Abstract
Background
The ongoing coronavirus disease 2019 pandemic has severely affected the United States. During infectious disease outbreaks, forecasting models are often developed to inform resource utilization. Pregnancy and delivery pose unique challenges, given the altered maternal immune system and the fact that most American women choose to deliver in the hospital setting.
Objective
This study aimed to forecast the first pandemic wave of coronavirus disease 2019 in the general population and the incidence of severe, critical, and fatal coronavirus disease 2019 cases during delivery hospitalization in the United States.
Study Design
We used a phenomenological model to forecast the incidence of the first wave of coronavirus disease 2019 in the United States. Incidence data from March 1, 2020, to April 14, 2020, were used to calibrate the generalized logistic growth model. Subsequently, Monte Carlo simulation was performed for each week from March 1, 2020, to estimate the incidence of coronavirus disease 2019 for delivery hospitalizations during the first pandemic wave using the available data estimate.
Results
From March 1, 2020, our model forecasted a total of 860,475 cases of coronavirus disease 2019 in the general population across the United States for the first pandemic wave. The cumulative incidence of coronavirus disease 2019 during delivery hospitalization is anticipated to be 16,601 (95% confidence interval, 9711–23,491) cases, 3308 (95% confidence interval, 1755–4861) cases of which are expected to be severe, 681 (95% confidence interval, 1324–1038) critical, and 52 (95% confidence interval, 23–81) fatal. Assuming similar baseline maternal mortality rate as the year 2018, we projected an increase in maternal mortality rate in the United States to at least 18.7 (95% confidence interval, 18.0–19.5) deaths per 100,000 live births as a direct result of coronavirus disease 2019.
Conclusion
Coronavirus disease 2019 in pregnant women is expected to severely affect obstetrical care. From March 1, 2020, we forecast 3308 severe and 681 critical cases with about 52 coronavirus disease 2019–related maternal mortalities during delivery hospitalization for the first pandemic wave in the United States. These results are significant for informing counseling and resource allocation.
Key words: coronavirus, COVID-19, forecasting, prediction model
In December 2019, an outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection ensued in Wuhan, Hubei Province, China.1 Since then, COVID-19 has spread across the globe and caused a total of 2,004,701 cases and 126,025 deaths as of April 15, 2020, resulting in a worldwide pandemic.2 In the United States, as of April 15, 2020, there were 615,302 COVID-19 cases and 26,114 deaths in the general population, and these numbers are expected to continue to rise.2
AJOG MFM at a Glance.
Why was this study conducted?
Coronavirus disease (COVID-19) has caused a global pandemic since its first case in December 2019 and is also expected to affect obstetrical care. This is the first study to forecast the impact of COVID-19 on delivery hospitalization.
Key findings
From March 1, 2020, we forecast 3308 severe and 681 critical COVID-19 cases among delivering women in the United States, with about 52 maternal mortalities for the first pandemic wave.
What does this add to what is known?
To the best of our knowledge, there is no existing forecast of the incidence of COVID-19 in pregnancy. Despite its limitations, this study might be able to guide resource allocation.
Infectious disease forecasting is a valuable tool in managing disease outbreaks.3 There are various models utilized in forecasting the incidence of infectious diseases with their benefits and limitations. Phenomenological growth models use early incidence counts to forecast future incidence, which has been found to be useful in forecasting diseases in situations with limited epidemiologic data.4 Previously, these models have been used to forecast the incidence of COVID-19 in several provinces in China.5
Pregnant women experience unique alterations in the immune system and are often more susceptible to severe respiratory infections.6 Furthermore, the unique risks of pregnancy and delivery prompt most women to deliver in the hospital setting, hence increasing exposure to other hospitalized patients and healthcare workers. Limited data are available on COVID-19 and pregnancy, although the available data do not seem to suggest increased severity of the disease among pregnant women.7, 8, 9 Apart from their physiological differences, inpatient management of pregnant women with respiratory diseases can be logistically challenging, given the need for fetal monitoring that is often not available in the intensive care unit (ICU) setting. Given these challenges, to better prepare for the peak of the pandemic, we aimed to forecast the incidence of COVID-19 in the general population and in pregnant women in the United States.
