Abstract
COVID-19, the disease caused by the novel Coronavirus, SARS-CoV-2, is increasingly being recognized as a systemic thrombotic and microvascular injury syndrome that may have its roots in complement activation. We had the opportunity to study the placental pathology of five full-term births to COVID-19 patients. All five exhibited histology indicative of fetal vascular malperfusion characterized by focal avascular villi and thrombi in larger fetal vessels. Vascular complement deposition in the placentas was not abnormal, and staining for viral RNA and viral spike protein was negative. While all cases resulted in healthy, term deliveries, these findings indicate the systemic nature of COVID-19 infection. The finding of vascular thrombosis without complement deposition may reflect the systemic nature of COVID-19's procoagulant effects unrelated to systemic complement activation.
Highlights
-
•
This paper explores thrombosis in the placentas COVID-19-positive patients at our hospital
-
•
Potential prothrombotic mechanisms are explored.
-
•
Direct infection of the placentas is ruled out as a cause.
1. Introduction
The severe acute respiratory distress syndrome-associated coronavirus-2 (SARS-CoV-2), etiologic agent of Coronavirus disease 2019 (COVID-19), emerged in December 2019 in Wuhan, China [1]. Now a global pandemic [2], the virus has infected over 2.2million people and claimed the lives of over 150,000 people [3]. The majority of people with COVID-19 have a self-limited illness; however, high mortality rates have been reported in the elderly and certain immune-suppressed populations [[4], [5], [6]]. Although there is evidence of vertical, intrauterine transmission [7,8], propitiously no maternal or neonatal mortalities have been reported to date.
The SARS-CoV-2 virus shares its name with the SARS-CoV virus, which caused the 2002 outbreak in south China, as well as symptomology and a common cellular entry point, angiotensin converting enzyme 2 (ACE2) [[9], [10], [11], [12]]. ACE2 is a zinc metalloprotease involved in the homeostatic balance of the renin-angiotensin-aldosterone axis, and is expressed in a variety of tissues including the nasopharynx, lung, and intestines, accounting for COVID-19's symptomatology of respiratory and digestive distress and diarrhea [4,13]. There are myriad mechanisms working in concert that seed the clinical and pathologic features of COVID-19; this virus is endotheliotropic, damaging endothelium primarily through complement activation and also causing vascular thrombosis [14].
There is emerging body of literature and earlier evidence from the SARS-CoV era that the ACE2 entry mechanism, and the subsequent post entry deactivation of ACE2 plays an important role in COVID19 morbidity [14,15]. The ACE2 loss results in a pathologic increase in Angiotensin II over Angiotensin (1–7) tone systemically that leads, through their respective receptors AT1 and MAS, to complement activation, vasoconstriction, and thrombosis [[16], [17], [18]]. When ACE2 proteins are internalized and destroyed, or the cell hosting many such proteins are destroyed by the virus, the resultant imbalance of Angiotensin II and Angiotensin (1–7) in the blood decreases the activity of endothelial nitrous oxide synthase (eNOS) [19]. eNOS, a potent down-regulator of the production of tissue factor (TF) through nitrous oxide(NO), is also a well-known vasodilator [20]. Another effect of this angiotensin subtype imbalance is increasing NOX-2 activity, which produces radical oxygen species that causes cellular damage [21]. These inflammatory radical oxygen species react with and thereby are a mechanism for disabling NO, further increasing vasoconstriction [22]. The now contracted vessels associated with higher levels of TF and concurrent cellular damage from ROS made by NOX-2 creates a microenvironment conducive for inflammation and thrombosis [23].
The same spike protein that gains entry to cells via ACE2 is suspected to activate the mannose-binding lectin (MBL) complement pathway via MASP-2, just as SARS-CoV was shown to do over a decade ago [24,25]. Complement deposition in major, blood-bathed organ systems can have systemic procoagulant effects. Complement activation product C3a activates platelets [26], and C5a increases the expression and activity of the potent coagulation initiator, tissue factor (TF), in both macrophages and the endothelium [[27], [28], [29]]. Reciprocally, there is the ability of FXa, thrombin, and FIXa to trigger the complement cascade by acting as independent C3 and C5 convertases [29] to create a feed forward mechanism. Uniquely fitting to the proposed MBL-pathway complement activation by SARS-CoV-2, MASP-2 can cleave prothrombin into thrombin [30].
