Abstract
Detection of the SARS-CoV-2 virus in stools and sewage has recently been reported, raising the hypothesis of faecal-oral transmission. If confirmed, this could have far-reaching consequences for public health and for pandemic control strategies. In this paper, we argue that a comprehensive and more nuanced analysis is required to test this hypothesis, taking into consideration both environmental dynamics and the persistence of viral infectivity. First, we examine the evidence regarding the presence of the virus in stools and sewage. Then we discuss the current framework of disease transmission through water and excreta and how the transmission of a respiratory disease fits into it. Against this background, we propose a framework to test the faecal-oral hypothesis, unpacking the different environmental routes from faeces to the mouth of a susceptible person. This framework should not be seen as a confirmation of the hypothesis but rather as an expanded view of its complexities, which could help shaping an agenda for research into a number of unanswered questions. Finally, the paper briefly discusses practical implications, based on current knowledge, for containment of the pandemic.
Keywords: Pandemic, COVID-19, SARS-CoV-2, Water, Sanitation, Faecal-oral transmission
Graphical abstract
Highlights
-
•
Presence of SARS-CoV-2 in stools and wastewater has recently been reported.
-
•
A possible faecal-oral transmission of SARS-CoV-2 has been raised.
-
•
Different routes from faeces to the mouth of a susceptible person exist.
-
•
Water, surfaces and places with vectors can be transmission routes.
-
•
A framework is proposed to help shaping a research agenda.
1. Introduction
The SARS-CoV-2 virus, the etiological agent of COVI-19, is primarily transmitted through respiratory droplets and contact routes (WHO, 2020). This virus uses angiotensin-converting enzyme ACE2 as a receptor to enter human cells (Xu et al., 2020) and ACE2 messenger RNA is highly expressed in the gastrointestinal system (Harmer et al., 2002). Detection of viable SARS-CoV-2 in stools of COVID-19 patients has been reported (Wang et al., 2020; Wu et al., 2020) and virus RNA has been found in sewage (Medema et al., 2020; Ahmed et al., 2020), raising the possibility of faecal-oral transmission.
COVID-19 patients can shed the virus in faeces for days after all respiratory symptoms have disappeared (Wu et al., 2020). However, persistence of viable SARS-Cov-2 in water and sewage has yet to be determined. A study with other coronaviruses demonstrated a 99.9% die-off of 10 days in tap water at 23 °C and over 100 days at 4 °C. In sewage, the time to achieve a 99.9% die-off ranged from 2 to 3 days at 23 °C (Gundy et al., 2009). Persistence of human coronaviruses on surfaces is highly variable (from 2 h to 9 days), depending on temperature, humidity, type of surface, and virus strain (Kampf et al., 2020). Moreover, several human coronaviruses have been shown to be particularly sensitive to water chlorination.
If confirmed, the “faecal-oral hypothesis” for COVID-19 may result in far-reaching consequences for public health and for pandemic control strategies. However, a comprehensive and more nuanced analysis is required to test this hypothesis, taking into consideration both environmental dynamics and the persistence of viral infectivity.
2. Faecal-oral routes of infectious diseases
Environmental classifications of infectious diseases can be traced back to the 1970s and are based on their environmental transmission routes and the life cycle of infectious agents. Those classifications differ from the traditional biological classification of diseases, focused on the etiological agent.
In a seminal study, White et al. (1972) assessed disease transmission routes for water-related hazards and classified them into four categories. Two may be of especial relevance for COVID-19: (i) WATER1: water-borne, in which transmission occurs by ingestion of a pathogen present in water, and water acts as a passive vehicle for the infecting agent; and (ii) WATER2: water-washed, in which infection can be prevented by the provision of sufficient water for domestic and personal hygiene.
