Abstract
Background:
Enteric nervous system (ENS) abnormalities have been implicated in delayed gastric emptying but studies exploring potential treatment options are limited by the lack of an experimental animal model. We examined the ENS abnormalities in the mouse stomach associated with ageing, developed a novel model of gastroparesis, and established a new approach to measure gastric emptying.
Methods:
A modified gastric emptying assay was developed, validated in nNOS −/− mice, and tested in mice at multiple ages. Age related changes in ENS structure were analyzed by immunohistochemistry. Gastric aganglionosis, was generated in Wnt1-iDTR mice using focal administration of diphtheria toxin (DT) into the anterior antral wall.
Key Results:
Older mice (>5 months) exhibit hypoganglionosis in the gastric antrum and a decreased proportion of nNOS neurons as compared to younger mice (age 5–7 weeks). This was associated with a significant age-dependent decrease in liquid and solid gastric emptying. A novel model of gastric antrum hypoganglionosis was established using neural crest-specific expression of diphtheria toxin receptor. In this model, a significant reduction in liquid and solid gastric emptying is observed.
Conclusions and Inferences:
Older mice exhibit delayed gastric emptying associated with hypoganglionosis and a reduction in nNOS-expressing neurons in the antrum. The causal relationship between antral hypoganglionosis and delayed gastric emptying was verified using a novel experimental model of ENS ablation. This study provides new information regarding the pathogenesis of delayed gastric emptying and provides a robust model system to study this disease and develop novel treatments.
Keywords: Enteric nervous system, Gastroparesis, Hypoganglionosis, Ageing, Gastric Emptying
Introduction
The emptying of solids and liquids from the stomach into the duodenum is a complex process that relies on intrinsic and extrinsic innervation, gastric smooth muscle, and interstitial cells of Cajal (ICC). These elements regulate gastric motor function, which includes relaxation of the fundus to allow food to be received and stored, grinding and propulsion in the antrum to break down food particles and propel them towards the pylorus, and pyloric relaxation to allow passage into the duodenum. Not surprisingly, given the complexity of this process, clinical disorders of gastric motility such as gastroparesis (delayed emptying of gastric contents in the absence of an anatomical obstruction), are common with a prevalence for men of 9.8 per 100,000 persons and 37.8 for women1.
Delayed gastric emptying often occurs in older patients, suggesting an age-related decline in gastric motor function2. O’Donovan et al3 compared gastric emptying in younger and older human subjects and showed that up to 3 hours after ingestion, older individuals exhibit a comparative delay at each thirty-minute time point. The underlying pathophysiology of this observation is complex, with the enteric nervous system (ENS) thought to play a major role. Changes in the size and appearance of myenteric ganglia, and alterations of ICCs with age have been reported in the stomach4–8 and in the small9,10 and large4,11–14 intestine of experimental animals5–11 and humans4,12–14. Abnormalities in enteric neuronal subtypes have been found to be associated with these changes. For example, several studies have identified a decrease in the number of nitrergic enteric neurons14–16 in aged mice, while other studies have shown specific loss of cholinergic subpopulations5,12,17. To date, the ENS in the aged stomach has not been carefully evaluated18. More importantly, despite the critical role of the pylorus in gastric emptying, the effects of ageing on the structure of enteric innervation in this region have not been previously described.
Gastroparesis has become an increasing problem, associated with greater rates of hospitalizations and increased costs in both adults19 and children20, but the pathophysiology of the disease remains incompletely understood. Broadly speaking, gastric emptying relies on intact function of smooth muscle, ICCs, and neurons in the stomach and pyloric region. Both intrinsic and extrinsic enteric neuropathies have been shown to delay gastric emptying21,22. In diabetic animals with gastroparesis, loss of CD206+ heme oxygenase-1-expressing M2 macrophages exhibit increased oxidative stress and loss of ICC number and function, leading to delayed emptying23–26. While the loss of gastric enteric neurons may play a role in delayed gastric emptying27–29, further investigation into potential treatment options have been limited by the lack of a tractable experimental model system.
In this study, we describe a technique to quantify gastric emptying for longitudinal analysis, report age-related changes in gastric emptying and in ENS structure in the gastric antrum, and develop a novel gastroparetic mouse model using diphtheria toxin receptor-mediated ablation of the enteric nervous system in the stomach. These studies reveal the important role of enteric antral innervation in gastric motor function and identify this as a potential target for enteric neuronal cell therapy.
Methods and Materials
Animals
All the animal protocols were conducted in accordance with the procedures reviewed and approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital, IACUC number 2009N000239. Male and female heterozygote nNOS (B6.129S4-Nos1tm1Plh/J, Stock # 002986) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and bred to obtain homozygote nNOS knockout mice (nNOS−/−). Wnt1Cre/+ mice (Tg(Wnt1Cre)11Rth Tg(Wnt1-GAL4)11Rth/J, Stock #003829 and B6.Cg-Tg(Wnt1-Cre)11Rth/MileJ, Stock #009107), were crossed with Cre-inducible DTR reporter (R26R-iDTR) mice (C57BL/6-Gt(ROSA)26Sortm1(HBEGF)Awai/J, Stock #007900) to generate Wnt1-Cre+;R26-iDTR mice (annotated as Wnt1-iDTR) and Wnt1-Cre−;R26-iDTR wild-type littermates (annotated as control).
DNA for genotyping was performed using Red Extract-N-Amp Tissue PCR kit (Sigma Aldrich) with the following primers: Cre (200 bp product) Forward 5`-ATTGCTGTCACTTGGTCGTGGC-3` and Reverse 5`-GGAAAATGCTTCTGTCCGTTTGC-3`; DTR (500 bp product) Forward 5`-GCCACCATGAA-3` and Reverse 5`-TCAGTGGGAAT-3`.
