Abstract
Introduction:
Pseudomonas aeruginosa are common in CRS and frequently resistant to antibiotic treatment. We recently described the ciprofloxacin and ivacaftor releasing biodegradable sinus stent (CISS): a drug delivery system that administers ciprofloxacin and the mucociliary activator (ivacaftor) at high local concentrations with prolonged mucosal contact time and sustained delivery. The objective of this study is to evaluate the efficacy of the CISS in a rabbit model of P. aeruginosa (PAO1 strain) sinusitis.
Methods:
Ciprofloxacin/ivacaftor (double layer) was coated on biodegradable poly-D/L-lactic acid. A total of 10 sinus stents (5 bare PLLA stent controls, 5 CISSs) were placed unilaterally in rabbit maxillary sinuses via dorsal sinusotomy after inducing infection for 1 week with PAO1. Animals were assessed 3 weeks after stent insertion with sinus culture, nasal endoscopy, CT scan, histopathology, and in vivo sinus potential difference (SPD) assay.
Results:
Rabbits treated with CISS had significant reductions in CT (Δ Kerschner scale, Control=0.55+/−0.92 vs. CISS=−5.92+/−1.69; p=0.024) and endoscopy scores (Control=4.0+/−0.0 vs. CISS=1.875+/−0.74; p=0.003). A 2-log reduction of PAO1 was observed (Control=−2.14+/−0.77 vs. CISS=1.84+/−1.52; p=0.047). SPD revealed significantly increased Cl− transport in the CISS group compared to controls (Cl−free+forskolin ΔPD (mV), Control=−4.23+/−1.04 vs. CISS=−18.36+/−6.31; p=0.026). Finally, marked improvements were noted in the histology of the mucosa and submucosa in treated animals.
Conclusion:
The CISS had robust clinical efficacy in treating P. aeruginosa rabbit sinusitis. The innovative design of double layered drug coating on the surface of the biodegradable stent may provide therapeutic advantages over current treatment strategies for P. aeruginosa sinusitis.
Keywords: Pseudomonas Aeruginosa, Ciprofloxacin, Ivacaftor, Sinus stent, Chronic Rhinosinusitis, recalcitrant sinusitis, sinusitis, bacterial biofilms
Introduction
Pseudomonas aeruginosa is one of the most common causes of chronic, biofilm-based sinus infections in patients with recalcitrant chronic rhinosinusitis (CRS).1 The existence of biofilm-forming P. aeruginosa strains has been associated with poor resolution of clinical symptoms and signs of CRS.2 Biofilm eradication strategies have been increasingly important due to paucity of antimicrobials under development by pharmaceutical companies and the concurrent propagation of antimicrobial resistance.3 At the same time, the eradication of biofilms using traditional methods of oral and intravenous antibiotics is challenging due to poor infiltration into biofilm structures.4,5
Topical antibiotics have been suggested due to their capability of delivering significantly higher doses of antibiotic to the sinonasal mucosal surface without systemic side effects.3,6,7 This approach allows for a larger dose and more efficient delivery of the drug to penetrate into biofilms, and thus result in a potent therapeutic effect. We initially developed a ciprofloxacin coated sinus stent that was shown to be effective in clearing P. aeruginosa biofilms in vitro.6 However, its clinical implications appeared to be inadequate due to an initial burst release of ciprofloxacin without sustained delivery in a single drug coated sinus stent based on pharmacokinetic assays. In addition, combining 2 or more distinct antibiotics represents a common strategy in treating multi-drug resistant bacterial infections to broaden the antimicrobial spectrum, generate synergistic effects, and counteract antibiotic resistance.8 Agents that enhance the antimicrobial activity of currently available antibiotics may also represent a valuable and cost-effective means for improving clinical efficacy.9 To improve the characteristics of the CSS, we recently developed a ciprofloxacin and ivacaftor coated sinus stent (CISS), which maintained a uniform coating and provided a marked reduction in P. aeruginosa biofilm formation in vitro.10 Furthermore, the dual coating (hydrophilic (inner - ciprofloxacin) and hydrophobic (outer - ivacaftor) layers) technique had the additional advantage of a sustained controlled release of the two drugs.
The objective of the current study was to evaluate the efficacy of a ciprofloxacin and ivacaftor releasing biodegradable sinus stent (CISS) in a preclinical model of rhinosinusitis infected with P. aeruginosa (in vivo).
