Abstract
Objective.
Elevated body weight in midlife is an established risk factor for accelerated cognitive decline, impairment, and dementia. Research examining the impact of later-life body mass index (BMI) on normal cognitive aging has produced mixed results. There is a need for longitudinal designs, replication across multiple cognitive domains, and consideration of BMI effects in the context of important moderators. The present research examined (a) BMI prediction of neuropsychological performance and decline in executive function (EF), neurocognitive speed, and memory and (b) sex stratification of BMI effects.
Method.
Participants (n = 869; 573 females; M age = 71.75, range = 53–85 years) were older adults from the Victoria Longitudinal Study. Latent growth modeling was used to examine BMI as a predictor of level and change in three latent variables of cognition. The data were then stratified by sex in order to test whether BMI effects differed for females and males. We adjusted for selected medical, psychosocial, and demographic characteristics.
Results.
Higher BMI predicted less decline in EF, neurocognitive speed, and memory. Interestingly, when the data were stratified by sex, higher BMI predicted less neuropsychological decline across domains for females only. BMI was unrelated to cognitive aging trajectories for males.
Conclusions.
We found that elevated BMI was a risk-reducing factor for cognitive decline only for females. Results may be used to enhance the precision with which intervention protocols may target specific subgroups of older adults.
Keywords: Victoria Longitudinal Study, aging, body mass index, obesity, cognition
Global prevalence rates of obesity have risen virtually unmitigated over the past 50 years (Hruby & Hu, 2015). A recent study pooled body mass index (BMI) data for 129 million participants in over 200 counties and found that, within the past four decades, we have transitioned from a world in which there was twice the number of underweight than overweight persons, to one in which there are more obese than underweight individuals (NCD Risk Factor Collaboration, 2017). This shift has been attributed to passive over-consumption of energy dense foods (e.g., items rich in fats, extracted sugars, and refined starches), together with progressive secular and age-related declines in physical activity levels (Haslam & James, 2005; Hruby & Hu, 2015). Obesity is increasingly understood to represent a complex, multifactorial disease with social, economic, lifestyle, environmental, behavioral, and genetic origins (Hruby & Hu, 2015). Mounting literature indicates that these factors often do not exert only direct effects, but rather have interactive or synergistic associations with obesity risk (Hruby & Hu, 2015). For example, modifiable lifestyle factors may be differentially associated with obesity as a function of age, sex, or ethnic background (Boardley & Pobocik, 2009; Hruby & Hu, 2015; Siddarth, 2013). That notwithstanding, obesity is recognized as one of the leading causes of preventable deaths (Hruby & Hu, 2015), and is expected to lead to the first decline in life expectancy in 100 years (Preston, Vierboom, & Stokes, 2018). This is due in large part to the fact that overweight and obese weight status are multisystem conditions that increase the risk of type 2 diabetes, cardiovascular disease, cancer, and other comorbidities (de Mutsert, Sun, Willett, Hu, & van Dam, 2014; Haslam & James, 2005; Vucenik & Stains, 2012). Although some of these diseases are themselves associated with cognitive decline in aging (Biessels, Strachan, Visseren, Kappelle, & Whitmer, 2014; Lutski, Weinstein, Goldbourt, & Tanne, 2017; Vega, Dumas, & Newhouse, 2017), emergent research suggests that BMI may be independently associated with adverse neurocognitive changes and outcomes.
Several recent reviews have established mid-life BMI as a risk factor for Alzheimer’s disease (AD) and related dementias (Anstey, Cherbuin, Budge, & Young, 2011; Pedditizi, Peters, & Beckett, 2016). Accordingly, BMI is a potential prognostic indicator of progression of mild cognitive impairment and AD (Besser et al., 2014; Kim, Kim, & Park, 2016). Neuroimaging studies have also linked elevated BMI with pathological brain changes prior to the onset of dementia (Shaw, Sachdev, Abhayaratna, Anstey, & Cherbuin, 2018). Findings from the limited literature examining the impact of later-life BMI on normal cognitive aging trajectories are equivocal: weight decline, weight increase, low BMI, and high BMI have all been linked with reduced performance and steeper decline, and yet other studies suggest that BMI does not affect cognition (for reviews see Bischof & Park, 2015; Smith, Hay, Campbell, & Trollor, 2011). The cognitive domains purported to be most sensitive to BMI effects also remains debatable (Prickett, Brennan, & Stolwyk, 2015). This inconsistency has led to a growing appreciation that BMI-cognition associations are more complex in older age than in early and mid-life (Smith, Hay, Campbell, & Trollor, 2011). These connections should therefore be studied with longitudinal designs, replicated across multiple cognitive domains, and examined in the context of important moderators. The present study was designed to fill in these gaps.
Aspects of methodological variability have been identified as a source of inconsistent findings. For instance, cross-sectional studies with a mean age up to 72 years report adverse effects of high BMI on cognitive function, whereas studies with a mean age of 73 years and above report risk-reducing effects (Smith et al., 2011). Contradictory findings from prospective research are often attributed to differences across studies in the definition of mid- and later-life, sex distribution, and sample size (Emmerzaal, Kiliaan, & Gustafson, 2015). Longitudinal studies may also have short follow-up times and/or inappropriate exclusionary criteria (e.g., do not exclude on the basis of dementia diagnosis or presence of conditions known to affect cognitive function; Dahl & Hassing, 2013; Prickett, Brennan, & Stolwyk, 2015). Such considerations make it difficult to differentiate cognitive decline as predicted by body weight from cognitive decline as predicted by preclinical dementia (Dahl & Hassing, 2013). We examined BMI-cognition relationships using longitudinal data spanning a 40-year band of aging (53–95 years) for a large sample of normally aging participants from the Victoria Longitudinal Study (VLS).
An additional methodological inconsistency in this area of research is measurement and analysis. Most studies have examined how body weight affects cognitive function using single neuropsychological indicators rather than latent variables (Dahl & Hassing, 2013). This can be problematic for several reasons, including variability across tasks in cognitive complexity and measurement error. In this study, we represent cognitive domains in a multi-indicator latent variable approach. Specifically, we used multiple measured neuropsychological indicators of each cognitive domain to represent and estimate scores on underlying cognitive constructs. Such latent variables have several advantages over single- or composite-variable approaches, including the ability to (a) correct for sources of measurement error that affect the reliability of measurement, and (b) establish the content, criterion, and construct validity of the latent variable under investigation (Little, 2013). Thus, this study addresses the challenging problem regarding classification and measurement of cognitive domains. Further, many studies employ neuropsychological outcome measures that are argued to be inappropriate for use with normally aging samples. For instance, the Mini-Mental State Exam and related measures of global cognitive function are primarily intended to serve as dementia screening tools, yet they have been widely employed in research conducted with normally aging older adults (Deckers, van Boxtel, Verhey, & Köhler, 2017; Prickett et al., 2015).
The present study examined the impact of BMI on three domains of cognition, including executive function (EF), neurocognitive speed (henceforth referred to as speed), and memory. We selected these domains on the basis of literature suggesting that differential effects associated with body weight may be more pronounced for EF and speed-related tasks (which may be sensitive to vascular compromise) as compared to memory tasks (which may be more sensitive to hippocampal dysfunction and early AD; Bischof & Park, 2015). Longitudinal studies that compare BMI effects across these domains are sparse and do not point to a consistent pattern (Clark, Xu, Callahan, & Unverzagt, 2016; Deckers, van Boxtel, Verhey, & Köhler, 2017), making this an important area of research attention.
Discrepant findings in BMI-aging research have also been attributed to variability across studies in the covariates or moderators that are selected for inclusion. For example, Bischof and Park (2015) noted in their review that several studies adjusted for comorbidities of obesity (e.g., type 2 diabetes), whereas others prioritized psychosocial (e.g., depression) and demographic (e.g., sex) characteristics (see also Dahl & Hassing (2013) for study-specific details on statistical controls). The authors argued that this inconsistency limits our ability to draw meaningful conclusions on obesity-cognition associations and suggest that future research should incorporate a range of potential covariates that may modify or mediate weight-cognition associations (e.g., hypertension, type 2 diabetes, metabolic abnormalities, education, and depression). In the present study, we controlled for the potential confounding effects of a wide and representative set of covariates, including: education, pulse pressure, type 2 diabetes, hardening of arteries, stroke, heart trouble, depressive symptoms, subjective health, alcohol use, smoking status, and everyday physical activity.
Notably, we did not control for potential sex-related effects, but rather specifically examined whether longitudinal BMI-cognition associations were moderated by sex. Our rationale for exploring sex moderation is threefold. First, it was recently noted that weight-cognition associations may be obscured in studies where sex is treated as a study covariate as opposed to a grouping variable (Andrew & Tierney, 2018; García-Ptacek, Faxén-Irving, Čermáková, Eriksdotter, & Religa, 2014). A possible reason for this is that the strength and direction of BMI effects may differ for females as compared to males. Second, limited research has explored sex differences and findings from available studies are mixed. For example, research from the Framingham Heart Study reported that, whereas obesity in females was unrelated to cognition, obesity in males was associated lower scores on a composite measure of global cognition (Elias, Elias, Sullivan, Wolf, & D’Agostino, 2005), as well as poorer learning and memory performance (Elias, Elias, Sullivan, Wolf, & D’Agostino, 2003). In constrast, other research found that obesity was associated with less global cognitive decline for males and accelerated global cognitive decline for females (Han et al., 2009). Interestingly, there are also studies that reported higher BMI was associated with a lower dementia risk for females, whereas BMI was less associated (Dahl, Löppönen, Isoaho, Berg, & Kivelä, 2008) or unassociated (Kim et al., 2016) with dementia risk for males. Importantly, these studies did not examine sex differences the context of comparisons across multiple cognitive domains (at the latent variable level) or in longitudinal trajectory analyses. Third, sex differences in brain and cognitive aging performance are evident across several domains (Ritchie et al., 2018; Weber, Skirbekk, Freund, & Herlitz, 2014). For instance, a recent cognitive trajectory analysis showed that females often demonstrate higher levels of performance and less susceptibility to age-related decline (McCarrey, Yang, Kitner-Triolo, Ferrucci, & Resnick, 2016). At the same time, however, females are disproportionately affected by AD in terms of disease severity, rate of progression, and prevalence (Mazure & Swendsen, 2016), and reviewers have called for increased investigation (Tierney, Curtis, Chertkow, & Rylett, 2017). When taken together, these studies suggest that sex may moderate BMI-cognition associations. Evidence for this moderation would be constituted by statistically significant differences in BMI-cognition trajectory predictions for males and females.