DT Injection
Diphtheria toxin (DT) (Sigma Aldrich, St. Louise, MO, USA, #D0564) was diluted to 1 μg mL−1 with saline. To achieve focal ENS ablation, 3-month-old Wnt1-iDTR and control mice were anesthetized using isoflurane inhalation. The stomach was exposed by laparotomy and a single dose of DT was injected into the wall of the anterior antrum of the stomach (7 μl of 1 μg mL−1 DT diluted in India ink).
Tissue preparation and Immunohistochemistry
Following euthanasia by inhalation of carbon dioxide, the stomach was dissected and opened along the greater curvature followed by removal of gastric contents. Stomach samples were pinned on Sylgard-coated plates and fixed in 4% paraformaldehyde overnight at 4 °C. Mucosa and submucosal layers were mechanically removed using forceps for wholemount preparations of the longitudinal muscle and myenteric plexus (LMMP). For cryosections, tissue samples were incubated in 15% sucrose at 4 °C overnight, and then in 15% sucrose containing 7.5% gelatin at 37 °C for 1 hour and rapidly frozen at −50 °C. Frozen sections were cut at 12 μm thickness with a Leica CM3050 S cryostat (Leica, Buffalo Grove, IL).
For immunohistochemistry, LMMP preparations and tissue cryosections were permeabilized with 0.1% Triton X-100 and blocked with 10% donkey serum and 4% bovine serum albumin (BSA) for 30 minutes. Primary antibodies were diluted in 10% donkey serum and 1% BSA and included mouse anti-neuronal class III β-tubulin (Tuj1; 1:400; Covance, Dedham, MA), human anti-Hu (Anna1, 1:16000, kindly gifted by Lennon lab), rabbit anti-S100β calcium-binding protein B (S100β; 1:100; Neomarkers, Fremont, CA), rabbit anti-smooth muscle actin (SMA; 1:100; Abcam, Cambridge, MA), rabbit anti-c-kit (CD117, 1:100, Dako Cytomation), rabbit anti-nNOS (1:150, Thermofisher Waltham, MA), goat anti-ChAT (Choline Acetyltransferase) (1:150, Millipore Ontario, Canada), Secondary antibodies included anti-rabbit IgG (1:500; Alexa Fluor 488 and 546; Fisher Scientific Life Technologies), anti-human IgG (1:200, Alexa Fluor 488 and 647; Fisher Scientific Life Technologies) and anti-goat IgG (1:500, Alexa Fluor 488; Fisher Scientific Life Technologies). Cell nuclei were stained with DAPI (Vector Labs, Burlingame, CA) and mounted with aqua-poly/mount (Fisher Scientific Polysciences Inc). Images were taken using a Nikon Eclipse 80i microscope or a Nikon A1R laser scanning confocal microscope (Nikon Instruments, Melville, NY).
Characterization of Antrum, Pylorus, and Duodenum:
Immunohistochemical characterization of the ENS in antrum, pylorus, and duodenum was performed. All wildtype mice had a C57BL/6 background and were separated into two groups (young and adult) by age, (5 weeks old and 5 months old), 3 mice per group. For each mouse, the stomach was removed, gastric contents evacuated, and tissue fixed and stained as above. The stomach was sectioned longitudinally and stained with Hu, ChAT, and nNOS. Three high power per field (HPF) (20×) images were taken for each area (antrum, pylorus, and duodenum), Quantitative analysis was achieved by a blinded reader using de-identified images to determine the number of neuronal cell bodies, number of ganglia per HPF, and neuronal cells per ganglia. The percentage of cells immunoreactive for ChAT or nNOS as a proportion of all Hu+ enteric neurons was examined and the ChAT/nNOS ratio calculated.
In Vivo Quantitative Measurement of Gastric Emptying
Gastric emptying was quantitatively analyzed in vivo in the following mice: adult (6–8 months old) nNOS+/+ and nNOS−/− mice; C57BL/6 mice of three different age groups: young (5–7 weeks), adult (5–9 months), and older (12–17 months); and 4–6 month-old Wnt1-iDTR mice. Mice were not fasted prior to gavage. Mice were briefly sedated with inhaled isoflurane then intragastrically gavaged 0.3 mL of barium sulfate (E-Z Paque, E-Z-EM Canada, Montreal, QC, Canada) and ten steel beads, (diameter = 0.81–0.9 mm; Bal-tec, Los Angeles, CA, USA). Mice were lightly anesthetized 90 or 120 minutes after gavage and placed prone on the radiation cassette. Radiographs of anteroposterior views were taken with a portable X-ray unit (50kV, 1.2 mAs, ScanX14 Portable Digital Imaging Systems, ALLPRO Imaging, Melville, NY, USA). Image files were visualized using Air Techniques (ALLPRO Imaging, NY).
Solid gastric emptying was calculated as the percentage of total beads outside the stomach. For liquid gastric emptying, beads were eliminated from the radiograph image using the remove outlier function in Image J. Barium within the stomach and within the rest of the GI tract was outlined. The integrated density (area under the curve) was measured for both areas. Liquid gastric emptying was calculated as the percentage of integrated density of barium outside of the stomach compared to the total integrated density within the GI tract.
