Abstract
Background
Coronavirus 2019 (COVID-19) is a worldwide pandemic, with many patients requiring prolonged mechanical ventilation. Tracheostomy is not recommended by current guidelines as it is considered a superspreading event owing to aerosolization that unduly risks health care workers.
Methods
Patients with severe COVID-19 who were on mechanical ventilation for 5 days or longer were evaluated for percutaneous dilational tracheostomy. We developed a novel percutaneous tracheostomy technique that placed the bronchoscope alongside the endotracheal tube, not inside it. That improved visualization during the procedure and continued standard mechanical ventilation after positioning the inflated endotracheal tube cuff in the distal trachea. This technique offers a significant mitigation for the risk of virus aerosolization during the procedure.
Results
From March 10 to April 15, 2020, 270 patients with COVID-19 required invasive mechanical ventilation at New York University Langone Health Manhattan’s campus; of those, 98 patients underwent percutaneous dilational tracheostomy. The mean time from intubation to the procedure was 10.6 ± 5 days. Currently, 32 patients (33%) do not require mechanical ventilatory support, 19 (19%) have their tracheostomy tube downsized, and 8 (8%) were decannulated. Forty patients (41%) remain on full ventilator support, and 19 (19%) are weaning from mechanical ventilation. Seven patients (7%) died as a result of respiratory and multiorgan failure. Tracheostomy-related bleeding was the most common complication (5 patients). None of health care providers has had symptoms or tested positive for COVID-19.
Conclusions
Our percutaneous tracheostomy technique appears to be safe and effective for COVID-19 patients and safe for health care workers.
Dr Kon discloses a financial relationship with Medtronic, Inc, and Breethe, Inc; Dr Mitzman with Genentech, Inc.
The Appendix can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2020.04.010] on http://www.annalsthoracicsurgery.org.
Coronavirus disease 2019 (COVID-19) is a worldwide pandemic, with more than 2.4 million cases diagnosed to date.1 Although most COVID-19 patients will not require supportive care, 10% to 15% of patients have acute respiratory distress that requires invasive ventilatory support.2 , 3 Mechanical ventilation for patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with prolonged airway intubation and high worldwide mortality of at least 50% to 67%.4, 5, 6 Based on the previous experience with severe acute respiratory syndrome (SARS) in 2003, aerosol-generating procedures such as tracheal intubation or performing tracheostomy were considered high-risk procedures for transmission of SARS-CoV-2 to health care workers.7 The American Academy of Otolaryngology and the Ears, Nose, Throat Surgery in the United Kingdom have stated providers should “avoid tracheotomy in COVID-19 positive or suspected patients” owing to the risks to health care providers.
These guidelines, based on limited evidence, recommend that tracheostomies should not be performed earlier than 2 to 3 weeks after intubation, preferably after negative COVID-19 testing,8 , 9 and recommend open tracheostomy placement in these circumstances as opposed to percutaneous dilational tracheostomy (PDT). An earlier tracheostomy for COVID-19 patients has potential advantages; therefore, we developed a novel modification of the standard PDT procedure designed to improve visualization and ventilation while minimizing the risk of aerosolization of SARS-CoV-2 to health care providers. In this paper, we describe the technical aspects of the procedure, detail early patient outcomes, and evaluate the early safety of our technique to our health care providers.
Patients and Methods
Patient Population
We conducted an institutional analysis of all patients admitted to the intensive care unit (ICU) at New York University Langone Health Care System’s Manhattan campus from March 10, 2020, to April 15, 2020, who had confirmed COVID-19 as documented by nasal pharyngeal swab for reverse transcriptase polymerase chain reaction assay and who had severe respiratory failure requiring mechanical ventilation. The New York University Langone Institutional Review Board approved this study (IRB #120-00475).
Patient Selection
The entry criteria for PDT were patients confirmed positive for COVID-19, on mechanical ventilation for 5 or more days with anticipated prolonged need for mechanical ventilation or placement on extracorporeal membranous oxygenation (ECMO) for more than 1 day; and isolated respiratory failure except acute renal failure on dialysis or continuous renal replacement therapy. Mechanical ventilation settings recommended for the procedure were a positive end-expiratory pressure of 12 mm Hg or less, fraction of inspired oxygen 60% or less, respiratory rate 25 or fewer breaths per minute, and partial pressure of carbon dioxide 60 mm Hg or less. Exclusion criteria included patients with uncorrected coagulopathy, body mass index more than 45 kg/m2, or multiorgan failure.