The overarching aims of this study were to explore BMI as a predictor of level (i.e., performance at a statistical centering age) and slope (i.e., longitudinal change) of three cognitive domains, specifically EF, speed, and memory. We performed analyses stratified by sex in order to ascertain whether these associations differed between females and males. We examined the following specific research goals (RG). RG1 examined whether continuous baseline BMI independently predicted performance and change in each of the latent cognitive variables. RG2 examined whether sex moderated the level and longitudinal BMI-cognition relationships. We hypothesized that BMI would predict cognitive performance and decline for EF and speed, whereas memory would be less affected or unaffected by BMI. We also anticipated that findings would differ for females relative to males; however, we did not make a prediction regarding the direction of these effects (i.e., risk or protection).
Methods
Participants
Participants were community dwelling adults from the VLS. The VLS is a large-scale, long-term investigation of neurocognitive aging as influenced by multiple biomarkers, including functional, health, genetic, metabolic, and lifestyle characteristics (Dixon & de Frias, 2004). The VLS and all data collection procedures were in full and certified compliance with prevailing human research ethics guidelines and boards. Written informed consent was obtained from all participants. The VLS includes longitudinal cohorts aged 53–85 at recruitment. Continuing participants were tested at an average of 4.4-year intervals. The source cohort for this study had three waves of longitudinal data. In line with established procedures for accelerated longitudinal designs (Galbraith, Bowden, & Mander, 2017; Little, 2013), we used age as the metric of longitudinal change. This has the advantage of allowing us to control for age-related effects and to improve interpretability of the results. The resulting design covers a 40-year band of aging (McFall, McDermott, & Dixon, 2019).
The following exclusionary criteria were applied at the first wave: concurrent (or history of) serious health conditions that may affect mortality or baseline cognitive health (e.g., self-reported serious cardiovascular, cerebrovascular, head injury, or psychiatric conditions), as well as a self-reported diagnosis of Parkinson’s disease or AD. We further applied the following exclusionary criteria to the source subsample at baseline: Mini Mental State Exam score < 24 (n = 4), missing data for BMI (n = 3), or BMI < 18.5 kg/m2 (n = 7), and missing data for over 50% of the neuropsychological tasks (i.e., more than 10; n = 17). Descriptive statistics for the remaining sample are outlined in Table 1 (n = 869; 573 females; Mage = 71.75, SD = 9.10, age range = 53 – 95 years; primarily White, non-Hispanic). Participant retention rates were 69% for W1 to W2 and 74% for W2 to W3.
Table 1.
Participant Characteristics at Baseline by Sex
| Characteristic | Females | Males |
|---|---|---|
| n (%) | 573 (66%) | 296 (34%) |
| Age (in years) | 71.16 (9.39) | 72.90 (8.39) |
| Body mass index (kg/m2) | 26.85 (4.56) | 26.85 (3.44) |
| Range | 18.58 – 48.61 | 18.91 – 38.22 |
| Education (in years) | 14.82 (2.85) | 15.80 (3.09) |
| Pulse pressure (mm Hg) | 52.22 (10.40) | 53.50 (9.59) |
| Type 2 diabetesa | 38 (7%) | 33 (11%) |
| Hardening of arteriesa | 51 (9%) | 31 (11%) |
| Strokea | 24 (4%) | 21 (7%) |
| Heart troublea | 106 (19%) | 85 (29%) |
| Depressionb | 111 (19%) | 32 (11%) |
| Health to perfectc | 4.14 (.76) | 4.18 (.72) |
| Health impact on recreationd | 4.18 (1.08) | 4.30 (1.00) |
| Alcohola | 481 (84%) | 266 (90%) |
| Smokinga | 22 (4%) | 15 (5%) |
| Everyday physical activitye | 15.19 (4.60) | 15.84 (6.09) |
Note. Results are presented as mean (standard deviation).
Results are reported as number (percent) of participants who responded yes to the question.
Participants indicated whether they experienced depressive symptoms or unhappiness over the past month.
Indicated health relative to perfect on a scale of 1 “very poor” to 5 “very good”.
Reported impact of health on ability to perform physical recreational activities on a scale of 1 “gave up” to 6 “improved”.
Indicated frequency of participation in everyday physical activities over a period of two years on a scale of 0 “never” to 8 “daily”. Responses were summed over four-items.
Measures
The neuropsychological tasks employed have (a) been widely used and documented with older adults in the VLS and related research, (b) established psychometric properties, and (c) demonstrated sensitivity to functional biomarkers and neurocognitive factors (de Frias, Dixon, & Strauss, 2009; McFall et al., 2019; McFall, Wiebe, Vergote, Anstey, & Dixon, 2015; Thibeau, McFall, Wiebe, Anstey, & Dixon, 2016). All tasks were (re)coded such that higher scores represented better performance.
Executive Function.
In order to establish a single-factor EF latent variable, we used the following eight manifest indicators that represented the inhibition (i.e., Hayling Sentence Completion Test and Stroop Test), shifting (i.e., Brixton Spatial Anticipation Test and Color Trails Test), and updating (i.e., Computation Span, Reading Span, Letter Sets, and Letter Series) aspects of EF.
Hayling Sentence Completion Test.
This task test measured initiation speed (Section 1; 15 items) and response suppression (Section 2; 15 items) in finding suitable words to complete a sentence. Standardized scores were based on errors from Section 2 and the speed of responses in both sections. These values were combined to obtain the final score which was then used as the target measure (Bielak, Mansueti, Strauss, & Dixon, 2006; Burgess & Shallice, 1997).
Stroop Test.
For this task, participants were required to ignore the automatic response of reading a printed word and instead name the color of ink in which the word was printed. This test consists of the standard three parts (Parts A, B and C), with the measures based on latencies (de Frias et al., 2009; McFall, Sapkota, McDermott, & Dixon, 2016; Regard, 1981). The target measure was the standardized Stroop interference index ([Part C- Part A]/ Part A).
Brixton Spatial Anticipation Test.
Participants were presented with 10 different circles, one of which was blue, while the remaining circles had no color. Participants were instructed to guess where the blue colored circle would appear on subsequent pages. The target measure was the total number of errors across 55 trials (Bielak et al., 2006; Burgess & Shallice, 1997).
Color Trails Test (Part 2).
This test comprises two different sections in which participants were asked to connect different attributes, such as numbered and colored circles. Latency scores in the second of two sections were computed and used as the target measure (D’Elia, Satz, Uchiyama, & White, 1996).
Computation Span.
Participants were instructed to solve a series of arithmetic problems and to hold the final digit from each problem solved in memory for later recall. The number of problems in a series increased from one to seven, with three trials at each series length. The highest span correctly recalled for two out of three trials was the target measure (Salthouse & Babcock, 1991).
Reading Span.
Participants answered questions about a series of short sentences and were asked to hold the final word from each sentence in memory for later recall. The number of sentences in a passage increased from one to seven. There were three trials at each series length. The target measure was the highest span correctly recalled for two out of three trials (Salthouse & Babcock, 1991).
Letter Sets.
Participants were presented with five sets of four letters (e.g., ABCD). They were instructed to find the rule that made each set of letters alike, and to then circle the set of letters that did not conform to this rule. The total number of correct responses out of 15 items was the target measure (Ekstrom, French, Harman, & Dermen, 1976).
Letter Series.
Participants were asked to identify the letter that would continue an established pattern in a series of letters (e.g., ABDA BDAB…). The target measure was the total correct out of 20 items (Thurstone, 1962).
Speed.
In order to establish a single-factor speed latent variable, we used eight manifest indicators as outlined below. Four of these tasks were multi-trial computer-based reaction time tasks. For these tasks, we used correction procedures validated by the VLS to trim extreme outliers from raw latency scores. We describe these techniques briefly below (for more detail see Dixon et al., 2007; McFall, Wiebe, Vergote, Anstey, & Dixon, 2015).
Digit Symbol Substitution.
Participants were given 90 seconds to match symbols and numbers in test boxes. The target measure was the number of correctly transcribed items (Wechsler, 1981).
Verbal Fluency.
The following three measures were drawn from the Kit of Factor Referenced Cognitive Tests (Ekstrom et al., 1976): Controlled Associations, Opposites, and Figures of Speech. In the Controlled Associations test, participants were given one of four target words and instructed to write as many words as they could within six minutes that have the same or similar meaning as the target word. In the Opposites task, participants were given one of four target words and were instructed to write as many words as possible within five minutes that had the opposite or nearly the opposite meaning of the target word. In the Figures of Speech task, participants were given one of five figures of speech and were instructed to write as many words or phrases that would complete the figure of speech. The target measure for each task was the total number of correct responses.
Simple Reaction Time.
Participants were presented with a warning stimulus followed by a signal stimulus in the middle of the screen. They were instructed to press a key with their preferred hand as quickly as possible when the signal stimulus appeared. A total of 10 practice trials followed by 50 test trials were administered. Ten randomly arranged trials were presented at each of the 5 intervals separating the warning and signal stimuli (i.e., 500, 625, 750, 875, and 1,000 ms). The target measure was the average latency across 50 trials (Dixon et al., 2007).
Choice Reaction Time.
A 2 × 2 grid matching the arrangement of keys on the response console was displayed on the screen. For each block of 10 trials, participants were required to attend to four plus signs. Following a 1000 ms delay, one of the plus signs was replaced by a square. Participants were instructed to press the key corresponding to the location of the square as quickly as possible. The target measure was the average latency across 20 trials (Dixon et al., 2007).
Lexical Decision.
Participants were presented with a string of five to seven letters on the computer screen and were asked to indicate as quickly as possible whether the letters formed an English word (e.g., island vs. nabion). A total of 3 practice trials and 60 test trials were presented (30 words and 30 nonwords). The target measure was the average response latency across 60 trials (Baddeley, Logie, Nimmosmith, & Brereton, 1985).
Sentence Verification.
A sentence was presented on the computer screen and participants were asked to identify as quickly as possible the plausibility of the sentence (e.g., The tree fell to the ground with a loud crash vs. The pig gave birth to a litter of kittens this morning). A total of 4 practice trials and 50 test trials were administered. The target measure was the average latency across 50 trials (Palmer, Macleod, Hunt, & Davidson, 1985).
Correction Procedures.
Validated correction procedures were applied to each of the four computer-based reaction-time tasks. Given that each task differed in cognitive complexity, we applied varying lower and upper limits as follows: (a) simple reaction time, 150 ms lower limit, 2500 ms upper limit, (b) choice reaction time, 150 ms lower limit, 4000 ms upper limit, (c) lexical decision, 400 ms lower limit, 10,000 ms upper limit, and (d) sentence verification, 1000 ms lower limit, 20,000 ms upper limit. We subsequently removed trials that fell three standard deviations above or below the sample mean.
