Supplemental Digital Content is Available in the Text.
Bariatric patients should be monitored to clarify the levels of serum vitamin A conferring deficiency in retina and establish the effectiveness of oral vitamin A supplementation. We present a noninvasive tool that can be used clinically to evaluate the status of vitamin A in relation to the visual cycle.
Key words: retina, vitamin A, bariatric surgery, fundus autofluorescence
Abstract
Background/Purpose:
Bariatric surgery is recognized as a treatment option for obesity. However, the cost-efficiency of screening for serum vitamin A and the effectiveness of its oral supplementation in these patients remain unclear. Here, we report a case in which vitamin A and carotenoid deficiency after bariatric surgery were monitored by noninvasive quantitative fundus autofluorescence imaging.
Methods:
Case report.
Results:
A 62-year-old man presented with a history of progressive night blindness. He had duodenal switch surgery 13 years earlier. One year before the initial visit, he had begun oral supplements of vitamins A. Short wavelength fundus autofluorescence images acquired for quantitative fundus autofluorescence revealed an intensity that was lower than the healthy-eye range. Scotopic rod-specific full-field electroretinograms were extinguished. These findings were consistent with vitamin A deficiency. The patient was given intramuscular vitamin A injections. At follow-up, quantitative fundus autofluorescence improved, ERG increased to normal, but macular pigment was unchanged.
Conclusion:
Oral vitamin A supplementation may not be sufficient after mal-absorptive surgery and a quantitative and noninvasive short wavelength fundus autofluorescence imaging technique may be useful to monitor the status of vitamin A and the carotenoids comprising macular pigment in the retina.
Obesity is a growing health problem and a major contributor to disease burden.1 Bariatric surgery is increasingly recognized as a treatment option. Nevertheless, these procedures are associated with risks of deficiencies in micronutrients, particularly vitamin A (all-trans-retinol).2,3 Mammals cannot synthesize retinol and thus depend on intestinal absorption of this micronutrient either as preformed dietary vitamin A (retinyl esters and retinol) or as the precursor β-carotene.4,5 The cost-efficiency of screening for serum vitamin A and the effectiveness of its oral supplementation in these patients are unclear.6
Vitamin A deficiency is perhaps most striking when it manifests as night blindness (delayed dark adaptation). This deficit reflects an insufficient supply of 11-cis-retinaldehyde, the vitamin A-derived chromophore of visual pigment in rods and cones. Here, we report a case in which vitamin A deficiency in a patient with previous bariatric surgery, was monitored by quantitative fundus autofluorescence (qAF) imaging.7
Case Report
A 62-year-old man presented to the Edward S. Harkness Eye Institute at Columbia University with a 24-month history of progressive night blindness. Two months earlier, he had undergone vitrectomy surgery to treat retinal detachment in his right eye. His history included LASIK surgery in both eyes and cataract surgery in the right eye, 27 and 2 years before, respectively. He also had bariatric surgery (duodenal switch) 13 years earlier. The patient had been noncompliant with respect to vitamin supplementation after the surgery; however, during the year before his initial visit, he had begun regular use of vitamin A, D, and E, multivitamin and calcium oral supplements.
Ophthalmologic examination revealed best-corrected visual acuities of 20/150 and 20/80 in the right and left eye, respectively. The anterior segment was quiet; the three-piece intraocular lens was well-positioned and clear in the right eye and a paracentral posterior polar cataract was detected in the left eye. Dilated fundus examination revealed a normal optic nerve with parapapillary atrophy in both eyes. The macula appeared normal except for reduced macular pigment. In the right eye, laser scars were seen temporally. The vessels appeared normal and no pigmentary clumps were seen.
By spectral domain optical coherence tomography, inner retinal layers and interfaces were discriminated. However, the ellipsoid zone and the interdigitation zone were less distinct (Figure 1A). Short wavelength fundus autofluorescence (SW-AF; 488 nm) images acquired by confocal scanning laser ophthalmoscopy and saved in normalized mode provided evidence of a marked reduction in macular pigment (Figure 2A). To enable measurement of fundus AF intensities, SW-AF images were also recorded using quantitative protocols (qAF).4 The mean qAF value (197 qAF-units) was lower than the range observed in age-similar healthy eyes (Figure 2, C and E; see Figure 1, Supplemental Digital Content 1, http://links.lww.com/ICB/A88).