Percutaneous Tracheostomy Technique
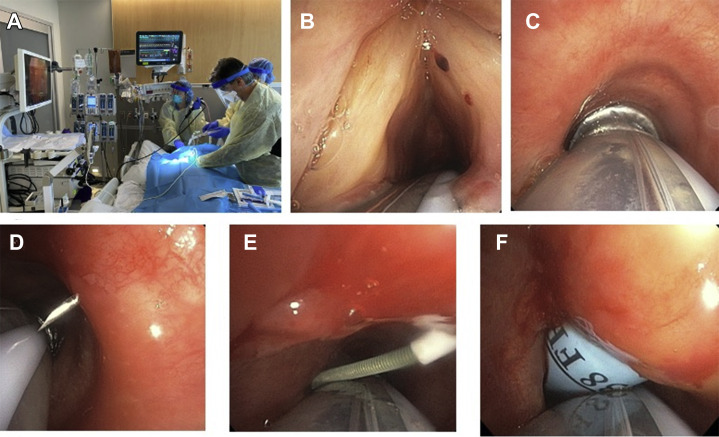
The PDT was performed at the bedside in the patient’s ICU room. All personnel wore full personal protection equipment per institutional policies (hair cover, N95 mask, surgical mask, face shield, gown, and two layers of gloves) on entering the room. Team members had three roles: (1) performing the bronchoscopy; (2) placing the tracheostomy; and (3) operating the ventilator, applying medication for sedation, and endotracheal tube management. The patient was placed supine with a shoulder roll in standard position for a percutaneous tracheostomy. Sedation and paralytics were given before the procedure (Figure 1 a; see Appendix for protocol).
Figure 1.
Percutaneous dilational tracheostomy in COVID-19 patients using novel visualization technique. (a) Three team members positioning in the patient’s room. (b) View of vocal cords using flexible bronchoscopy. (c) Visualization of trachea and cartilaginous rings anterior to endotracheal tube through bronchoscope. (d) Bronchoscopic visualization of needle insertion. (e) Wire insertion under bronchoscopic visualization. (f) Technique of dilation with Ciaglia Blue Rhino (Cook Medical, Bloomington, IN) dilator with endotracheal tube in place.
Anterior neck Doppler ultrasonography was performed with a linear probe to identify anatomic landmarks and vascular structures, and to mark the site of insertion between the first and second tracheal rings. Maximal oxygenation was performed before the procedure. The endotracheal tube (ETT) was freed from the holder, and the bronchoscope was inserted adjacent to the ETT and advanced to the level of the vocal cords (Figure 1b). The bronchoscope was advanced past the vocal cords, anterior to the ETT (Figure 1c). With the ventilator on standby mode, we deflated the ETT cuff and advanced the ETT further into the distal trachea. The ETT cuff was reinflated and the ventilator restarted. We then performed the PDT. The needle and catheter were inserted into the airway under direct visualization (Figure 1d). The guidewire was threaded into the airway and directed caudally (Figure 1e). A superficial, small (0.5 cm) skin incision was made after the wire was in place. The track was dilated with a short dilator, then with the Ciaglia Blue Rhino (Cook Medical, Bloomington, IN) dilator (Figure 1f). Before insertion of the tracheostomy tube, the ventilator was put on standby mode again, and the ETT cuff was deflated. The ETT was withdrawn under direct visualization until it was proximal to the tracheostomy site. A percutaneous tracheostomy tube (Shiley flexible tracheostomy tube 8.5; Covidien, Dublin, Ireland) was inserted into the trachea, the cuff was inflated, and the bronchoscope was advanced through the tracheostomy tube to confirm position and to remove any blood or secretions from the airway. The ventilator was then connected and restarted once the circuit was closed.
To improve access to the subglottic space, we used a therapeutic bronchoscope (Olympus America, Chelmsford, MA). This scope ensures enough torque and rigidity to maneuver around the ETT and push through the anterior space of the vocal cords. Secretions were suctioned from the mouth and retropharynx before bronchoscope insertion to improve visualization. When the ETT is seen in the posterior aspect of the vocal cords, a “jaw thrust” maneuver with a posterior push on the ETT helped open the space to facilitate passage of the bronchoscope.
Endpoints
The endpoints for this study were the safety and feasibility of our bedside PDT in mechanically ventilated patients with COVID-19, early patient outcomes, and early health care providers outcomes for COVID-19 symptoms or SARS-CoV-2 positive testing.