Memory.
In order to establish a single-factor memory latent variable, we used four manifest indicators as follows.
Rey Auditory Verbal Learning Test.
For this task, participants were read 15 nouns and asked to immediately recall as many nouns as possible. Participants completed five trials using this same list. A second list of 15 unrelated nouns was then read aloud to participants who were again asked to immediate recall as many nouns as possible (Trial B1). Finally, participants were asked to recall nouns from the first list (Trial A6). The number of nouns recalled from Trial B1 and A6 were used as the target measures (Lezak, 1983).
Word Recall.
Participants were presented with two categorized lists of 30 English nouns and asked to remember as many words possible. They were given two minutes to study the words and then had a five-minute period in which they wrote down as many words as they could remember. The average number of correctly recalled words across the two lists was the target measure (Dixon, Wahlin, Maitland, Hultsch, Hertzog, & Backman, 2004).
Digit Symbol Substitution.
Participants were asked to recall what number corresponded to each of the symbols in the foregoing task. The target measure was the total number of correct responses out of nine (Wechsler, 1981).
Body Mass Index.
Height and weight measurements were taken at each wave in accordance with a standardized protocol. BMI was calculated for each participant by dividing weight in kg by height in m2. Because general consensus on the definition of obesity in the elderly has yet to be reached (Decaria, Sharp, & Petrella, 2012), we used continuous BMI as the focal predictor in all analyses reported below. Results from an unconditional latent growth model indicated that there was no variability across study participants in the rate of change in BMI over the study duration (e.g., subgroups of individuals with increased BMI, stability, or precipitous decline; see Table 3). For this reason, we used BMI at baseline for all analyses reported below (see Table 1 for descriptive statistics).
Table 3.
Goodness-of-Fit Indices for Body Mass Index, Executive Function, Speed, and Memory Unconditional Latent Growth Models
| Model | −2LL | Parameters free | AIC | BIC | D | Δdf |
|---|---|---|---|---|---|---|
| Body Mass Index | ||||||
| Fixed intercept | 10,650.81 | 4 | 10,658.81 | 10,677.81 | -- | -- |
| Random intercept | 9,068.69 | 5 | 9,078.69 | 9,102.44 | 1,582.11* | 1 |
| Random intercept, fixed slopea | 9,018.14 | 6 | 9,030.14 | 9,058.64 | 50.55* | 1 |
| Random intercept, random slope | 9,015.58 | 8 | 9,031.58 | 9,069.58 | 2.57 | 2 |
| Executive Function | ||||||
| Fixed intercept | 5,572.72 | 4 | 5,580.72 | 5,599.79 | -- | -- |
| Random intercept | 3,852.99 | 5 | 3,862.99 | 3,886.82 | 1,719.73* | 1 |
| Random intercept, fixed slope | 2,650.38 | 6 | 2,662.38 | 2,690.98 | 1,202.61* | 1 |
| Random intercept, random slopea | 2,333.39 | 8 | 2,349.39 | 2,387.53 | 316.99* | 2 |
| Speed | ||||||
| Fixed intercept | 5,592.43 | 4 | 5,600.43 | 5,619.50 | -- | -- |
| Random intercept | 4,362.82 | 5 | 4,372.82 | 4,396.66 | 1,229.61* | 1 |
| Random intercept, fixed slope | 3,444.05 | 6 | 3,456.05 | 3,484.65 | 918.77* | 1 |
| Random intercept, random slopea | 3,254.02 | 8 | 3,270.02 | 3,308.16 | 190.03* | 2 |
| Memory | ||||||
| Fixed intercept | 5,457.77 | 4 | 5,475.65 | 5,494.72 | -- | -- |
| Random intercept | 3,793.33 | 5 | 3,835.51 | 3,859.35 | 1,664.44* | 1 |
| Random intercept, fixed slope | 2,569.23 | 6 | 2,638.94 | 2,667.55 | 1,224.10* | 1 |
| Random intercept, random slopea | 2,113.30 | 8 | 2,184.52 | 2,222.66 | 455.93* | 2 |
Note. −2LL = −2 log likelihood; AIC = Akaike information criterion; BIC = Bayesian information criterion; D = difference statistic (using −2LL).
Best fitting model.
p ≤ .001
p ≤ .01
p ≤ .05
Covariates.
We selected potential baseline covariates on the basis of earlier BMI-aging research. Potential covariates included self-reports of the following variables: total years of education, type 2 diabetes (no or yes), hardening of arteries (no or yes), history of stroke (no or yes), heart trouble (no or yes), experience of depressive symptoms or severe unhappiness over the past month (no or yes; derived from the Bradburn Affect Balance Scale; Bradburn, 1969), current alcohol use (no or yes), current smoking status (no or yes), health relative to perfect (1 = very poor, 5 = very good), and impact of health on ability to perform physical recreational activities (1 = gave up, 6 = improved). Participants also responded to four questions related to how often they participated in everyday physical activities over a period of two years (0 = never, 8 = daily). Responses were summed over the four-items (Thibeau et al., 2016). Finally, we included a clinical assessment of pulse pressure (a proxy for arterial stiffness; McFall et al., 2015) as a potential study covariate.
Statistical Analyses
Analyses included confirmatory factor analysis (CFA), longitudinal measurement invariance testing, and latent growth modeling (LGM). These analyses were conducted using Mplus 8.0 (Muthén & Muthén, 2017). We centered age at 75 years for all LGM based on the following criteria (a) this is the approximate mean of the 40-year span of data, (b) cognitive aging research has established this as a common inflection point for such age spans (Dixon et al., 2012), and (c) this practice mirrors earlier VLS research (e.g., McFall et al., 2015). We used multiple imputations to estimate (a) age and factor scores for EF, speed, and memory that were missing due to attrition and (b) between 3–15% of covariate data that were missing. We selected this approach to handling missing data on the basis of literature indicating that generalizations from studies using multiple imputations are superior to those from studies using classical approaches to handling missing data (e.g., list-wise or pair-wise deletion; Little, 2013). Data were assumed to either be missing at random (attrition) or missing completely at random (item non-response; Little, 2013). In accordance with prevailing conventions (Enders, 2011; Little, 2013), we generated 20 imputations of the data set and pooled these for all conditional LGM.
Foundational Analyses.
In order to test and confirm basic characteristics of the data, we performed a series of foundational analyses as outlined below. Analyses were performed separately for each latent variable.
First, we verified that EF, speed, and memory could each be represented by a single-factor latent variable using CFA. In brief, CFA allows researchers to test how well each indicator represents the latent construct. This information is gleaned from statistical model fit and factor loadings. Statistical model fit was determined using the following standard fit indexes: (a) χ2 for which a good fit would produce a non-significant result (i.e., p > .05; indicates that the data do not significantly differ from the model-based estimates), (b) the comparative fit index (CFI) for which fit is judged by a value of ≥ .95 as good and ≥ .90 as adequate, (c) root mean square error of approximation (RMSEA) for which fit is judged by a value of ≤ .05 as good and ≤ .08 as adequate, and (d) standardized root mean square residual (SRMR) for which fit is judged by a value of ≤ .08 as good (Little, 2013). Neuropsychological tasks were determined to be strong indicators of the corresponding latent construct if factor loadings were > .30 (Little, 2013).
Second, we tested these models for longitudinal measurement invariance. Specifically, we evaluated (a) configural invariance, which constrains the latent variable at each time point to be represented by the same set of indicators; (b) metric invariance, which constrains the factor loadings of each indicator onto the latent variable to be equal across time points; and (c) scalar invariance, which constrains the intercepts for each indictor to be equal across time. The tenability of invariance assumptions were tested by comparing models with unconstrained and constrained parameters using change in CFI, for which changes of ≤ .01 suggest the assumption is reasonable (Little, 2013). We then estimated factor scores for each latent variable and employed these in all subsequent LGM.
Third, we established the functional form of change for each latent variable using unconditional LGM. We identified the best model for each latent variable by testing the following models in sequence (a) a fixed intercept model, which assumes that there is no variability between or within persons; (b) a random intercept model, which allows for variability across persons in overall level but assumes no intraindividual change; (c) a random intercept, fixed slope model, which allows for variability across persons in level but assumes that each person changes at the same rate; and (d) a random intercept, random slope model, which allows for variability across persons in both level and change (Singer & Willett, 2003). Analyses for our focal RGs were subsequently performed. We describe these in greater detail below.
RG1: BMI prediction of EF, speed, and memory growth models.
We used the best-fitting unconditional LGM identified in foundational analyses as the baseline model against which we tested a conditional LGM for which continuous baseline BMI predicted performance and change in EF, speed, and memory. Analyses were performed separately for each latent variable. Models were covariate adjusted using a three-step approach (Bäckman et al., 2015). In the first step, we performed a conditional LGM for each latent variable that included the entire pool of potential covariates, together with baseline BMI. Covariates that were associated with level of performance and/or change at the criterion of p < .10 were retained and included in a subsequent conditional LGM (step two). Covariates that showed a significant association (p ≤ .05) with level of performance and/or change were retained and included in a final conditional LGM (step three).
RG2: Sex moderation of BMI-cognition relationships.
We examined whether sex moderated BMI effects by testing two models for each latent variable. First, we stratified the data by sex and tested a multiple-group model where baseline BMI predicted level and change. Second, we tested a nested model where these effects were constrained to be equal across sex. Evidence of moderation was inferred from a significant −2 log likelihood (−2LL) difference statistic (D at p < .10), which compared the unconstrained model to the constrained model. For this analysis, we retained the same set covariates as in RG1. However, in the event that a covariate was no longer a significant predictor for females or males, it was dropped from the model. This approach allowed us to arrive at a parsimonious model for each latent variable that adjusted only for the effect of significant covariates.
We also explored our data for an age x sex x BMI interaction. To do this, we stratified the data into young-old (aged ≤ 72 years) and old-old (aged > 72 years) age groups. We controlled for potential confounds using the same approach as outlined above. Results of these exploratory analyses were not significant and are thus not described in greater detail below.