Viral-specific thrombus-promoting pathways aside, the placenta has many defenses against hemorrhage that predispose it to thrombosis, most notably high levels of TF in placental trophoblasts and Plasminogen Activator Inibitor-2 (PAI-2) production. After central nervous system (CNS) astrocytes and ahead of lung alveolar cells, placental trophoblasts are the most densely packed TF-expressing cells [27]. PAI-2 is a procoagulant suicide-inhibitor of tissue plasminogen activator (tPA) that is unique to the placental trophoblasts and to a far lesser extent, macrophages [31]. The inhibitor to fibrinolysis is nearly undetectable before pregnancy, and rises over the gravid course [32]. PAI-2 forms dense polymers in the placenta, and may be fragmented and activated by redox potential [33,34], and an important connection to the previously-discussed effect of Angiotensin II predominance on NOX-2 which produces oxidizing free radicals.
In combination, the inherent thrombophilic state of pregnancy state including increased FVIII and vWF activities [35,36], the prothrombotic effects SARS-CoV-2 infection pathology, and placental physiography and molecular mechanics, provides a logical explanation for why placentas in COVID19 infected patients could potentially be susceptible to thrombus formation, as illustrated in the following 5 cases. Key placental histology is reviewed compared to healthy, normal-vaginal-delivery placental as a control. Components of complement activation and viral staining are explored to see whether or not there is direct infection of the placenta.
2. Materials and methods
2.1. Complement immunohistochemistry
Routine light microscopy and immunohistochemical assessment for the deposition of C5b-9 (membrane attack complex, MAC), C3d, and C4d via a diaminobenzidene technique was conducted on formalin-fixed tissue. Identification of C5b-9, C3d, or C4d within epithelial basement membrane zones, elastic fibers, or the elastic lamina of vessels was considered nonspecific staining. Immunohistochemical (IHC) staining was performed using C3d (Cell Marque, Rocklin CA, 403A-78), C4d (Alpco, Salem NH, BI-RC4d), C5b-9 (Agilent, Santa Clara CA, M077701-5) antibodies on paraffin embedded sections using a modified Leica protocol. Heat-mediated antigen retrieval with Tris-EDTA buffer (pH = 9, epitope retrieval solution 2) was performed for 20 min followed by incubation with each antibody for 15 min. Targeted proteins were detected using an HRP-conjugated compact polymer system and DAB as chromogen. Hematoxylin counterstain was mounted with Leica Micromount. The absence of involvement of these complement components in placental tissue was documented by the author (CMM).
2.2. Spike immunohistochemistry
Our immunohistochemistry protocol has been previously published [37,38]. The five placental cases and five normal controls obtained prior to 2019 were each tested for the covid-19 spike and envelope protein in a blinded fashion (ProSci, Poway, CA). Optimal pretreatment conditions included EDTA antigen retrieval solution (pH 9.0) for 30 min with dilutions of 1:4000 and 1:500, respectively. The analyses were done on the automated Leica Bond platform with the modification that the Enzo Life Sciences peroxidase anti-mouse/rabbit conjugate (catalogue # ADI-950-113-0100) was used in place of the equivalent Leica conjugate as this reduced background [38]. Each placenta yielded a strong signal for CD59 for the internal positive control.
2.3. Viral RNA In-situ hybridization
Our in situ protocol for RNA viruses has been previously published [39]. In brief, the probe and detection kit were provided by ACD (RNAscope 2.5) (Newark, CA) and the assay was done per the manufacturer's recommendations. Positive viral controls for each assay included autopsy tissue from the lung of people who died of covid-19.
3. Results
3.1. Maternal history summary
All 5 placentas from confirmed COVID-19 patients were delivered at full-term (range: 38 to 40 weeks gestational age). Maternal ages ranged from 26 to 40-years-old. Maternal medical histories were largely unremarkable – there were no diagnoses of known thrombotic diseases. Antenatal histories were also unremarkable – there were no cases of gestational hypertension, pre-eclampsia, or diabetes (Table 1). Coagulation tests were sent in only one case (Case # 1), which showed minimal acceleration of APTT and normal PT, INR, and fibrinogen, typical for gravid state.
Table 1.