Results
Patients Undergoing Tracheostomy
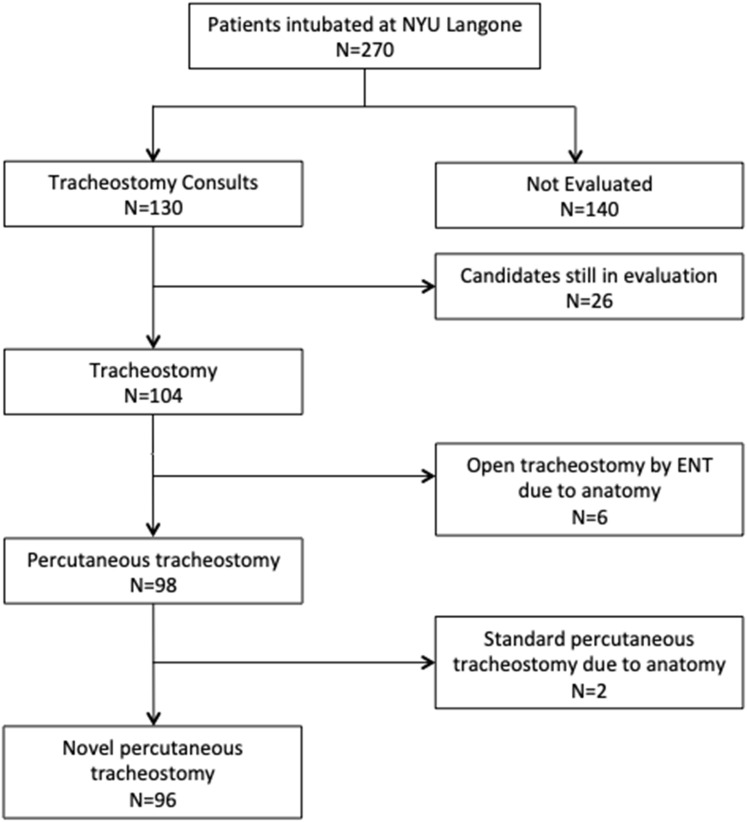
From March 10 to April 15, 2020, 270 patients were intubated and started on mechanical ventilation at the New York University Langone Health Manhattan campus. We had tracheostomy consults on 130 patients; 98 patients underwent a bedside PDT with our technique (Figure 2 ). In the overall study population, the mean age was 57 years; 80 patients (82%) were men, the mean body mass index was 31 kg/m2, and at the time of intubation they had severe respiratory failure with a mean partial pressure of arterial oxygen/fraction of inspired oxygen ratio of 132 (Table 1 ). The described technique was successful in 96 patients (98%). In 2 patients receiving ECMO support with severe upper airways and subglottic edema, we could not provide adequate visualization during the procedure, and they required a standard PDT approach; however, as they were on ECMO support, we performed the PDT under apneic conditions. In the study population, when we had accessed the subglottic space, the ETT cuff was in the subglottic space in 75% of patients, with frequent findings of inflammation and mucosal ischemic changes. Six other patients were not candidates for PDT and underwent an open tracheostomy, at the bedside, by two otolaryngology attending physicians (2 patients with tracheal stenosis, 1 redo tracheostomy with calcified stoma, 2 patients with body mass index more than 50 kg/m2, and 1 patient with innominate artery overlying the trachea superior to the sternal notch).
Figure 2.
Number of patients diagnosed with coronavirus disease 2019 (COVID-19) and intubated at New York University (NYU) Langone Health between March 15 and April 15, 2020, those evaluated for tracheostomy and deemed appropriate for bedside percutaneous tracheostomy, and number successfully placed by the novel technique. (ENT = ear, nose, throat.)
Table 1.
Clinical Characteristics of COVID-19 Patients Undergoing Percutaneous Dilational Tracheostomy
| Variable | PDT Patients (n = 98) |
|---|---|
| Sex | |
| Male | 80 (82) |
| Female | 18 (18) |
| Age, y | 57 ± 15 |
| Body mass index, kg/m2 | 31 ± 6 |
| Comorbidities | |
| Cardiaca | 43 (44) |
| Diabetes mellitus | 30 (31) |
| Asthma/COPD | 3 (3) |
| Chronic kidney disease | 9 (9) |
| Stroke | 6 (6) |
| HIV/AIDS | 3 (3) |
| MV and oxygenation values | |
| Plateau pressure | 26 ± 6 |
| Positive end expiratory pressure | 12 ± 4 |
| Pao2/Fio2 ratio | 132 ± 58 |
| Deep sedation and paralysis | 56 (57) |
| Days on ventilator before tracheostomy | 11 ± 5 |
| Follow-up after starting MV, d | 18 ± 5 |
| Follow-up after PDT, d | 11 ± 6 |
Values are n (%) or mean ± SD.