Statistics
Values are represented as mean ± SEM (standard error of mean). Statistical analysis was performed using Prism 7 (GraphPad software, Inc., La Jolla, CA, USA). Statistical significance was assessed using Student’s t-test. p-values <0.05 were regarded as significant.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
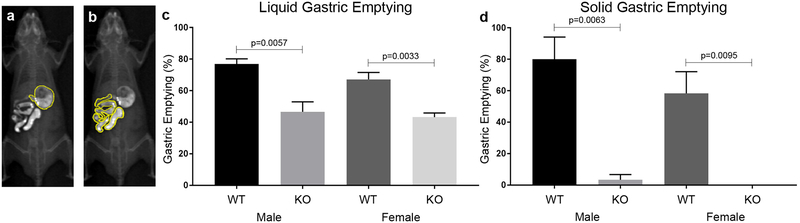
We adapted a previously described method30 to quantitatively measure gastric emptying in vivo in mice. To validate this technique, gastric emptying was measured in adult nNOS−/− mice, an established model of gastroparesis22. Mice were gavaged with 10 steel beads and 0.3 ml of barium sulfate, with radiographs taken 2 hours later (Fig. 1a, b). Given their size and composition, the steel beads represent indigestible material, rather than food, and are used to quantify solid gastric emptying, as previously described22. Solid and liquid emptying of beads and barium, respectively, was quantitatively compared between male and female wild-type and nNOS−/− animals. Liquid gastric emptying (Fig. 1c) was significantly delayed in nNOS−/− mice, both in males (46.6±6.4% vs. 76.9±3.3% in WT) and females (43.2±2.6% vs. 67.2±4.3% in WT). Solid gastric emptying (Fig. 1d) was also markedly delayed in nNOS−/− mice, in males (3.3±3.3% vs. 80.0±14.1% in WT) and females (0±0% vs. 58.3±13.8% in WT). No gender-specific differences were observed in either genetic background. These findings confirm that this radiologic assay can quantify liquid and solid gastric emptying in mice. Moreover, it confirms the presence of delayed gastric emptying in nNOS−/− mice and shows that gender differences are not present.
Figure 1: Validation of a radiographic gastric emptying assay using nNOS−/− mice.
A radiologic-based assay using gavaged radio-opaque beads and contrast was used to measure gastric emptying in WT mouse (a, b). nNOS−/− mice exhibit a significant delay in liquid (c, male WT (n=4) vs male KO (n=3), p<0.01 and female WT (n=6) vs female KO (n=4), p<0.01). Solid gastric emptying in nNOS−/− mice was also significantly delayed (d, male WT (n=4) vs male KO (n=3), p<0.01 and female WT (n=6) vs female KO (n=4), p<0.01). No difference was observed between genders within the same genotypes in liquid or solid gastric emptying. KO, knockout; WT, wild-type
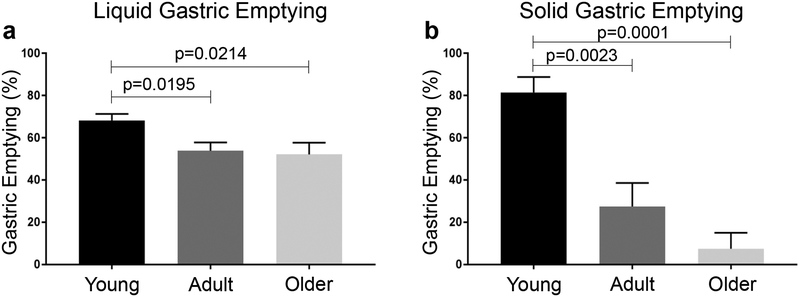
Human ageing is associated with delayed gastric emptying2 and represents a cause of significant morbidity. We examined whether mice exhibit age-related gastroparesis using three distinct age groups: young (5–7 weeks), adult (5–9 months), and older (12–17 months). In young mice, liquid gastric emptying was 68.2±3.1%, while adult and older mice were significantly delayed (Fig. 2a). This decline was also observed in solid gastric emptying, where young mice exhibited 81.4±7.4% emptying at 2 hours. This is markedly faster than in adult and older mice (Fig. 2b). These findings demonstrate that, as is seen in humans, ageing in mice is similarly associated with a significant delay in gastric emptying.
Figure 2: Age is associated with a decrease in liquid and solid gastric emptying.
Using the radiographic gastric emptying assay, we measured emptying in WT mice at three different ages: young (5–7 weeks, n=7), adult (5–9 months, n=4), and older (12–17 months, n=4) mice. A significant delay in liquid (a, Adult (n=4) vs Young (n=7), p<0.05 and Older (n=4) vs Young (n=7), p<0.05) and solid (b, Adult (n=4) vs Young (n=7), p<0.01 and Older (n=4) vs Young (n=7), p<0.001) emptying was observed in both older age groups as compared to young mice.
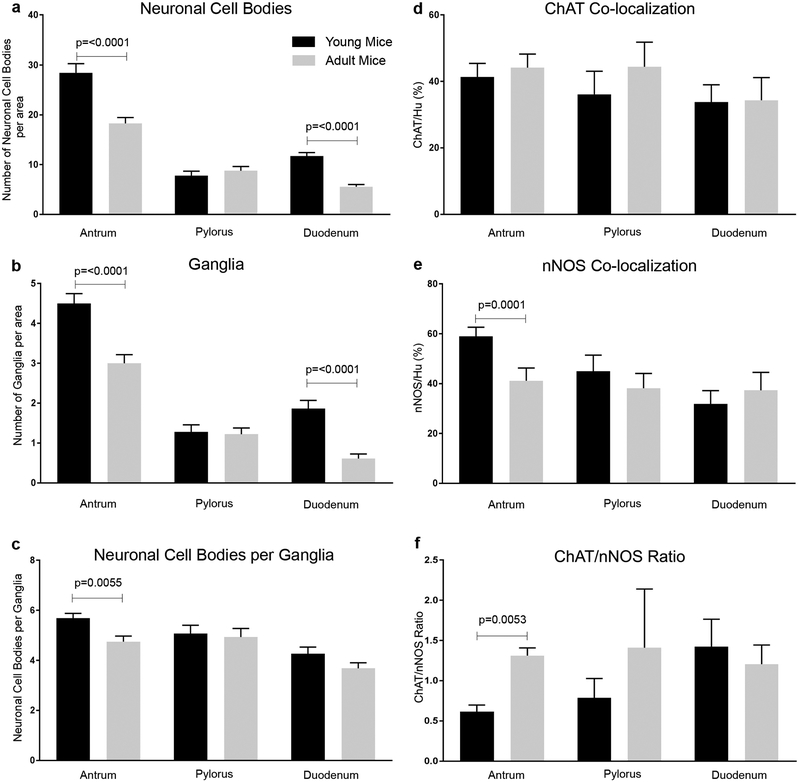
Changes in ENS structure and in neurotransmitter subtypes have been postulated to contribute to delayed emptying in the ageing human stomach28. However, comprehensive analysis of the ENS in the antro-pyloro-duodenal region has not previously been performed. We analyzed the myenteric plexus in young (5 weeks old) and adult (5 months old) WT mice in this region, including the distal gastric antrum, pylorus, and proximal duodenum. We did not include older (12–17 months) mice for analysis here as no statistical differences in gastric emptying were seen between adult and older mice (Fig. 2). The number of neuronal cell bodies and enteric ganglia were determined, as well as the proportion of nNOS- and ChAT-expressing neurons. As shown in Fig. 3a, adult mice exhibit a 35.6% decrease in enteric neuronal density in the antrum and a 52.1% decrease in the duodenum in comparison to young mice. No difference was observed in the pylorus. Similarly, a 33.3% and 68.4% reduction was observed in the density of enteric ganglia in the antrum and duodenum of adult mice, respectively (Fig. 3b). Again, no difference was seen in the pylorus. To further understand ENS structure in this region, neuronal packing density was calculated. This refers to the number of neuronal cell bodies per ganglion. Adult antrum exhibited a 15.8% decrease compared to young mice, with no difference observed in the pylorus or duodenum (Fig. 3c).