Patient information for five RT-PCR confirmed COVID-19 mothers.
| Case | GA | Maternal age | Mode of delivery | Medical and antenatal history | Intrapartum course | Laboratory, abnormal only |
|---|---|---|---|---|---|---|
| 1 | 39 | 35 | NSVD | Focal accreta ×2 | Febrile to 38.2 °C | aPTT:26s [27.6–36.6] |
| Long umbilical cord | Albumin 2.4 g/dL [3.2–4.8] |
|||||
| 2 | 38 | 30 | NSVD | Pelvic fracture | Febrile to 38.1 °C | None |
| 3 | 40 | 29 | NSVD | Polycystic Ovary Syndrome, Iron Deficiency Anemia | Unremarkable | None |
| 4 | 39 | 40 | Repeat C-section | Hypothyroidism, C-section | 1088 mL blood loss due to uterine atony | None |
| 5 | 38 | 26 | NSVD | C-section, history of IUFD | Unremarkable | None |
GA – gestational age (w), NSVD – normal spontaneous vaginal delivery, IUFD – intrauterine fetal demise.
Nasopharyngeal swabs were collected and sent for SARS-CoV-2 RT PCR testing in the intrapartum period, and all cases resulted positive. Of the 5 cases, 2 mothers had fevers (max temp: 38.2 C) which resolved following administration of acetaminophen. The mothers were otherwise mostly asymptomatic, with only one endorsing a sore throat and a mild cough. None of the mothers required supplemental oxygen and were discharged without complications. The testing for SARS-CoV-2 in asymptomatic patients was due to direct exposure to a person infected with COVID-19.
3.2. Routine histopathology and complement staining
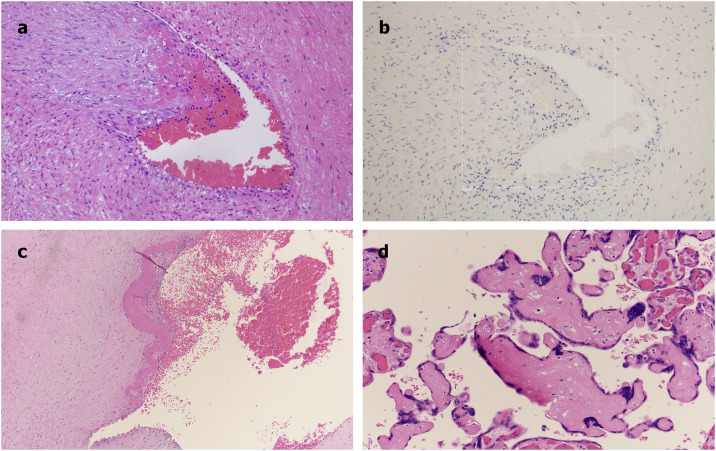
All five cases showed fetal vascular malperfusion. Specific lesions and other pathology are organized in Table 2 . In all cases, there was evidence of thrombosis in larger vessels in the fetal circulation. These thrombosed vessels were found within the chorionic plate and stem villi (Fig. 1a). While there was evidence of complement deposition within the villi and perivillous areas and decidua similar to the normal placental controls, there was no evidence of complement staining within the larger thrombosed vessels (Fig. 1b). Frank thrombosis of fetal chorionic plate vessels was seen in 3 cases while in two cases the larger vessel thrombosis was confined to the stem villi (Fig. 1c). Distal lesions in villi indicative of fetal malperfusion was seen in 2 cases represented in one case as foci of avascular villi (Fig. 1d) and in another case villous stromal-vascular karyorrhexis (not illustrated). Other findings not relative to thrombosis are shown in Table 2.
Table 2.
Histology.
| Case | Histology FVM | Other histological findings |
|---|---|---|
| 1 | Thrombosis, intramural fibrin deposition | Focal increase in perivillous fibrin focal chorangiosis, furcate insertion of umbilical cord |
| 2 | Thrombosis, intramural fibrin deposition | Meconium |
| 3 | Thrombosis, Villous stromal-vascular karyorrhexis | Meconium |
| 4 | Thrombosis intramural fibrin deposition, avascular villi | Meconium |
| 5 | Thrombosis, intramural fibrin deposition |
Fig. 1.
Thrombosis in placental chorionic plate vessels (a). The thrombosed vessel was devoid of complement deposition. Illustrated is C5b-9 stained (b), adluminal thrombus in an artery (c), and downstream effects of occlusion on avascular villi (d).