Results
Foundational Analyses
Details on model fit indices and model comparisons are presented in Table 2 and 3, respectively. Results of the CFA demonstrated that a single-factor latent variable model for EF (as depicted in Figure 1), speed, and memory fit the data adequately and all indicators had strong factor loadings (i.e., > .50) on the corresponding latent construct. Results for tests of measurement invariance for EF showed full metric and partial scalar invariance (i.e., intercepts for Brixton were unconstrained, while the remaining intercepts were constrained across time; final model fit indices: RMSEA = .04; CFI = .96; SRMR = .05). The speed model showed full metric and full scalar invariance (final model fit indices: RMSEA = .07; CFI = .91; SRMR = .10). The memory model had full metric and partial scalar invariance (i.e., intercepts for Rey B1 were unconstrained while the remaining intercepts were constrained across time; final model fit indices: RMSEA = .04; CFI = .98; SRMR = .07). These results indicated that it was reasonable to proceed with analyses related to the key parameters of each construct (Little, 2013).
Table 2.
Goodness-of-Fit Indices for Executive Function, Speed, and Memory Confirmatory Factor Analysis Models and Measurement Invariance Testing
| Model | AIC | BIC | χ2 | df | p | RMSEA | CFI | SRMR | ΔCFI |
|---|---|---|---|---|---|---|---|---|---|
| One-factor EF | |||||||||
| Configural invariance | 45,246.58 | 45,747.15 | 410.84 | 219 | <.001 | .03 (.03 - .04) | .97 | .04 | -- |
| Metric invariance | 45,287.90 | 45,721.73 | 480.16 | 233 | <.001 | .04 (.03 - .04) | .97 | .05 | 0 |
| Scalar invariance | 46,155.68 | 46,518.00 | 1,377.94 | 248 | <.001 | .07 (.07 - .08) | .84 | .09 | .13 |
| Partial scalar invarianceac | 45,305.07 | 45,681.69 | 521.332 | 245 | <.001 | .04 (.03 - .04) | .96 | .05 | .01 |
| One-factor speed | |||||||||
| Configural invariance | 59,510.81 | 60,054.29 | 1,086.82 | 210 | <.001 | .07 (.06 - .07) | .92 | .09 | -- |
| Metric invariance | 59,526.91 | 60,003.65 | 1,130.93 | 224 | <.001 | .07 (.06 - .07) | .92 | .09 | 0 |
| Scalar invariancec | 59,556.10 | 59,966.10 | 1,188.12 | 238 | <.001 | .07 (.06 - .07) | .91 | .10 | .01 |
| One-factor memory | |||||||||
| Configural invariance | 32,089.27 | 32,332.40 | 67.08 | 39 | .003 | .03 (.02 - .04) | .99 | .03 | -- |
| Metric invariance | 32,112.17 | 32,326.70 | 101.98 | 45 | <.001 | .04 (.03 - .05) | .98 | .07 | .01 |
| Scalar invariance | 32,156.20 | 32,342.12 | 158.01 | 51 | <.001 | .05 (.04 - .06) | .96 | .07 | .02 |
| Partial scalar invariancec | 32,129.23 | 32,324.69 | 127.04 | 49 | <.001 | .04 (.03 - .05) | .98 | .07 | 0 |
Note. EF = executive function; AIC = Akaike information criterion; BIC = Bayesian information criterion; χ2 = chi-square test of model fit; df = degrees of freedom for model fit; RMSEA = root mean square error of approximation; RMSEA is shown with 90% confidence intervals; CFI = comparative fit index; SRMR = standardized root mean square residual; ΔCFI = change in CFI.
Brixton free to vary.
Rey B1 free to vary.
Best fitting model.
Figure 1.
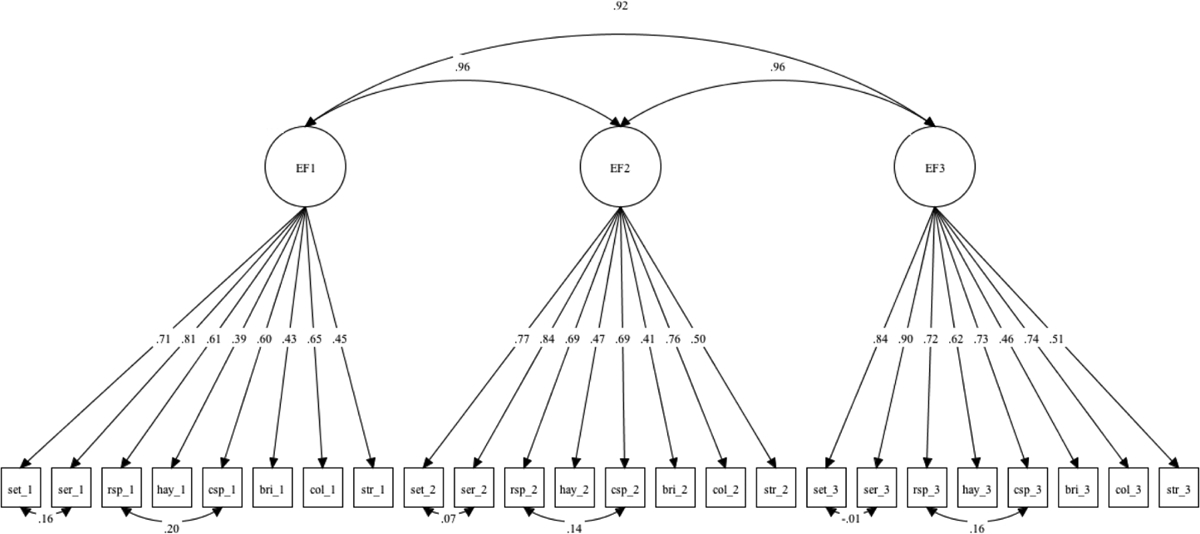
Longitudinal confirmatory factor analysis model of executive function (EF). SET, Letter Sets; SER, Letter Series; RSP, Reading Span; CSP, Computation Span. Standardized loadings are shown. All loadings were significant at < .05. Residuals for each indicator were correlated across waves (not depicted). Indicators with similar methodology were correlated. Variance of the latent variable was fixed at 1.00 for scale setting.
We subsequently determined that the best-fitting unconditional LGM for each latent variable was a random intercept, random slope model. These results permit three overall conclusions. First, participants demonstrated significant variability in their level of EF (b = .752, p < .001), speed (b = .734, p < .001), and memory performance (b = .812, p < .001). Second, individuals showed significant decline in EF (M = −.068, p < .001), speed (M = −.070, p < .001), and memory performance (M = −.074, p < .001). Third, there were significant individual differences in the rate of decline observed for EF (b = .001, p < .001), speed (b = .002, p < .001), and memory (b = .002, p < .001). Results for our two main RGs were as follows.
RG1: BMI prediction of EF, speed, and memory growth models
Covariate adjusted results (see Table 4 for covariates that were significant predictors of cognitive performance and/or decline) revealed that BMI did not predict level of performance for EF (b = .003, p = .950), speed (b = .016, p = .722), or memory (b = .010, p = .816). However, BMI was related to change in EF (b = .006, p = .007), speed (b = .009, p = .004), and memory (b = .007, p = .002). For all cognitive domains, higher BMI values predicted less decline.
Table 4.
Adjusted Conditional Latent Growth Models for Executive Function, Speed, and Memory
| Baseline BMI plus covariates | Level β | Change β |
|---|---|---|
| EF model | ||
| Body mass index (kg/m2) | .003 | .006* |
| Education (in years)b | .069** | .003** |
| Pulse pressure (mm Hg)b | -- | −.015** |
| Type 2 diabetesa | −.198* | -- |
| Hardening of arteriesa | -- | −.022** |
| Health to perfectb | .101* | -- |
| Health impact on recreationb | -- | .009** |
| Alcohola | .260* | .014* |
| Speed model | ||
| Body mass index (kg/m2) | .024 | .009* |
| Education (in years)b | .049** | .003* |
| Pulse pressure (mm Hg)b | -- | −.022** |
| Type 2 diabetesa | −.328* | -- |
| Strokea | -- | −.033* |
| Depressiona | -- | .012* |
| Alcohola | .228* | -- |
| Memory model | ||
| Body mass index (kg/m2) | .010 | .007* |
| Education (in years)b | .036* | .002** |
| Pulse pressure (mm Hg)b | .132** | −.010** |
| Hardening of arteriesa | -- | −.014* |
| Heart troublea | -- | −.014** |
| Depressiona | -- | .009* |
| Everyday physical activityb | -- | .001** |
Note. Performance at age 75 (level) and 9-year change (slope) were only adjusted for the effect of significant covariates. BMI = body mass index; EF = executive function.
Responses coded such that 0 = no and 1 = yes.
Grand mean centered.
p ≤ .001
p ≤ .01
p ≤ .05
RG2: Sex moderation of BMI-cognition relationships
Analyses were performed using continuous BMI; however, for ease of interpretation, we discuss significant results in this subsection in reference to the following values for BMI: 30 (i.e., within the obese range), 25 (i.e., within the overweight range), 20 (i.e., within the normal weight range). Results for study covariates that were significant predictors of cognitive performance and/or decline are outlined in Table 5.
Table 5.
Adjusted Conditional Latent Growth Models as Stratified by Sex for Executive Function, Speed, and Memory
| Females | Males | |||
|---|---|---|---|---|
| Baseline BMI plus covariates | Level β | Change β | Level β | Change β |
| EF model | ||||
| Body mass index (kg/m2) | .021 | .008* | −.039 | .001 |
| Education (in years)b | .072** | .003** | .068** | .003* |
| Pulse pressure (mm Hg)b | -- | −.016** | -- | −.010* |
| Type 2 diabetesa | −.247* | -- | −.118 | -- |
| Hardening of arteriesa | -- | −.023** | -- | −.020 |
| Health to perfectb | .113* | -- | .071 | -- |
| Health impact on recreationb | -- | .008** | -- | .011* |
| Alcohola | .299* | .015* | .151 | .015 |
| Speed model c | ||||
| Body mass index (kg/m2) | .065 | .012** | −.075 | −.002 |
| Education (in years)b | .053** | .002* | .055* | .005* |
| Pulse pressure (mm Hg)b | -- | −.023** | -- | −.016* |
| Type 2 diabetesa | −.415** | -- | −.224 | -- |
| Strokea | -- | −.042** | -- | −.016 |
| Alcohola | .341** | -- | −.065 | -- |
| Memory modelcd | ||||
| Body mass index (kg/m2) | −.018 | .007* | .107 | .007 |
| Education (in years)b | .052* | .003** | .046* | .003* |
| Pulse pressure (mm Hg)b | .140* | −.010** | .159* | −.010* |
| Heart troublea | -- | −.017** | -- | −.015* |
| Everyday physical activityb | -- | .001** | -- | .001* |
Note. Performance at age 75 (level) and 9-year change (slope) were only adjusted for covariates that were significant for either sex. BMI = body mass index; EF = executive function.