METHODS
Materials and Preparation of CISS
Ciprofloxacin HCl (99.5% purity) was purchased from GenHunter Corporation (Nashville, TN). Ivacaftor (VX-770) was obtained from Selleckchem (Houston, TX). Poly (D, L-lactide-co-glycolide) (PLGA) was purchased from PolySciTech (West Layfeyette, IN). All other chemicals and reagents used in this study were procured from Sigma-Aldrich (St. Louis, MO). As previously described, biodegradable poly-D/L-lactic acid (PLLA) tubes were obtained from Zeus Inc (Orangeburg, SC) and laser fine cutting (Laserage Technology Corporation, Waukegan, IL) was employed to create porous stents for the experiments, conforming to the size of the rabbit maxillary sinus, 1 cm in length and 0.3 cm in height.6,11 Eudragit RS 100 (a copolymer of ethyl acrylate, methyl methacrylate and a low content of methacrylic acid ester with quaternary ammonium groups) was used for time-controlled drug release by sustained release formulations.12 Ciprofloxacin (hydrophilic) and ivacaftor (hydrophobic) PLGA nanoparticles were coated in the inner and outer layer respectively.11,13 A total of 0.6mg of ciprofloxacin (10 times higher than the in vitro stent with the AUC0–24/MIC far in excess of the minimum goal (>100)) and 1 mg of ivacaftor (3 times higher than the in vitro stent) were successfully coated on the PLLA stents for the in vivo assay.10
Induction of PAO-1 Sinusitis in the Rabbit Model
This study was approved by the institutional animal care and use committee (IACUC) at the University of Alabama at Birmingham. Pasteurella-free, female, New Zealand white rabbits (3–4 kg) were used for the study. Before study initiation, rabbits were acclimatized to the animal facility for at least 1 week. Rabbits were anesthetized with [ketamine (20 mg/kg) (MWI, Boise, ID), dextomitor (0.25 mg/kg) (Zoetis Inc., Kalamazoo, MI), buprenorphine (0.03 mg/kg) (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA), and carprofen (5 mg/kg) (Zoetis Inc., Kalamazoo, MI)] in a warm room. No rabbits were intubated or provided any inhalational anesthetic agents. Nasal endoscopy (1.7mm 30-degree scope, Karl Storz, Tuttlingen, Germany) was performed bilaterally to exclude pre-existing infection or abnormal lesions. A synthetic sponge (Merocel ®, Medtronics, FL) was inserted into the middle meatus to occlude the maxillary sinus outflow tract. The ipsilateral maxillary sinus was inoculated directly into the maxillary sinus laterally with 0.5 mL of wild-type P. aeruginosa (PAO-1) using a 22-guage spinal needle (Becton-Dickinson, Franklin Lakes, NJ). The concentration was adjusted to an OD600 = 0.6 in sterile saline (4.0 × 108 colony forming units (CFUs)).
Insertion of the Ciprofloxacin Ivacaftor Coated Sinus Stents (CISS)
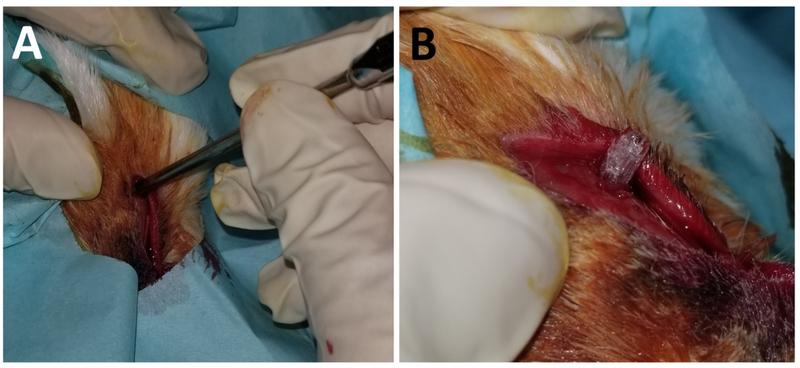
One week after inoculation, the middle meatal sponge was removed and 10 New Zealand white rabbits were randomized to receive either a CISS (n = 5) or sham (PLLA bare control) stent (n = 5). The surgical procedure to insert the stent was as follows: 1) a dorsal nasal, vertical, midline incision was created, 2) the unilateral maxillary sinus was entered by creating a small 8mm × 8mm dorsal hole using a trocar (Figure 1A), 3) the stent was inserted through the opening (Figure 1B), and 4) the periosteum, subcutaneous tissue, and skin were closed with 4–0 Vicryl suture (Ethicon, Inc., Somerville, NJ). Rabbits did not receive any oral or parenteral antibiotics during or after the surgical procedure.
Figure 1.
Placement of CISS
A. Creating a small dorsal hole using a trocar
B. Inserting the stent through the opening
CISS: CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
Outcome Measures
A sham version of the stent served as the control to differentiate effects related to the drug from the consequences related to the physical presence of the stent.
Micro computed tomography (CT) scanning - All rabbits were scanned at week 1 to confirm the presence of sinusitis after the inoculation of PAO-1. After implantation, micro CT scanning was repeated at week 4. Micro CT scanning was performed at the UAB small animal imaging shared facility using SPECT/CT (X-SPECT system, Gamma Medica, Northridge, CA). Sinus opacification grading was performed using Kerschner’s rabbit Sinus CT grading system (scoring each imaging study based on estimated percent (%) opacification of the maxillary sinus: 1 for <10%, 2 for 10%−19%, 3 for 20%−29%, 4 for 30%−39%, 5 for 40%−49%, 6 for 50%−59%, 7 for 60%−69%, 8 for 70%−79%, 9 for 80%−89%, and 10 for 90% ≤).14 Opacification % was measured using the ImageJ version 1.50i (National Institutes of Health, Bethesda, MD) by two blinded judges.