Excreta-related diseases are classified into six categories (Feachem et al., 1983). Two may be relevant to the current pandemic. SANITATION1 includes faecal-oral (non-bacterium) infections, characterised by agents of low infective doses, including enteroviruses (polio-, echo-, and coxsackievirus), hepatitis A and rotavirus, which can easily spread when domestic or personal hygiene is inadequate. For this route, excreta disposal has limited effect on the incidence of infections if it is disconnected from thorough changes in personal cleanliness, which requires major improvements in water supply, housing, and health education. Another worrisome route is transmission by excreta-related insect vectors (category SANITATION6). Vectors such as flies and cockroaches circulate in environments where faeces are present and may carry viruses on their body and in their intestinal tract, contaminating surfaces (Dehghani and Kassiri, 2020).
The role of water in the transmission of respiratory infectious diseases was suggested decades after the original classification of water-related diseases. However, the focus was mainly on the protective effect of handwashing (Cairncross, 2003; Fung and Cairncross, 2006). Recently, a fifth category of water-related diseases was proposed (Bartram and Hunter, 2015): WATER5: engineered water system-associated transmission, including inhalation of droplets or aerosols generated in plumbing systems (e.g. Legionella). This category may be of interest for a possible faecal-inhalation route of SARS-CoV-2, since researches have been raising the possibility of virus spread via wastewater plumbing systems, such as the reported spread of SARS coronavirus in sewage aerosols in a 50-storey residential building in Hong Kong (WHO, 2003) and later on controlled experiments in a full-scale plumbing test-rig (Gormley et al., 2020). However, these more recent developments in associating water to respiratory diseases have not fully captured the complex routes possibly involved in SARS-CoV-2 transmission from faeces to the mouth of a susceptible person.
3. A framework for faecal-oral transmission of SARS-CoV-2
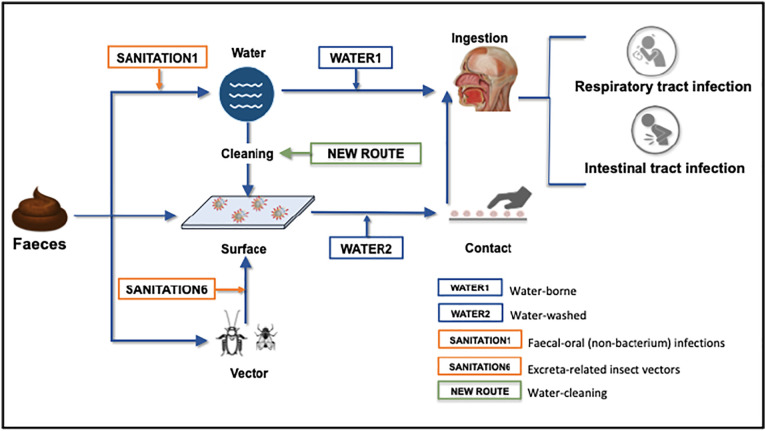
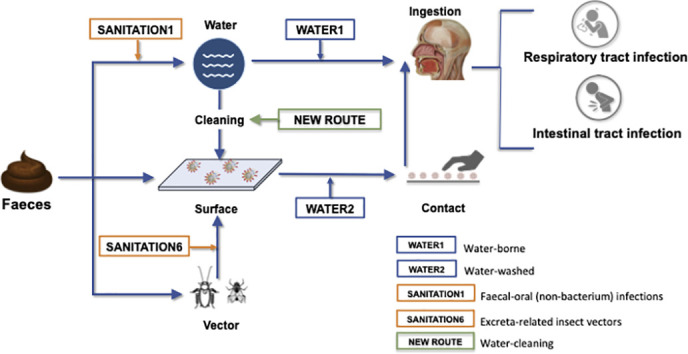
Based on the environmental routes of excreted diseases, and the state-of-knowledge of SARS-CoV-2 persistence and infectiousness, Fig. 1 shows a proposed framework for the faecal-oral hypothesis, unpacking the possible environmental routes from faeces to mouth. This framework should not be seen as a confirmation of this hypothesis but rather as an expanded view of its complexities, which could help shaping an agenda for research into a number of unanswered questions. From faeces, there are three primary routes for the virus: to water, to surfaces or to places where insect vectors are present. From these environments, through different pathways, viruses may reach the mouth and infect both the intestinal and the respiratory tracts of a suceptible host. The four aforementioned environmental categories of water- and excreta-related diseases (WATER1, WATER2, SANITATION1 and SANITATION6) play a role in this framework. Additionally, a fifth category emerges: a “water-cleaning” category, in which contaminated water used to clean surfaces may, via hand contact, bring the virus to the mouth. Validation of this framework will require significant research efforts to better understand SARS-CoV-2 persistence and infectivity in stools, sewage, and untreated water; the role of vectors in transporting the virus, and the appropriate investigation of the “water-cleaning” route.