Responses coded such that 0 = no and 1 = yes.
Grand mean centered.
Depression was no longer a significant predictor and was dropped from the model.
Hardening of arteries was no longer a significant predictor and was dropped from the model.
p ≤ .001
p ≤ .01
p ≤ .05
Sex moderation of BMI predicting EF.
Adjusted results for the unconstrained model stratified by sex showed that BMI did not predict EF performance level for females (b = .021, p = .670) or males (b = −.039, p = .605). However, BMI significantly predicted change in EF for females (b = .008, p = .003) and not for males (b = .001, p = .858). Sex was a significant moderator (D = 12.418, Δdf = 7, p = .090). As shown in Figure 2a, females with a BMI of 30 (M = −.081) exhibited less decline relative to females with a BMI of 25 (M = −.089) or 20 (M = −.097). Non-significant results for males are shown in Figure 2b.
Figure 2.
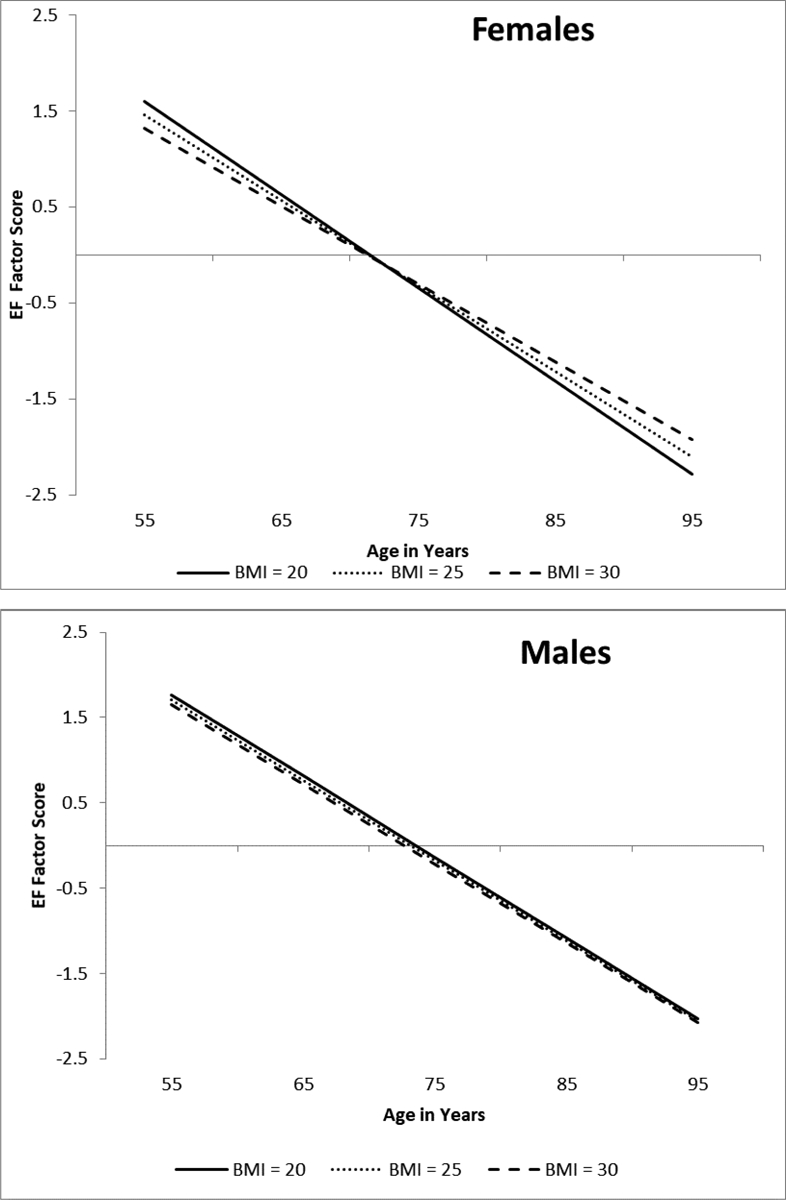
Predicted growth curve model for executive function (EF) factor scores by sex using continuous baseline body mass index (BMI) as a predictor. The three levels of BMI are depicted for ease of interpretation. Age in years was used as the metric of change and centered at 75. BMI predicted cognitive decline for females and not for males. Sex was a significant moderator.
Sex moderation of BMI predicting speed.
Adjusted results for the unconstrained speed model stratified by sex showed that BMI was not a significant predictor of performance level for females (b = .065, p = .228) or males (b = −.075, p = .365). However, in line with the EF results, BMI significantly predicted change in speed for females (b = .012, p < .001) and not for males (b = −.002, p = .814). Sex was a significant moderator (D = 21.044, Δdf = 7, p = .004). As depicted in Figure 3a, females with a BMI of 30 (M = −.067) showed less decline relative to females with a BMI of 25 (M = −.079) or 20 (M = −.091). Non-significant results for males are shown in Figure 3b.
Figure 3.
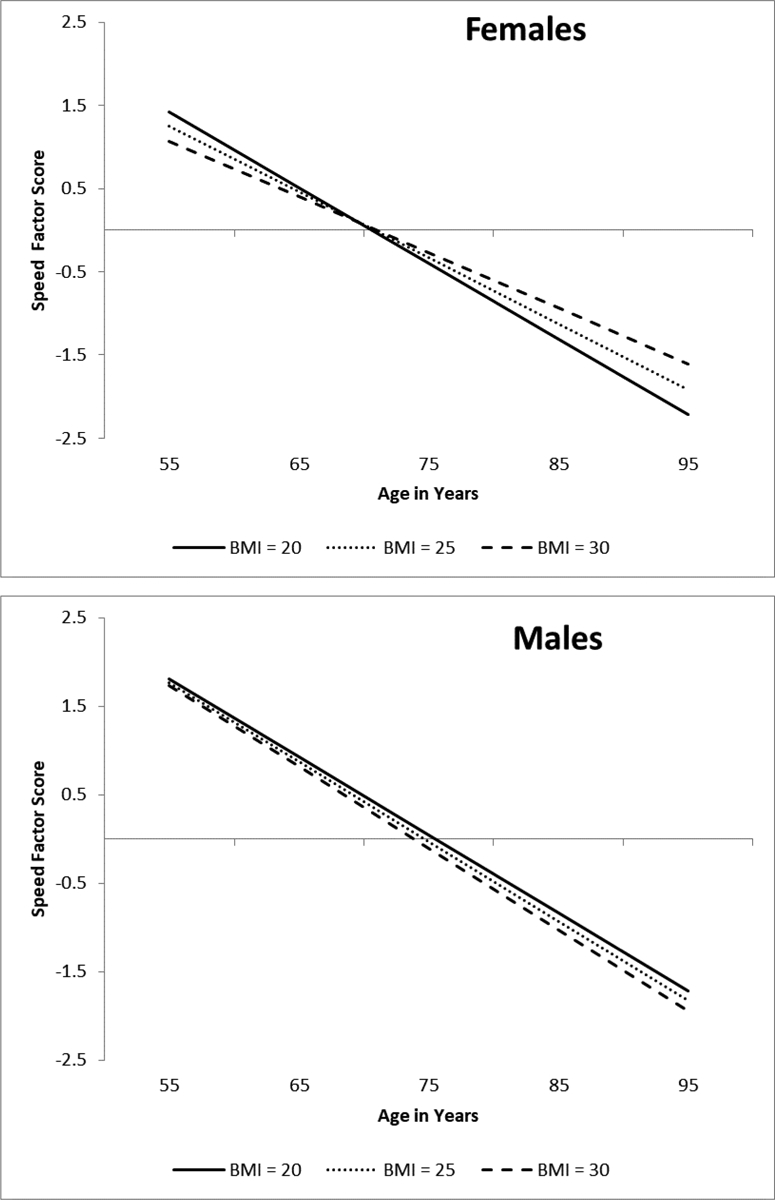
Predicted growth curve model for speed factor scores by sex using continuous baseline body mass index (BMI) as a predictor. The three levels of BMI are depicted for ease of interpretation. Age in years was used as the metric of change and centered at 75. BMI predicted cognitive decline for females and not for males. Sex was a significant moderator.
Sex moderation of BMI predicting memory.
Adjusted results for the unconstrained model stratified by sex showed that BMI did not predict memory performance level for females (b = −.018, p = .707) or males (b = .107, p = .192). Consistent with the above described results, BMI significantly predicted memory change for females (b = .007, p = .010) and not for males (b = .007, p = .137). These results were moderated by sex (D = 64.844, Δdf = 7, p < .001). As shown in Figure 4a, females with a BMI of 30 (M = −.069) exhibited less decline relative to females with a BMI of 25 (M = −.076) or 20 (M = −.083). Non-significant results for males are shown in Figure 4b.
Figure 4.
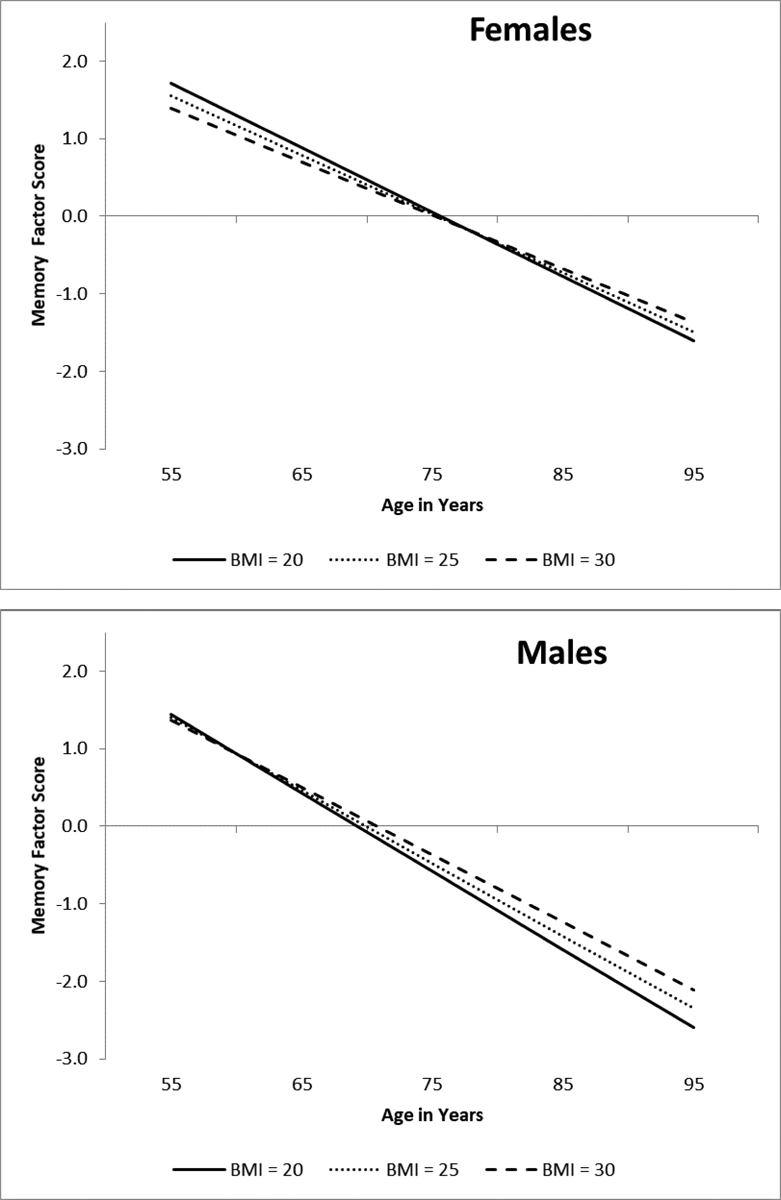
Predicted growth curve model for memory factor scores by sex using continuous baseline body mass index (BMI) as a predictor. The three levels of BMI are depicted for ease of interpretation. Age in years was used as the metric of change and centered at 75. BMI predicted cognitive decline for females and not for males. Sex was a significant moderator.