Objective
This study aimed to forecast the first wave of incidence of COVID-19 in the US general population and the incidence of severe, critical, and fatal COVID-19 cases during delivery hospitalization in the United States.
Methods
Coronavirus disease 2019 incidence forecasting model
We utilized a phenomenological model previously used to forecast the incidence of COVID-19 locally in several provinces in China and broadly in the entire country of China.5 , 10 The generalized logistic growth model (GLM) extends the simple logistic growth model to accommodate subexponential growth dynamics with an additional scaling parameter.11 GLM is defined by the differential equation:
where C(t) is the cumulative cases at time t, r is the early growth rate, p is the scaling of growth parameter, and K is the carrying capacity and final epidemic size. Values of p=1 correspond to exponential growth, p=0 represents constant growth, and 0<p<1 defines subexponential growth.
Incidence data of the reported confirmed cases of COVID-19 in the general population in the United States were obtained from the Centers for Disease Control and Prevention (CDC). Data from March 1, 2020, to April 14, 2020, were used to estimate the best-fit solution using nonlinear least squares fitting. A Parametric bootstrap approach was used to generate uncertainty bounds around the best-fit solution assuming a Poisson error structure. Based on this model, we generated the daily incidence of the first wave of COVID-19 in the United States for the first pandemic wave.
Monte Carlo simulation
We reviewed the available published data on incidence of COVID-19 in the general population and its severity based on age group. We used overall data from the United States, Italy, and China because these are the countries with the most data.12, 13, 14, 15, 16 Some of these data contain asymptomatic cases; however, most reported cases were symptomatic. Lowest and highest data from each report were used as the lower and upper bound of the inputs. We incorporated the study by Breslin et al in determining the ranges for pregnancy-specific severe and critical cases, although it was only incorporated into the 20–29 years and 30–39 years age groups to match the population in their study.9 In cases where the age ranges did not match those of our study age group, we assumed equal distribution among each year in an age group and estimated the proportion of our desired age range. From collected literature, critical cases were defined as cases requiring critical care admission.16 , 17 Severe cases were defined as requiring hospitalization in 2 studies that were derived from the general population.16 , 17 However, in only 1 series of pregnant women, severity was defined on the basis of clinical criteria described by Wu et al.9 , 18
We first identified the probability of COVID-19 in females, and then we estimated the incidence of COVID-19 in reproductive-age women (10–49 years) on the basis of age group incidence. Because available evidence does not suggest increased susceptibility, we assumed that the incidence of COVID-19 and the incidence of pregnancy for each age group are independent of each other in the lowest incidence range.7, 8, 9 With this assumption, we calculated the incidence of COVID-19 in pregnancy for each age group according to the multiplicative rule of probability. To account for the possibility of an increased likelihood of COVID-19 during delivery, based on previous reports of severe outcomes in other respiratory viruses, we modified the upper range of delivering women proportion by a factor of 2.87, which was the increased odds ratio of contracting influenza in pregnancy.6 , 19 Our Monte Carlo model inputs are listed in Table 1 .
Table.