Figure 3: Analysis of enteric neuronal density and neurotransmitter expression in the stomach and duodenum.
ENS was examined in the distal antrum, pylorus, and proximal duodenum of young (5-week-old) and adult (5-month-old) mice. Neuronal density was decreased in aged mice in the antrum (n=3 per group, p<0.0001) and duodenum (n=3 per group, p<0.0001). These regions also exhibited fewer enteric ganglia (b, n=3 per group, p<0.0001). Number of neuronal cell bodies per ganglion was reduced in the antrum (c, n=3 per group, p<0.01). The proportion of ChAT+ (d) and nNOS+ (e) neurons was not significantly altered with age, except that the antrum in older mice had a significantly smaller proportion of nNOS+ neurons (e, n=3 per group, n<0.001), resulting in a significantly increased ChAT to nNOS ratio (f, n=3 per group, p<0.01.
The proportions of cholinergic (ChAT+) and nitrergic (nNOS+) neurons were also calculated in the same region. As shown in Fig. 3d, young and adult mice had the same proportion of cholinergic neurons in all three anatomic regions. In contrast, nitrergic neurons were decreased by 30% in the adult antrum compared to young mice (Fig. 3e). Calculation of the ChAT:nNOS ratio showed a significant increase in the adult antrum, with no difference between the two groups in the pylorus or duodenum (Fig. 3f).
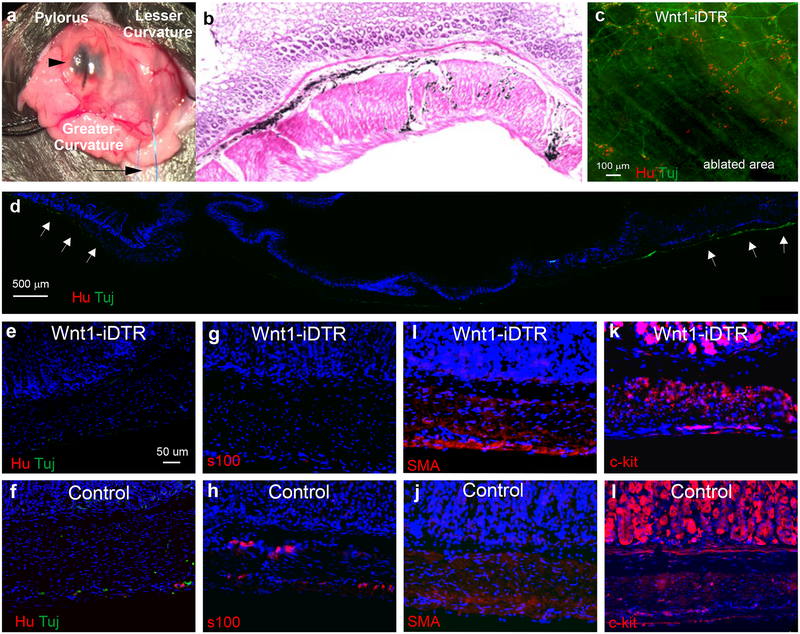
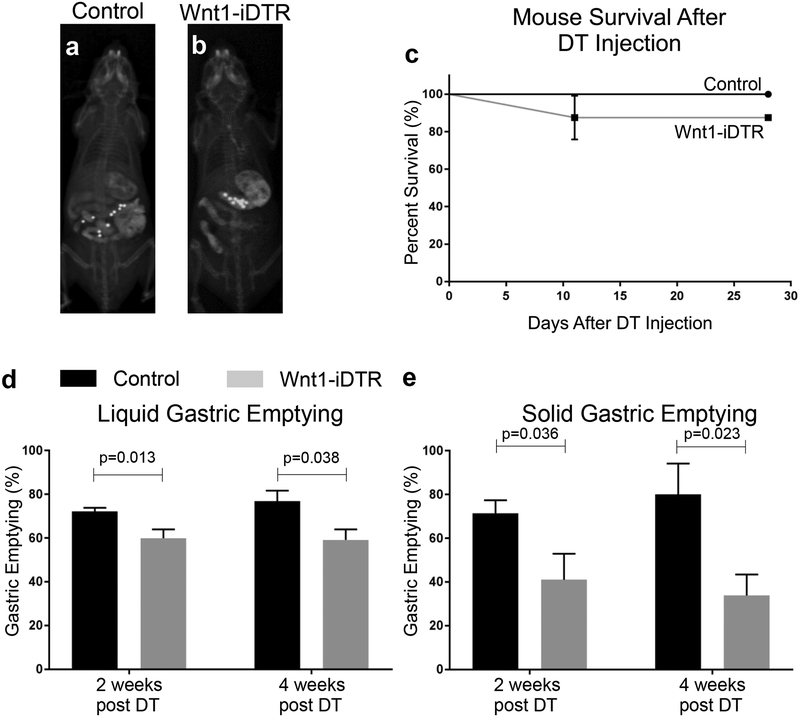
To determine whether the observed decreases in antral neuronal density contribute to the observed delay in gastric emptying, we established a novel mouse model of enteric neuronal loss in the antrum. This was achieved by inducing focal aganglionosis in the antrum using Wnt1-iDTR transgenic mice. In this model, Cre recombination renders Wnt1-expressing neural crest cells susceptible to diphtheria toxin (DT)-mediated cell death. Focal ENS ablation was achieved by injection of DT into the anterior wall of the gastric antrum via laparotomy (Fig. 4a). India ink was added to the DT to allow visualization both grossly (Fig. 4a) and on H&E staining (Fig. 4b). A single injection of 7 μl of 1 ng μL−1 DT to Wnt1-iDTR mice resulted in focal loss of the ENS in the antrum, as confirmed by immunostaining for the pan-neuronal markers, Tuj1 and Hu, on wholemount preparation of the stomach (Fig. 4c). Longitudinal sections of DT-injected stomach also showed focal ablation of the ENS (Fig. 4d), with normal endogenous Tuj1+ and Hu+ neurons present in the surrounding area (Fig. 4d, arrows). Aganglionosis persisted for at least 2 months after DT injection (Fig. 4c, d). Further immunohistochemical characterization at higher magnification demonstrates successful ablation of enteric neurons (Fig. 4e) and glia (Fig. 4g). Importantly, focal injection of DT did not result in loss of these cell types in control (Cre-negative) mice (Fig. 4f, h). Immunostaining of longitudinal sections of DT-treated antrum of Wnt1-iDTR and control mice demonstrate no loss of either smooth muscle cells (Fig. 4i, j) or interstitial cells of Cajal (Fig. 4k, l), suggesting minimal, if any, off-target effects to non-neural-crest derived cells.