Discussion
Few studies have examined the impact of body weight and obesity on normal cognitive aging trajectories, and our review indicated that results from available research are contradictory. We sought to determine whether there were underlying consistencies in the pattern of results by consolidating these dimensions of concern (i.e., longitudinal, measurement, and analytical). Therefore, the two main goals of this study were to explore the independent association between continuous BMI and neuropsychological performance and decline across three latent variables and to determine whether these associations were moderated by sex. We observed an identical pattern of effects across domains, in that BMI was inversely related to cognitive decline. However, these associations were largely driven by females. When the data were stratified by sex, elevated BMI predicted slower decline for females, whereas males evinced comparable trajectories of cognitive decline regardless of BMI. We discuss these findings in greater detail below.
RG1: BMI prediction of EF, speed, and memory growth models
Results indicated that higher BMI was associated with less decline in EF, speed, and memory. Although these findings ran in contrast to our expectations that BMI effects would be more pronounced for EF and speed-related tasks as compared to memory tasks (Bischof & Park, 2015), these findings extend an earlier systematic review concluding that cognitive flexibility, speed, and memory domains are the most reliably affected, though the direction of weight effects varied across studies (van den Berg, Kloppenborg, Kessels, Kappelle, & Biessels, 2009).
It is interesting that we found higher BMI predicted less neuropsychological decline across cognitive domains. The Baltimore Longitudinal Study of Aging also indicated that higher BMI was associated with better performance and less decline on Trail Making Test A (Gunstad, Lhotsky, Wendell, Ferrucci, & Zonderman, 2010). Later research reported that increased adiposity predicted less decline on the Digit Symbol Substitution Test for participants in the Cardiovascular Health Study (Luchsinger et al., 2013). More recently, Arvanitakis and colleagues (2018) combined data from the Minority Aging Research Study and the Memory Aging Project and reported that higher BMI predicted slower decline on composite measures of semantic and episodic memory. Our study is one of the first to use a latent variable approach to systematically examine BMI effects on cognitive aging trajectories across each of these age-sensitive domains. These results thus make an important contribution to the ongoing debate surrounding the obesity paradox (i.e., whether or not it truly exists; Oreopoulos & Kalantar-Zadeh, 2009), and suggest that BMI may in fact be inversely related to risk for adverse neurocognitive changes and outcomes in aging.
Although the pathophysiologic mechanisms of the obesity paradox remain largely unknown, several researchers have argued that low BMI and weight loss may be secondary to pathophysiological changes accompanying preclinical dementia (Atti et al., 2008). Indeed, a recent study found that lower baseline memory scores predicted steeper decline in BMI (Suemoto, Gilsanz, Mayeda, & Glymour, 2015). Subsequent research investigated the neural substrates of later-life BMI-cognition associations and reported that decreasing BMI was associated with cortical thinning in a number of regions across the frontal cortex bilaterally, as well as in the left precuneus (Shaw et al., 2018). Although these findings buttress a reverse causation hypothesis, this explanation is unlikely to account for the present findings given that participants did not show variability in BMI decline. Specifically, it is possible that participants with elevated BMI also had larger white matter volumes, possibly due to increased density of the lipid-based myelin sheath (Walther, Birdsill, Glisky, & Ryan, 2010). This in turn may have reduced the risk for cognitive decline. Further, elevated BMI likely reflects accumulation of fat in various region of the body, including in the legs, and increased leg fat mass in later life promotes glucose metabolism, which in turn has positive effects on cognition (Snijder et al., 2004). It is also possible that selective mortality effects may have given rise to this pattern of results. That is, individuals who are obese in later life may represent hardy-survivors who possess other traits that are especially protective (Oreopoulos et al., 2009). Although these mechanisms are largely speculative, these ideas may spur future research efforts (e.g., longitudinal studies with neuroimaging, biological, psychological, and social markers).
RG2: Sex moderation of BMI-cognition associations
Analysis of change trajectories revealed significant BMI-cognition relationships as moderated by sex. Specifically, we found that higher BMI predicted less decline in EF, speed, and memory for females, whereas BMI was unrelated to neuropsychological decline for males. We conducted a post hoc check to verify that these results could not be attributed to the potential confounding effects of including participants who had experienced a stroke (n = 44; 5% of sample) or eventual diagnosis of neurodegenerative disease (i.e., AD or PD; n = 13; 1% of sample). Exclusion of these participants revealed findings that were concordant (although slightly attenuated, which is arguably to be expected when using a smaller, restricted study sample) as those we originally reported.
Earlier research also reported that weight loss in postmenopausal females was associated with worse performance on measures of EF, processing speed, and memory (Bojar et al., 2015; Driscoll et al., 2011). However, these studies did not include male participants, nor did they examine actual trajectories of cognitive change. Other research indicated that older adults with higher continuous BMI had a lower dementia risk compared to those with lower continuous BMI (Dahl, Löppönen, Isoaho, Berg, & Kivelä, 2008). Interestingly, when the data were stratified on the basis of sex, this association was significant for females and not for males. The relationship between elevated BMI in later-life and reduced risk for cognitive decline and impairment is also reported to be stronger for females as compared to males (Kim et al., 2016). Our study extends these findings by examining the period of normal cognitive aging, especially the trajectories preceding clinical impairment.
There are several potential explanations for the selective effects of BMI on cognitive decline for females. First, bioactive insulin-like growth factor-1 levels are higher in females with elevated BMI (Frystyk, Brick, Gerweck, Utz, & Miller, 2009), which may in turn promote better cognitive function (Okereke, Kang, Ma, Hankinson, Pollak, & Grodstein, 2007). Second, the hormone leptin is largely synthesized by adipocytes and circulates in the plasma at levels commensurate with body fat (Considine et al., 1996; Maffei et al., 1995). Mounting literature suggests that leptin has far reaching effects on central nervous system function, including regulation of hippocampal synaptic plasticity and inhibition of cell death (Harvey, Solovyova, & Irving, 2006; Morrison, 2009). Third, following menopause, the principal estrogen (i.e., estrone) is largely produced in adipose tissue, and is therefore found circulating at higher levels in females with elevated BMI (Rannevik et al., 2008). Estrogen has several neuroprotection effects, including promotion of synaptic plasticity, nerve growth, and cholinergic function, as well as reduced oxidative stress (Hara, Waters, McEwen, & Morrison, 2015; McEwen, 2002). Important for the present research, there are a handful of studies that link weight-cognition associations to estrogen. For example, Patel and colleagues (2004) found that obese postmenopausal females had higher levels of estrogen (i.e., estrone) and better neuropsychological function relative to non-obese females. The Betula Project reported a positive association between BMI and estrogen (i.e., estradiol) and reported that overweight postmenopausal females exhibited less cognitive decline relative to normal weight comparisons (Thilers, MacDonald, Nilsson, & Herlitz, 2010). Taken together, these studies suggest that estrogen may mediate the impact of BMI on cognitive aging trajectories for older females. This question should be explored in future longitudinal trajectory research.
We acknowledge several methodological limitations. First, it is possible that participants may not be representative of the broadest population of older adults— the VLS initially selects individuals who are relatively healthy and free from neurodegenerative disease at baseline. Although limited in global generalizability, this sample may reflect a growing proportion of older adults in Western countries. Further, this positive selection fades with aging (and time in study). Second, because participants in our study did not show significant variability in BMI decline, we could not explore bidirectional effects. Rather, we tested baseline BMI as a predictor of cognitive performance and decline. This is an important limitation. Other researchers (Suemoto et al., 2015) have speculated that the risk-reducing effect of BMI may be due in part to weight loss accompanying preclinical dementia (Stewart et al., 2005) or secondary to incipient neuropathological changes that cause declines in both weight and cognition (Sperling, Mormino, & Johnson, 2014). These possibilities should be explored in samples for which BMI varies longitudinally. Third, because of unavailability, our study did not incorporate additional measures of adiposity (e.g., waist-to-hip ratio and waist circumference). As individuals age, fat mass increases and lean mass decreases. BMI may therefore be a less reliable indicator of fat accumulation in older age (Pedditizi et al., 2016). Although we cannot rule out this possibility, BMI remains a well-accepted surrogate measure of body fat in epidemiological studies and in routine clinical practice (Decaria et al., 2012). Further, BMI shows comparable cognitive effects relative to other measures of adiposity (Gunstad et al., 2010; Waldstein & Katzel, 2006). Fourth, we do not have measures related to diet or nutritional status in the VLS, and thus could not control for the potential confounding effects of these characteristics. These variables should be considered in future research.