Nasal endoscopic examination - Nasal endoscopies were performed in all rabbits (n = 10) on week 0, week 1 (induction of acute sinusitis with PAO-1, before the placement of stents), and week 4 (after insertion of the stents). The inferior and middle meati were inspected bilaterally. Endoscopic findings in the middle meatus were graded according to the following system: 1) Edema (0: No edema, 1: Mild to moderate edema, 2: Polypoid degeneration); 2) (0: Absent, 1: Hyaline, 2: Thick/purulent) by two independent blinded reviewers (Table 1).
Bacterial counts (colony forming units (CFUs)) – Mucus was collected at week 1 (before stent insertion) and week 4 (at sacrifice) using the suction method previously described.15 This was performed by serial dilution in Luria broth (LB) and then removing 10μl of the serially diluted culture and spreading with sterile glass beads onto an agar plate (Pseudomonas Isolation Agar) in quadruplicate. The plates were then incubated at 37°C for 24 hours and CFUs were counted.
Sinus Potential Difference (PD) measurement - The measurement of sinus PD provides a direct and sensitive evaluation of Na+ and Cl− ion transport in airway epithelial cells via assessment of trans-epithelial bioelectric properties in in vivo subjects.16 Although PD measurements have not been validated to diagnose sinusitis, in vivo direct measurement of epithelial ion transport in sinus epithelium allows objective characterization of ion transport capabilities in the pathogenesis of sinusitis and to assess the efficacy of new drug trials. Sinus PD measurement was performed prior to euthanasia (n = 10) as described previously.16 Following disinfection with a disposable swabstick dipped in 10% povidone-iodine solution (Betadine® solution swabstick, Purdue Products L.P., Stamford, CT), access to the sinus was obtained by releasing the previous incision in the skin overlying the dorsum of the nose and entering the hole created to place the sinus stent. A polyethylene 90 tubing was inserted into the sinus cavity to measure the PD. The solutions perfused include Ringer’s lactate plus amiloride (100 uM), a low chloride solution containing [K2HPO4 (2.4 mM), KH2PO4 (0.4 mM), Na Gluconate (115 mM), NaHCO3 (25 mM), Ca Gluconate2 (1.24 mM)], CFTR agonist (forskoin 20 μM), and CFTR blocker (Inh-172 10 μM + Glyh-101 10 μM). Each perfused condition was maintained for at least 5 mins.
Histology – After micro CT scanning at week 4, rabbits were euthanized and heads were harvested for histologic sectioning with hematoxylin and eosin (H&E) staining. Ten representative sections of the maxillary sinus were selected and stained with H&E. Slides were evaluated by a veterinary pathologist blinded to the specimen and side for inflammatory characteristics and measurement of epithelial and subepithelial thickness.
Table 1.
Endoscopic Grading System used in this preclinical model of Pseudomonas sinusitis.
| Characteristics | Edema in Middle Meatus | Discharge from Middle Meatus |
|---|---|---|
| 0 | Absent | No discharge |
| 1 | Mild | Clear or thin discharge |
| 2 | Severe | Thick or purulent discharge |
Statistical Analysis
Statistical analyses were conducted using Excel 2016 and GraphPad Prism 6.0 software (La Jolla, Ca) with significance set at P < 0.05. Statistical evaluation utilized unpaired Student t tests. Data is expressed +/− standard error of the mean.
RESULTS
Micro CT
Micro CT examination was also performed in rabbits (n = 10) before and after insertion of the sham stents and CISS at week 1 and week 4. All rabbits demonstrated complete or near-complete opacification of the ipsilateral sinuses at week 1 before placing sinus stents and the average CT scores (based on Kerschner’s rabbit Sinus CT grading system) from sinusitis rabbits were 9.74 +/− 0.21 (n = 10). At week 4 after 3 weeks of having sinus stents, the CISS group had marked improvement of opacification by week 4 compared to the sham group at week 4 (Figure 2A). Scores were significantly higher in rabbits with control stents (Δ Kerschner scale, n = 5, 7.34 +/− 1.17) compared to those rabbits treated with CISS (n = 5, 2.95 +/− 1.28) (p = 0.032) at week 4. When comparing the CT score changes (Δ) of each individual rabbit between week 1 and week 4, statistically significant improvements were noticed in rabbits treated with CISS (Δ Kerschner scale, Control (n = 5) = −0.55 +/− 0.92, CISS (n = 5) = −5.92 +/− 1.69, p = 0.024) (Figure 2B).
Figure 2.
Radiographic (CT) Findings
A. CISS group had marked improvement of opacification by week 4 compared to the control group at week 4. CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
B. When comparing the CT score changes (Δ) of each individual rabbit between week 1 and week 4, statistically significant improvements were noticed in rabbits treated with CISS (Δ Kerschner scale, Control = 0.55+/−0.92, CISS = −5.92+/−1.69, p = 0.024).
CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
Endoscopic Grading
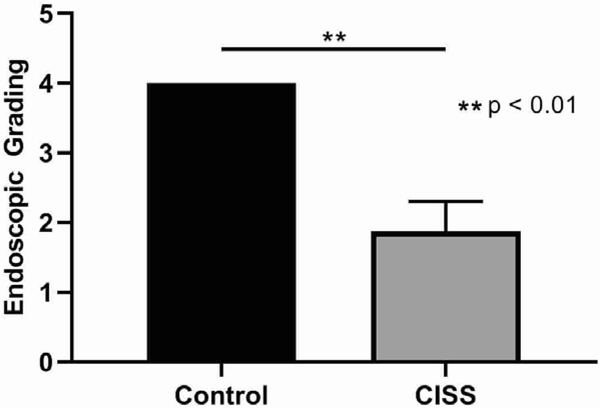
There was no evidence of purulent drainage or signs of infection on week 0. All rabbits demonstrated significant purulent drainage from the nostril where PAO-1 was inoculated by week 1. By week 4, a marked difference was noticed between the 2 groups (Control = 4.0 +/− 0.0, CISS = 1.88 +/− 0.43, p = 0.003). For those rabbits who had CISS at week 1 (n = 5), purulent drainage from the middle meatus was almost cleared in the CISS cohort when compared to the sham stent cohort (n = 5) by week 4 (Figure 3).
Figure 3.
Endoscopic Grading System at Week 4
The CISS group had significantly improved endoscopic grade at 4 weeks (Control = 4.0+/−0.0 vs CISS = 1.88+/−0.74, p = 0.003)
Bacterial Load
Colonies of PAO1 strains of P. aeruginosa were counted after three weeks with control stents or CISS (n = 5 each group). To estimate the magnitude of the change in CFUs before and after the treatment, log reduction was calculated17:
Where: A is the number of viable microorganisms before the treatment; B is the number of viable microorganisms after the treatment
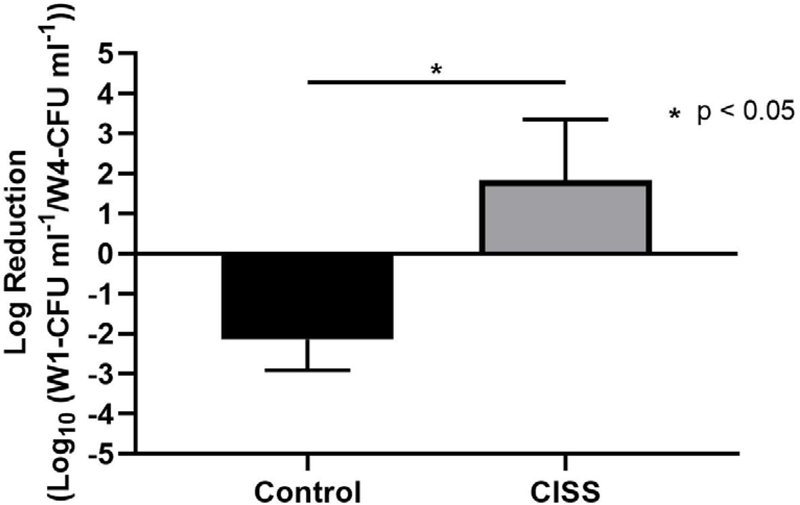
An average of 2-log reduction of PAO1 strains was observed for CISS groups (CISS = 1.84 +/− 1.52 log reduction, n = 5), which was statistically higher than controls (Control = −2.14 +/− 0.77 log reduction, n = 5) (p = 0.047). CISS appeared to eradicate approximately between 90% and 99% of PAO strains in this preclinical model. Log reduction values below 0 showed a proliferation of PAO1 strains, as observed in control stents (Figure 4).
Figure 4.
Bacterial Load
An average of 2-log reduction of PAO1 strains was observed for CISS groups (CISS = 1.84 +/− 1.52 log reduction, n = 5), which was statistically higher than controls (Control = −2.14 +/− 0.77 log reduction, n = 5) (p = 0.047).
CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
Sinus Potential Difference Measurements
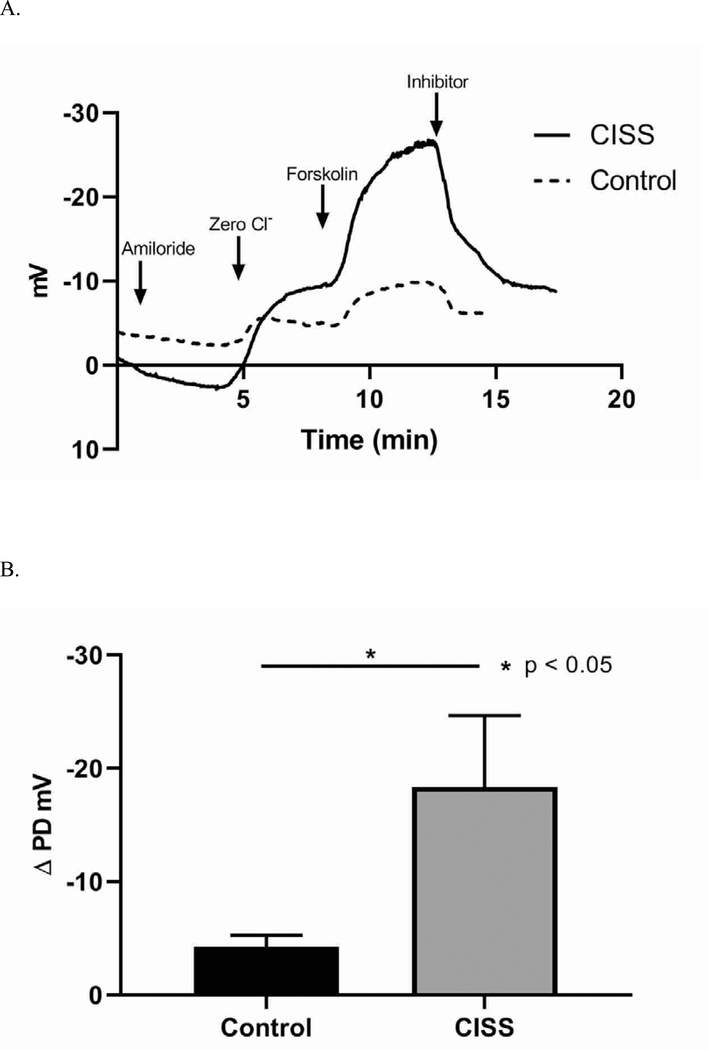
After 3 weeks of treatment with CISS, the ion transport characteristics were significantly different from those rabbits who had control stents (Figure 5). Sinus PD measurements revealed significantly decreased response to Cl− free ringers + forskolin in those rabbits that had control stents compared to those treated with CISS (Cl− free + forskolin ΔPD (mV), Control = −4.23 +/− 1.04, CISS = −18.36 +/− 6.31, p = 0.026), suggesting that CISS improved acquired ion transport dysfunction caused by infection in a cyclic AMP dependent fashion (indicative of improved CFTR activity). Robust baseline potential difference in all three groups indicated the presence of epithelial integrity and preserved tight junctions.
Figure 5.
Characteristics of Ion Transport
A. After 3 weeks of treatment with CISS, the ion transport characteristics were significantly different from those rabbits who had control stents. Sinus PD measurements revealed significantly increased response to Cl− free ringers + forskolin in sinusitis rabbits treated with CISS compared to those that had control stents
CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
B. CISS promoted transepithelial Cl− secretion in a cyclic AMP dependent fashion, indicative of improved CFTR activity (Cl− free + forskolin ΔPD (mV), Control = −4.23 +/− 1.04, CISS = −18.36 +/− 6.31, p = 0.026).
CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
Histology
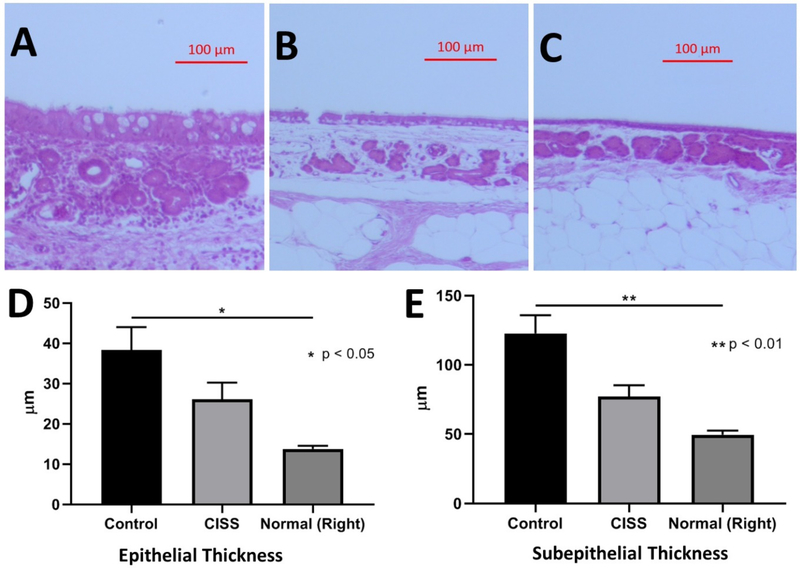
Histologic analysis was performed at identical sites in rabbits at the lateral wall of the maxillary sinus after staining with H&E on 5 rabbits from each group (total, n = 10) at week 4 (Figure 6). The thickness of the epithelial and submucosal layer was measured and the contralateral non-infected right maxillary sinuses (from those rabbits treated with control stents) served as internal control measurements (n = 5). In those rabbits who received non-drug eluting control stents (n = 5), significant infiltration of inflammatory cells was noted with significant thickening of the epithelial layer and the loss of differentiated epithelial cells. The lamina propria (just underneath the epithelium) was edematous with dilated capillaries, inflammatory cells (neutrophils and lymphocytes), and significant hyperplastic submucous glands. Purulent inflammatory mucus (pus) adherent to the sinus mucosa was also noticed just above the sinus epithelium. Once treated with CISS, there were less inflammatory responses in the submucosal area. When measuring the height of the epithelial cell layer, sinus epithelia from those rabbits treated with non-drug eluting stents were the thickest (38.31 +/− 5.7 μm), followed by CISS (26.07 +/− 4.2 μm) and normal controls (13.75 +/− 0.80 μm) (Welch’s ANOVA test, p = 0.02). There was a statistical significance among these groups with a trend toward improvement of epithelial thickness with CISS. Submucosal layer was also the deepest in those sinusitis rabbits who had non-drug eluting (control) stents (122.6 +/− 13.16 μm), followed by sinusitis rabbits treated with CISS (77.08 +/− 8.23 μm) and placebo (49.60 +/− 3.0 μm) (Welch’s ANOVA, p = 0.008). Those rabbits treated with CISS demonstrated significantly thinner submucosal edema compared to non-drug eluting control stents, although did not decrease to a level of thickness comparable to the contralateral normal non-infected sinuses.
