DDS less than 2 or higher than 5) above and below which there was no significant increase in the multitude of hazard ratio (HR) for mortality (Figure 1; Appendix 1 in the Supplement). Thus, Cox proportional hazard models were performed to examine the relationship of mortality with DDS, categorized as scores <2, 2, 3, 4, 5, ≥6, with adjustment for age (continuous variable), sex, residence, occupation, educational background, source of income, current marital status, living pattern, tobacco smoking, alcohol drinking, regular exercise, number of teeth (continuous variable), use of artificial denture, hypertension, heart disease, cerebrovascular disease, respiratory disease, digestive system diseases, cognitive impairment, and disability in activities of daily living. Cox proportional hazard models were also performed to examine the relationship of mortality with nine major food groups: meat, fish and seafood, eggs, beans, fruits, salty vegetables, tea, garlic, and fresh vegetables, adjusted with referred covariates.
Figure 1. The adjusted hazard ratio of mortality for dietary scores in Cox models with penalized splines.
The lines depict the adjusted hazard ratio for risk of mortality; the dotted lines indicate the 95% confidence intervals. Two cut-offs were identified in DDS scores (<2 and ≥6) based on hazard ratios for mortality that did not significantly change further for scores below or above these cut-offs respectively.
Adjusting for age (continuous variable), sex, residence, occupation, educational background, source of income, current marital status, living pattern, tobacco smoking, alcohol drinking, regular exercise, number of teeth (continuous variable), use of artificial denture, hypertension, heart disease, cerebrovascular disease, respiratory disease, digestive system diseases, cognitive impairment, and disability in ADL.
In sensitivity analysis, participants lost to follow-up in the second or later follow-up survey were removed to examine possible attrition bias; Cox proportional hazard models were re-estimated excluding deaths in the first year; or excluding the participants with comorbidity (including heart disease, cerebrovascular disease, respiratory disease, and digestive system diseases); two other potentially confounding factors, body mass index (BMI, was continuous variable) and sufficient income (yes or no, based on the judgment of the participants), were further adjusted in the models. Subgroup analyses were also performed in three age groups (octogenarians, nonagenarians, and centenarians), men and women, participants living in urban or rural areas, participants with different occupation (farmer or non-farmer), participants with different source of income (pension or other), non-smokers or smokers, non-drinkers or drinkers, participants with different teeth number (≥5 or <5), participants using artificial denture or not, participants with cognitive impairment or not, participants with disability in ADL or not. Statistical interaction was performed for building Cox model including 2 interaction terms and their product. The maximum of likelihood ratios test was used to detect the significance of the interaction effect. The I² statistic, which describes the percentage of variation across studies that is due to heterogeneity rather than chance, was calculated in the heterogeneity tests. P <0.05 (two-sided) was considered statistically significant. SAS (version 9.4, SAS Institute Inc, Cary, NC) and R version 3.4.2 (R foundation for Statistical Computing, Vienna, Austria) were used for the statistical analyses.
Results
Baseline characteristics of participants
Baseline DDS was less than 2 points in 19.8% (5707/28,790) of participants and higher than 6 points in 16.6% (4789/28,790) of participants. The mean age was 92.9 (standard deviation, 7.5) years; 61.8% were women. More description was present in Table 1 and Appendix 2 in the Supplement.
Table 1.