Our study has several notable strengths. First, we tested and observed novel sex differences in BMI as it relates to cognitive decline. These findings contribute to and extend earlier research suggesting that BMI-cognition associations may be distorted in studies that do not stratify on the basis of sex (García-Ptacek et al., 2014). Future studies should also be sufficiently powered to test and confirm sex moderation. Second, we used a substantial and well-characterized longitudinal sample tested across a 40-year band of aging. Third, we used an accelerated longitudinal design with age as the metric of change. This allowed us to control for potential age-related effects, and in doing so, address an important limitation of extant research (Deckers et al., 2017). Fourth, we investigated BMI biomarker effects using contemporary statistical methods (i.e., structural equation modeling). This approach to analysis is largely absent in weight-obesity research. Fifth, we used a large battery of well-established manifest EF, speed, and memory neuropsychological tasks. This contributed to validated, invariant, longitudinal latent variables. This approach has several advantages over single and composite variable approaches, including the ability to (a) correct for measurement error and (b) establish the content, criterion, and construct validity of the cognitive domains under investigation (Little, 2013). It is important to note, however, that these features of latent variables may serve to mask differential single variable results. Sixth, we controlled for the potential confounding effects a wide range of medical, psychosocial, and demographic characteristics. While this methodological approach addressed a prominent and recurring criticism of BMI-aging research, it may have obscured or attenuated some of the pathways by which BMI affects cognition in aging. Because participants in the VLS are relatively healthy and free from comorbid conditions at baseline, we cannot rule out this possibility. This question should be examined with longitudinal samples that vary considerably in terms of health status.
In conclusion, this study showed that, in a longitudinal sample representing a 40-year band of aging, BMI was inversely related to cognitive neuropsychological decline across three age-sensitive domains for females and was unrelated to cognitive trajectories for aging males. These findings mark an important contribution to the sparse longitudinal literature on domain-specific effects of BMI on normal cognitive aging. Future research would profitably be directed towards replicating and extending these results (e.g., by considering additional modifying risk and protection factors, such as genotype). Continued research efforts are needed in order to generate practical recommendations regarding ideal body weight in older age (Decaria et al., 2012). For now, our data suggest that older females may benefit from maintaining higher BMI (i.e., lean body mass, perhaps through aerobic and resistance training) and avoiding weight loss and nutritional deficit (Smith et al., 2011). Moreover, clinicians should tailor weight loss recommendations to characteristics of the individual patient.
Public significance statement:
Across the globe, the proportion of older adults is on the rise. Research that examines modifiable risk and protection factors for age-related cognitive decline is therefore important. We report in this study that higher body mass index (BMI) was associated with less decline in executive function, speed, and memory selectively for older females, whereas BMI was unrelated to cognitive decline for older males.
Acknowledgments
Linzy Bohn’s participation in this research was supported by the Social Sciences and Humanities Research Council of Canada.
The VLS is supported by a grant from the National Institutes of Health (National Institute on Aging) to Roger A. Dixon (R01 AG008235), who is also supported by the Canada Research Chairs program. We acknowledge additional support from the Canadian Consortium on Neurodegeneration in Aging (with funding from Canadian Institutes of Health Research and partners).
References
- Andrew MK, & Tierney MC (2018). The puzzle of sex, gender and Alzheimer’s disease: Why are women more often affected than men? Women’s Health, 14, 1–8. 10.1177/1745506518817995 [DOI] [Google Scholar]
- Anstey KJ, Cherbuin N, Budge M, & Young J (2011). Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obesity Reviews, 12, e426–e437. 10.1111/j.1467-789X.2010.00825.x [DOI] [PubMed] [Google Scholar]
- Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, & Fratiglioni L (2008). Late-life body mass index and dementia incidence: Nine-year follow-up data from the Kungsholmen Project. Journal of the American Geriatrics Society, 56, 111–116. 10.1111/j.1532-5415.2007.01458.x [DOI] [PubMed] [Google Scholar]
- Bäckman K, Joas E, Waern M, Östling S, Guo X, Blennow K, … Gustafson DR (2015). 37 years of body mass index and dementia: Effect modification by the APOE genotype: Observations from the prospective population study of women in Gothenburg, Sweden. Journal of Alzheimer’s Disease, 48, 1119–1127. 10.3233/JAD-150326 [DOI] [PubMed] [Google Scholar]
- Baddeley A, Logie R, Nimmosmith I, & Brereton N (1985). Components of fluent reading. Journal of Memory and Language, 24(1), 119–131. [Google Scholar]
- Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus D, Kukull W, & Gustafson DR (2014). Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer’s disease. Alzheimer Disease and Associated Disorders, 28(1), 36–43. 10.1097/WAD.0000000000000005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak AAM, Mansueti L, Strauss E, & Dixon RA (2006). Performance on the Hayling and Brixton tests in older adults: Norms and correlates. Archives of Clinical Neuropsychology, 21, 141–149. 10.1016/j.acn.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, & Whitmer RA (2014). Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. The Lancet Diabetes and Endocrinology, 2, 246–255. 10.1016/S2213-8587(13)70088-3 [DOI] [PubMed] [Google Scholar]
- Bischof GN, & Park DC (2015). Obesity and aging: Consequences for cognition, brain structure, and brain function. Psychosomatic Medicine, 77, 697–709. 10.1097/PSY.0000000000000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardley D, & Pobocik RS (2009). Obesity on the Rise. Primary Care - Clinics in Office Practice, 36, 243–255. 10.1016/j.pop.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Bojar I, Owoc J, Wójcik-Fatla A, Raszewski G, Stančiak J, & Raczkiewicz D (2015). Cognitive functions, lipid profile, and Apolipoprotein E gene polymorphism in postmenopausal women. Annals of Agricultural and Environmental Medicine, 22(2), 313–319. 10.5604/12321966.1152086 [DOI] [PubMed] [Google Scholar]
- Bradburn NM (1969). The structure of psychological well-being. Chicago, IL: Aldine. [Google Scholar]
- Burgess PW, & Shallice T (1997). The Hayling and Brixton tests. Thurston, England: Thames Valley Test Company. [Google Scholar]
- Clark DO, Xu H, Callahan CM, & Unverzagt FW (2016). Does body mass index modify memory, reasoning, and speed of processing training effects in older adults. Obesity (Silver Spring), 24(11), 2319–2326. 10.1002/oby.21631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, … Caro JF (1996). Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England Journal of Medicine, 334(5), 292–295. 10.1097/00019616-199607000-00020 [DOI] [PubMed] [Google Scholar]
- D’Elia LA, Satz P, Uchiyama CL, & White T (1996). Color Trails Test: Professional manual. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Dahl AK, & Hassing LB (2013). Obesity and cognitive aging. Epidemiologic Reviews, 35, 22–32. 10.1093/epirev/mxs002 [DOI] [PubMed] [Google Scholar]
- Dahl AK, Löppönen M, Isoaho R, Berg S, & Kivelä SL (2008). Overweight and obesity in old age are not associated with greater dementia risk. Journal of the American Geriatrics Society, 56, 2261–2266. 10.1111/j.1532-5415.2008.01958.x [DOI] [PubMed] [Google Scholar]
- de Frias CM, Dixon RA, & Strauss E (2009). Characterizing executive functioning in older special populations: From cognitively elite to cognitively impaired. Neuropsychology, 23(6), 778–791. https://doi.org/doi: 10.1037/a0016743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mutsert R, Sun Q, Willett WC, Hu FB, & van Dam RM (2014). Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: A cohort study. American Journal of Epidemiology, 179(11), 1353–1365. 10.1093/aje/kwu052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaria JE, Sharp C, & Petrella RJ (2012). Scoping review report: Obesity in older adults. International Journal of Obesity, 36, 1141–1150. 10.1038/ijo.2012.29 [DOI] [PubMed] [Google Scholar]
- Deckers K, van Boxtel MPJ, Verhey FRJ, & Köhler S (2017). Obesity and cognitive decline in adults: Effect of methodological choices and confounding by age in a longitudinal study. Journal of Nutrition, Health and Aging, 21(5), 546–553. 10.1007/s12603-016-0757-3 [DOI] [PubMed] [Google Scholar]
- Dixon RA, & de Frias CM (2004). The Victoria Longitudinal Study: From characterizing cognitive aging to illustrating changes in memory compensation. Aging, Neuropsychology, and Cognition, 11(2), 346–376. 10.1080/13825580490511161 [DOI] [Google Scholar]
- Dixon RA, Garrett DD, Lentz TL, MacDonald SWS, Strauss E, & Hultsch DF (2007). Neurocognitive markers of cognitive impairment: Exploring the roles of speed and inconsistency. Neuropsychology, 21(3), 381–399. 10.1037/0894-4105.21.3.381 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Small BJ, MacDonald SWS, & McArdle JJ (2012). Yes, memory declines with aging-- but when, how, and why? In Naveh-Benjamin M & Ohta N (Eds.), Memory and aging: Current issues and future directions (pp. 325–347). New York, NY: Psychology Press. [Google Scholar]
- Dixon RA, Wahlin Å, Maitland SB, Hultsch DF, Hertzog C, & Bäckman L (2004). Episodic memory change in late adulthood: Generalizability across samples and performance indices. Memory and Cognition, 32(5), 768–778. 10.3758/BF03195867 [DOI] [PubMed] [Google Scholar]
- Driscoll I, Espeland MA, Wassertheil-Smoller S, Gaussoin SA, Ding J, Granek IA, … Resnick SM (2011). Weight change and cognitive function: Findings from the Women’s Health Initiative Study of Cognitive Aging. Obesity, 19(8), 1595–1600. 10.1038/oby.2011.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, & Dermen D (1976). Manual for kit of factor-referenced cognitive tests. Princeton, NJ: Educational Testing Service. [Google Scholar]
- Emmerzaal TL, Kiliaan AJ, & Gustafson DR (2015). 2003–2013: A decade of body mass index, Alzheimer’s disease, and dementia. Journal of Alzheimer’s Disease, 43, 739–755. 10.3233/JAD-141086 [DOI] [PubMed] [Google Scholar]
- Enders CK (2011). Analyzing longitudinal data with missing values. Rehabilitation Psychology, 56(4), 267–288. 10.1037/a0025579 [DOI] [PubMed] [Google Scholar]
- Frystyk J, Brick DJ, Gerweck AV, Utz AL, & Miller KK (2009). Bioactive insulin-like growth factor-I in obesity. Journal of Clinical Endocrinology & Metabolism, 98(4), 3093–3097. 10.1210/jc.2009-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith S, Bowden J, & Mander A (2017). Accelerated longitudinal designs: An overview of modelling, power, costs and handling missing data. Statistical Methods in Medical Research, 26(1), 374–398. 10.1177/0962280214547150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ptacek S, Faxén-Irving G, Čermáková P, Eriksdotter M, & Religa D (2014). Body mass index in dementia. European Journal of Clinical Nutrition, 68, 1204–1209. 10.1038/ejcn.2014.199 [DOI] [PubMed] [Google Scholar]