Monte Carlo model inputs
| Variable | Base case (%) | Citation |
|---|---|---|
| Proportion of affected women | 40.2–48.6 | 13,16 |
| Proportion of affected by age group (y) | 13,15, 16, 17 | |
| 10–19 | 0.7–5.0 | |
| 20–29 | 3.9–8.1 | |
| 30–39 | 6.7–17.0 | |
| 40–49 | 12.0–19.2. | |
| Proportion of women delivering by age groupa (y) | 19,20 | |
| 10–19 | 0.9–2.6 | |
| 20–29 | 8.3–23.8 | |
| 30–39 | 7.6–21.8 | |
| 40–49 | 5.3–15.2 | |
| Severe cases by age group (y) | 16,17 | |
| 10–19 | 1.6–2.5 | |
| 20–29 | 9.3–20.8 | |
| 30–39 | 9.3–24.8 | |
| 40–49 | 24.8–28.3 | |
| Critical cases by age group (y) | 9,17 | |
| 10–19 | 0 | |
| 20–29 | 2.0–4.7 | |
| 30–39 | 2.0–4.7 | |
| 40–49 | 3.1–7.9 | |
| Fatal cases by age group (y) | 12, 13, 14,17 | |
| 10–19 | 0 | |
| 20–29 | 0.1–0.2 | |
| 30–39 | 0.1–0.2 | |
| 40–49 | 0.4–0.8 |
Putra et al. Forecasting COVID-19 infection in pregnancy. AJOG MFM 2020.
Upper bound was modified using influenza OR in pregnancy 2.87.
The following formulas were used to estimate the outcomes:
iCW refers to the incidence of COVID-19 in women, iCG refers to the incidence of COVID-19 in the general population, and CW refers to COVID-19 in women.
Based on the projected incidence of COVID-19 derived from the GLM model, we performed Monte Carlo simulation for each weekly cumulative incidence of COVID-19 from March 1, 2020. Model inputs with ranges were varied using flat distribution probability in 10,000 trials for each week. Based on the Monte Carlo simulation, we estimated the overall incidence of COVID-19 and incidence of severe COVID-19 requiring extended hospitalization, critical COVID-19, and fatal COVID-19 during delivery hospitalization in the first wave of the pandemic in the US.
Results
Coronavirus disease 2019 forecast in the general population
We generated daily forecasts for the reported incidence of COVID-19 for the first pandemic wave in the United States from April 15, 2020, based on the incidence data from March 1, 2020, to April 14, 2020. Figure 1 presents the reported and cumulative incidence data in the general population. From March 1, 2020, a total of 860,475 COVID-19 cases are expected in the United States during the first wave of the pandemic. Peak daily incidence had occurred during the first 2 weeks of April, and the number of new cases is expected to continue to decline with the number plateauing after the end of June. We emphasize that these projections are for the first wave only, and thus do not represent long-term predictions of cumulative incidence. Daily forecasted incidence of COVID-19 in the United States is available in Supplemental Material 1.
Figure 1.
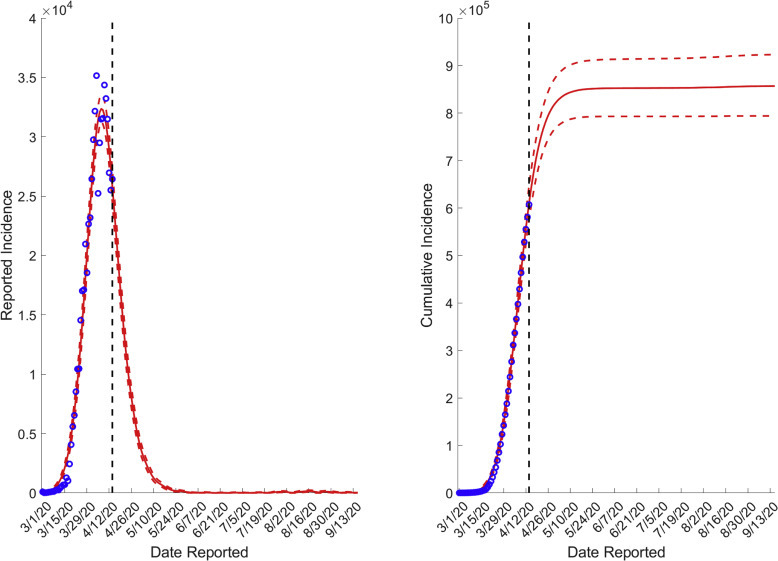
Reported incidence and cumulative incidence of COVID-19 in the general population in the United States, including GLM projections for the first pandemic wave
COVID-19, coronavirus disease 2019.