Fig. 1.
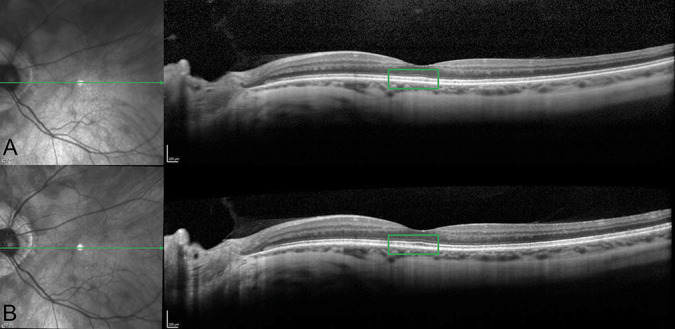
Spectral domain optical coherence tomography at first presentation (A) and follow-up visit (B). Left eye. Ellipsoid and interdigitation zone were not well-defined at the first visit; however, they improved after vitamin A treatment (green square in A and B).
Fig. 2.
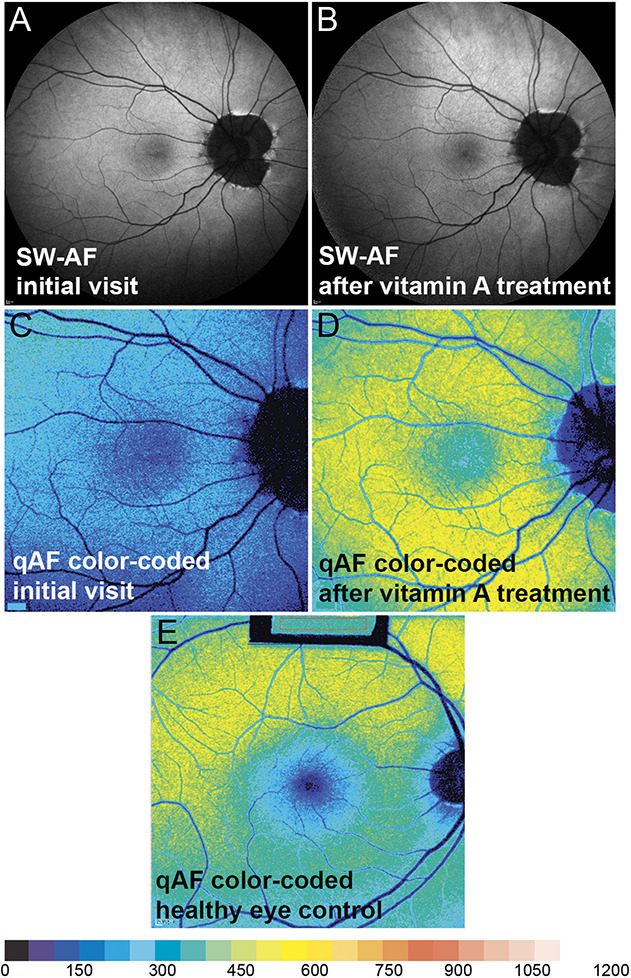
Short wavelength fundus autofluorescence and color-coded qAF. Short wavelength fundus autofluorescence (488 nm; normalized, right eye) image acquired at the initial visit (A) and after vitamin A treatment (B). Quantitative fundus autofluorescence color-coded image before (C) and after (D) vitamin A treatment. Quantitative fundus autofluorescence color-coded image of healthy control eye, 55 year-old subject (E). Note that macular pigment (blue central zone) is appreciably reduced initially and at follow-up (C and D).
