Abstract
Background
Distention of the esophagus elicits a unique pattern of repetitive contractions in healthy controls. We aimed to assess the rhythm and rate of distension-induced contractile patterns between achalasia and controls and identify factors that distinguish the normal contractile response to distension.
Methods
20 asymptomatic controls and 140 adult patients with treatment-naïve achalasia defined by HRM (29 type I, 81 type II, 30 type III) were prospectively evaluated with functional luminal imaging probe (FLIP) during sedated endoscopy. 16-cm FLIP balloons were positioned within the distal esophagus during stepwise balloon distension. FLIP panometry studies were retrospectively analyzed using a customized program.
Key Results
All controls had contractility in a repetitive antegrade contraction (RAC) pattern with a rate of mean (10–90th) 6 (4–8) contractions per minute. 19/20 controls had > 6 consecutive antegrade contractions (ACs), i.e. duration >6 ACs, >6-cm in length, at a rate of 6+/−3 contractions per minute (met the ‘Rule-of-6s’). 50 achalasia patients had repetitive contractions that occurred at a rates of 11 (7 – 15) ACs per minute; P < 0.001 compared with controls, or 12 (8–15) repetitive retrograde contractions per minute. Only 1/140 achalasia patients had a contractile response that met the ‘Rule-of-6s’.
Conclusion
The normal contractile response to sustained distention is associated with >6 RACs with a consistent rate of 6+/−3 per minute, which was exceptionally rare in achalasia. These findings support that the RAC pattern is disrupted in achalasia and the faster rate may be a manifestation of abnormal inhibition and/or a reduced refractory period.
Keywords: achalasia, dysphagia, FLIP, impedance, manometry, peristalsis
Introduction
The response of the esophagus to distention is complex and varied based on the pattern of distention. The typical response to proximal segmental esophageal balloon distention is distal relaxation of the distal esophageal body and the lower esophageal sphincter (LES).1 There is typically an aboral atropine sensitive contraction above the balloon and a descending inhibitory discharge mediated by nitric oxide (NO) which leads to hyperpolarization. Upon deflation, contraction occurs and this is likely due to a combination of rebound depolarization and excitatory action generated from extrinsic reflexes and myogenic activity. The direction of contraction is modulated by the latency gradient across the esophagus and this progresses distally along the gradient. When distention is sustained in the proximal esophagus, the esophageal body remains relaxed and a contraction will only occur when the balloon is deflated.2 These secondary peristaltic events likely serve a protective role in clearing refluxate after a reflux event and a food bolus after an ineffective swallow.
Recently, our team has described a different response to distention using the functional luminal imaging probe (FLIP).3 After applying a sustained pan-esophageal volumetric distention across the esophagus, a repetitive contractile response is elicited in normal subjects that appears to defy descending inhibition noted during segmental sustained contraction. There have been reports of phasic and repetitive contractions in the esophagus and the exact mechanism behind these contractions is unclear.4–6 During FLIP distention, there is no sustained inhibition of LES tone or distal esophageal contractile activity and instead contractions proceed down the esophagus in a rhythm that is similar to the migrating motor complex (MMC), but at double the rate. These findings suggest that this pattern may be related to some type of underlying pacemaker-slow wave stimulus or a pattern generated by a constant stimulus and the inherent latency gradient or intrinsic myenteric inhibition of esophageal peristalsis.
Although absent peristalsis is a defining manometric feature of achalasia, we also previously demonstrated that esophageal contractility can be observed in patients with achalasia during evaluation with FLIP panometry.7, 8 Presence of non-occluding esophageal contractions in achalasia was also observed using high-frequency, intraluminal ultrasound and these contractions were associated with the pan-esophageal pressurization of the type II achalasia sub-type.9 Although antegrade contractions can be seen in achalasia, patients with achalasia tend to have a chaotic pattern consisting of sporadic focal contractions and retrograde propagation.
We hypothesize that the rate of contractions will be different in achalasia patients and controls due to issues related to disrupted inhibitory function in achalasia. Thus, the aim of this study was to assess the rhythm and rate of distension-induced contractile patterns among patients with achalasia and controls. Additionally, we also sought to refine the definition of RACS based on our comparison between normal controls and achalasia patients.
Methods
Subjects
Adult patients presenting to the Esophageal Center of Northwestern for evaluation of dysphagia between November, 2012 and October, 2017 that completed HRM and FLIP during upper endoscopy were prospectively evaluated. 140 consecutive patients newly diagnosed with achalasia by HRM were included for this study. Achalasia was diagnosed and sub-classified by HRM (details below).10 None of the patients had previously been treated with pneumatic dilation or LES myotomy. Patients with previous upper gastrointestinal surgery, significant medical co-morbidities, eosinophilic esophagitis, severe reflux esophagitis (LA-classification C or D), or large hiatal hernia were also excluded. There is overlap in this study cohort with previous reports.7, 8
For comparison, 20 healthy, asymptomatic, adult volunteers were also included. These subjects were previously described.11 Informed consent was obtained from all subjects. The study protocol was approved by the Northwestern University Institutional Review Board.