Figure 6.
Histologic Characteristics
A. H&E staining of maxillary sinus at week 4 with non-drug eluting (control) stent (x10, Scale bar = 100μm)
B. H&E staining of maxillary sinus at week 4 with CISS (x10, Scale bar = 100μm)
C. H&E staining of maxillary sinus at week 4 on right normal side (x10, Scale bar = 100μm)
D. The height of the epithelial cell layer: Sinus epithelia from those rabbits treated with non-drug eluting stents were the thickest (38.31 +/− 5.7 μm), followed by CISS (26.07 +/− 4.2 μm) and normal controls (13.75 +/− 0.80 μm) (Welch’s ANOVA test, * p = 0.02)
E. The thickness of the submucosal layer: Submucosal layer was also the thickest in those sinusitis rabbits who had non-drug eluting (control) stents (122.6 +/− 13.16 μm), followed by sinusitis rabbits treated with CISS (77.08 +/− 8.23 μm) and placebo (49.60 +/− 3.0 μm) (Welch’s ANOVA, ** p = 0.008)
CISS: Ciprofloxacin Ivacaftor coated Sinus Stent
DISCUSSION
Topical drug delivery is a mainstay in the management of CRS especially in reducing biofilm mass as it allows for higher dosing and direct administration.18,19 Previous studies have demonstrated the utility of topical administration of steroid using bioabsorbable stents in the sinus cavities.20 Building on these prior studies utilizing steroid releasing stents, we evaluated the utility of ciprofloxacin and ivacaftor releasing stents for the management of Pseudomonas sinusitis in a preclinical model. We believe that this innovative new stent design (inner: hydrophilic, outer: hydrophobic) is a ground-breaking therapeutic for recalcitrant CRS treatment for the following reasons: 1) utilizes a novel and innovative approach (nanotechnology) to deliver two drugs in a controlled manner, 2) targets delivery locally at the site of pathology (the affected mucosa in sinusitis), thus minimizing systemic toxicity, 3) delivers both ciprofloxacin and ivacaftor for synergistic bactericidal effects, 4) provides long-term delivery over at least 3 weeks for bacterial biofilm eradication, and 5) supplies ivacaftor to enhance MCC.10,21 After inserting the CISS for 3 weeks, radiographic, functional and histologic improvements were significantly different between the CISS and controls.
Antibiotic overuse has led to an increase in bacterial resistance and extremely recalcitrant sinus infections. Several small molecules that activate CFTR22,23 for treatment of respiratory diseases of mucus clearance have been identified for the purpose of restoring Cl− secretion in patients with CF. Several studies have evaluated the use of ivacaftor (a non-specific CFTR channel potentiator) for therapy of other chronic inflammatory airway disease with poor mucus clearance, including COPD and CRS.21,24–29 Ivacaftor activates MCC in normal subjects with wild type CFTR and has weak off-target effects on P. aeruginosa.10,21,30,31 Thus, ivacaftor as a therapeutic approach for CRS represents a shift in treatment paradigm.32 However, the drug is only approved by the U.S. Food and Drug Administration (FDA) for patients with CF with specific CFTR genetic mutations.33 Even though ciprofloxacin and ivacaftor delivery should translate well to human clinical trials, commercialization and non-formulary applications of ivacaftor in CRS without CF will be challenging.