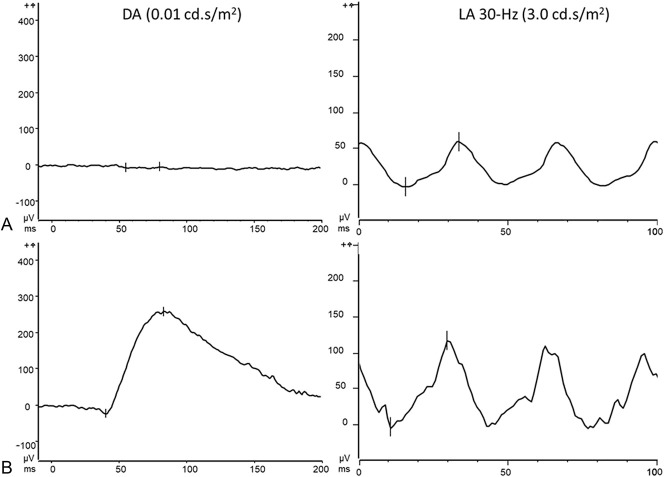
Full field electroretinography was performed according to the International Society for Clinical Electrophysiology of Vision (ISCEV) standard protocol. Scotopic rod-specific ERG was extinguished in both eyes. Photopic 30 Hz flicker ERG showed a normal amplitude, but a mild delay (Figure 3A).
Fig. 3.
Electroretinograms (ERG). Left eye. No rod-specific response was detected at the baseline visit (A, left). Thirty-Hertz flicker ERG revealed a mild delay (A, right). At the follow-up visit, after vitamin A treatment, dark-adapted b-wave amplitude and implicit times were normal (B, left) and the 30-Hz flicker ERG (B, right). DA, dark-adapted; LA, light-adapted.
The absence of rod function together with reduced qAF in a patient with previous mal-absorptive bariatric surgery suggested vitamin A deficiency. Indeed, vitamin A serological levels were 8 µg/dL (normal range: 24–85 µg/dL). Thus, the patient was given intramuscular injections of vitamin A (300,000 International Units) weekly for 1 month followed by bi-weekly injections for 3 months.
At the 4-month follow-up visit, best-corrected visual acuities improved to 20/50 both eyes and rod-specific ERG amplitudes were in the normal range as was the 30-Hz flicker ERG (Figure 3B). In spectral domain optical coherence tomography scans, ellipsoid zone and interdigitation zone reflectivity bands were better defined at the follow-up visit (Figure 1B). Short wavelength fundus autofluorescence imaging with acquisition of normalized images did not reveal differences in the pre and posttreatment image intensities (Figure 2, A and B). However, calculation of qAF using nonnormalized SW-AF images to avoid histogram stretch together with adjustment for sensitivity, ocular media absorbance, and magnification revealed that posttreatment mean qAF was 503 qAF-unit. The treatment effect was also apparent with color-coded qAF display (Figure 2, C and D). In addition, comparison of the qAF color-coded image after vitamin A treatment with the qAF map acquired from an age-similar healthy eye (Figure 2E) revealed that the topographic distribution of qAF had been altered, with intensities being noticeably higher in inferior hemiretina of the patient. No change in macular pigment was observed.
Discussion
We have shown here that in a patient that was vitamin A deficient after bariatric surgery, the vitamin A status of retina could be monitored by qAF protocols that included disabling the normalization mode of the cSLO that otherwise scales grey levels in the image to the full greyscale range (0–255). The autofluorescence emission that is recorded noninvasively as SW-AF originates in vitamin A-aldehyde adducts that form randomly and nonenzymatically as a result of the constant cycling of reactive retinaldehyde for vision.8 These bisretinoid fluorophores form in photoreceptor outer segments and are transferred to retinal pigment epithelial cells where they accumulate with age as lipofuscin. Bisretinoids are subject to photodegradation, a damaging process.7 Thus at any given time, levels of bisretinoid accumulation measured as qAF together with the fundus distribution of SW-AF intensities reflect the balance between synthesis and loss of the fluorophores due to photoregradation. Supplementation with vitamin A is known to accelerate the accumulation of bisretinoid lipofuscin,9 but the full capability of elevated systemic vitamin A to drive the formation of bisretinoid is not fully understood. This is an issue we are currently investigating.