FLIP Study Protocol and Analysis
Evaluation was completed after a minimum 6-hour fast. Subjects underwent upper endoscopy in the left lateral decubitus position. Conscious sedation with 2–15 mg midazolam and 50–300 mcg fentanyl was administered during the procedure. Other sedative medications, e.g. propofol, (in addition to midazolam and fentanyl) were used with anesthesiologist assistance at the discretion of the performing endoscopist in some cases.
The 16-cm FLIP (EndoFLIP® EF-322N; Medtronic, Inc, Shoreview, MN) was calibrated to atmospheric pressure prior to trans-oral probe placement. With the endoscope withdrawn, the FLIP was positioned within the esophagus such that 1–3 impedance sensors were observed beyond the EGJ with this positioning maintained throughout the FLIP study. Stepwise 5-ml or 10-ml balloon distensions beginning with 20 ml and increasing to target volume of 60ml or 70 ml were then performed (variations in FLIP study protocol evolved during the course of this study); each stepwise distension volume was maintained for 30–60 seconds.
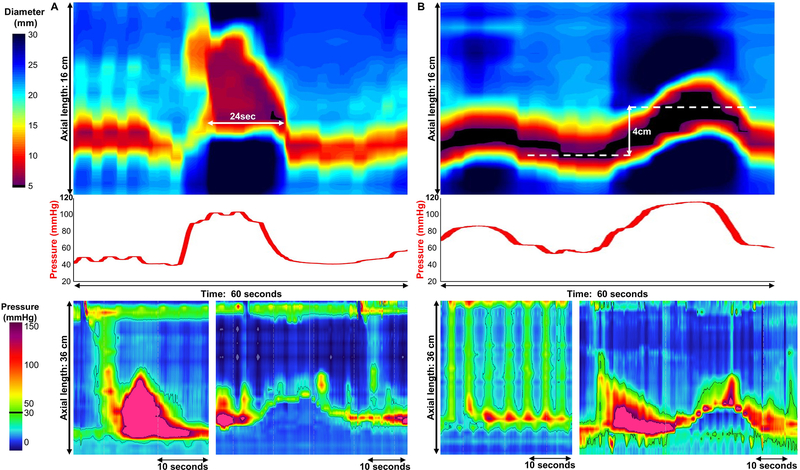
FLIP data including distension volume, intra-balloon pressure, and 16 channels of luminal diameter were exported to a customized program as previously described.3, 7, 8, 11 Esophageal body contractions and contractility patterns were identified similarly to previous studies.7, 8, 11 Esophageal body contractions were identified by a transient decrease of ≥ 5 mm in the luminal diameter in ≥ 3 adjacent impedance planimetry channels using FLIP topography plots and 16-channel diameter line-tracing output. The axial length of contractions was determined by the number of consecutive impedance planimetry channels (1-cm spacing) with a decrease in luminal diameter. The direction of contractions (antegrade or retrograde) was categorized based on a tangent line placed at the onset of contraction. Specific patterns of the contractile response to distension were further categorized similar to our original descriptions as repetitive if ≥ 3 contractions of similar directionality occurred consecutively at a consistent time interval and further by contraction direction: Repetitive, antegrade contractions (RACs) and repetitive, retrograde contractions (RRCs); Figure 1.3, 7 We sought to further refine the RAC pattern by determining the best discriminatory cut-offs between controls and achalasia. When a RAC or RRC pattern occurred, the duration of the RAC or RRC pattern was assessed both by the number of consecutive contractions that occurred and also by the time over which they occurred. The rate of repetitive contractions was then derived by dividing the number of repetitive contractions by duration (time) of repetitive contraction pattern and then normalized to reflect the rate of contractions as the number of contractions per minute (Figure 2).