Fig. 1.
Framework of possible SARS-CoV-2 faecal-oral transmission routes.
4. Conclusions
Due to the current lack of evidence on the relevance of the faecal-oral transmission of SARS-Cov-2, this paper raises the need for more in-depth research to ascertain the actual role of water and sanitation interventions in preventing this route of transmission. If the faecal–oral hypothesis is confirmed, interventions related to the provision of safe water and adequate sanitation should be immediately added to the current strategies for COVID 19 pandemic control, on top of the already recognised key role of water for hand washing. Yet, considering that worldwide 2.2 billion people lack access to safely managed drinking water and 4.2 billion to safely managed sanitation, the possible containment of the COVID-19 through the access to these services is per se a justification to establish immediate measures to mitigate the exposure of people living in the most vulnerable situations to faecal-oral diseases. This at the same time will respond to the call of the 2030 Agenda and reinforce the urgent need for realising the human rights to safe drinking water and sanitation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the Instituto Nacional de Ciência e Tecnologia em Estações Sustentáveis de Tratamento de Esgoto – INCT ETEs Sustentáveis (INCT Sustainable Sewage Treatment Plants) and the Oswaldo Cruz Foundation for creating a stimulating environment for the discussion of the topic.
References
- Ahmed W., Angel N., Edson J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram J., Hunter P. Bradley Classification of disease transmission routes for water-related hazards. In: Bartram J., with Baum R., Coclanis P.A., Gute D.M., Kay D., McFadyen S., Pond K., Robertson W., Rouse M.J., editors. Routledge Handbook of Water and Health. Routledge; London and New York: 2015. [Google Scholar]
- Cairncross S. Editorial: handwashing with soap – a new way to prevent ARIs? Trop. Med. Int. Health. 2003;8:677–679. doi: 10.1046/j.1365-3156.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- Dehghani R., Kassiri H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19) Arch. Clin. Infect. Dis. 2020;15:e102863. doi: 10.5812/archcid.102863. (COVID-19) [DOI] [Google Scholar]
- Feachem R.G., Bradley D.J., Garelick H., Mara D. World Bank Studies in Water Supply and Sanitation; No. 3. John Wiley & Sons; New York, NY: 1983. Sanitation and disease: health aspects of excreta and wastewater management (English)http://documents.worldbank.org/curated/en/704041468740420118/Sanitation-and-disease-health-aspects-of-excreta-and-wastewater-management [Google Scholar]
- Fung I.C.-H., Cairncross S. Effectiveness of handwashing in preventing SARS: a review. Trop. Med. Int. Health. 2006;11:1749–1758. doi: 10.1111/j.1365-3156.2006.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley COVID-19: mitigating transmission via wastewater plumbing systems. Lancet Glob. Health. 2020 doi: 10.1016/S2214-109X(20)30112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundy P., Gerba C.P., Pepper I.L. Survival of coronaviruses in water and wastewater. Food Environ. Virol. 2009;1:10–14. [Google Scholar]
- Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R. Medrxiv; 2020. Presence of SARS-Coronavirus-2 in Sewage. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G., Bradley D., White A. University of Chicago Press; Chicago, IL, USA: 1972. Drawers of Water; p. 1972. [Google Scholar]
- WHO World Health Organization; 2003. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) https://apps.who.int/iris/handle/10665/70863
- WHO Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- Wu Y., Guo C., Tang L., Hong Z. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]