Baseline characteristics of the 28,790 study participants according to DDSa
| Variable | DDS<2 | DDS=2 | DDS=3 | DDS=4 | DDS=5 | DDS>5 | Total | P Value |
|---|---|---|---|---|---|---|---|---|
| Number of participants | 5707 (19.8) | 5256 (18.3) | 5059 (17.6) | 4492 (15.6) | 3487 (12.1) | 4789 (16.6) | 28790(100) | |
| Age, mean (SD), y | 93.8±7.3 | 93.2±7.5 | 93.0±7.5 | 92.7±7.5 | 92.3±7.6 | 91.8±7.5 | 92.9±7.5 | <.001 |
| Sex | <.001 | |||||||
| Men | 1963 (34.4) | 1857 (35.3) | 1899 (37.5) | 1760 (39.2) | 1437 (41.2) | 2095 (43.7) | 11011 (38.2) | |
| Women | 3744 (65.6) | 3399 (64.7) | 3160 (62.5) | 2732 (60.8) | 2050 (58.8) | 2694 (56.3) | 17779 (61.8) | |
| Residence | <.001 | |||||||
| Urban | 1097 (19.2) | 1085 (20.6) | 1167 (23.1) | 1169 (26.0) | 992 (28.4) | 1530 (31.9) | 7040 (24.5) | |
| Rural | 4610 (80.8) | 4171 (79.4) | 3892 (76.9) | 3323 (74.0) | 2495 (71.6) | 3259 (68.1) | 21750 (75.5) | |
| Occupation | <.001 | |||||||
| Non-farmer | 2389 (41.9) | 2189 (41.6) | 2203 (43.5) | 1982 (44.1) | 1599 (45.9) | 2298 (48.0) | 12660 (44.0) | |
| Farmer | 3318 (58.1) | 3067 (58.4) | 2856 (56.5) | 2510 (55.9) | 1888 (54.1) | 2491 (52.0) | 16130 (56.0) | |
| Educational background | <.001 | |||||||
| Illiteracy | 4456 (78.1) | 3955 (75.2) | 3653 (72.2) | 3151 (70.1) | 2311 (66.3) | 3106 (64.9) | 20632 (71.7) | |
| Literacy | 1251 (21.9) | 1301 (24.8) | 1406 (27.8) | 1341 (29.9) | 1176 (33.7) | 1683 (35.1) | 8158 (28.3) | |
| Source of income | <.001 | |||||||
| Pension | 380 (6.7) | 439 (8.4) | 584 (11.5) | 611 (13.6) | 615 (17.6) | 1014 (21.2) | 3643 (12.7) | |
| Other | 5327 (93.3) | 4817 (91.6) | 4475 (88.5) | 3881 (86.4) | 2872 (82.4) | 3775 (78.8) | 25147 (87.3) | |
| Sufficient incomeb | <.001 | |||||||
| No | 1567 (35.4) | 1265 (28.0) | 1084 (24.1) | 825 (19.8) | 527 (15.9) | 743 (16.0) | 6011 (23.5) | |
| Yes | 2860 (64.6) | 3259 (72.0) | 3417 (75.9) | 3339 (80.2) | 2780 (84.1) | 3896 (84.0) | 19551 (76.5) | |
| Marital status | <.001 | |||||||
| In marriage | 812 (14.2) | 804 (15.3) | 835 (16.5) | 790 (17.6) | 693 (19.9) | 1073 (22.4) | 5007 (17.4) | |
| Not in marriage | 4895 (85.8) | 4452 (84.7) | 4224 (83.5) | 3702 (82.4) | 2794 (80.1) | 3716 (77.6) | 23783 (82.6) | |
| Living pattern | <.001 | |||||||
| With family members | 4601 (80.6) | 4306 (81.9) | 4272 (84.4) | 3778 (84.1) | 2973 (85.3) | 4079 (85.2) | 24009 (83.4) | |
| Alone or at nursing home | 1106 (19.4) | 950 (18.1) | 787 (15.6) | 714 (15.9) | 514 (14.7) | 710 (14.8) | 4781 (16.6) | |
| Tobacco smoking status | <.001 | |||||||
| Non-smoker | 4139 (72.5) | 3773 (71.8) | 3571 (70.6) | 3170 (70.6) | 2349 (67.4) | 3221 (67.3) | 20223 (70.2) | |
| Current smoker | 872 (15.3) | 820 (15.6) | 793 (15.7) | 677 (15.1) | 554 (15.9) | 806 (16.8) | 4522 (15.7) | |
| Former smoker | 696 (12.2) | 663 (12.6) | 695 (13.7) | 645 (14.4) | 584 (16.7) | 762 (15.9) | 4045 (14.1) | |
| Alcohol drinking status | <.001 | |||||||
| Non-drinker | 4081 (71.5) | 3696 (70.3) | 3554 (70.3) | 3132 (69.7) | 2393 (68.6) | 3207 (67.0) | 20063 (69.7) | |