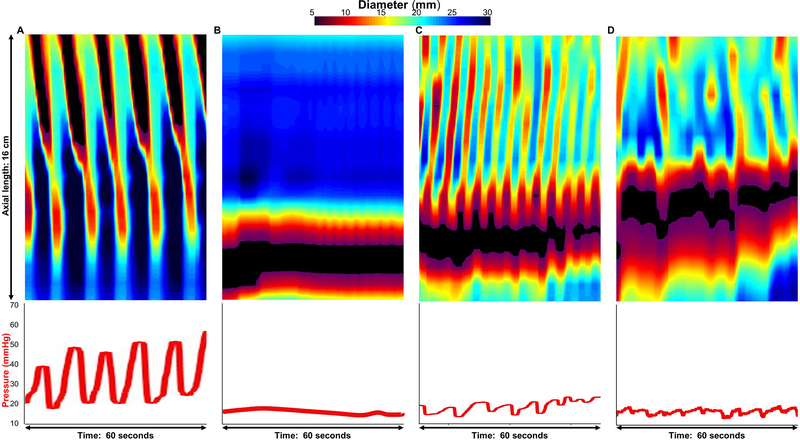
Figure 1. Distension-induced contractility patterns.
FLIP panometry with 16-cm of interpolated esophageal diameter topography (top panels) and intra-balloon pressure (bottom panel) by time. A.) Repetitive antegrade contractions (RAC) pattern from an asymptomatic control. B.) Absent contractile response and C) repetitive retrograde contractions (RRCs; C) from patients. Some patients also had distension-induced contractility observed, but the contractility did not occur in one of the other distinct patterns (D). Figure used with permission from the Esophageal Center at Northwestern.
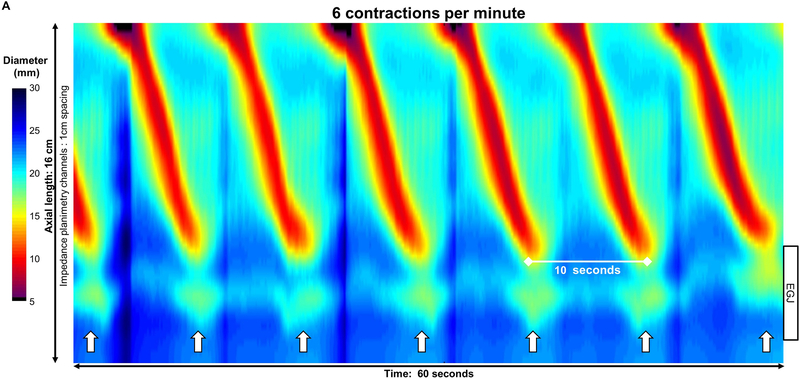
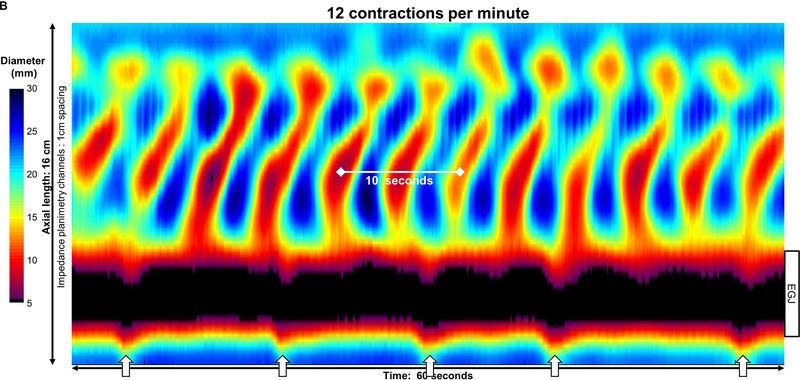
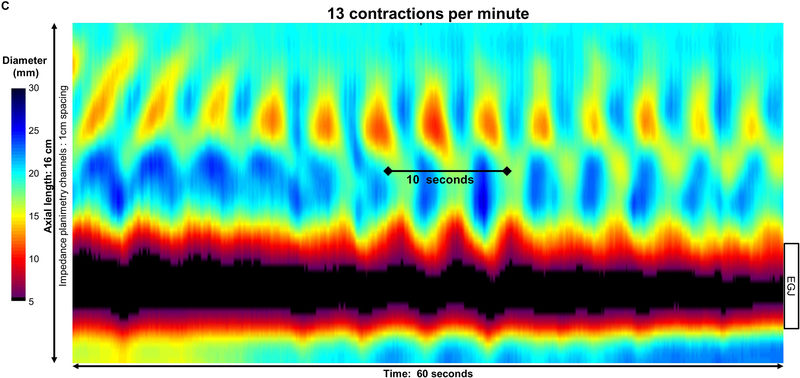
Figure 2: Pace of distension-induced esophageal contractions.
A) An asymptomatic control with repetitive antegrade contraction (RAC) pattern B) type III achalasia with repetitive retrograde contraction (RRC) pattern; C) type II achalasia with repetitive contractions occurring with both retrograde and short antegrade contractions. Contractions appeared synchronized with respiration, as noted by crural contractions (white arrows) in the control, but not in this achalasia patient; unfortunately, the crural contraction could not be reliably identified in all subjects (such as in C). Figure used with permission from the Esophageal Center at Northwestern.