Figure 4. Antral aganglionosis can be generated using focal of diphtheria toxin (DT).
DT was injected into the anterior antrum of Wnt1-iDTR mice via laparotomy. Caudal retraction of the grater curvature of the stomach with a suture (a, arrow) facilitates exposure. Site of DT injection is marked by India ink (a, arrowhead). H&P-staining 8 weeks after injection shows India ink within the submucosal (b, arrow) and myenteric (b, arrowhead) layers (b). Immunofluorescence one month after injection shows focal loss of myenteric neurons in the antrum by staining for Hu and Tuj1 on wholemount (c) and on longitudinal section (d, arrows mark endogenous neurons at margins of DT ablation). DT injection led to focal ablation of neurons (e) and glia (g), but no gross change in smooth muscle (i) or interstitial cells of Cajal (k) as compared to DT-injected Cre-negative controls (f, h, j, l)
The functional consequence of DT-mediated ENS ablation in the gastric antrum was tested using the radiographic assay described above. One Wnt1-iDTR mouse did not have focal ablation on immunohistochemistry at 8 weeks post-injection and thus was excluded from the analysis. For the 4-week time point, 3 mice in the control group aspirated during gavage, leaving 4 mice for analysis. A total of 7 control (Fig. 5a) and 8 Wnt1-iDTR (Fig. 5b) mice were injected with 7 μl of 1 ng μl−1 DT into the anterior antrum. This resulted in mild enlargement of the stomach in Wnt-iDTR mice (Fig. 5b), without any other gross anatomical abnormality. At two and four weeks, animals were gavaged with steel beads and barium, and radiographs obtained 90 minutes later, when the contrast is still in the small intestine and has not yet reached the colon. Survival was excellent, with only one Wnt1-iDTR mouse dying due to aspiration. This mouse was excluded from analysis (Fig, 5c). Wnt1-iDTR mice exhibited a significant delay in liquid gastric emptying: 60±4.0% versus 72±1.7% for controls at 2 weeks; 59.2±4.8% versus 76.8±4.9% at 4 weeks (Fig. 5d). A marked delay was also observed for solid gastric emptying: 41.0±11.9% versus 71.4±5.9% for controls at 2 weeks; 33.8±9.6% versus 80.0±14.1% at 4 weeks (Fig. 5e). Experimentally induced neuronal ablation in the anterior antrum thus induces a significant delay in both liquid and solid gastric emptying.
Figure 5: DT-induced antral aganglionosis causes delayed gastric emptying.
Gastric emptying was quantitatively assessed in control (a) and Wnt1-iDTR (b) mice 4 weeks after DT injection. No significant difference in survival was observed (c). Both liquid (d) and solid (e) emptying in Wnt1-iDTR mice were delayed at 2 weeks following DT injection (d, e, Control (n=6) vs Wnt1-iDTR (n=7), p<0.05). These changes persisted at 4 weeks following DT injection (d, e, Control (n=6) vs Wnt1-iDTR (n=4), p<0.05).