Putra et al. Forecasting COVID-19 infection in pregnancy. AJOG MFM 2020.
Monte Carlo analysis
Based on the Monte Carlo analysis, we estimated the weekly and cumulative incidence of COVID-19 during delivery in the United States (Figures 2 and 3 ). During the study period, the cumulative incidence was found to be 16,601 (95% confidence interval [CI], 9711–23,491) cases of delivery admission with COVID-19 infection. Among those, 13,308 (95% CI, 1755–4861) cases are expected to be severe and 681 (95% CI, 1324–1038) critical. A total of 52 (95% CI, 23–81) cases of COVID-19–related maternal deaths were expected. Weekly Monte Carlo analysis results are available in Supplemental Material 2.
Figure 2.
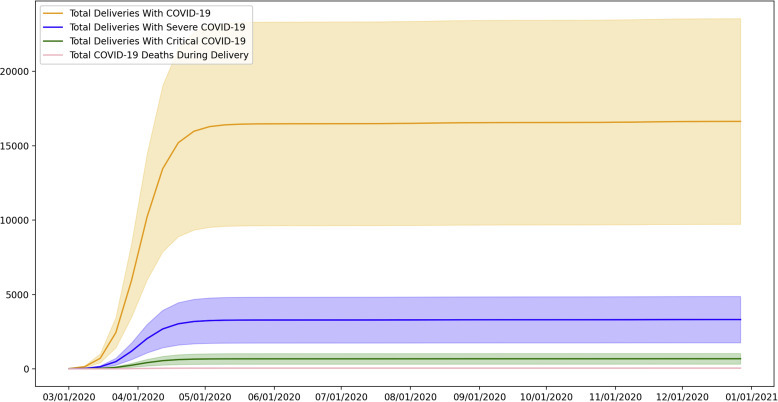
Cumulative incidence of COVID-19 during delivery hospitalization in the United States
COVID-19, coronavirus disease 2019.
Putra et al. Forecasting COVID-19 infection in pregnancy. AJOG MFM 2020.
Figure 3.
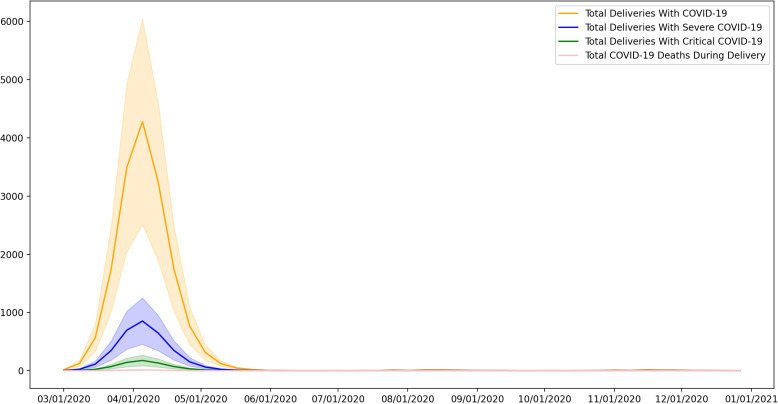
Weekly incidence of COVID-19 during delivery hospitalization in the United States
COVID-19, coronavirus disease 2019.
Putra et al. Forecasting COVID-19 infection in pregnancy. AJOG MFM 2020.
In 2018, there were 3,791,712 deliveries; 658 women died of maternal causes, yielding a maternal mortality rate of 17.4 deaths per 100,000 live births in the United States. Assuming similar number of deliveries and baseline maternal mortality rate in 2020, additional 52 (95% CI, 23–81) cases will result in a maternal mortality rate of 18.7 (95% CI, 18.0–19.5) deaths per 100,000 live births.