Contractions were also categorized as occluding if they achieved a minimal esophageal diameter < 6mm or non-occluding contractions if they did not.7 Additionally, the pressure-changes associated with contractions were assessed and patient studies were dichotomized as occurring with pressure changes ≥ 10 mmHg versus pressure changes occurring < 10 mmHg. When occluding contractions occurred spontaneously and not in a repetitive pattern (i.e. not RAC nor RRC) and were associated with a prolonged contraction greater than the typical AC duration and a ≥ 10 mmHg pressure increase, they were designated as sustained occluding contractions (SOCs; Figure 3). Another pattern found in achalasia patients and not seen in controls was the LES-lift (LES-L) that was associated with a ≥10 mmHg increase in pressure without evidence of associated SOC(Figure 3).
Figure 3. Abnormal contractile activity observed in achalasia.
FLIP topography (top), FLIP intra-balloon pressure (middle), and high-resolution manometry (HRM; bottom) from two patients are presented. A). A Sustained occluding contractions (SOC) SOC in a patient with type III achalasia is displayed. SOCs occurred with or without lift of the lower esophageal sphincter (LES) though generally were observed with continuity with the sphincter and a >6 cm segment of esophageal contraction. These events also typically resulted in large increases in intra-balloon pressure. A supine test swallow (left) on HRM had an integrated relaxation pressure (IRP) of 22 mmHg and a distal latency of 4-seconds; an esophageal shortening event with LES-lift (right) was also observed during the HRM study.. B.A LES lift (LES-L) in a patient with type II achalasia is displayed.. These events were also associated with increases in intra-balloon pressure. Displayed from the HRM are a supine test swallow (left) with IRP 18mmHg and pan-esophageal pressurization and an esophageal shortening event with LES-lift (right) that occurred independently of test swallows. Figure used with permission from the Esophageal Center at Northwestern.
HRM protocol and analysis
After a minimum 6-hour fast, HRM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). The HRM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. After a 2-minute baseline recording, the HRM protocol was performed with ten, 5-ml liquid swallows in a supine position.10, 12
Manometry studies were analyzed using ManoView version 3.0 analysis software (Medtronic) to measure basal EGJ pressure (end-expiration), the integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency according to the Chicago Classification. 10, 12 A median IRP of > 15 mmHg was applied as the upper-limit of normal, thus all patients had a median IRP > 15 mmHg as a criterion the achalasia diagnosis. Type I achalasia was defined by absent contractility in 100% of swallows. Type II achalasia was defined by pan-esophageal pressurization at an isobaric contour of 30 mmHg in > 20% of swallows. Type III (spastic) achalasia was defined by ≥20% of premature swallows (i.e. distal latency < 4.5s).
Statistical analysis
Results were expressed as mean (SD) or median and interquartile range (IQR) depending on data distribution. Statistical comparisons were made between normal controls and all achalasia patients. Comparison of dichotomous and categorical variables between groups was assessed with Χ2 test or Exact tests. Continuous variables were compared via t-test, ANOVA or Mann-Whitney U or Kruskal-Wallis tests, depending on data distribution. Statistical significance was considered at a two-tailed p-value < 0.05.
Results
Subjects
The controls had a mean (SD) age of 30 (6) years; 70% were female. The HRM-classification among the controls was normal motility in 17 (85%), EGJ outflow obstruction in 2 (10%: both had normal peristalsis with median IRPs of 16 and 18mmHg), and 1 (5%) with ineffective esophageal motility. The mean (SD) dosages of midazolam and fentanyl used during endoscopy with FLIP were 9.0 (8.0 – 10.0) mg and 200 (200 – 200) mcg, respectively.
The achalasia patients had a mean (SD) age of 52 (18) years (P < 0.001 compared with controls); 39% were female (P = 0.014). Achalasia sub-classification on HRM included 29 (21%) patients with type I, 81 patients (58%) with type II, and 30 patients (21%) with type III achalasia); thus 110 (76%) patients had absent contractility (with or without pressurization) on HRM. The median (IQR) dosages of midazolam and fentanyl used during endoscopy with FLIP were 6.0 (4.0 – 8.8) mg and 150 (100 – 194) mcg, respectively; P < 0.001 for both compared with controls. Twenty-six patients (19%) had sedation with propofol, 16 of whom also were administered ketamine. No differences in presence or pattern of distension-induced contractility were detected with relationship to endoscopic sedation or chronic opioid use, Supplementary Table 1.