| Current drinker | 1076 (18.9) | 1019 (19.4) | 973 (19.2) | 878 (19.5) | 714 (20.5) | 1063 (22.2) | 5723 (19.9) | |
| Former drinker | 550 (9.6) | 541 (10.3) | 532 (10.5) | 482 (10.7) | 380 (10.9) | 519 (10.8) | 3004 (10.4) | |
| Regular exercise | <.001 | |||||||
| Yes | 2625 (46.0) | 2109 (40.1) | 1918 (37.9) | 1604 (35.7) | 1261 (36.2) | 1748 (36.5) | 11265 (39.1) | |
| No | 3082 (54.0) | 3147 (59.9) | 3141 (62.1) | 2888 (64.3) | 2226 (63.8) | 3041 (63.5) | 17525 (60.9) | |
| Number of teeth, mean (SD) | 1(6) | 2(7) | 2(7) | 2(7) | 2(8) | 2(8) | 2(7) | <.001 |
| Use of artificial denture | <.001 | |||||||
| No | 4891 (85.7) | 4330 (82.4) | 4031 (79.7) | 3460 (77.0) | 2603 (74.6) | 3335 (69.6) | 22650 (78.7) | |
| Yes | 816 (14.3) | 926 (17.6) | 1028 (20.3) | 1032 (23.0) | 884 (25.4) | 1454 (30.4) | 6140 (21.3) | |
| BMI, mean (SD), kg/m2c | 18.7±3.7 | 18.7±3.6 | 19.0±3.7 | 19.2±3.7 | 19.4±3.6 | 19.8±3.5 | 19.1±3.6 | <.001 |
| Hypertension | <.001 | |||||||
| No | 2256 (39.5) | 2268 (43.2) | 2338 (46.2) | 2060 (45.9) | 1624 (46.6) | 2298 (48.0) | 12844 (44.6) | |
| Yes | 3451 (60.5) | 2988 (56.8) | 2721 (53.8) | 2432 (54.1) | 1863 (53.4) | 2491 (52.0) | 15946 (55.4) | |
| Heart disease | <.001 | |||||||
| No | 5373 (94.1) | 4951 (94.2) | 4770 (94.3) | 4176 (93.0) | 3207 (92.0) | 4406 (92.0) | 26883 (93.4) | |
| Yes | 334 (5.9) | 305 (5.8) | 289 (5.7) | 316 (7.0) | 280 (8.0) | 383 (8.0) | 1907 (6.6) | |
| Cerebrovascular disease | 0.003 | |||||||
| No | 5511 (96.6) | 5050 (96.1) | 4887 (96.6) | 4307 (95.9) | 3352 (96.1) | 4559 (95.2) | 27666 (96.1) | |
| Yes | 196 (3.4) | 206 (3.9) | 172 (3.4) | 185 (4.1) | 135 (3.9) | 230 (4.8) | 1124 (3.9) | |
| Digestive diseases | ||||||||
| No | 5505(96.5) | 5050(96.1) | 4872(96.3) | 4308(95.9) | 3340(95.8) | 4586(95.8) | 27661(96.1) | |
| Yes | 202(3.5) | 206(3.9) | 187(3.7) | 184(4.1) | 147(4.2) | 203(4.2) | 1129(3.9) | |
| Respiratory disease | 0.28 | |||||||
| No | 5072 (88.9) | 4672 (88.9) | 4461 (88.2) | 4028 (89.7) | 3096 (88.8) | 4278 (89.3) | 25607 (88.9) | |
| Yes | 635 (11.1) | 584 (11.1) | 598 (11.8) | 464 (10.3) | 391 (11.2) | 511 (10.7) | 3183 (11.1) | |
| Cognitive impairment | <.001 | |||||||
| No | 3332 (58.4) | 3299 (62.8) | 3221 (63.7) | 2931 (65.2) | 2312 (66.3) | 3367 (70.3) | 18462 (64.1) | |
| Yes | 2375 (41.6) | 1957 (37.2) | 1838 (36.3) | 1561 (34.8) | 1175 (33.7) | 1422 (29.7) | 10328 (35.9) | |
| Disability in ADL | <.001 | |||||||
| No | 2781 (48.7) | 2785 (53.0) | 2724 (53.8) | 2476 (55.1) | 1934 (55.5) | 2767 (57.8) | 15467 (53.7) | |
| Yes | 2926 (51.3) | 2471 (47.0) | 2335 (46.2) | 2016 (44.9) | 1553 (44.5) | 2022 (42.2) | 13323 (46.3) |
ADL: activities of daily living; BMI: body mass index DDS: dietary diversity score.
Among participants with different DDS scores, differences of categorical variables were examined with the Cochran-Armitage test for trends; differences of continuous variables were examined with analysis of variance
3228/28790 participants without the information on the sufficient income.