We have previously established the 1) in vitro efficacy of the CISS in the treatment of P. aeruginosa biofilms and 2) anti-biofilm activity of ciprofloxacin coated sinus stents in the preclinical model of P. aeruginosa sinusitis.10,11 The in vitro study revealed sustained release of drug over a 21-day period and demonstrated significant reduction in biofilm formation and inhibition of preformed biofilm.10 In the current study, rabbit sinuses were cultured and grown in an agar plate for 24 hours to evaluate CFU’s. This plate count method is a sensitive technique that provides semi-quantitative assessment of CISS antimicrobial activity by determining an estimate of the overall concentration of live bacteria cells before and after exposure.17 CISS markedly reduced the PAO1 strain of P. aeruginosa approximately 98.4% (1.8-log reduction), but did not completely eradicate (>8-log reduction) the infection. Future studies using higher doses of drug could perhaps provide improved bacterial log reduction that eliminates adherent P. aeruginosa biofilms.
There are several limitations to the current study. This is an in vivo preclinical investigation and this rabbit model may not represent human recalcitrant CRS. CRS is a heterogeneous disease, but dysfunctional MCC is considered a unifying mechanism prevalent in all forms of the chronic disease.34 In this preclinical model, obstructive (anaerobic) respiratory pathologies associated with P. aeruginosa superinfection reflect the histologic changes exemplified by subepithelial edema and increased epithelial thickness with subepithelial edema and glandular (SMG) hyperplasia.15 The sinuses of CISS treated rabbits did not completely return to normal according to radiographic and histologic findings. Because there is incomplete contact of the CISS to the complete mucosal surface infected with Pseudomonas, future studies will need to investigate parameters that impact drug release, including saline irrigations or other stent designs that may improve mucosal contact. The dosage of those drugs and the interval of placement will require further study to optimize clinical efficacy. For this study, the bare stent served as the control to measure the impact of the drug’s release and does not take into account any impact of the bare stent on the sinusitis. However, the PLLA biocompatible polymer has been recognized as a safe biocompatible material and in our previous study, no negative effects on the histology of normal rabbit sinuses was observed with prolonged 3 week insertion of the stent.6 Finally, the rabbit’s contralateral (right) maxillary sinus (from those rabbits who had non-drug eluting stents) served as the negative normal control for histology, and thus may be impacted from local or systemic crossover effects from the diseased sinus.
CONCLUSION
The current study revealed robust clinical efficacy of the CISS in treating PAO1-induced rabbit maxillary sinusitis. The innovative design using a double layered drug coating on the surface of the sinus stent may provide therapeutic advantages over current treatment strategies for recalcitrant bacterial infections in CRS.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006–04) to B.A.W.; National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482–02) to S.M.R.; and NIH/National Institutes of Allergy and Infectious disease (K08AI146220), John W. Kirklin Research and Education Foundation Fellowship Award, UAB Faculty Development Research Award, American Rhinologic Society New Investigator Award, and Cystic Fibrosis Foundation Research Development Pilot grant (ROWE15R0) to D.Y.C.
This manuscript was presented at the American Rhinologic Society Annual Meeting in New Orleans, LA, September2019.
Footnotes
Bradford A. Woodworth, M.D. is a consultant for Cook Medical and Smith and Nephew.
References
- 1.Foreman A, Boase S, Psaltis A, Wormald PJ. Role of bacterial and fungal biofilms in chronic rhinosinusitis. Curr Allergy Asthma Rep 2012; 12:127–135. [DOI] [PubMed] [Google Scholar]
- 2.Bendouah Z, Barbeau J, Hamad WA, Desrosiers M. Biofilm formation by Staphylococcus aureus and Pseudomonas aeruginosa is associated with an unfavorable evolution after surgery for chronic sinusitis and nasal polyposis. Otolaryngol Head Neck Surg 2006; 134:991–996. [DOI] [PubMed] [Google Scholar]
- 3.Fastenberg JH, Hsueh WD, Mustafa A, Akbar NA, Abuzeid WM. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World Journal of Otorhinolaryngology-Head and Neck Surgery 2016; 2:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Moser C, Wang HZ, Hoiby N, Song ZJ. Strategies for combating bacterial biofilm infections. Int J Oral Sci 2015; 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fastenberg JH, Hsueh WD, Mustafa A, Akbar NA, Abuzeid WM. Biofilms in chronic rhinosinusitis: Pathophysiology and therapeutic strategies. World J Otorhinolaryngol Head Neck Surg 2016; 2:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho DY, Hoffman K, Skinner D et al. Tolerance and pharmacokinetics of a ciprofloxacin-coated sinus stent in a preclinical model. Int Forum Allergy Rhinol 2017; 7:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh A, Anand U, Ugwu MC, Feridooni T, Massoud E, Agu RU. Drug-eluting nasal implants: formulation, characterization, clinical applications and challenges. Pharmaceutics 2014; 6:249–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2014; 