The Normal Rate and Rhythm of Repetitive Distension-Induced Contractions
All 20 controls exhibited distension-induced contractility that included a RAC pattern; none of the controls exhibited an RRC pattern. The rate of contractions in a RAC pattern across the entire duration of the FLIP study was mean (SD) 6 (1) contractions per minute in the controls (Figure 1 and 4).
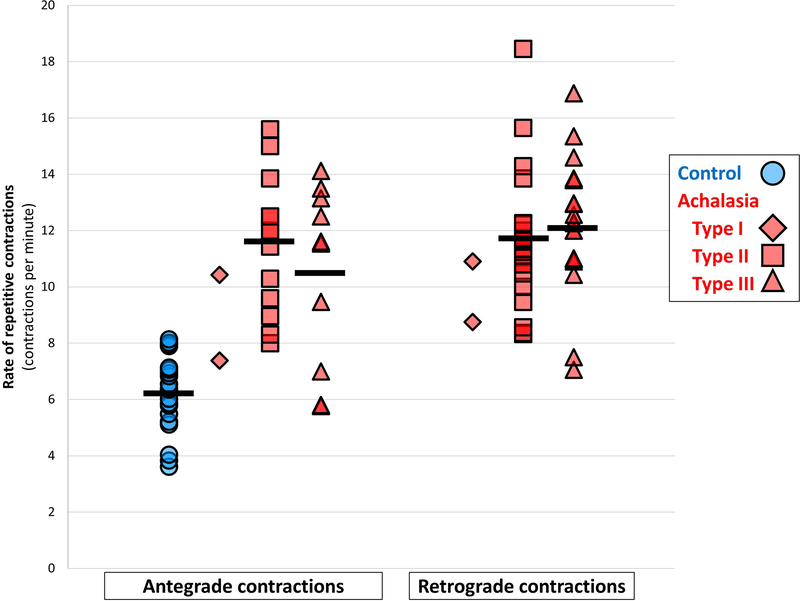
Figure 4. Rate of repetitive contractions differs between achalasia and controls.
Group means are indicated with horizontal lines. Darker colored symbols indicate overlapping data points. Rates of repetitive contractions were similar when compared between patients with non-spastic achalasia (types I and II) and spastic (type III) achalasia with both antegrade (P = 0.523) and retrograde contractile patterns (, P = 0.461). Figure used with permission from the Esophageal Center at Northwestern.
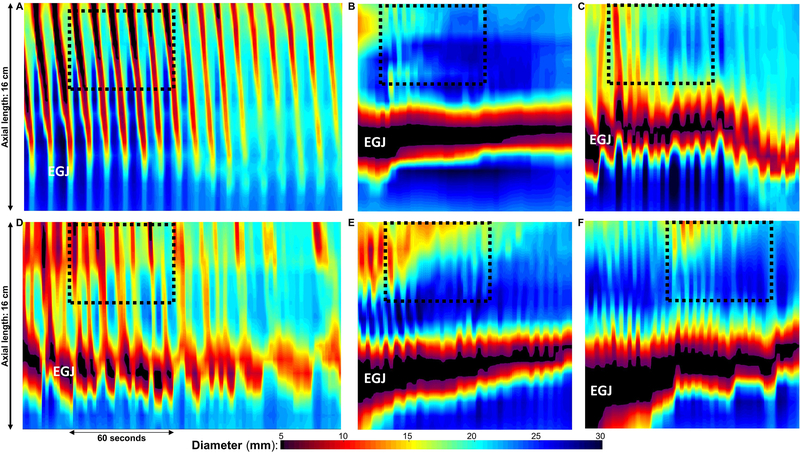
Among the controls, the RAC pattern persisted for a duration (i.e. number of contractions) of median (IQR) of 24 (18–30) antegrade contractions; this RAC pattern duration was dependent on the FLIP study duration in the 17/20 controls in which the RAC pattern persisted through the entirety of the FLIP distension protocol (i.e. completion of the 70-ml fill volume), while the other three controls had cessation of the RAC pattern at the 60-ml fill volume. The RAC duration was > 6 consecutive antegrade contractions in 19/20 (95%) controls, and in 19/20 (95%), > 6 antegrade contractions had an axial length of > 6cm. Thus, in 19/20 (95%) of controls, the RAC pattern met each of the following three criteria: 1) duration > 6 antegrade contractions that were 2) >6-cm in axial length, and occurred at a 3) rate of 6 (+/−3) antegrade contractions per minute (Figure 5). This Rule-of-6s was therefore proposed for application to a RAC pattern observed during a FLIP panometry study to aid designation of the normal contraction response. Additionally, all 20 controls had contraction-associated pressure-amplitudes > 10-mmHg. None of the controls has an LES-lift event nor a SOC observed.