3389/28790 participants without the information on BMI
DDS and all-cause mortality
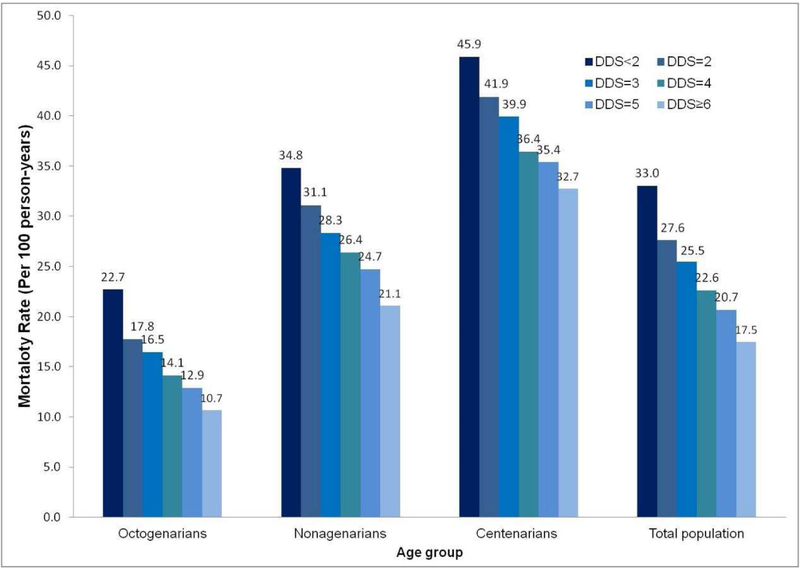
Follow-up time was a mean of 3.4 years, with a standard deviation of 2.7 years, ranging from 0.1 years to 16.3 years. There were 23,503 deaths during 96,739 person-years of follow-up. Kaplan-Meier curves revealed an inverse association of DDS and mortality for six categories of the DDS (log rank test: P < 0.001 for <2, 2, 3, 4, 5, ≥6 DDS points). For the whole cohort, the mortality rate was 21.2 per 100 person-years; 15.2, 27.8, and 39.2 per 100 person-years for octogenarians, nonagenarians, and centenarians; and 33.0, 27.6, 25.4, 22.6, 20.7, and 17.5 per 100 person-years for participants with <2, 2, 3, 4, 5, and ≥6 DDS points (Figure 2).
Figure 2. Mortality rate (person-years) in participants with different dietary diversity scores.
For the whole cohort, the mortality rate was 21.2 per 100 person-years, and 33.0, 27.6, 25.4, 22.6, 20.7, and 17.5 per 100 person-years for participants with less than 2, 2, 3, 4, 5, and ≥6 points of DDS.
DDS was inversely associated with mortality, each one score increase in DDS corresponded to a 10% lower risk of mortality, with a crude hazard ratio (HR) of 0.90(0.89–0.90), P<0.001. After adjusting a number of confounding factors between DDS and mortality, there was slight attenuation of risk estimates, with an HR of 0.91 (0.90–0.92), P<0.001. Compared to participants whose DDS was less than 2 points, those with a DDS of 2, 3, 4, 5, and higher than 5 scores had a lower mortality risk, the HRs were 0.86 (0.82–0.89), 0.78 (0.75–0.81), 0.69 (0.66–0.72), 0.65 (0.62–0.68), and 0.56 (0.53–0.58) respectively, and a significant trend emerged (p<0.001). Protein-rich food items were associated with prominent beneficial effects on mortality including meat (HR and 95% CI for high vs low frequency: 0.70 (0.68–0.72), P<0.001), fish and sea food (HR, 0.74 (0.72–0.77), P<0.001), egg (HR, 0.75 (0.73–0.77), P<0.001), and bean (HR, 0.80 (0.78–0.82), P<0.001).(Figure 3)
Figure 3. Association of dietary diversity scores with all-cause mortality among the oldest old.
Adjusting for age (continuous variable), sex, residence, occupation, educational background, source of income, current marital status, living pattern, tobacco smoking, alcohol drinking, regular exercise, number of teeth (continuous variable), use of artificial denture, hypertension, heart disease, cerebrovascular disease, respiratory disease, digestive system diseases, cognitive impairment, and disability in ADL.