During the peak incidence in the first 2 weeks of April, a total of 7718 (95% CI, 4521–10,916) cases were predicted by the model to happen during delivery hospitalization, of which 1538 (95% CI, 818–2259) cases were predicted to be severe, 317 (95% CI, 151–482) cases were critical, and 24 (95% CI, 11–38) cases of COVID-19–related maternal deaths. Assuming weekly deliveries of 72,918, COVID-19 had affected 9.5% of all deliveries during this period.20 Total COVID-19 critical care admission in the first 2 weeks of April will be approximately 2 in 1000 deliveries.
Comment
Principal findings
From March 1, 2020, we projected 3308 severe cases and 681 critical COVID-19 cases with about 52 maternal mortalities during delivery hospitalization for the first wave of the pandemic in the United States. Our study predicted that the United States had experienced the first peak of COVID-19 cases in the first 2 weeks of April both in the general population and among delivering women. This forecast for the peak of incidence was similar to that in data from the Institute for Health Metrics and Evaluation (IHME).21 During this period, more than 50% of expected total hospital beds and resource burden of COVID-19 cases during the first wave had occurred.
Although we do not forecast state-level data, we anticipate the national numbers for delivering women will share a similar bicoastal distribution observed in IHME forecasting model.21 To further help project these data into state-level and local estimates, we have created a simple calculator to estimate the local incidence of COVID-19 in pregnancy. Using this HTML tool, a user can input the expected incidence of COVID-19 during a certain period of time, and the tool will estimate the number of pregnant women with total, severe, and critical COVID-19 cases during that particular time. We also provided a rough estimate of future cases assuming similar trajectory as national trend in the United States. Certainly, future numbers will strongly vary depending on how close a certain region trend correlates to the national trend. This tool is available at bit.ly/COVID19Del. Through this link, readers will be able download the HTML file, and the program will run natively in their web browser.
Strengths and limitations
To the best of our knowledge, this is the first study to estimate the number of COVID-19 severe, critical, and fatal cases during delivery hospitalization in the United States. Our prediction is based on a phenomenological model which is utilized when the epidemiologic characteristics of an outbreak are not clearly delineated as in the case of COVID-19.4 The GLM is a purely empirical model using correlations among observed data to forecast statistically similar trends in the future.22 As opposed to mechanistic models, our model did not directly account for interventions (eg, social distancing or changing in testing pattern). However, this was accounted for indirectly because the pattern of data resulted from intervention done previously would affect the forecasted results. Practically, the model would assume increasing interventions in the first half of the curve and gradual relaxing in the second half of the curve. Nonetheless, abrupt changes in future interventions cannot be accounted for using this model. Further, this model only allows for one peak, so again we emphasize that these projections are for the first wave of the pandemic.
Most of the reported cases in the United States are symptomatic, and we do not yet have the epidemiologic data to forecast specifically for asymptomatic carriers.17 Although asymptomatic cases may fuel the transmission of COVID-19, reported, severe, and critical cases are likely to put stress on healthcare resources. Hence, we opted not to estimate the number of asymptomatic cases.
We only forecasted the incidence of COVID-19 during delivery hospitalization. Given the difficulty in estimating the number of pregnant women across gestational ages, we anticipate the number of pregnant women to be larger than our current estimate. Forecasting the incidence of COVID-19 severe and critical cases are especially challenging given the lack of standardization in testing algorithms and definition of severe and critical cases. Furthermore, the definition of critical and severe could be different in the setting of delivery hospitalizations given the varying monitoring capability of each labor and delivery unit. Although we were able to use all available data of COVID-19 in pregnancy, including the largest case series to date, the lack of a large number of previous studies on COVID-19 in pregnancy makes it difficult to obtain an accurate range in delivering women.7, 8, 9 , 23
Monte Carlo analyses have an inherent limitation that they can only perform according to the accuracy of underlying assumptions. Similar to the phenomenological model, abrupt changes in testing pattern or interventions could affect the performance of this model. Some labor and delivery units are performing universal testing for all admitted women. It is unclear how many units implement this practice; as such, we were not able to incorporate this in the model.24 Universal testing is expected to increase the total number of confirmed cases by adding asymptomatic women. Finally, there are reports suggesting the possibility of resurgence of COVID-19.25 As of now, it is not possible for us to model this because we do not have the epidemiologic data for the resurgence. Therefore, our results are useful for short-term projections and should only be considered with the underlying assumptions and limitations of both the data and models.