COPD, chronic obstructive pulmonary disease; Fio2, fraction of inspired oxygen; HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; MV, mechanical ventilation; PDT, percutaneous dilational tracheostomy.
Includes coronary artery disease and hypertension.
Early Patient Outcomes
The mean time from the start of invasive mechanical ventilation to the PDT was 10.6 ± 5 days. The mean follow-up for all the patients after starting mechanical ventilation was 18 ± 5 days, and after PDT, it was 11 ± 6 days. Of the 98 patients who underwent a PDT, 40 (41%) remain on full ventilator support, 19 (19%) are undergoing weaning from mechanical ventilation with continuous positive airway pressure or pressure support ventilation. Thirty-two patients (33%) are not requiring mechanical ventilatory support, with 4 (4%) of them discharged from the hospital, 17 (17%) on the noncritical care floors, and 11 (11%) remaining in the ICU with oxygen by tracheostomy collar. The tracheostomy tubes of 19 patients (19%) were downsized, and 8 (8%) have had the tracheostomy tubes removed. Seven patients (7%) died as result of respiratory and multiorgan failure.
Complications
Bleeding after tracheostomy was present in 5 patients, all of whom were receiving anticoagulation therapy and 3 were on ECMO support. Of those 5 patients, 1 required surgical exploration, which was a standard PDT. Two accidental tracheostomy tube removals occurred during the study, 1 by a patient and another by a health care provider.
Health Care Provider Exposure
The tracheostomy team included six physicians and two nurses who participated across the 98 bedside percutaneous tracheostomies. Four of these team members agreed to testing, and all have been negative by nasal pharyngeal swab for reverse transcriptase polymerase chain reaction assay testing. None has missed any days of work, and to this date, all continue to take care of patients and all are performing PDT in additional patients. To date, none of these eight team members or additional nurses, respiratory, and physical therapist providing care to these COVID-19 patients after tracheostomy has had any symptoms of fever, general malaise, cough, or shortness of breath, nor has any tested positive for COVID-19.
Comment
From March 10 to April 15, 2020, we had 270 patients with SARS-CoV-2 infection admitted to the ICU with severe respiratory failure requiring invasive mechanical ventilation. Their respiratory failure, as previously reported, was prolonged, with an average duration to this date of 18 days. We believed that an earlier tracheostomy provided the optimal care to these patients, who in addition to the severe respiratory failure, were also receiving high levels of sedation or paralysis, had major issues with increased airway secretions, and limited access to respiratory and physical therapy as a result of the overwhelming number of patients on mechanical ventilation and the risk presented by SARS-CoV-2 to health care workers.
This study reports the feasibility of performing this novel technique for PDT in patients stricken with COVID-19. For the 98 patients who fulfilled the inclusion criteria appropriate for PDT, 96 PDTs (98%) were successfully performed. The other 2 patients had severe subglottic edema that required a standard approach to PDT; these 2 patients were on ECMO support and did not require ventilation during the procedure.
The early outcomes appear to support the many benefits of tracheostomy placement. As shown in other series, tracheostomy may reduce the duration of mechanical ventilation requirement and lead to shorter ICU stays.10 , 11 The placement of a tracheostomy enables better management of secretions due to easier suctioning and the ability to change the inner cannula. That is a major benefit for patients with COVID-19. Owing to improved comfort, patients with tracheostomy can be weaned from heavy sedation,5 which has the added benefit of decreased use of the paralytic agents and sedatives that were required in more than 56% of these patients. Moreover, patients with a tracheostomy undergoing ventilator support weaning are more easily able to participate in respiratory therapy, physical therapy, and to sit in a chair, contributing to faster recovery. Although there was a 7% mortality rate, this early mortality report is significantly lower than the 50% to 67% reported in the literature on COVID-19 patients with respiratory failure requiring invasive mechanical respiratory support.4, 5, 6 This finding reflects that our inclusion criteria selected the patients with the highest potential benefit from an early tracheostomy and survival. Indeed, 32 patients (33%) have already been weaned entirely off ventilator support, with 19 (19%) of those patients downsized and 8 (8%) decannulated from their tracheostomy tubes.