Sensitivity analyses and subgroup analyses
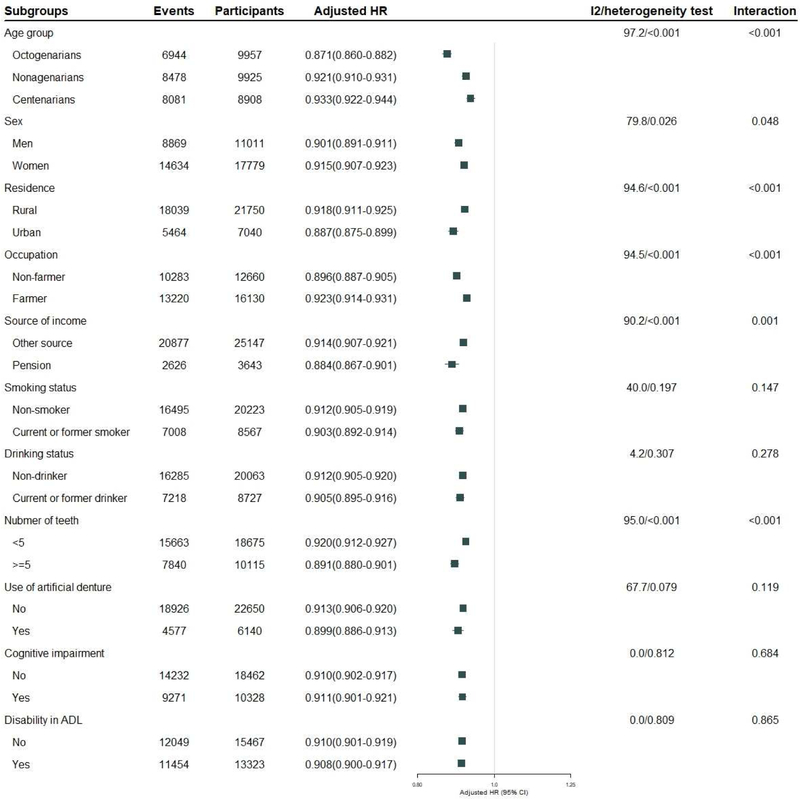
Sensitivity analyses, excluding deaths during the first year (Appendix Figure 2 in the Supplement), or excluding the participants lost to follow-up (Appendix Figure 3 in the Supplement), or excluding the participants with comorbidity (Appendix Figure 4 in the Supplement), or further adjusting for sufficient income and BMI (Appendix Figure 5 in the Supplement), showed that the inverse association of DDS with mortality risk was robust. With respect to mortality risk, there were significant interactions of DDS with age group, sex, residence, occupation, exercise practice and cognitive function. The beneficial effects of higher DDS for mortality risk decreased with increments of age (heterogeneity test: P <0.001); the HR was 0.87 (0.86–0.88) for octogenarians, P<0.001, 0.92 (0.91–0.93) for nonagenarians, P<0.001, 0.93 (0.92–0.94) for centenarians, P<0.001. Heterogeneity tests showed that the inverse association of DDS with mortality was more prominent for men, participants living in urban areas, non-farmer, participants having pension, participants having ≥ 5 teeth. These estimated effects were similar in smokers and non-smokers, alcohol drinkers and non-drinkers, participants using artificial denture or not, participants with and without cognitive impairment, and participants with and without disability in ADL (Figure 4).
Figure 4. Subgroup analyses for the association of dietary diversity score with all-cause mortality among the oldest old (DDS as continuous variables, ranged from 0 to 9).
Adjusting for age (continuous variable), sex, residence, occupation, educational background, source of income, current marital status, living pattern, tobacco smoking, alcohol drinking, regular exercise, number of teeth (continuous variable), use of artificial denture, hypertension, heart disease, cerebrovascular disease, respiratory disease, digestive system diseases, cognitive impairment, and disability in ADL.
Discussion
To our knowledge, this is the first cohort study in a nationally representative sample of Chinese oldest old providing support for a relationship between higher DDS and lower mortality risk in the oldest old. After adjustment for potential confounders, a 44% lower risk of mortality was observed among the participants with the highest DDS than the lowest. Very few studies to date have explored the association of DDS with survival for the population aged 80+. Owing to the high mortality rate of this population and long duration of follow-up, most of the survival status was documented. Cox models with penalized splines were done to investigate potential non-linear association of DDS with mortality, which made it possible to identify the optimal cut-offs of DDS. Additionally, a number of identified confounder of DDS with all-cause mortality were adjusted, sensitivity analyses or subgroup analyses were performed to explore the reverse casualty and selection bias, which made our finding more confident.
Although higher consumption of protein has been associated with an increased mortality risk in the general population [13], our data in Chinese oldest old reveal a protective effect of higher protein consumption. It has been reported that the best way to optimize health span and longevity is likely to be low protein consumption during the younger age and then moderate to high protein consumption in older age [28]. A high prevalence of insufficient protein intake has been reported in Chinese elderly [29]. Our results are consistent with the common belief that the elderly need more protein. A notable difference of the Chinese relative to other populations, is that as many as 40% of the Chinese oldest old consumed beans often or almost every day. Bean products have protective effects on breast cancer, and decrease mortality risk [30].