Clinical implications
With cases peaking in the beginning of April, we are bracing for the worst impact in obstetrical resource utilization. Although state-level data are not available, the peak incidence is expected to vary by state in relation to severity and mitigation provided by each state.21 With the numbers provided in this study, we hope that we could provide and prioritize obstetrical care providers with appropriate personal protective equipment. This is especially important because nearly all women with COVID-19 will elect to deliver in the hospital setting, regardless of the severity of disease. Thus, obstetrical care providers will be facing a unique and unavoidable exposure to COVID-19. In addition, previous reports have suggested increased risks for preterm birth, preeclampsia, cesarean delivery, and perinatal death.23 , 26 This might suggest that we will also see an increase in resource utilization not directly related to COVID-19 or delivery alone.
Historical data show that 2–4 peripartum critical care admissions occur for every 1000 deliveries, but during the peak of COVID-19, we estimated that an additional of 2 ICU admissions per 1000 deliveries had happened. Although the peak has passed, the number of critical cases will continue to linger and potentially continue to increase the strain in the medical resources. Given the unique changes in physiology and the potential logistical hurdles, critical care for pregnant women with COVID-19 could be challenging. Thus, obstetrical care providers should prepare themselves to provide insight into the multidisciplinary care of critically ill pregnant women.27 The Society for Maternal-Fetal Medicine has graciously provided their obstetrical critical care resources for free through https://education.smfm.org/.
In terms of maternal mortality rate, COVID-19 is projected to increase the maternal mortality modestly from 17.4 deaths to 18.7 deaths per 100,000 live births. However, the actual maternal mortality rate for the year 2020 may be even higher than our projected number. This is because it is possible that resource reallocation, reduction in face-to-face prenatal visits, and COVID-19 economic impact could also cause an increase in maternal mortality. Most recently, United Nations has found an approximately 30% increase in domestic violence against women worldwide.28 Finally, although currently hypothetical, the resurgence of COVID-19 remains a possibility. Another outbreak this winter could further increase the maternal mortality rate. All these factors could result in the highest maternal mortality in modern history.
Research implications
Once epidemiologic parameters of COVID-19 in various circumstances are well defined, a robust mechanistic model could provide a better insight into the possibility and prediction of future outbreaks. Our study was limited by the availability of data from pregnant women with COVID-19. We encourage obstetrical care providers nationally to participate in registries for pregnant women affected by COVID-19. Although we were able to estimate the impact of COVID-19 on delivery hospitalization, the impact of COVID-19 could extend beyond the disease itself. Multiple guidelines have suggested to scale down antenatal testing and prenatal visits.6 , 26 Studies are needed to assess the impact of resource reallocation to non–COVID-19-affected women, including the impact of domestic violence which was known to affect pregnant women at higher rates.29
Conclusion
In summary, COVID-19 is also expected to severely affect obstetrical care. Despite their younger age, we still projected an increase in critical care admission during delivery hospitalization and maternal mortality rate among pregnant women. From March 1, 2020, we projected 3308 severe and 681 critical cases with about 52 COVID-19–related maternal mortalities during delivery hospitalization in the United States during the first pandemic wave. Continuing efforts in mitigating the downstream effects of COVID-19 need to be made to prevent worsening of these projected numbers.