Five normal control placentas were analyzed in the same manner as the pathologic placentas. None showed fetal vascular malperfusion, avascular villi, nor thrombosis in larger vessels. Complement staining of C3d, C4d, and C5b-9 was found within the decidua and in a number of villi and amidst perivillous fibrin deposition. Overall, the pattern of complement staining was similar between the COVID positive placentas and the COVID negative placentas.
Viral spike protein and viral RNA staining within the COVID-19 placentas was rare, suggesting that direct viral infection of the placentas did not occur and that the effects of thrombosis were due to systemic, not local effects of the virus.
4. Discussion
While perivillous fibrin deposition and intervillous fibrin deposition is normal in every placenta, thrombosed larger vessels in the fetal circulation of delivered placentas is abnormal, as was found in these cases.
Given the prothrombotic nature of the pregnancy state, the high placental expression of TF and PAI-2, and the SARS-CoV-2-related-destruction of ACE2 with the resultant inability of Angiotensin (1–7) to challenge outsized activity of Angiotensin II, placental thrombosis in these 5 patients is not surprising. Since, at the time of writing these patients represent 100% of the confirmed COVID-19 deliveries at New York Presbyterian Hospital, Cornell Campus, we suspect this may be a wide-spread phenomenon among COVID-19 patients. Case 1 featured a long umbilical cord and furcate insertion, which are thrombotic risk factors. None of the other patients had other known causes for thrombotic (VMP) such as cord abnormalities coagulopathies, diabetes or anti-phospholipid syndrome, [40,41].
Though, all five patients delivered without significant morbidity or mortality to them or their newborns, findings of thrombotic fetal vascular malperfusive pathology or placental thrombosis result in placental insufficiency. All patients were approaching term at the time they developed COVID-19; infection earlier in the gestational course may be less benign, potentially leading to placental insufficiency and associated miscarriages or low birth weight infants.
One point of interest in this pandemic has been irregularities to the pattern of mortality in COVID-19 patients. Some young patients do very poorly without predisposing factors, and Italy suffered a particularly high death rate. One potential reason may be mutations in thrombotic pathways or in complement inhibitory factors like complement factor H which are thought to exist in ~1% of the population [42]. Italians have a significant prevalence of a complement factor H mutation that redisposes them to complement activation [43]. The absence of findings in this paper of direct infection or complement deposition in the placentas of patients with non-severe COVID-19 infections is comforting. However, we may not yet have encountered a gravid woman with a predisposing thrombotic or complement regulating mutation that would likely have a more severe presentation.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020 doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong H., Wang Y., Chung H.T., Chen C.J. Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children. Pediatr Neonatol. 2020 doi: 10.1016/j.pedneo.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan T. Novel coronavirus: from discovery to clinical diagnostics. Infect Genet Evol. 2020 doi: 10.1016/j.meegid.2020.104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan M., Wu N.C., Zhu X., Lee C.-C.D., So R.T.Y., Lv H. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020:eabb7269. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J. 2020. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaduganathan M, Vardeny O, Michel T, McMurray JJ V, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020:NEJMsr2005760. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed]
- 16.Murugan D., Lau Y.S., Lau W.C., Mustafa M.R., Huang Y. Angiotensin 1-7 protects against angiotensin II-induced endoplasmic reticulum stress and endothelial dysfunction via mas receptor. PLoS One. 2015 doi: 10.1371/journal.pone.0145413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousif M.H.M., Benter I.F., Diz D.I., Chappell M.C. Angiotensin-(1–7)-dependent vasorelaxation of the renal artery exhibits unique angiotensin and bradykinin receptor selectivity. Peptides. 2017;90:10–16. doi: 10.1016/j.peptides.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava P., Badhwar S., Chandran D.S., Jaryal A.K., Jyotsna V.P., Deepak K.K. Imbalance between Angiotensin II - Angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab Syndr Clin Res Rev. 2019 doi: 10.1016/j.dsx.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Xiao X., Zhang C., Ma X., Miao H., Wang J., Liu L. Angiotensin-(1-7) counteracts angiotensin II-induced dysfunction in cerebral endothelial cells via modulating Nox2/ROS and PI3K/NO pathways. Exp Cell Res. 2015 doi: 10.1016/j.yexcr.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y., Loscalzo J. Regulation of tissue factor expression in human microvascular endothelial cells by nitric oxide. Circulation. 2000;101:2144–2148. doi: 10.1161/01.CIR.101.18.2144. [DOI] [PubMed] [Google Scholar]