Although a variety of diet quality indices and health outcomes have been used in different previous studies; most studies reported an association of a healthier diet with better health outcomes within the general population or younger elderly [31–34]. A recent meta-analysis focusing on elderly in the United States and southern Europe showed that greater adherence to the WHO dietary guidelines was associated with lower cardiovascular disease (CVD) mortality [5]. Among 492,823 Americans between the ages of 50 and 71 years, older adults with a higher dietary index scores reported a 12–28% lower risk of cancer, CVD, and all-cause mortality [6]. In 1743 Taiwanese elderly aged 65 or older, DDS higher than 4 were predictors of lower all-cause and cause-specific mortality [33]. In 826 Dutch elderly aged 65–84 years, a high quality diet was associated with a 40% lower mortality risk and 2.5 years longer life expectancy in elderly men [34].
There is no standardized measure for dietary diversity, while the relationship of dietary diversity with health outcome may differ by inconsistencies in the types and numbers of food groups included. A recent science advisory declared that dietary diversity, composed of a wide range of unhealthy and healthy foods and food groups, does not contribute to a healthy dietary pattern or promote a healthy body weight for the general population [9]. In the present study the index of DDS, mainly composed of healthy food items, may indicate higher diet quality scores, which was developed to assess adequate food consumption and diet healthfulness [35]. It underlines that understanding different measures of dietary diversity and stratification by food healthfulness is critical to assess the role of dietary diversity in health outcome and survival.
There are several ways in which an increased DDS might improve survival. DDS has been found to be a useful proxy of nutrient intake in the elderly [36]. Higher dietary diversity is associated with a lower likelihood of toxic ingredients in food, optimized telomere length and maintenance of muscle strength [37–39]. A lower DDS may lead to dietary inadequacies of essential nutrients, micronutrients that are a particular concern in elderly people. Both the 2016 Dietary Guidelines for Chinese Residents and the 2015–2020 Dietary Guidelines for Americans included messages about dietary variety [11, 12]. After adjusting for various confounders, such as social-economic status, oral health, and health status in the present study, there was little change in the HR [40, 41]. It shows that confounding factors were not likely explanations for the results observed here.
It is known that dietary variety declines with advancing age [42]. Interestingly, with respect to mortality, an interaction of age with DDS was observed. It was not surprising that a stronger association of DDS and survival was observed for octogenarians than centenarians consistent with a higher capability for nutrient digestion and absorption for this age group. A similar explanation may also be possible for the more prominent protective effect of DDS on mortality in men, participants living in urban areas, non-farmer, participants having pension, and participants having ≥ 5 teeth.
The associations of DDS with mortality in the oldest old may be influenced by methodological issues, for instance, survival bias or selection bias. In the elderly aged 80+, diseases or health deterioration likely lead to a change of lifestyle. The effects of DDS on mortality can be diluted by the misclassification of lifelong exposure levels. Additionally, adults with unhealthier lifestyles or dietary behaviors may have died before the age of 80 [43]. The healthier survivor biases may have caused an underestimation of the effects of DDS in the population aged 80+ [44]. In our sensitivity analyses, exclusion of participants who died within the first year of follow-up, and exclusion of prevalent cases of identified disease or functional impairment made reverse causation or selection bias unlikely.
There were several limitations with regard to the study design and measurements in this study. Firstly, only self-reported information on DDS was collected, which serves as a potential source of recall bias. Social desirability bias may also have occurred due to the subjective and crude categorization of the food items; given that the means age of the participants was as high as 92.9 years and the low education level, we deemed such categorizations to be the most sensible choices. Secondly, a detailed quantitative dietary intake evaluation was not performed; this made it impossible to adjust for energy intake in the analyses. However, a number of key determinants of energy intake were taken into account, such as age, sex, BMI, comorbidities and physical activity. Thirdly, information on different types of cereals and oils, as two of the most important food categories contribute to dietary diversity, was not collected; this may attenuate the association of DDS with mortality risk. Fourthly, the association of DDS and cause-specific mortality was not explored, due to missing data on death records reviewed by doctors. Fifthly, chronic diseases were self-reported thus residual confounding may still exist.
Conclusions
In the Chinese oldest old, a clear dose-response relationship of DDS with mortality was revealed; higher DDS was associated with significant lower mortality risk. Even among those elderly aged 80 and older, DDS is a simple and straightforward tool for potentially identifying and screening for individuals at higher risk for mortality. This study provides support for recommending dietary diversity, a modifiable behavioral factor, especially consumption of protein-rich food items, for the oldest old to reduce mortality risk.
Supplementary Material
Highlights.
In this prospective cohort, 23,503 deaths were documented during 96,739 person-years of follow-up, higher DDS was associated with significant lower mortality risk.
Protein-rich food items were associated with prominent beneficial effects on mortality.
Even after the age of 80, the DDS tool may offer a simple and straightforward mean of identifying and screening individuals at high risk for mortality.