Acknowledgments
The authors thank Wesley Verne for his help in developing the simulation model.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
Cite this article as: Putra M, Kesavan M, Brackney K, et al. Forecasting the impact of coronavirus disease during delivery hospitalization: an aid for resource utilization. Am J Obstet Gynecol MFM 2020;2:100127.
Supplementary Material
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biocomplexity Institute, University of Virginia COVID-19 surveillance dashboard 1.1.2. https://nssac.bii.virginia.edu/covid-19/dashboard/ Available at: Published March 19, 2020. Accessed March 30, 2020.
- 3.Lutz C.S., Huynh M.P., Schroeder M., et al. Applying infectious disease forecasting to public health: a path forward using influenza forecasting examples. BMC Public Health. 2019;19:1659. doi: 10.1186/s12889-019-7966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pell B., Kuang Y., Viboud C., Chowell G. Using phenomenological models for forecasting the 2015 Ebola challenge. Epidemics. 2018;22:62–70. doi: 10.1016/j.epidem.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Roosa K., Lee Y., Luo R., et al. Short-term forecasts of the COVID-19 epidemic in Guangdong and Zhejiang, China: February 13–23, 2020. J Clin Med. 2020;9:596. doi: 10.3390/jcm9020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon L.C., Yang H., Lee J.C.S., et al. ISUOG Interim Guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H., Guo J., Wang C., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslin N., Baptiste C., Gyamfi-Bannerman C., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roosa K., Lee Y., Luo R., et al. Real-time forecasts of the COVID-19 epidemic in China from February 5th to February 24th, 2020. Infect Dis Model. 2020;5:256–263. doi: 10.1016/j.idm.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viboud C., Simonsen L., Chowell G. A generalized-growth model to characterize the early ascending phase of infectious disease outbreaks. Epidemics. 2016;15:27–37. doi: 10.1016/j.epidem.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livingston E., Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4344. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19)-China, 2020. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 14.Onder G., Rezza G., Brusaferro S. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Dowd J.B., Rotondi V., Andriano L., et al. Demographic science aids in understanding the spread and fatality rates of COVID-19. medRxiv. 2020 doi: 10.1073/pnas.2004911117. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verity R., Okell L.C., Dorigatti I., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30243-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) — United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Trushakova S., Kisteneva L., Guglieri-López B., et al. Epidemiology of influenza in pregnant women hospitalized with respiratory illness in Moscow, 2012/2013–2015/2016: a hospital-based active surveillance study. BMC Pregnancy and Childbirth. 2019:19–72. doi: 10.1186/s12884-019-2192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin J., Hamilton B., Osterman M.J.K., Driscoll A.K. Births: Final data for 2018. Natl Vital Stat Rep. 2019;68:1–46. [PubMed] [Google Scholar]
- 21.COVID I., Murray C.J. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator-days and deaths by US state in the next 4 months. medRxiv. 2020 [Epub ahead of print] [Google Scholar]
- 22.Transtrum M.K., Qiu P. Bridging mechanistic and phenomenological models of complex biological systems. PLoS Comput Biol. 2016;12 doi: 10.1371/journal.pcbi.1004915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal Screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020 doi: 10.1056/NEJMc2009316. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020 doi: 10.1126/science.abb5793. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boelig R.C., Saccone G., Bellussi F., Berghella V. MFM Guidance for COVID-19. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeomans E.R., Gilstrap L.C. Physiologic changes in pregnancy and their impact on critical care. Crit Care Med. 2005;33:S256–S258. doi: 10.1097/01.ccm.0000183540.69405.90. [DOI] [PubMed] [Google Scholar]
- 28.UN Women. COVID-19 and Ending Violence Against Women and Girls [press release]. United Nations Publications. Published April 6, 2020.
- 29.Cook J., Bewley S. Acknowledging a persistent truth: domestic violence in pregnancy. J R Soc Med. 2008;101:358–363. doi: 10.1258/jrsm.2008.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