Figure 5. The RAC Rule-of-6s.
FLIP topography plots of an asymptomatic control (A) and patients with achalasia that had multiple antegrade contractions (B-F) and met the original definition for repetitive antegrade contractions (RACs). Positioning of the FLIP can be noted by the narrowing at the esophagogastric junction (EGJ). The black-dashed boxes represent 60-seconds duration and 6-cm of axial length, and thus the normal RAC pattern would involve 6 +/− 3 antegrade contractions that span both the length and duration (i.e. number of consecutive contractions) of this ‘Rule-of-6s’ box. Thus, even achalasia patients with multiple antegrade contractions generally had contractions that were shorter in length than normal (B, E, F), did not persist for as long (i.e. had a small number of consecutive contractions) as normal (B, C, E, F), and occurred at a more rapid rate than normal (F). The patient in D was the only achalasia patient that met the RAC ‘Rule-of-6s,’ however, the contractile response still did not appear normal; additional information on this patient is included in the supplemental figure. Figure used with permission from the Esophageal Center at Northwestern.
Distension-induced contractility parameters in achalasia and comparison with controls
An absent contractile response (ACR), i.e. no distension-induced contractility observed during a FLIP panometry study, was observed in 48/140 (34%) patients and 0 controls. None of the type III patients had an ACR, while 76% of the Type I and 32% of the Type II patients had an ACR. Distension-induced contractility was observed in 92 (66%) of achalasia patients; (P < 0.001 compared with controls): 24% of type I achalasia, 68% of type II achalasia, and 100% of type III achalasia; (P < 0.001 between subtypes). Some form of repetitive ACs (not a normal RAC pattern) were observed in 25 (18%) patients (P<0.001 compared with controls) and occurred at a rate of mean (SD) 11 (3) contractions per minute; P< 0.001 compared with controls (Figure 4). A RRC pattern was observed in 41 (29%) of patients (P=0.002 compared with controls) and the rate of contractions in a RRC pattern was mean (SD; range) 12 (2; 7–18). The duration of the repetitive contraction pattern
Sixteen patients had both repetitive ACs and a RRC pattern observed over the course of the FLIP study, thus only 9/140 (6%) patients had repetitive ACs without RRCs. Of those 9 patients, (1 type I, 5 type II and 3 type III by HRM), the median (IQR) number of ACs was 5 (4 – 9) contractions, and the median (IQR) rate of those short bursts of ACs was 10 (9 – 14) contractions per minute (Figure 5). One patient had a SOC with LES-lift event. Only 3/9 had an AC pattern duration > 6 antegrade contractions, and only two had an AC rate < 9 contractions per minute, and only one of these patients (type I achalasia on HRM) met the RAC Rule-of-6s (with 8, >6-cm antegrade contractions at a rate of 7 per minute). This patient had cessation of the RAC pattern prior to the 50ml fill volume and a 60ml EGJ-distensibility index of 2.7 mm2/mmHg supporting that this patient may have some non-occluding contractions not detected with manometry (Figure 5 and Supplemental Figure).
In summary, 1/140 (<1%) of patients with achalasia exhibited a contractile response to distension of a RAC pattern meeting the Rule-of-6s on FLIP panometry (though still did not have a normal esophageal response to distension), compared with 95% of the controls.
Discussion
We demonstrated that the rate at which repetitive esophageal contractions occur in response to sustained esophageal distension differed between healthy volunteers and patients with achalasia. The rate of contractions during RACs were on average 6 per minute in healthy volunteers and the average rate was approximately double in achalasia patients with antegrade (11 per minute) or retrograde contractions (12 per minute). These findings have interesting physiologic implications as the rate and number of contractions appear to distinguish disease from normal physiology. Further, we incorporated the rate of contractions into a Rule-of-6s definition of RACs [6 or more repetitive contractions that extend at least 6 cm occurring at 6 +/− 3 contractions per minute] that is consistently observed in asymptomatic controls, but almost never in achalasia.
The physiology of the RAC pattern is extremely interesting as it is very different from segmental proximal distention. Proximal segmental distention typically will induce a cholinergic mediated proximal aboral contraction with distal inhibition and the contraction will only progress down the esophagus after the distention resolves. This supports that the peristaltic wave is partially related to rebound depolarization as it progresses down the latency gradient. In contrast, the sustained pan-esophageal distention elicits contraction during the distention and this activity will begin at varying trigger volumes. It is conceivable that the same excitatory aboral cholinergic response is being propagated down the esophagus in a repetitive fashion that is modulated by the latency gradient and refractoriness of the muscle.13, 14 The rate of propagation of the contraction is similar to primary peristalsis and the rate of repetitive contractions is consistent with a 10–12 second refractory period that would allow another contraction to be triggered. Contractions at a rate of 5 seconds or less would not be triggered to propagate down the normal esophagus due to the refractory period. The fact that achalasia patients have contractions every 5 seconds could be explained by the defects in inhibitory function that have been previously described. Thus, the increased rate may be a physiomarker of impaired inhibition.