6:532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider EK, Azad MA, Han ML et al. An “Unlikely” Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect Dis 2016; 2:478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho DY, Lim DJ, Mackey C et al. In-vitro evaluation of a ciprofloxacin- and ivacaftor-coated sinus stent against Pseudomonas aeruginosa biofilms. Int Forum Allergy Rhinol 2019; 9:486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho DY, Lim DJ, Mackey C et al. Preclinical therapeutic efficacy of the ciprofloxacin-eluting sinus stent for Pseudomonas aeruginosa sinusitis. Int Forum Allergy Rhinol 2018; 8:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HS, Singh B, Park TE et al. Mannan-decorated thiolated Eudragit microspheres for targeting antigen presenting cells via nasal vaccination. Eur J Pharm Sci 2015; 80:16–25. [DOI] [PubMed] [Google Scholar]
- 13.Garhwal R, Shady SF, Ellis EJ et al. Sustained ocular delivery of ciprofloxacin using nanospheres and conventional contact lens materials. Invest Ophthalmol Vis Sci 2012; 53:1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerschner JE, Cruz MJ, Beste DJ, Donahue KM, Kehl KS. Computed tomography vs. magnetic resonance imaging of acute bacterial sinusitis: a rabbit model. Am J Otolaryngol 2000; 21:298–305. [DOI] [PubMed] [Google Scholar]
- 15.Cho DY, Mackey C, Van Der Pol WJ et al. Sinus Microanatomy and Microbiota in a Rabbit Model of Rhinosinusitis. Front Cell Infect Microbiol 2017; 7:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho DY, Skinner D, Mackey C et al. Herbal dry extract BNO 1011 improves clinical and mucociliary parameters in a rabbit model of chronic rhinosinusitis. Int Forum Allergy Rhinol 2019; 9:629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bankier C, Cheong Y, Mahalingam S et al. A comparison of methods to assess the antimicrobial activity of nanoparticle combinations on bacterial cells. PLoS One 2018; 13:e0192093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desrosiers M, Bendouah Z, Barbeau J. Effectiveness of topical antibiotics on Staphylococcus aureus biofilm in vitro. Am J Rhinol 2007; 21:149–153. [DOI] [PubMed] [Google Scholar]
- 19.Orlandi RR, Kingdom TT, Hwang PH et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 2016; 6 Suppl 1:S22–209. [DOI] [PubMed] [Google Scholar]
- 20.Li PM, Li PF, Downie D, Hwang PH. Controlled steroid delivery via bioabsorbable stent: safety and performance in a rabbit model. Am J Rhinol Allergy 2009; 23:591–596. [DOI] [PubMed] [Google Scholar]
- 21.Cho DY, Lim DJ, Mackey C et al. Ivacaftor, a Cystic Fibrosis Transmembrane Conductance Regulator Potentiator, Enhances Ciprofloxacin Activity Against Pseudomonas aeruginosa. Am J Rhinol Allergy 2019; 33:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Illek B, Fischer H, Santos GF, Widdicombe JH, Machen TE, Reenstra WW. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol 1995; 268:C886–893. [DOI] [PubMed] [Google Scholar]
- 23.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol 2004; 124:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho DY, Lim DJ, Mackey C et al. l-Methionine anti-biofilm activity against Pseudomonas aeruginosa is enhanced by the cystic fibrosis transmembrane conductance regulator potentiator, ivacaftor. Int Forum Allergy Rhinol 2018; 8:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju SV, Lin VY, Liu L et al. The Cystic Fibrosis Transmembrane Conductance Regulator Potentiator Ivacaftor Augments Mucociliary Clearance Abrogating Cystic Fibrosis Transmembrane Conductance Regulator Inhibition by Cigarette Smoke. Am J Respir Cell Mol Biol 2017; 56:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloane PA, Shastry S, Wilhelm A et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 2012; 7:e39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho DY, Woodworth BA. Acquired Cystic Fibrosis Transmembrane Conductance Regulator Deficiency. Adv Otorhinolaryngol 2016; 79:78–85. [DOI] [PubMed] [Google Scholar]
- 28.Cho DY, Zhang S, Lazrak A et al. Resveratrol and ivacaftor are additive G551D CFTR-channel potentiators: therapeutic implications for cystic fibrosis sinus disease. Int Forum Allergy Rhinol 2019; 9:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tipirneni KE, Grayson JW, Zhang S et al. Assessment of acquired mucociliary clearance defects using micro-optical coherence tomography. Int Forum Allergy Rhinol 2017; 7:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernarde C, Keravec M, Mounier J et al. Impact of the CFTR-potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS One 2015; 10:e0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reznikov LR, Abou Alaiwa MH, Dohrn CL et al. Antibacterial properties of the CFTR potentiator ivacaftor. J Cyst Fibros 2014; 13:515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Goor F, Hadida S, Grootenhuis PD et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009; 106:18825–18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durmowicz AG, Lim R, Rogers H, Rosebraugh CJ, Chowdhury BA. The U.S. Food and Drug Administration’s Experience with Ivacaftor in Cystic Fibrosis. Establishing Efficacy Using In Vitro Data in Lieu of a Clinical Trial. Ann Am Thorac Soc 2018; 15:1–2. [DOI] [PubMed] [Google Scholar]
- 34.Alexander NS, Blount A, Zhang S et al. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope 2012; 122:1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]