Recommendation of dietary diversity, especially consumption of protein-rich food, may be advocated to reduce mortality risk and promote longevity in the oldest old.
Acknowledgments
We thank all investigators and participants who conducting and participating the survey.
Funding sources
This work was supported by National Science and Technology Planning Project (2018YFC2000300), National Natural Sciences Foundation of China (812760, 81573247, and 71490732), the U.S. National Institute on Aging (2P01AG031719, 5P30AG028716) and United Nations Fund for Population Activities. The sponsor(s) have no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Abbreviations:
- ADL
activities of daily living (ADL)
- BMI
body mass index
- CLHLS
Chinese Longitudinal Healthy Longevity Survey
- CI
confidence interval
- DDS
dietary diversity score
- HR
hazard ratios
- MMSE
Mini-Mental State Examination
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- 1.Micha R, Peñalvo JL, Cudhea F, et al. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017; 317(9): 912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao JG, Zeng XT, Wang J, et al. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017;318(24):2466–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeanandin G1, Molato O, Le Duff F, et al. Impact of restrictive diets on the risk of undernutrition in a free-living elderly population. Clin Nutr 2012;31(1):69–73. [DOI] [PubMed] [Google Scholar]
- 4.Morris MC, Brockman J, Schneider JA, et al. Association of Seafood Consumption, Brain Mercury Level, and APOE ε4 Status With Brain Neuropathology in Older Adults. JAMA 2016;315(5):489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jankovic N, Geelen A, Streppel MT, et al. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr. 2015;102(4):745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher Diet Quality Is Associated with Decreased Risk of All-Cause, Cardiovascular Disease, and Cancer Mortality among Older Adults. J Nutr. 2014;144(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNaughton SA, Bates CJ, Mishra GD. Diet quality is associated with all-cause mortality in adults aged 65 years and older. J Nutr. 2012;142(2):320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krebs-Smith SM, Smiciklas-Wright H, Guthrie HA, et al. The effects of variety in food choices on dietary quality. J Am Diet Assoc. 1987;87(7):897–903. [PubMed] [Google Scholar]
- 9.Otto MCO, Anderson CAM, Dearborn JF, et al. Dietary diversity: implications for obesity prevention in adult populations: a science advisory from the American heart association. Circulation. 2018; 138(11):e160–e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Z, Fei Z, Qiu C, et al. Dietary Diversity and Cognitive Function among Elderly People: A Population-Based Study. J Nutr Health Aging. 2017;21(10):1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy G, Ballard T, Dop MC. Guidelines for Measuring Household and Individual Dietary Diversity. Rome: FAO of the UN; 2013. [Google Scholar]
- 12.World Health Organization (WHO). Preparation and Use of Food-Based Dietary Guidelines: Report of a Joint Fao/Who Consultation. WHO; Geneva, Switzerland, 1998. [PubMed] [Google Scholar]
- 13.Yu D, Zhang X, Xiang YB, et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. 2014; 100(2):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US department of agriculture and US department of health and human services. 2015–2020 Dietary Guidelines for Americans. 2015. https://health.gov/dietaryguidelines/2015/.Accessed September 8, 2018.
- 15.Yang YC, Boen C, Gerken K, et al. Social relationships and physiological determinants of longevity across the human life span. Proc Natl Acad Sci USA. 2016;113(3):578–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015; 161(1): 106–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jankovic N, Geelen A, Streppel MT, et al. Adherence to a healthy diet according to the world health organization guidelines and all-cause mortality in elderly adults from Europe and the United States. Am J Epidemiol. 2014; 180(10): 978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nybo H, Petersen HC, Gaist D, et al. Predictors of mortality in 2249 Nonagenarians-The Danish 1905-cohort survey. J Am Geriatr Soc. 2003;51(10):1365–73. [DOI] [PubMed] [Google Scholar]
- 19.Newson RS, Witteman JC, Franco OH, et al. Predicting survival and morbidity-free survival to very old age. Age (Dordr). 2010;32(4):521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivlin RS. Keeping the young elderly healthy: is it too late to improve our health through nutrition? Am J Clin Nutr. 2007;86(5):1572S–6S. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Hu X, Xie L, et al. The Prevalence of Sarcopenia in Chinese Elderly Nursing Home Residents: A Comparison of 4 Diagnostic Criteria. J Am Med Dir Assoc. 2018;19(8):690–5. [DOI] [PubMed] [Google Scholar]