Another intriguing theory on the genesis of the RAC pattern could be related to an underlying pacemaker and entrainment of a baseline slow wave. There are definite morphologic similarities between the RAC pattern and the MMC and it is intriguing to think that this could be somehow related to a pacemaker governed by interstitial cells of Cajal (ICCs) and PDGFR alpha cells. A slow wave frequency of 6 per minute is within the range of other GI slow wave rates and also fits within the construct of the refractory period of the esophagus. Previously, Chen et al described rhythmic simultaneous contractions on manometry in early achalasia.5 The contraction rate was approximately 7 per minute and was similar to the rate we saw in normal controls. Based on inspection of the figure in their paper, the pressure changes were simultaneous isobaric contractions that likely represent repetitive contractions in type II achalasia. Thus, these may be consistent with conversion from normal peristalsis to the erratic pattern seen in type II and III achalasia.
It is interesting that the RAC pattern appears to synchronize with respiration in healthy controls and the frequency of contractions in achalasia is similar to the quiet respiratory rate generated by respiratory pacemaker believed to involve the dorsal respiratory group (DRG) in the medulla [12 per minute]. It is possible that extrinsic influence from the DRG or other respiratory pacemaker sites in the brainstem may modulate the frequency of contractions through interactions with ICCs. Theoretically, the baseline slow wave rate could be 12 per minute and the normal intrinsic latency interval could slow the rate to 6 per minute while achalasia patients with reduced latency gradients could generate contractions at a rate of 12 per minute.15 Once again, this is supported by the fact that contractions are also spaced approximately 10 seconds apart to avoid the refractory period and the preceding descending inhibitory latency gradient.14, 15
Although not the focus of this study, we were able to identify patterns of contraction that seemed to have a logical relationship with the subtypes of achalasia. The ACR was only found in 76% of Type I patients and 32% of Type II patients and no Type III patients. Thus, the ACR pattern could be used to classify Type I achalasia and may also rule out the possibility of a spastic motor disorder. Similarly, we also noted that LES-L could be seen in Type II achalasia while SOCs were predominantly seen in type III achalasia.16–18 Although these findings suggest that the pattern of contractions could help subtype achalasia, a more detailed assessment is required that focuses on comparison with radiographic findings and clinical outcomes.
In conclusion, the rate of repetitive contractions during distention appears to distinguish healthy controls and achalasia patients. This pattern of contraction may have important physiologic implications as it provides a unique model to study secondary peristalsis and the neural circuitry that controls peristalsis. Further work focused on pharmacologic triggers and effects of electrical stimulation would be helpful in further defining this unique contractile response as it is likely a marker of latency and intrinsic triggering of myogenic responses. Although the morphologic similarities between the RAC pattern and MMC and the potential synchrony with respiration are intriguing findings, further work focused on correlating this response to respiratory rate in both the quiet and stimulated state need to be performed to develop a better understanding of this unique contractile pattern.
Supplementary Material
Supplemental Figure. Achalasia patient with distension-induced contractility. FLIP topography demonstrating 8 consecutive antegrade contractions with 8 spanning >6-cm in axial length at a rate of 7 antegrade contractions per minute (thus meeting the ‘Rule-of-6s’) is displayed in Figure 5D. The patient presented with dysphagia and regurgitation of 10 years duration and had an unrevealing upper endoscopy. A) Three representative test swallows (black arrows) from the high-resolution manometry (HRM) are displayed. The HRM was classified as type I achalasia with a median IRP across 10 supine 5-ml liquid swallows of 17 mmHg and 100% failed swallows. Pan-esophageal pressurization was observed at 22 – 28 mmHg, thus falling below the diagnostic threshold for type II achalasia. Further, a propagating contraction was observed following inadvertent dry swallows (B). C.) The esophagram (not performed via a timed protocol) did show delayed esophageal passage of liquid barium and retention of a barium tablet at a narrowed esophagogastric junction. The patient was successfully treated with a laparoscopic Heller’s myotomy. Figure used with permission from the Esophageal Center at Northwestern.
Acknowledgments
Grant support: This work was supported by P01 DK117824 (JEP) from the Public Health service.