Discussion
Gastroparesis, or the delay of emptying gastric contents into the small bowel, has become an increasing issue for the United States healthcare system with an increase in hospitalization rates and healthcare costs in both adults and children19,20. Studying gastric emptying in rodent models requires a robust, quantitative, and reliable methodology. While quantitative testing for gastric emptying has been standardized in humans31, no such standard approach exists for rodents, limiting the ability to study this important aspect of gastrointestinal (GI) function. Classically, gavage of a fluorescent dye followed by measuring absorbance is used to measure transit, but this requires sacrificing the animals for analysis and thus prohibits longitudinal studies22. Another technique, utilizing nuclear magnetic resonance, is highly effective but requires equipment not readily available at many institutions32. Other methods use a subjective scoring system to quantify GI transit30 and gastric emptying33. We therefore modified an existing methodology30 into a validated and quantitative approach that would not require sacrificing the animals and thus allow repetitive, longitudinal analysis of individual mice over time.
An important aspect of our method is that it is quantitative and can identify small differences between groups and within individual mice. Moreover, materials that are required, including barium sulfate, steel beads, and radiography, are easily accessible. Using this method, we found 71% liquid emptying and 65.5% solid emptying at 2 hours in wild-type mice. Mashimo et al22 found 70% liquid emptying at 30 minutes and 82% solid emptying of indigestible material at 2 hours in WT, while Baudry et al34 showed roughly 50% solid food emptying at 90 minutes. These results are similar to ours with regard to the emptying of solid indigestible material, but our results are slower when comparing liquid emptying. It is possible that our brief use of isoflurane during gavage may contribute to this mild delay as this has been reported previously in rodents35. Nevertheless, using the same protocol for all animals allowed us to control for these potential variables.
We identified a quantitative delay in gastric emptying in nNOS−/− mice, as shown previously21,22. Interestingly, the significant delay seen in both liquid and solid emptying did not differ between genders in either wild-type or mutant animals. This is distinct from studies in patients with gastroparesis, where a female predominance is consistently observed1. Knight et al36 observed an attenuated postprandial antral contractile activity in the follicular phase of premenopausal women. Animal studies have shown that gastric emptying is slower during the estrus cycle while circulatory estrogen levels are elevated37 and administration of progesterone to ovariectomized female rats for 6 days increased gastric emptying38. More recent studies have reported that estrogen may be responsible for increased gastric nNOS expression and nitrergic relaxation of gastric muscle39,40. Moreover, the differences in nNOS alpha dimerization between females and males have been proposed as an alternative reason for the greater susceptibility of females to diabetic gastroparesis41,42, which may account for a vanishing gender differences in gastric emptying in nNOS-null mice observed in the current study. While a decrease in nitric oxide contributes to abnormal gastric accommodation, pyloric dysfunction is also involved in abnormal gastric emptying. Sivarao et al measured intraluminal pressure at the level of pylorus of nNOS-null mice while stimulating the vagus nerve and demonstrated impaired pyloric relaxation mediated by extrinsic vagus fibers21. The steel beads used in our assay may be more consistent with indigestible material given their relatively larger diameter and, unlike solid food, their inability to be pulverized into smaller material. The emptying of indigestible material is regulated by the migrating motor complexes (MMCs) produced by ICCs. Decreased neuronal nitric oxide has been associated with a decreased volume of ICCs within the gastric body43, suggesting a complex network of cellular interactions and a possible additional reason for significant solid delay in nNOS−/− mice.
Delayed gastric emptying is commonly associated with ageing2, and changes in the enteric nervous system have been implicated7. We analyzed the ENS in the pylorus and adjacent areas at different ages. Interestingly, we found that the ENS in the pylorus did not change over time. The pylorus receives extensive extrinsic innervation and is regulated largely by vagal stimulation to promote pyloric relaxation and gastric emptying44. The pre-pyloric distal antrum had the largest age-associated change, with a significant decrease in the number of neurons and ganglia within the myenteric plexus as well as a decreased proportion of nNOS positive neurons, relative to cholinergic neurons, in older mice. Loss of nNOS neurons has commonly been observed in patients with gastroparesis45–48 as well as mice with diabetic gastroparesis49–51. Interestingly, significant increase in excitatory neurons have been reported in mice and human patients with diabetes52,53. Therefore, an imbalance of inhibitory and excitatory neurons in the stomach, which we seen in the current study, is known to cause gastroparesis. The underlying mechanism is not fully unveiled. However, Jenkinson and Reid have reported that gastric smooth muscle isolated from diabetic rats demonstrate increased contractility that can cause impaired gastric relaxation54. ENS neurodegeneration has been reported during the ageing process throughout the gastrointestinal tract5, including the stomach55, but whether it represents the cause of delayed gastric emptying has been unknown.
In order to test whether loss of antral neurons leads to delayed gastric emptying, we established a novel model of focal aganglionosis through ablation of neural crest cells by administration of DT. Cell ablation using Cre-inducible human DTR was first described in the ablation of T and B cells56, and subsequently used to model human diseases57–59. Systemic administration of DT in our Wnt1-iDTR mice resulted in the mortality of animals within 48 hours. To ensure ablation of the enteric nervous system and to avoid off-target injuries, we focally injected DT into the wall of the anterior gastric antrum. We validated our specific and focal ablation of the anterior antrum via immunohistochemistry and found a significant functional delay in both liquid and solid gastric emptying up to 4 weeks after DT injection. The delay in solid emptying may be directly related to neuronal loss or indirectly due to ICC dysfunction, which can be caused by degeneration of nitrergic neurons60 via a Klotho-dependent mechanism61.
Modeling gastroparesis using a DT-induced ENS ablation model has several advantages. The first is that animals survive after focal ablation of the enteric nervous system of the anterior antrum. Second, the ablation is specific to neural crest-derived enteric neurons and glia with no injury to non-neural crest-derived cells. This suggests that the functional outcome is specifically due to loss of the ENS in the anterior antrum. Furthermore, the focal ablation of the ENS lasts for at least 8 weeks without reinnervation over that time period, allowing time for possible therapeutic interventions, such as drug administration or cell therapy. The stomach’s ability ultimately to adapt and regain partial function may be due to hormonal changes based on dietary changes62 or to the ability of the ENS to migrate in from the periphery of the ablated areas as occurs in BAC mouse models63. This adaptation merits additional analyses. Most importantly, by modeling focal antral aganglionosis and demonstrating delayed emptying in this model, our findings lend support to the hypothesis that age-associated loss of enteric neurons in the antrum leads to gastroparesis. This offers a valuable model system to explore cell-based treatment options for gastric hypoganglionosis and to understand the role of the ENS in gastric emptying and its interactions with other cell types within the gut wall.