- 21.Bendall J.K., Rinze R., Adlam D., Tatham A.L., De Bono J., Channon K.M. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007 doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- 22.Lynch S.M., Frei B., Morrow J.D., Roberts L.J., Xu A., Jackson T. Vascular superoxide dismutase deficiency impairs endothelial vasodilator function through direct inactivation of nitric oxide and increased lipid peroxidation. Arterioscler Thromb Vasc Biol. 1997 doi: 10.1161/01.ATV.17.11.2975. [DOI] [PubMed] [Google Scholar]
- 23.Chu A.J. Tissue factor mediates inflammation. Arch Biochem Biophys. 2005 doi: 10.1016/j.abb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Ip W.K.E., Chan K.H., Law H.K.W., Tso G.H.W., Kong E.K.P., Wong W.H.S. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2005 doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniam S., Jurk K., Hobohm L., Jackel S., Saffarzadeh M., Schwierczek K. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood. 2017 doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu A.J. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:1–30. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruf W. Links between complement activation and thrombosis. Blood. 2019;134 doi: 10.1182/blood-2019-121113. SCI-40-SCI-40. [DOI] [Google Scholar]
- 29.Nilsson H.-L., Gebhard F., Lambris J.D., Klos M., Chen H., Acker B. Complement and coagulation systems molecular intercommunication between the. J Immunol Ref. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krarup A., Wallis R., Presanis J.S., Gál P., Sim R.B. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroder W.A., Le T.T.T., Major L., Street S., Gardner J., Lambley E. A physiological function of inflammation-associated SerpinB2 is regulation of adaptive immunity. J Immunol. 2010;184:2663–2670. doi: 10.4049/jimmunol.0902187. [DOI] [PubMed] [Google Scholar]
- 32.Åstedt* B., Lindoff C., Lecander I. Significance of the plasminogen activator inhibitor of placental type (PAI-2) in pregnancy. Semin Thromb Hemost. 1998;24:431–435. doi: 10.1055/s-2007-996035. [DOI] [PubMed] [Google Scholar]
- 33.Lobov S., Wilczynska M., Bergström F., Johansson L.B., Ny T. Structural bases of the redox-dependent conformational switch in the serpin PAI-2. J Mol Biol. 2004;344:1359–1368. doi: 10.1016/j.jmb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Mikus P., Ny T. Intracellular polymerization of the serpin plasminogen activator inhibitor type 2. J Biol Chem. 1996;271:10048–10053. doi: 10.1074/jbc.271.17.10048. [DOI] [PubMed] [Google Scholar]
- 35.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004 doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Katz D., Beilin Y. Disorders of coagulation in pregnancy. Br J Anaesth. 2015 doi: 10.1093/bja/aev374. [DOI] [PubMed] [Google Scholar]
- 37.Nuovo G., Schwartz Z., Magro C. A comparison of the detection of biomarkers in infections due to low risk versus high-risk human papillomavirus types. Ann Diagn Pathol. 2019 doi: 10.1016/j.anndiagpath.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Nuovo G. False-positive results in diagnostic immunohistochemistry are related to horseradish peroxidase conjugates in commercially available assays. Ann Diagn Pathol. 2016 doi: 10.1016/j.anndiagpath.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Nuovo G.J., de Andrade C.V., Thirukkumaran C., Nicol A.F. Importin-β and exportin-5 are indicators of acute viral infection: correlation of their detection with commercially available detection kits. Ann Diagn Pathol. 2018 doi: 10.1016/j.anndiagpath.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Heider A. Vol. 141. College of American Pathologists; 2017. Fetal vascular malperfusion. Arch. Pathol. Lab. Med; pp. 1484–1489. [DOI] [PubMed] [Google Scholar]
- 41.Shamonki J.M., Salmon J.E., Hyjek E., Baergen R.N. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007 doi: 10.1016/j.ajog.2006.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez-Barricarte R., Pianetti G., Gautard R., Misselwitz J., Strain L., Fremeaux-Bacchi V. The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2007080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonelli F., Frisso G., Testa F., Fiore D., Vitale F., Manitto M.P. Polymorphism p.402Y.H in the complement factor H protein is a risk factor for age related macular degeneration in an Italian population. Br J Ophthalmol. 2006;90:1142–1145. doi: 10.1136/bjo.2006.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]