Conflicts of interest:
Dustin A. Carlson and John E. Pandolfino hold shared intellectual property rights and ownership surrounding FLIP panometry systems, methods, and apparatus with Medtronic Inc.
Dustin A. Carlson: Medtronic (Speaking. Consulting)
Wenjun Kou: Crospon, Inc (Consulting)
John E. Pandolfino: Crospon, Inc (stock options), Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking), Medtronic (Speaking. Consulting), Torax (Speaking, Consulting), Ironwood (Consulting), Impleo (Grant).
References
- 1.Paterson WG, Anderson MA, Anand N. Pharmacological characterization of lower esophageal sphincter relaxation induced by swallowing, vagal efferent nerve stimulation, and esophageal distention. Can J Physiol Pharmacol 1992;70(7):1011–5. [DOI] [PubMed] [Google Scholar]
- 2.Paterson WG, Rattan S, Goyal RK. Esophageal responses to transient and sustained esophageal distension. Am J Physiol 1988;255(5 Pt 1):G587–95. [DOI] [PubMed] [Google Scholar]
- 3.Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterol Motil 2015;27(7):981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preiksaitis HG, Diamant NE. Phasic contractions of the muscular components of human esophagus and gastroesophageal junction in vitro. Can J Physiol Pharmacol 1995;73(3):356–63. [DOI] [PubMed] [Google Scholar]
- 5.Chen JH, Wang XY, Liu LW, et al. On the origin of rhythmic contractile activity of the esophagus in early achalasia, a clinical case study. Front Neurosci 2013;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mujica VR, Mudipalli RS, Rao SS. Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol 2001;96(5):1371–7. [DOI] [PubMed] [Google Scholar]
- 7.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology 2015;149(7):1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of Esophageal Motility Utilizing the Functional Lumen Imaging Probe. Am J Gastroenterol 2016;111(12):1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Zifan A, Kumar D, et al. Genesis of Esophageal Pressurization and Bolus Flow Patterns in Patients with Achalasia Esophagus. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27(2):160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Kou W, Lin Z, et al. Normal Values of Esophageal Distensibility and Distension-Induced Contractility Measured by Functional Luminal Imaging Probe Panometry. Clin Gastroenterol Hepatol 2019;17(4):674–81 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol 2008;103(1):27–37. [DOI] [PubMed] [Google Scholar]
- 13.Vanek AW, Diamant NE. Responses of the human esophagus to paired swallows. Gastroenterology 1987;92(3):643–50. [DOI] [PubMed] [Google Scholar]
- 14.Meyer GW, Gerhardt DC, Castell DO. Human esophageal response to rapid swallowing: muscle refractory period or neural inhibition? Am J Physiol 1981;241(2):G129–36. [DOI] [PubMed] [Google Scholar]
- 15.Gidda JS, Goyal RK. Regional gradient of initial inhibition and refractoriness in esophageal smooth muscle. Gastroenterology 1985;89(4):843–51. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatek MA, Post J, Pandolfino JE, et al. Transient lower oesophageal sphincter relaxation in achalasia: everything but LOS relaxation. Neurogastroenterol Motil 2009;21(12):1294–e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaban DH, Yamamoto Y, Liu J, et al. Sustained esophageal contraction: a marker of esophageal chest pain identified by intraluminal ultrasonography. Gastroenterology 1999;116(1):29–37. [DOI] [PubMed] [Google Scholar]
- 18.Mittal RK, Karstens A, Leslie E, et al. Ambulatory high-resolution manometry, lower esophageal sphincter lift and transient lower esophageal sphincter relaxation. Neurogastroenterol Motil 2012;24(1):40–6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Achalasia patient with distension-induced contractility. FLIP topography demonstrating 8 consecutive antegrade contractions with 8 spanning >6-cm in axial length at a rate of 7 antegrade contractions per minute (thus meeting the ‘Rule-of-6s’) is displayed in Figure 5D. The patient presented with dysphagia and regurgitation of 10 years duration and had an unrevealing upper endoscopy. A) Three representative test swallows (black arrows) from the high-resolution manometry (HRM) are displayed. The HRM was classified as type I achalasia with a median IRP across 10 supine 5-ml liquid swallows of 17 mmHg and 100% failed swallows. Pan-esophageal pressurization was observed at 22 – 28 mmHg, thus falling below the diagnostic threshold for type II achalasia. Further, a propagating contraction was observed following inadvertent dry swallows (B). C.) The esophagram (not performed via a timed protocol) did show delayed esophageal passage of liquid barium and retention of a barium tablet at a narrowed esophagogastric junction. The patient was successfully treated with a laparoscopic Heller’s myotomy. Figure used with permission from the Esophageal Center at Northwestern.