Acknowledgments
CB, KC, EA, NW and SB performed the research. MA analyzed the data. CB, AMG, and RH wrote the manuscript. AMG and RH designed the research study.
Funding
This work is supported by the National Institutes of Health (R01DK103785) and by the American Neurogastroenterology and Motility Research Grant (2016D000927).
Abbreviations-
- BAC
Benzalkonium chloride
- ChAT
choline acetyltransferase
- CM
circular muscle
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- ENS
enteric nervous system
- GI
gastrointestinal
- HPF
high power field
- HSCR
Hirschsprung disease
- ICC
interstitial cells of Cajal
- LM
longitudinal muscle
- LMMP
longitudinal muscle myenteric plexus
- MM
muscularis mucosa
- nNOS
neuronal nitric oxide synthase
- WT
wild-type
Footnotes
Disclosure
No competing interests declared.
References:
- 1.Jung HK, Choung RS, Locke GR 3rd, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136(4):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gidwaney NG, Bajpai M, Chokhavatia SS. Gastrointestinal Dysmotility in the Elderly. J Clin Gastroenterol. 2016;50(10):819–827. [DOI] [PubMed] [Google Scholar]
- 3.O’Donovan D, Hausken T, Lei Y, et al. Effect of aging on transpyloric flow, gastric emptying, and intragastric distribution in healthy humans--impact on glycemia. Dig Dis Sci. 2005;50(4):671–676. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Pinilla PJ, Gibbons SJ, Sarr MG, et al. Changes in interstitial cells of cajal with age in the human stomach and colon. Neurogastroenterol Motil. 2011;23(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci. 2003;106(2):69–83. [DOI] [PubMed] [Google Scholar]
- 6.Phillips RJ, Powley TL. As the gut ages: timetables for aging of innervation vary by organ in the Fischer 344 rat. J Comp Neurol. 2001;434(3):358–377. [DOI] [PubMed] [Google Scholar]
- 7.El-Salhy M, Sandstrom O, Holmlund F. Age-induced changes in the enteric nervous system in the mouse. Mech Ageing Dev. 1999;107(1):93–103. [DOI] [PubMed] [Google Scholar]
- 8.Kwon YH, Kim N, Nam RH, et al. Change in the Interstitial Cells of Cajal and nNOS Positive Neuronal Cells with Aging in the Stomach of F344 Rats. PLoS One. 2017;12(1):e0169113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thrasivoulou C, Soubeyre V, Ridha H, et al. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging cell. 2006;5(3):247–257. [DOI] [PubMed] [Google Scholar]
- 10.Abalo R, Jose Rivera A, Vera G, Isabel Martin M. Ileal myenteric plexus in aged guinea-pigs: loss of structure and calretinin-immunoreactive neurones. Neurogastroenterol Motil. 2005;17(1):123–132. [DOI] [PubMed] [Google Scholar]
- 11.Peck CJ, Samsuria SD, Harrington AM, King SK, Hutson JM, Southwell BR. Fall in density, but not number of myenteric neurons and circular muscle nerve fibres in guinea-pig colon with ageing. Neurogastroenterol Motil. 2009;21(10):1075–e1090. [DOI] [PubMed] [Google Scholar]
- 12.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil. 2009;21(7):746–e746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanani M, Fellig Y, Udassin R, Freund HR. Age-related changes in the morphology of the myenteric plexus of the human colon. Auton Neurosci. 2004;113(1–2):71–78. [DOI] [PubMed] [Google Scholar]
- 14.Sun T, Li D, Hu S, et al. Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging (Albany NY). 2018;10(12):3851–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abalo R, Vera G, Rivera AJ, Martin MI. Age-related changes in the gastrointestinal tract: a functional and immunohistochemical study in guinea-pig ileum. Life Sci. 2007;80(26):2436–2445. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Qoubaitary A, Owyang C, Wiley JW. Decreased expression of nitric oxide synthase in the colonic myenteric plexus of aged rats. Brain Res. 2000;883(1):15–21. [DOI] [PubMed] [Google Scholar]
- 17.Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut. 2000;47(5):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Dev Biol. 2013;382(1):344–355. [DOI] [PubMed] [Google Scholar]
- 19.Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103(2):313–322. [DOI] [PubMed] [Google Scholar]
- 20.Lu PL, Moore-Clingenpeel M, Yacob D, Di Lorenzo C, Mousa HM. The rising cost of hospital care for children with gastroparesis: 2004–2013. Neurogastroenterol Motil. 2016;28(11):1698–1704. [DOI] [PubMed] [Google Scholar]
- 21.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS−/− and W/Wv mutant mice: animal models of gastroparesis and duodenogastric reflux. GASTROENTEROLOGY. 2008;135(4):1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. GASTROENTEROLOGY. 2000;119(3):766–773. [DOI] [PubMed] [Google Scholar]
- 23.Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055–2064, 2064 e2051–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi KM, Kashyap PC, Dutta N, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–2409, 2409 e2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipriani G, Gibbons SJ, Miller KE, et al. Change in Populations of Macrophages Promotes Development of Delayed Gastric Emptying in Mice. Gastroenterology. 2018;154(8):2122–2136 e2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrugia G. Histologic changes in diabetic gastroparesis. Gastroenterol Clin North Am. 2015;44(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BJ, Kuo B. Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment. J Neurogastroenterol Motil. 2019;25(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grover M, Bernard CE, Pasricha PJ, et al. Clinical-histological associations in gastroparesis: results from the Gastroparesis Clinical Research Consortium. Neurogastroenterol Motil. 2012;24(6):531–539, e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moshiree B, Potter M, Talley NJ. Epidemiology and Pathophysiology of Gastroparesis. Gastrointest Endosc Clin N Am. 2019;29(1):1–14. [DOI] [PubMed] [Google Scholar]