- Gunstad J, Lhotsky A, Wendell CR, Ferrucci L, & Zonderman AB (2010). Longitudinal examination of obesity and cognitive function: Results from the Baltimore Longitudinal Study of Aging. Neuroepidemiology, 34, 222–229. 10.1159/000297742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Jo SA, Seo JA, Kim BG, Kim NH, Jo I, … Park KW (2009). Adiposity parameters and cognitive function in the elderly: Application of “Jolly Fat” hypothesis to cognition. Archives of Gerontology and Geriatrics, 49(2), 133–138. 10.1016/j.archger.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, & Morrison JH (2015). Estrogen effects on cognitive and synaptic health over the lifecourse. Physiological Reviews, 95(3), 785–807. 10.1152/physrev.00036.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, & Irving A (2006). Leptin and its role in hippocampal synaptic plasticity. Progress in Lipid Research, 45, 369–378. 10.1016/j.plipres.2006.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam DW, & James WP (2005). Obesity. Lancet, 366, 1197–1209. 10.7326/AITC201903050 [DOI] [PubMed] [Google Scholar]
- Hruby A, & Hu FB (2015). The epidemiology of obesity: A big picture. Pharmacoeconomics, 33(7), 673–689. 10.1007/s40273-014-0243-x.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim Y, & Park SM (2016). Body mass index and decline of cognitive function. PLoS ONE, 11(2), 1–14. 10.1371/journal.pone.0148908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD (1983). Neuropsychological assessment. New York, NY: Oxford University Press. [Google Scholar]
- Little TD (2013). Longitudinal Structural Equation Modeling. New York: NY: Guilford Press. [Google Scholar]
- Luchsinger JA, Biggs ML, Kizer JR, Barzilay J, Fitzpatrick A, Newman A, … Kuller L (2013). Adiposity and cognitive decline in the cardiovascular health study. Neuroepidemiology, 40(4), 274–281. 10.1159/000345136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutski M, Weinstein G, Goldbourt U, & Tanne D (2017). Insulin resistance and future cognitive performance and cognitive decline in elderly patients with cardiovascular disease. Journal of Alzheimer’s Disease, 57, 633–643. 10.3233/JAD-161016 [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, … Friedman JM (1995). Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature Medicine, 1(11), 1155–1161. [DOI] [PubMed] [Google Scholar]
- Mazure CM, & Swendsen J (2016). Sex differences in Alzheimer’s disease and other dementias. Lancet Neurology, 15(5), 461–452. 10.1016/S1474-4422(16)00067-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, Yang A, Kitner-Triolo MH, Ferrucci L, & Resnick SM (2016). Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging, 31(2), 166–175. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B (2002). Estrogen actions throughout the brain. Recent Progress in Hormone Research, 57, 357–384. [DOI] [PubMed] [Google Scholar]
- McFall GP, McDermott KL, & Dixon RA (2019). Modifiable risk factors discriminate memory trajectories in non-demented aging: Precision factors and targets for promoting healthier brain aging and preventing dementia. Journal of Alzheimer’s Disease, 70(s1), S101–S118. 10.3233/JAD-180571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Sapkota S, McDermott KL, & Dixon RA (2016). Risk-reducing Apolipoprotein E and Clusterin genotypes protect against the consequences of poor vascular health on executive function performance and change in non-demented older adults. Neurobiology of Aging, 42, 91–100. 10.1016/j.neurobiolaging.2016.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Anstey KJ, & Dixon RA (2015). Alzheimer’s genetic risk intensifies neurocognitive slowing associated with diabetes in nondemented older adults. Alzheimer’s and Dementia: Diagnosis, Assessment and Disease Monitoring, 1, 395–402. 10.1016/j.dadm.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall GP, Wiebe SA, Vergote D, Westaway D, Jhamandas J, Bäckman L, & Dixon RA (2015). ApoE and pulse pressure interactively influence level and change in the aging of episodic memory: Protective effects among ε2 carriers. Neuropsychology, 29(3), 388–401. 10.1037/neu0000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD (2009). Leptin signaling in the brain: A link between nutrition and cognition? Biochimica et Biophysica Acta, 1792(5), 401–408. 10.1016/j.bbadis.2008.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (n.d.). Mplus User’s Guide. Eigth Edition. Muthén & Muthén Los Angeles, CA: Muthén & Muthén; 10.1111/j.1600-0447.2011.01711.x [DOI] [Google Scholar]
- NCD Risk Factor Collaboration. (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet, 390, 2627–2642. 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke O, Kang JH, Ma J, Hankinson SE, Pollak MN, & Grodstein F (2007). Plasma IGF-I levels and cognitive performance in older women. Neurobiology of Aging, 28, 135–142. 10.1016/j.neurobiolaging.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Oreopoulos A, Kalantar-Zadeh K, Sharma AM, & Fonarow GC (2009). The obesity paradox in the elderly: Potential mechanisms and clinical implications. Clinics in Geriatric Medicine, 25, 643–659. 10.1016/j.cger.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Palmer J, Macleod C, Hunt E, & Davidson J (1985). Information-processing correlates of reading. Journal of Memory and Language, 24(1), 59–88. [Google Scholar]
- Pedditizi E, Peters R, & Beckett N (2016). The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age and Ageing, 45, 14–21. 10.1093/ageing/afv151 [DOI] [PubMed] [Google Scholar]
- Preston SH, Vierboom YC, & Stokes A (2018). The role of obesity in exceptionally slow US mortality improvement. Proceedings of the National Academy of Sciences, 115(5), 957–961. 10.1073/pnas.1716802115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickett C, Brennan L, & Stolwyk R (2015). Examining the relationship between obesity and cognitive function: A systematic literature review. Obesity Research & Clinical Practice, 9, 93–113. 10.1016/j.orcp.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, & Svanberg L (2008). Reprint of a longitudinal study of the perimenopausal transition: Altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas, 61(1–2), 67–77. 10.1016/0378-5122(94)00869-9 [DOI] [PubMed] [Google Scholar]
- Regard M Unpublished doctoral dissertation. University of Victoria; Victoria, British Columbia, Canada: 1981. Cognitive rigidity and flexibility: A neuropsychological study. [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, … Deary IJ (2018). Sex differences in the adult human brain: Evidence from 5216 UK biobank participants. Cerebral Cortex, 28(8), 2959–2975. 10.1093/cercor/bhy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, & Babcock RL (1991). Decomposing adult age differences in working memory. Developmental Psychology, 27(5), 763–776. 10.1037/0012-1649.27.5.763 [DOI] [Google Scholar]
- Shaw ME, Sachdev PS, Abhayaratna W, Anstey KJ, & Cherbuin N (2018). Body mass index is associated with cortical thinning with different patterns in mid- and late-life. International Journal of Obesity, 42, 455–461. 10.1038/ijo.2017.254 [DOI] [PubMed] [Google Scholar]
- Siddarth D (2013). Risk factors for obesity in children and adults. Journal of Investigative Medicine, 61(6), 1039–1042. 10.2310/JIM.0b013e31829c39d0 [DOI] [PubMed] [Google Scholar]
- Singer JD, & Willett J (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press. [Google Scholar]
- Smith E, Hay P, Campbell L, & Trollor JN (2011). A review of the association between obesity and cognitive function across the lifespan: Implications for novel approaches to prevention and treatment. Obesity Reviews, 12, 740–755. 10.1111/j.1467-789X.2011.00920.x [DOI] [PubMed] [Google Scholar]
- Snijder MB, Mekker JM, Visser M, Bouter LM, Stehouwer CDA, Yudken JS, … Seidell JC (2004). Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels. Diabetes Care, 27(2), 372–377. [DOI] [PubMed] [Google Scholar]
- Sperling R, Mormino E, & Johnson K (2014). The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron, 84(3), 608–622. 10.1002/9781118596005.ch65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, & Launer LJ (2005). A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Archives of Neurology, 62(1), 55–60. 10.1001/archneur.62.1.55 [DOI] [PubMed] [Google Scholar]
- Suemoto CK, Gilsanz P, Mayeda ER, & Glymour MM (2015). Body mass index and cognitive function: The potential for reverse causation. International Journal of Obesity, 39(9), 1383–1389. 10.1038/ijo.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeau S, McFall GP, Wiebe SA, Anstey KJ, & Dixon RA (2016). Genetic factors moderate everyday physical activity effects on executive functions in aging: Evidence from the Victoria Longitudinal Study. Neuropsychology, 30(1), 6–17. 10.1037/neu0000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilers PP, MacDonald SWS, Nilsson LG, & Herlitz A (2010). Accelerated postmenopausal cognitive decline is restricted to women with normal BMI: Longitudinal evidence from the Betula Project. Psychoneuroendocrinology, 35, 516–524. 10.1016/j.psyneuen.2009.08.018 [DOI] [PubMed] [Google Scholar]
- Thurstone TG (1962). Primary mental abilities: Grades 9–12, 1962 revision. Chicago, IL: Science Research Associates. [Google Scholar]
- Tierney MC, Curtis AF, Chertkow H, & Rylett RJ (2017). Integrating sex and gender into neurodegeneration research: A six-component strategy. Alzheimer’s & Dementia: Translational Research and Clinical Interventions, 3, 660–667. 10.1016/j.trci.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Kloppenborg RP, Kessels RPC, Kappelle L, & Biessels GJ (2009). Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochimica et Biophysica Acta, 1792, 470–481. 10.1016/j.bbadis.2008.09.004 [DOI] [PubMed] [Google Scholar]
- Vega JN, Dumas J, & Newhouse P (2017). Cognitive effects of chemotherapy and cancer-related treatments in older adults. American Journal of Geriatric Psychiatry, 25(12), 1415–1426. 10.1016/j.jagp.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucenik I, & Stains JP (2012). Obesity and cancer risk: Evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences, 1271, 37–43. 10.1111/j.1749-6632.2012.06750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, & Katzel LI (2006). Interactive relations of central versus total obesity and blood pressure to cognitive function. International Journal of Obesity, 30, 201–207. 10.1038/sj.ijo.0803114 [DOI] [PubMed] [Google Scholar]
- Walther K, Birdsill AC, Glisky EL, & Ryan L (2010). Structural brain differences and cognitive functioning related to body mass index in older females. Human Brain Mapping, 31, 1052–1064. 10.1002/hbm.20916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D, Skirbekk V, Freund I, & Herlitz A (2014). The changing face of cognitive gender differences in Europe. Proceedings of the National Academy of Sciences, 111(32), 11673–11678. 10.1073/pnas.1319538111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981). WAIS–R manual. New York, NY: Psychological Corporation. [Google Scholar]