- 22.Lv YB, Yuan JQ, Mao C, et al. Association of Body Mass Index with Disability in Activities of Daily Living Disability Among Chinese Adults. JAMA Network Open.2018; 1(5):e181915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford DW1, Jensen GL, Hartman TJ, et al. Association between dietary quality and mortality in older adults: a review of the epidemiological evidence. J Nutr Gerontol Geriatr. 2013;32(2):85–105. [DOI] [PubMed] [Google Scholar]
- 24.Lv YB, Gao X, Yin ZX, et al. Revisiting the association of blood pressure with mortality in oldest old people in China: community based, longitudinal prospective study. BMJ. 2018;361:k2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y, Feng Q, Hesketh T, et al. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017; 389 (10079): 1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu L, Sautter J, Gu D. Associations between frequency of tea consumption and health and mortality: evidence from old Chinese. Br J Nutr. 2012;108(9):1686–97. [DOI] [PubMed] [Google Scholar]
- 27.Malloy EJ, Spiegelman D, Eisen EA. Comparing measures of model selection for penalized splines in Cox models. Comput Stat Data Anal. 2009;53(7):2605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine ME, Suarez JA, Brandhorst S, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Byles JE, Shi Z, et al. Evaluation of older chinese people’s macronutrient intake status: Results from the China health and nutrition survey. Br J Nutr. 2015;113(1):159–71. [DOI] [PubMed] [Google Scholar]
- 30.Namazi N, Saneei P, Larijani B, et al. Soy product consumption and the risk of all-cause, cardiovascular and cancer mortality: a systematic review and meta-analysis of cohort studies. Food Funct. 2018;9(5):2576–88. [DOI] [PubMed] [Google Scholar]
- 31.Milte CM, Mcnaughton SA. Dietary patterns and successful ageing: a systematic review. Eur J Nutr. 2016;55(2):423–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milte CM, Thorpe MG, Crawford D, et al. Associations of diet quality with health-related quality of life in older Australian men and women. Exp Gerontol. 2015;64:8–16. [DOI] [PubMed] [Google Scholar]
- 33.Lee MS, Huang YC, Su HH, et al. A simple food quality index predicts mortality in Elderly Taiwanese. J Nutr Health Aging. 2011; 15(10):815–21. [DOI] [PubMed] [Google Scholar]
- 34.Sijtsma FP, Soedamahmuthu SS, de Hoon SE, et al. Healthy eating and survival among elderly men with and without cardiovascular-metabolic diseases. Nutr Metab Cardiovasc Dis. 2015;25(12):1117–24. [DOI] [PubMed] [Google Scholar]
- 35.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavakoli S, Dorosty-Motlagh AR, Hoshiar-Rad A, et al. Is dietary diversity a proxy measurement of nutrient adequacy in Iranian elderly women? Appetite. 2016; 105:468–76. [DOI] [PubMed] [Google Scholar]
- 37.Nettleton JA, Diez-Roux A, Jenny NS, et al. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008; 88(5):1408–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley J, Tucker G, Hugo G, Wittert G, et al. The Australian baby boomer population–factors influencing changes to health-related quality of life over time. J Aging Health. 2013; 25(1):29–55 [DOI] [PubMed] [Google Scholar]
- 39.Danquah I, Galbete C, Meeks K, et al. Food variety, dietary diversity, and type 2 diabetes in a multi-center cross-sectional study among Ghanaian migrants in Europe and their compatriots in Ghana: the RODAM study. Eur J Nutr. 2017: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchi CM, Egnell M, Huneau JF, et al. Plant protein intake and dietary diversity are independently associated with nutrient adequacy in French adults. J Nutr. 2016;146(11):2351–60. [DOI] [PubMed] [Google Scholar]
- 41.Stringhini S, Zaninotto P, Kumari M, et al. Socio-economic trajectories and cardiovascular disease mortality in older people: the English Longitudinal Study of Ageing. Int J Epidemiol. 2018; 47(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YC, Wahlqvist ML, Lee MS. Appetite predicts mortality in free-living older adults in association with dietary diversity. A NAHSIT cohort study. Appetite. 2014; 83: 89–96. [DOI] [PubMed] [Google Scholar]
- 43.Benetos A, Thomas F, Bean KE, et al. Role of modifiable risk factors in life expectancy in the elderly. J Hypertens. 2005;23(10):1803–8. [DOI] [PubMed] [Google Scholar]
- 44.Mayeda ER, Filshtein TJ, Tripodis Y, et al. Does selective survival before study enrolment attenuate estimated effects of education on rate of cognitive decline in older adults? A simulation approach for quantifying survival bias in life course epidemiology. Int J Epidemiol. 2018; 47(5) :1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.