- 30.Reed DE, Pigrau M, Lu J, Moayyedi P, Collins SM, Bercik P. Bead study: a novel method to measure gastrointestinal transit in mice. Neurogastroenterol Motil. 2014;26(11):1663–1668. [DOI] [PubMed] [Google Scholar]
- 31.Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am J Gastroenterol. 2008;103(3):753–763. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz R, Kaspar A, Seelig J, Kunnecke B. Gastrointestinal transit times in mice and humans measured with 27Al and 19F nuclear magnetic resonance. Magn Reson Med. 2002;48(2):255–261. [DOI] [PubMed] [Google Scholar]
- 33.Cabezos PA, Vera G, Martin-Fontelles MI, Fernandez-Pujol R, Abalo R. Cisplatin-induced gastrointestinal dysmotility is aggravated after chronic administration in the rat. Comparison with pica. Neurogastroenterol Motil. 2010;22(7):797–805, e224–795. [DOI] [PubMed] [Google Scholar]
- 34.Baudry C, Reichardt F, Marchix J, et al. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. J Physiol. 2012;590(3):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torjman MC, Joseph JI, Munsick C, Morishita M, Grunwald Z. Effects of isoflurane on gastrointestinal motility after brief exposure in rats. International journal of pharmaceutics. 2005;294(1–2):65–71. [DOI] [PubMed] [Google Scholar]
- 36.Knight LC, Parkman HP, Brown KL, et al. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92(6):968–975. [PubMed] [Google Scholar]
- 37.Gangula PR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: is nitric oxide the answer? Dig Dis Sci. 2011;56(9):2520–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol. 1995;268(1 Pt 1):G171–176. [DOI] [PubMed] [Google Scholar]
- 39.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1546–1554. [DOI] [PubMed] [Google Scholar]
- 40.Al-Shboul OA, Nazzal MS, Mustafa AG, et al. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: A sex-associated differential effect. Exp Ther Med. 2018;16(3):1685–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Showkat Ali M, Tiscareno-Grejada I, Locovei S, et al. Gender and estradiol as major factors in the expression and dimerization of nNOSalpha in rats with experimental diabetic gastroparesis. Dig Dis Sci. 2012;57(11):2814–2825. [DOI] [PubMed] [Google Scholar]
- 43.Choi KM, Gibbons SJ, Roeder JL, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19(7):585–595. [DOI] [PubMed] [Google Scholar]
- 44.Lu KH, Cao J, Oleson S, et al. Vagus nerve stimulation promotes gastric emptying by increasing pyloric opening measured with magnetic resonance imaging. Neurogastroenterol Motil. 2018;30(10):e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41(11):1076–1087. [DOI] [PubMed] [Google Scholar]
- 46.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. GASTROENTEROLOGY. 2001;121(2):427–434. [DOI] [PubMed] [Google Scholar]
- 47.Zarate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52(7):966–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grover M, Farrugia G, Lurken MS, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–1585 e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. GASTROENTEROLOGY. 1997;113(5):1535–1544. [DOI] [PubMed] [Google Scholar]
- 50.Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42(10):2106–2110. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19(12):951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spangeus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes). Histol Histopathol. 2001;16(1):159–165. [DOI] [PubMed] [Google Scholar]
- 53.LePard KJ. Choline acetyltransferase and inducible nitric oxide synthase are increased in myenteric plexus of diabetic guinea pig. Auton Neurosci. 2005;118(1–2):12–24. [DOI] [PubMed] [Google Scholar]
- 54.Jenkinson KM, Reid JJ. Effect of diabetes on relaxations to non-adrenergic, non-cholinergic nerve stimulation in longitudinal muscle of the rat gastric fundus. Br J Pharmacol. 1995;116(1):1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips RJ, Powley TL. Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci. 2007;136(1–2):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buch T, Heppner FL, Tertilt C, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature methods. 2005;2(6):419–426. [DOI] [PubMed] [Google Scholar]
- 57.Guo JK, Marlier A, Shi H, et al. Increased tubular proliferation as an adaptive response to glomerular albuminuria. J Am Soc Nephrol. 2012;23(3):429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demircik F, Buch T, Waisman A. Efficient B cell depletion via diphtheria toxin in CD19-Cre/iDTR mice. PLoS One. 2013;8(3):e60643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bi R, Fan Y, Lauter K, et al. Diphtheria Toxin- and GFP-Based Mouse Models of Acquired Hypoparathyroidism and Treatment With a Long-Acting Parathyroid Hormone Analog. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2016;31(5):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izbeki F, Asuzu DT, Lorincz A, et al. Loss of Kitlow progenitors, reduced stem cell factor and high oxidative stress underlie gastric dysfunction in progeric mice. J Physiol. 2010;588(Pt 16):3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asuzu DT, Hayashi Y, Izbeki F, et al. Generalized neuromuscular hypoplasia, reduced smooth muscle myosin and altered gut motility in the klotho model of premature aging. Neurogastroenterol Motil. 2011;23(7):e309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi G, Leray V, Scarpignato C, et al. Specific adaptation of gastric emptying to diets with differing protein content in the rat: is endogenous cholecystokinin implicated? Gut. 1997;41(5):612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanani M, Ledder O, Yutkin V, et al. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J Comp Neurol. 2003;462(3):315–327. [DOI] [PubMed] [Google Scholar]