No intraprocedural complications were noted, and bleeding was minimized as we always performed ultrasonography with Doppler in the selected access area. Seventy-one patients (72%) who underwent PDT were receiving therapeutic anticoagulation drugs; 5 of those patients (5.1%) had bleeding within the first 48 hours of the procedure that required bedside packing around the tracheostomy, with 1 patient requiring surgical management. Another potential benefit of tracheostomies is the decreased incidence of tracheal stenosis in the subglottic space.11 Two patients already had granulation tissue and subglottic stenosis at the time of bronchoscopy. After discharge from the ICU, all patients are followed by speech pathology and otolaryngology for final decannulation and independent evaluation of long-term complications.
A key finding of this study is that all providers who have participated in the bedside percutaneous tracheostomy procedures remain COVID-19 negative by symptoms or by testing. There was no conversion rate in the four providers (50%) who agreed to testing, and no providers have had any symptoms develop or have missed any work days. All providers wore standard personal protection equipment. We decreased the number of health care providers in the room to three team members, minimized the amount of time with an open-air system to a few seconds, and had no time with active ventilation and aerosolization during the open-air system. Compared with a standard bedside PDT, which has an open-air system with active ventilation and aerosolization through most of the procedure, this modified technique is beneficial for COVID-19 patients because of the high risk for transmission with aerosolization of the virus.12 The benefits to the patient must always be balanced against the risk to the provider; this technique moves toward optimizing the former without unnecessarily risking the latter.
Our paper has strengths and weaknesses. It is the largest report in the literature with 98 COVID-19 patients on mechanical ventilation undergoing PDT. We also demonstrate a high success rate in performing this novel technique, with safe patient and health care provider outcomes and minimal postprocedural complications. In addition, this study is the first to demonstrate how to perform a PDT that mitigates the aerosolization of SARS-CoV-2 during the procedure. Most important, the early outcomes reported support that tracheostomies are beneficial for these patients and that PDT can be safely performed with low health care worker exposure to the virus. There are several flaws, however, including the short duration of this initial report, with only early outcomes being available with an average follow-up of 18 days after starting mechanical ventilation. Also, not all health care workers providers involved both in the procedure and in the postprocedure care have been tested by reverse transcriptase polymerase chain reaction or SARS-CoV-2 antibodies, and a certain percent of COVID-19 infected people are asymptomatic; however, it is impossible to determine the value of a positive test, as daily testing was not feasible and the continuous high level of exposure during the 5 weeks of providing critical care to COVID-19 patients outside the PDT procedures.
The described technique allows optimal COVID-19 patient care in the ICU while minimizing the risk of infection for health care workers. The technique is described in detail and is easy to perform. This study supports that, at early review, both can be achieved. On a larger scale, the earlier weaning from mechanical ventilation may increase the number of available ICU beds and ventilators needed to adequately treat the large demands from these COVID-19 patients. Further follow-up studies on this cohort will evaluate late outcomes of this technique and evaluate its efficacy and value in patients with COVID-19.
Supplementary Data
References
- 1.Center for Systems Science and Engineering. Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html Available at:
- 2.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 in New York City [preprint]. MedRXiv.https://doi.org/10.1101/2020.04.08.20057794, accessed April 16, 2020.
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-2019) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Intensive Care National Audit and Research Center Report on COVID-19 in critical care, April 10, 2020. https://www.icnarc.org/our-audit/audits/cmp/reports Available at:
- 5.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol general procedures and risk of transmission of acute respiratory infections to health care workers: a systematic review. PloS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker N.P., Schiff B.A., Fritz M.A. Tracheotomy recommendations during the COVID-19 pandemic. Airway and Swallowing Committee of the American Academy of Otolaryngology-Head and Neck Surgery. https://www.entnet.org/content/tracheotomy-recommendations-during-covid-19-pandemic Available at:
- 9.Ear Nose Throat United Kingdom (ENTUK) Guidance for surgical tracheostomy and tracheostomy tube change during the COVID-19 pandemic. https://www.entuk.org/tracheostomy-guidance-during-covid-19-pandemic Available at:
- 10.Tong C.C., Kleinberger A.J., Paolino J., Altman K.W. Tracheotomy timing and outcomes in the critically ill. Otolaryngol Head Neck Surg. 2012;147:44–51. doi: 10.1177/0194599812440262. [DOI] [PubMed] [Google Scholar]
- 11.Andriolo B.N., Andriolo R.B., Saconato H., Atallah A.N., Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2015;1:CD007271. doi: 10.1002/14651858.CD007271.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel Z.M., Fernandez-Miranda J., Hwang P.H. Letter: Precautions for endoscopic transnasal skull base surgery during the COVID-19 pandemic. Neurosurg. 2020;87:e66–e67. doi: 10.1093/neuros/nyaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.