Reduced fundus autofluoresccence can also be found in other retinal diseases associated with the vitamin A metabolism. For instance, patients with homozygous or compound heterozygous mutations in the RPE65 gene are not able to produce 11-cis-retinal and present with severely reduced fundus autofluorescence intensity.10
Macular pigment consists of the carotenoids lutein and zeaxanthin and the conjugated double-bond structure of carotenoids is a feature shared with vitamin A.4 Indeed, beta-carotene has a pro-Vitamin A function. Given that the optical density of macular pigment varies widely amongst healthy individuals11 we cannot be certain that the macular pigment presentation in our patient signaled the effects of bariatric surgery. However, it would not be surprising if malabsorption of vitamin A after bariatric surgery extended also to carotenoids. Macular pigment is considered to improve visual function by reducing light scatter and chromatic aberration.12 Thus, the persistent macular pigment deficiency may explain at least in part, why visual acuity had not recovered to 20/20.
In conclusion, we report that oral vitamin A supplementation may not be sufficient after mal-absorptive surgery. A quantitative and noninvasive SW-AF imaging technique may be useful to monitor the status of vitamin A and the carotenoids comprising macular pigment in the retina of these patients.
Supplementary Material
Footnotes
J. R. Lima de Carvalho, S. H. Tsang and J. R. Sparrow had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. Concept and design: J. R. Lima de Carvalho, S. H. Tsang and J. R. Sparrow. Acquisition, analysis and interpretation of data: J. R. Lima de Carvalho and J. R. Sparrow. Drafting of the manuscript: J. R. Lima de Carvalho and J. R. Sparrow. Critical revision of the manuscript for important intellectual content: J. R. Lima de Carvalho, S. H. Tsang and J. R. Sparrow. Statistical analysis: not relevant. Administrative technical or material support: S. H. Tsang and J. R. Sparrow. Study supervision: S. H. Tsang and J. R. Sparrow.
Supported by grants from the National Eye Institute/NIH EY024091 (J.R.S.); the Global Ophthalmology Awards Program, a Bayer-sponsored initiative committed to supporting ophthalmic research across the world (J.R.L.C.); Edward N. & Della L. Thome Memorial Foundation (S.H.T.); Jonas Children's Vision Care (S.H.T., J.R.S.); and a grant from Research to Prevent Blindness to the Department of Ophthalmology, Columbia University.
None of the authors has conflicting interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aasheim ET, Björkman S, Søvik TT, et al. Vitamin status after bariatric surgery: a randomized study of gastric bypass and duodenal switch. Am J Clin Nutr 2009;90:15–22. [DOI] [PubMed] [Google Scholar]
- 3.Guerreiro RA, Ribeiro R. Ophthalmic complications of bariatric surgery. Obes Surg 2015;25:167–173. [DOI] [PubMed] [Google Scholar]
- 4.O'Byrne SM, Blaner WS. Retinol and retinyl esters: biochemistry and physiology. J Lipid Res 2013;54:1731–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeed A, Dullaart RPF, Schreuder TCMA, et al. Disturbed vitamin A metabolism in non-alcoholic fatty liver disease (NAFLD). Nutrients 2017;10:E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis CA, de Jersey S, Hopkins G, et al. Does bariatric surgery cause vitamin A, B1, C or E deficiency? A systematic review. Obes Surg 2018;28:3640–3657. [DOI] [PubMed] [Google Scholar]
- 7.Delori FA, Duncker T, Sparrow JR. The measurement of fundus autofluorescence levels. In: Lois N, Forrester JV, eds. Fundus Autofluorescence . Philadelphia, PA: Wolters Kluwer; 2016:52–58. [Google Scholar]
- 8.Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res 2012;31:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radu RA, Yuan Q, Hu J, et al. Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following Vitamin A supplementation. Invest Ophthalmol Vis Sci 2008;49:3821–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenz B, Poliakov E, Schambeck M, et al. A comprehensive clinical and biochemical functional study of a novel RPE65 hypomorphic mutation. Invest Ophthalmol Vis Sci 2008;49:5235–5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curran Celentano J, Burke JD, Hammond BR, Jr. In vivo assessment of retinal carotenoids: macular pigment detection techniques and their impact on monitoring pigment status. J Nutr 2002;132:535S–539S. [DOI] [PubMed] [Google Scholar]
- 12.Hammond BR, Jr, Fletcher LM, Elliott JG. Glare disability, photostress recovery, and chromatic contrast: relation to macular pigment and serum lutein and zeaxanthin. Invest Ophthalmol Vis Sci 2013;54:476–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.